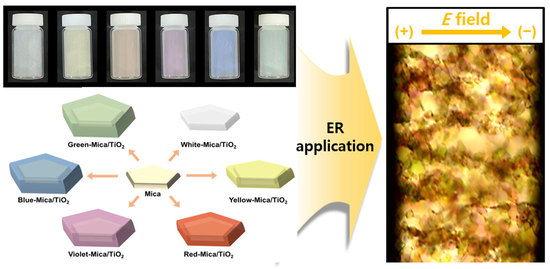
Development of Novel Colorful Electrorheological Fluids
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
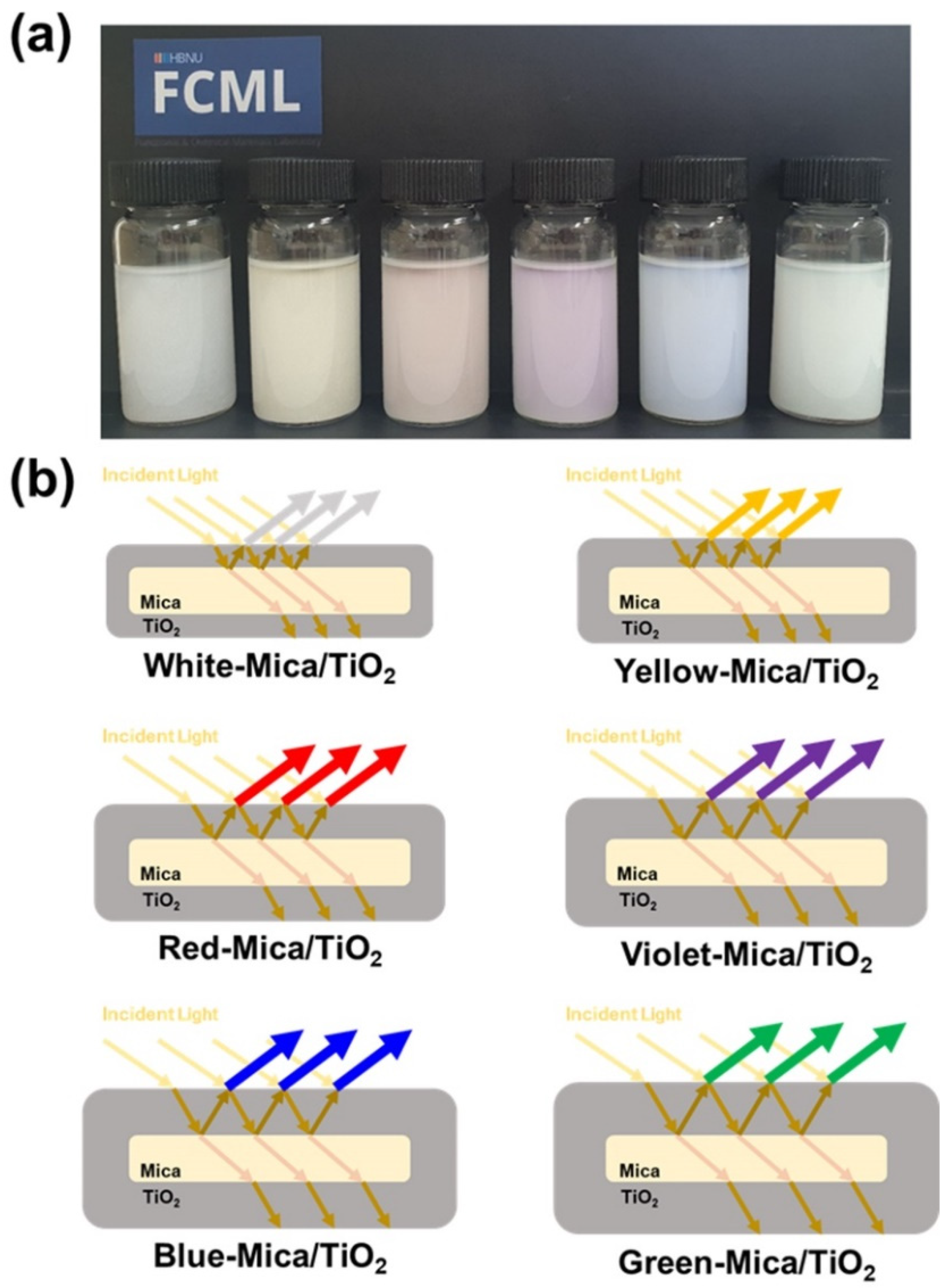
2.2. Synthesis of Color-Mica/TiO2 Materials
2.3. Characterization
2.4. Investigation of ER Properties
3. Results and Discussion
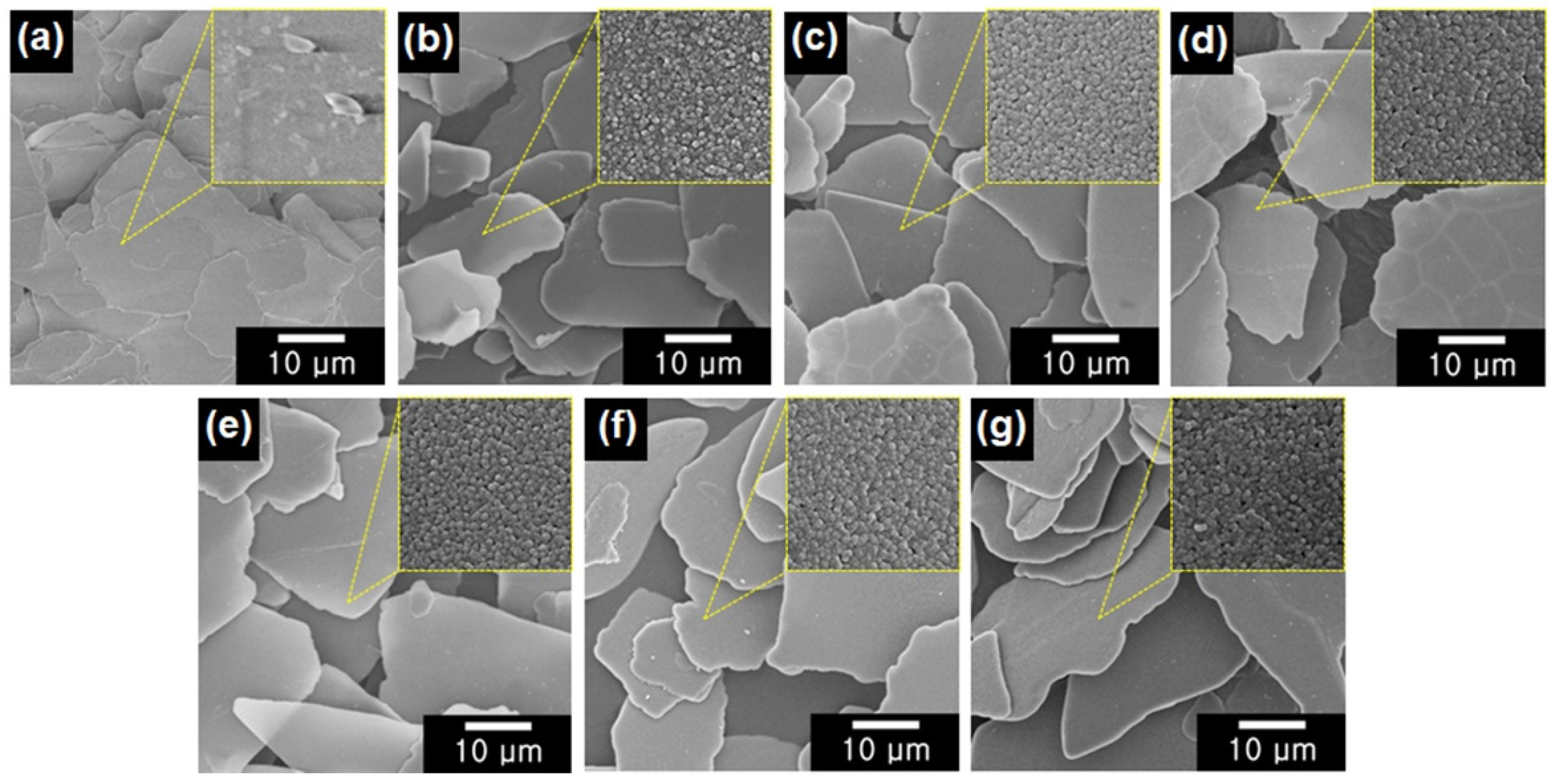
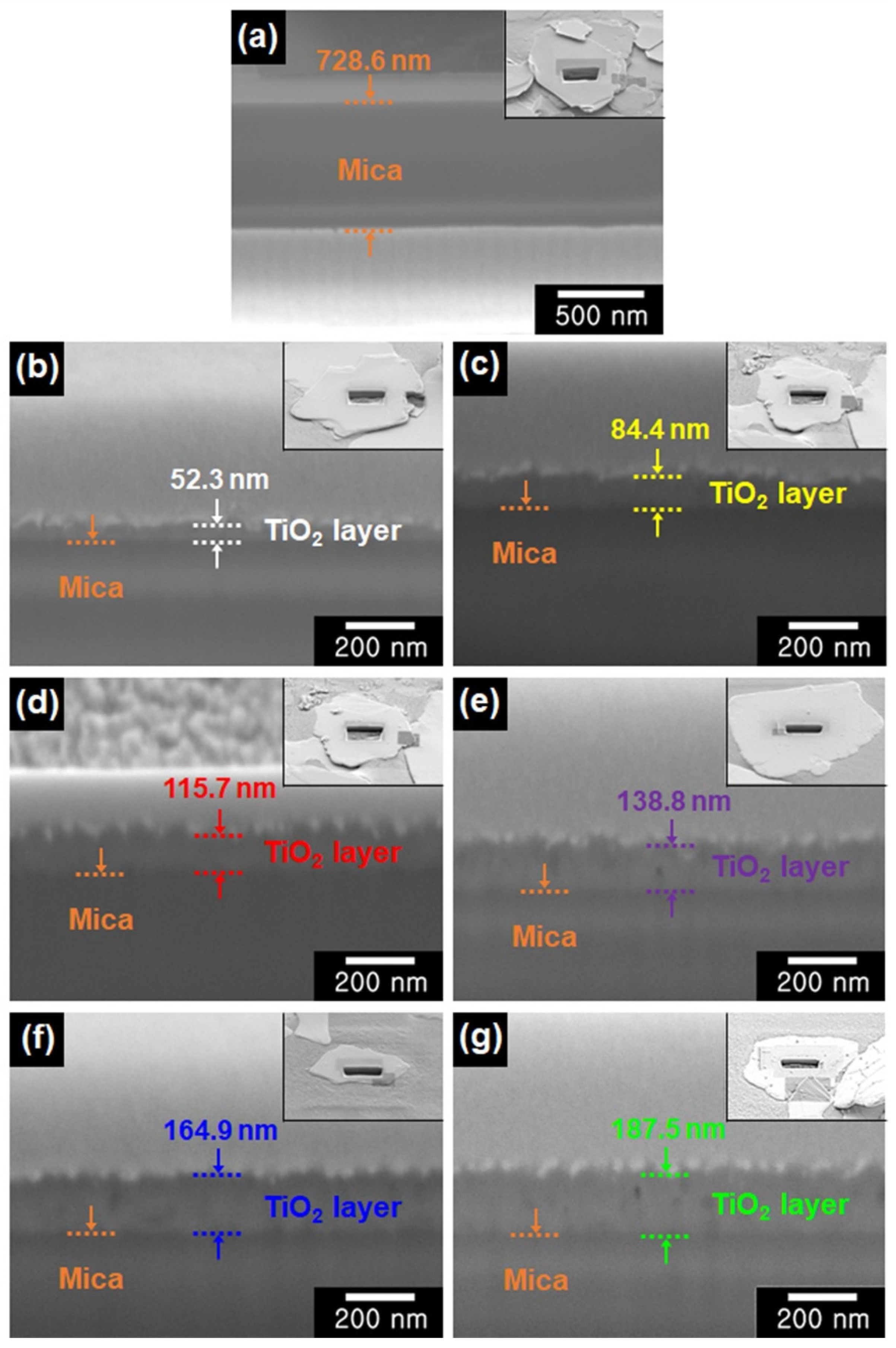
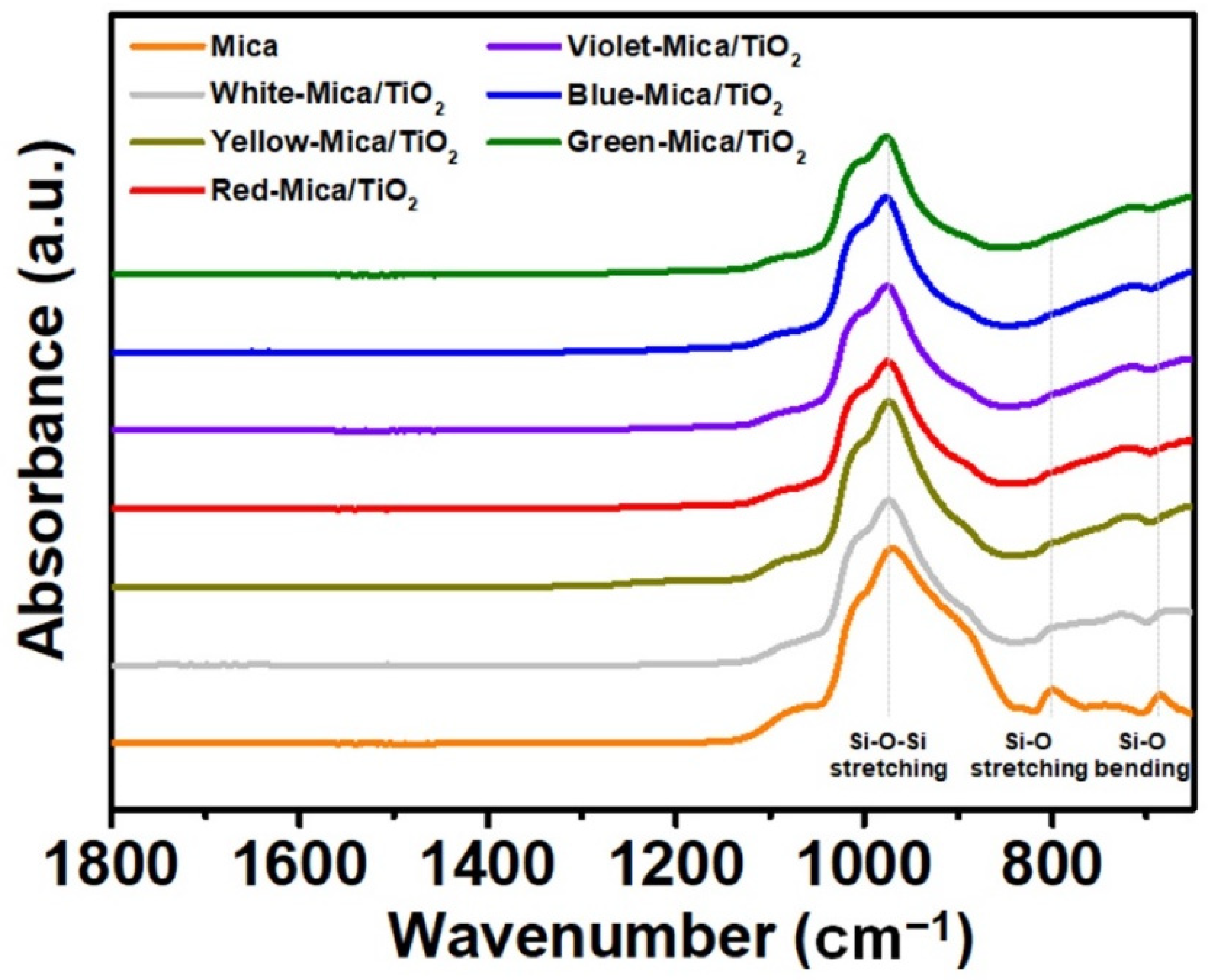
3.1. Structure and Morphology of Color-Mica/TiO2 Materials
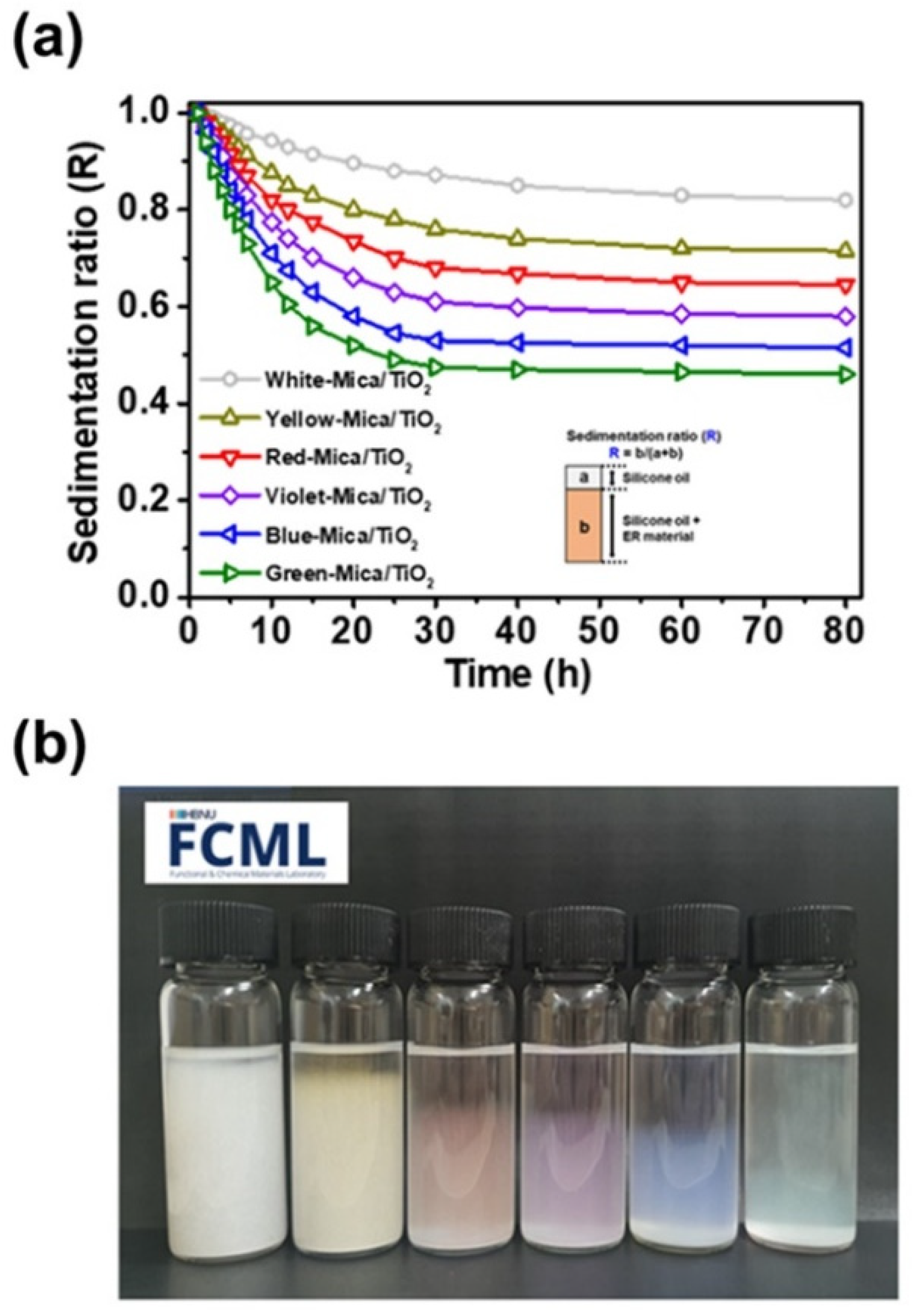
3.2. Suitability Tests of Color-Mica/TiO2 Materials for ER Application
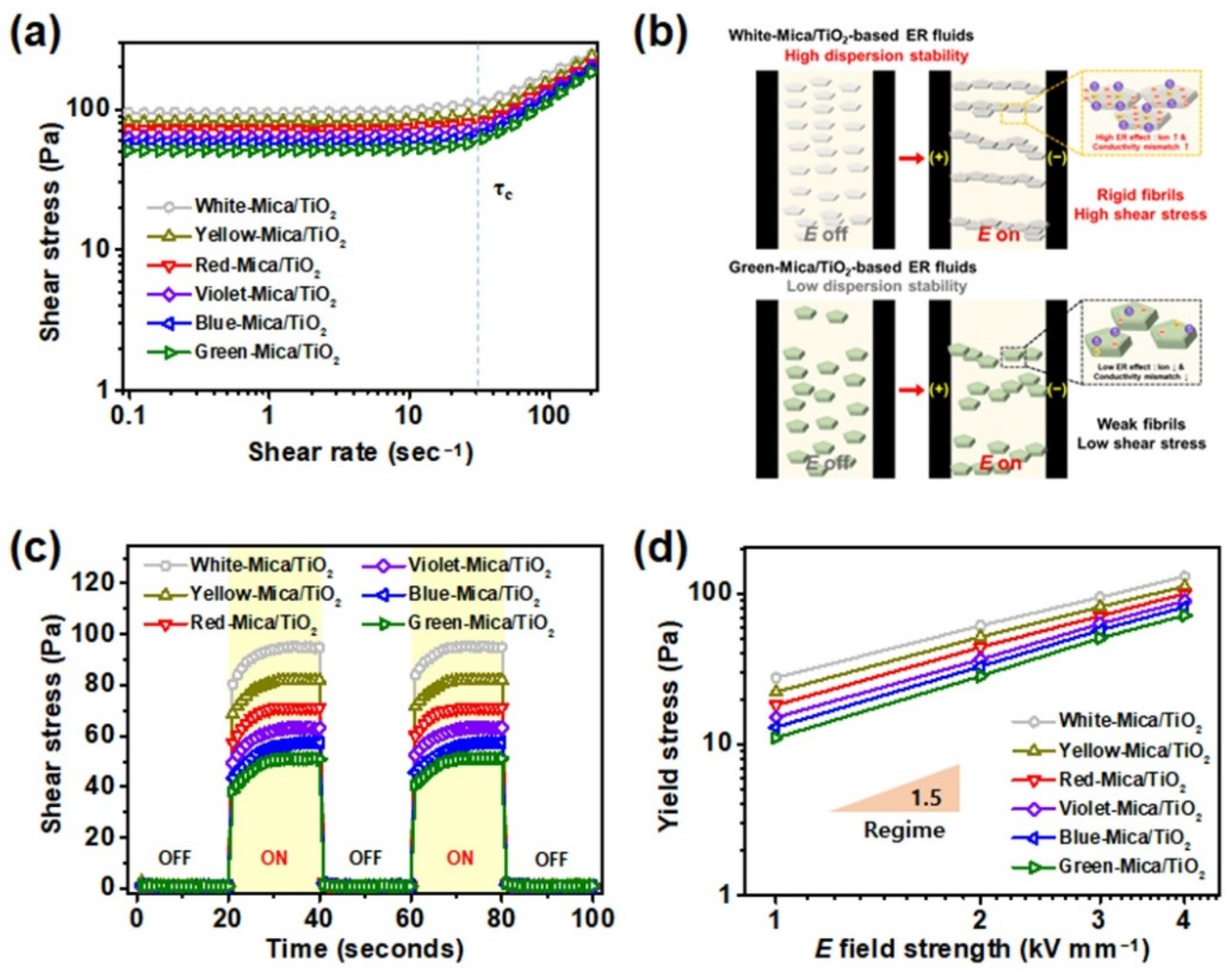
3.3. ER Performances of Color-Mica/TiO2-Based ER Fluids
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lee, S.; Yoon, C.-M.; Hong, J.-Y.; Jang, J. Enhanced electrorheological performance of a graphene oxide-wrapped silica rod with a high aspect ratio. J. Mater. Chem. C 2014, 2, 6010–6016. [Google Scholar] [CrossRef]
- Negita, K.; Misono, Y.; Yamaguchi, T.; Shinagawa, J. Dielectric and Electrical Properties of Electrorheological Carbon Suspensions. J. Colloid Interface Sci. 2008, 321, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Hao, T. Electrorheological Fluids. Adv. Mater. 2001, 13, 1847–1857. [Google Scholar] [CrossRef]
- Wu, J.; Xu, G.; Cheng, Y.; Liu, F.; Guo, J.; Cui, P. The Influence of High Dielectric Constant Core on the Activity of Core-Shell Structure Electrorheological Fluid. J. Colloid Interface Sci. 2012, 378, 36–43. [Google Scholar] [CrossRef]
- Zhang, Y.; Lu, K.; Rao, G.; Tian, Y.; Zhang, S.; Liang, J. Electrorheological fluid with an extraordinarily high yield stress. Appl. Phys. Lett. 2002, 80, 880–890. [Google Scholar] [CrossRef]
- Yoon, C.-M.; Lee, S.; Hong, S.H.; Jang, J. Fabrication of Density-Controlled Graphene Oxide-Coated Mesoporous Silica Spheres and Their Electrorheological Activity. J. Colloid Interface Sci. 2015, 438, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Mclntyre, C.; Yang, H.; Green, P.F. Electrorheology of Suspensions Containing Interfacially Active Constituents. ACS Appl. Mater. Interfaces 2013, 5, 8925–8931. [Google Scholar] [CrossRef]
- Liu, Y.D.; Quan, X.; Hwang, B.; Kwon, Y.K.; Choi, H.J. Core-Shell-Structured Monodisperse Copolymer/Silica Particle Suspension and Its Electrorheological Response. Langmuir 2014, 30, 1729–1734. [Google Scholar] [CrossRef]
- Linzhi, L.I.; Shujuan, G.A.O. Polyaniline (PANI) and BaTiO3 Composite Nanotube with High Suspension Performance in Electrorheological Fluid. Mater. Today Commun. 2020, 24, 100993. [Google Scholar] [CrossRef]
- Kutalkova, E.; Mrlik, M.; Ilcikova, M.; Osicka, J.; Sedlacik, M.; Mosnacek, J. Enhanced and Tunable Electrorheological Capability Using Surface Initiated Atom Transfer Radical Polymerization Modification with Simultaneous Reduction of the Graphene Oxide by Silyl-Based Polymer Grafting. Nanomaterials 2019, 9, 308. [Google Scholar] [CrossRef] [Green Version]
- Kamelreiter, M.; Kemmetmüller, W.; Kugi, A. Digitally Controlled Electrorheological Valves and Their Application in Vehicle Dampers. Mechatronics 2012, 22, 629–638. [Google Scholar] [CrossRef]
- Gong, X.; Wu, J.; Huang, X.; Wen, W.; Sheng, P. Influence of Liquid Phase on Nanoparticle-Based Giant Electrorheological Fluid. Nanotechnology 2008, 19, 165602. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Song, Z.; Liu, F.; Guo, J.; Cheng, Y.; Ma, S.; Xu, G. Giant Electrorheological Fluids with Ultrahigh Electrorheological Efficiency Based on a Micro/Nano Hybrid Calcium Titanyl Oxalate Composite. NPG Asia Mater. 2016, 8, e322. [Google Scholar] [CrossRef]
- Noh, J.; Yoon, C.-M.; Jang, J. Enhanced Electrorheological Activity of Polyaniline Coated Mesoporous Silica with High Aspect Ratio. J. Colloid Interface Sci. 2016, 470, 237–244. [Google Scholar] [CrossRef]
- Noh, J.; Hong, S.; Yoon, C.-M.; Lee, S.; Jang, J. Dual External Field-Responsive Polyaniline-Coated Magnetite/Silica Nanoparticles for Smart Fluid Applications. Chem. Commun. 2017, 53, 6645–6648. [Google Scholar] [CrossRef]
- Yoon, C.-M.; Jang, Y.; Noh, J.; Kim, J.; Jang, J. Smart Fluid System Dually Responsive to Light and Electric Fields: An Electrophotorheological Fluid. ACS Nano 2017, 11, 9789–9801. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zhao, X. Core/Shell Nanocomposite Based on the Local Polarization and Its Electrorheological Behavior. Langmuir 2005, 21, 6553–6559. [Google Scholar] [CrossRef]
- Yoon, C.-M.; Jang, Y.; Noh, J.; Kim, J.; Lee, K.; Jang, J. Enhanced Electrorheological Performance of Mixed Silica Nanomaterial Geometry. ACS Appl. Mater. Interfaces 2017, 9, 36358–36367. [Google Scholar] [CrossRef]
- Plachy, T.; Sedlacik, M.; Pavlinek, V.; Trchová, M.; Morávková, Z.; Stejskal, J. Carbonization of aniline oligomers to electrically polarizable particles and their use in electrorheology. Chem. Eng. J. 2014, 256, 398–406. [Google Scholar] [CrossRef]
- Yoon, C.-M.; Lee, K.; Noh, J.; Lee, S.; Jang, J. Electrorheological Performance of Multigram-Scale Mesoporous Silica Particles with Different Aspect Ratios. J. Mater. Chem. C 2016, 4, 1713–1719. [Google Scholar] [CrossRef]
- Shin, K.-Y.; Lee, S.; Hong, S.; Jang, J. Graphene Size Control via a Mechanochemical Method and Electroresponsive Properties. ACS Appl. Mater. Interfaces 2014, 6, 5531–5537. [Google Scholar] [CrossRef] [PubMed]
- Yoon, C.-M.; Lee, G.; Noh, J.; Lee, C.; Cheong, O.J.; Jang, J. A Comparative Study of the Electrorheological Properties of Various N-Doped Nanomaterials Using Ammonia Plasma Treatment. Chem. Commun. 2016, 52, 4808–4811. [Google Scholar] [CrossRef] [PubMed]
- Yoon, C.-M.; Noh, J.; Jang, Y.; Jang, J. Fabrication of a Silica/Titania Hollow Nanorod and Its Electroresponsive Activity. RSC Adv. 2017, 7, 19754–19763. [Google Scholar] [CrossRef]
- Kim, S.; Kim, C.; Hong, J.-Y.; Hwang, S.H. Enhanced electroheological performance of barium-doped SiO2/TiO2 hollow mesoporous nanospheres. RSC Adv. 2014, 4, 6821–6824. [Google Scholar] [CrossRef]
- Yoon, C.-M.; Jang, Y.; Lee, S.; Jang, J. Dual Electric and Magnetic Responsivity of Multilayered Magnetite-Embedded Core/Shell Silica/Titania Nanoparticles with Outermost Silica Shell. J. Mater. Chem. C 2018, 6, 10241–10249. [Google Scholar] [CrossRef]
- Chen, J.-F.; Wang, J.-X.; Liu, R.-J.; Shao, L.; Wen, L.-X. Synthesis of Porous Silica Structures with Hollow Interiors by Templating Nanosized Calcium Carbonate. Inorg. Chem. Commun. 2004, 7, 447–449. [Google Scholar] [CrossRef]
- Yin, J.; Zhao, X. Titanate Nano-Whisker Electrorheological Fluid with High Suspended Stability and ER Activity. Nanotechnology 2006, 17, 192–196. [Google Scholar] [CrossRef]
- Hua, W.; Kar, P.; Roy, P.; Bu, L.; Shoute, L.C.T.; Kumar, P.; Shankar, K. Resistance of Superhydrophobic Surface-Functionalized TiO2 Nanotubes to Corrosion and Intense Cavitation. Nanomaterials 2018, 8, 783. [Google Scholar] [CrossRef]
- Wei, M.; Li, Z.; Chen, P.; Sun, L.; Kang, S.; Dou, T.; Qu, Y.; Jing, L. N-Rich Doped Anatase TiO2 with Smart Defect Engineering as Efficient Photocatalysts for Acetaldehyde Degradation. Nanomaterials 2022, 12, 1564. [Google Scholar] [CrossRef]
- Fan, J.; Wang, T.; Wu, B.; Wang, C. Highly Active Amino-Fullerene Derivative-Modified TiO2 for Enhancing Formaldehyde Degradation Efficiency under Solar-Light Irradiation. Nanomaterials 2022, 12, 2366. [Google Scholar] [CrossRef]
- Oh, S.Y.; Oh, M.K.; Kang, T.J. Characterization and Electrorheological Response of Silica/Titania-Coated MWNTs Synthesized by Sol-Gel Process. Colloids Surfaces A Physicochem. Eng. Asp. 2013, 436, 354–362. [Google Scholar] [CrossRef]
- Yin, J.B.; Zhao, X.P. Electrorheological Fluids Based on Glycerol-Activated Titania Gel Particles and Silicone Oil with High Yield Strength. J. Colloid Interface Sci. 2003, 257, 228–236. [Google Scholar] [CrossRef]
- Choi, M.; Kim, C.; Jeon, S.O.; Yook, K.S.; Lee, J.Y.; Jang, J. Synthesis of titania embedded silica hollow nanospheres via sonication mediated etching and re-deposition. Chem. Commun. 2011, 47, 7092–7094. [Google Scholar] [CrossRef] [PubMed]
- Yoon, C.-M.; Lee, S.; Cheong, O.J.; Jang, J. Enhanced Electroresponse of Alkaline Earth Metal-Doped Silica/Titania Spheres by Synergetic Effect of Dispersion Stability and Dielectric Property. ACS Appl. Mater. Interfaces 2015, 7, 18977–18984. [Google Scholar] [CrossRef]
- Jang, Y.; Yoon, C.-M.; Kim, S.; Lee, I.; Jang, J. Single/Dual Alkaline Earth Metal-Doped Hollow Nanoparticles as Nanocarrier for Accelerating Neurite Development by Activating pERK and pJNK. Part. Part. Syst. Char. 2018, 35, 1800132. [Google Scholar] [CrossRef]
- Tian, Y.; Meng, Y.; Wen, S. Electrorheology of a zeolite/silicone oil suspension under dc fields. J. Appl. Phys. 2001, 90, 493–496. [Google Scholar] [CrossRef]
- Ma, N.; Zhang, Z.; Dong, X.; Wang, Q.; Niu, C.; Han, B. Dynamic viscoelasticity and phenomenological model of electrorheological elastomers. J. Appl. Polym. Sci. 2017, 134, 45407. [Google Scholar] [CrossRef]
- Méheust, Y.; Parmar, K.P.S.; Schjelderupsen, B.; Fossum, J.O. The electrorheology of suspensions consisting of Na-Fluorohectorite synthetic clay particles in silicon oil. J. Rheol. 2011, 55, 809–833. [Google Scholar] [CrossRef]
- Ohta, S.-I. Synthetic Mica and Its Applications. J-Stage 2006, 12, 119–124. [Google Scholar]
- Ahmad, R.; Mirza, A. Application of Xanthan gum/ n-acetyl cysteine modified mica bionanocomposite as an adsorbent for the removal of toxic heavy metals. Groundw. Sustain. Dev. 2018, 7, 101–108. [Google Scholar] [CrossRef]
- Ke, S.; Chen, C.; Fu, N.; Zhou, H.; Ye, M.; Lin, P.; Yuan, W.; Zeng, X.; Chen, L.; Huang, H. Transparent Indium Tin Oxide Electrodes on Muscovite Mica for High-Temperature-Processed Flexible Optoelectronic Devices. ACS Appl. Mater. Interfaces 2016, 8, 28406–28411. [Google Scholar] [CrossRef] [PubMed]
- Junru, T.; Yunfang, H.; Wenxiang, H.; Xiuzeng, C.; Xiansong, F. The preparation and characteristics of cobalt blue mica coated titania pearlescent pigment. Dyes Pigm. 2002, 52, 215–222. [Google Scholar] [CrossRef]
- Junru, T.; Xiansong, F.; Wenxiang, H.; Xiuzeng, C.; Li, W. The preparation and characteristics of a multi-cover-layer type, blue mica titania, pearlescent pigment. Dyes Pigm. 2003, 56, 93–98. [Google Scholar] [CrossRef]
- Guillén, I.; Marco, J.; Gutierrez, D.; Jakob, W.; Jarabo, A. A General Framework for Pearlescent Materials. ACM Trans. Graph. 2020, 39, 253. [Google Scholar] [CrossRef]
- Nadal, M.E.; Early, E.A. Color Measurements for Pearlescent Coatings. Color Res. Appl. 2002, 29, 38–42. [Google Scholar] [CrossRef]
- Bayat, N.; Baghshahi, S.; Alizadeh, P. Synthesis of white pearlescent pigments using the surface response method of statistical analysis. Ceram. Int. 2008, 34, 2029–2035. [Google Scholar] [CrossRef]
- Du, H.; Liu, C.; Sun, J.; Chen, Q. An investigation of angle-dependent optical properties of multi-layer structure pigments formed by metal-oxide-coated mica. Powder. Technol. 2008, 185, 291–296. [Google Scholar] [CrossRef]
- Cho, K.-D.; Lee, I.; Han, J.-H. Dynamic Characteristics of ER Fluid-filled Composite Plate Using Multielectrode Configuration. J. Intel. Mat. Syst. Str. 2005, 16, 411–419. [Google Scholar] [CrossRef]
- Bailey, A.I.; Kay, S.M. Measurement of refractive index and dispersion of mica, employing multiple beam interfaces techniques. Brit. J. Appl. Phys. 1965, 16, 39–44. [Google Scholar] [CrossRef]
- Taherniya, A.; Raoufi, D. The annealing temperature dependence of anatase TiO2 thin films prepared by the electron-beam evaporation method. Semicond. Sci. Technol. 2016, 31, 125012. [Google Scholar] [CrossRef]
- Chen, J.; Lu, H. Generalized Laws of Reflection and Refraction from Real Valued Boundary Conditions. Opt. Commun. 2011, 284, 3802–3807. [Google Scholar] [CrossRef]
- Luhn, S.; Hentschel, M. Analytical Fresnel Laws for Curved Dielectric Interfaces. J. Opt. 2020, 22, 015605. [Google Scholar] [CrossRef]
- Guan, W.; Ji, F.; Chen, Q.; Yan, P.; Pei, L. Synthesis and Enhanced Phosphate Recovery Property of Porous Calcium Silicate Hydrate Using Polyethyleneglycol as Pore-Generation Agent. Materials 2013, 6, 2846–2861. [Google Scholar] [CrossRef] [PubMed]
- Busigny, V.; Cartigny, P.; Philippot, P.; Javoy, M. Ammonium quantification in muscovite by infrared spectroscopy. Chem. Geol. 2003, 198, 21–31. [Google Scholar] [CrossRef]
- Anbalagan, G.; Prabakaran, A.R.; Gunasekaran, S. Spectroscopic Characterization of Indian Standard Sand. J. Appl. Spectrosc. 2010, 77, 86–94. [Google Scholar] [CrossRef]
- Cho, M.S.; Cho, Y.H.; Choi, H.J.; Jhon, M.S. Synthesis and Electrorheological Characteristics of Polyaniline-Coated Poly(methyl methacrylate) Microsphere: Size Effect. Langmuir 2003, 19, 5875–5881. [Google Scholar] [CrossRef]
- Lee, H.-J.; Chin, D.B.; Yang, S.-M.; Park, O.O. Surfactant Effect on the Stability and Electrorheological Properties of Polyaniline Particle Suspension. J. Colloid Interface Sci. 1998, 206, 424–438. [Google Scholar] [CrossRef]
- Liu, Y.D.; Fang, F.F.; Choi, H.J. Core-Shell Structured Semiconducting PMMA/Polyaniline Snowman-like Anisotropic Microparticles and Their Electrorheology. Langmuir 2010, 26, 12849–12854. [Google Scholar] [CrossRef]
- Seo, Y.P.; Choi, H.J.; Seo, Y. Analysis of the flow behavior of electrorheological fluids with the aligned structure reformation. Polymer 2011, 52, 5695–5698. [Google Scholar] [CrossRef]
- Kim, M.H.; Choi, H.J. Core-shell structured semiconducting poly(diphenylamine)-coated polystyrene microspheres and their electrorheology. Polymer 2017, 131, 120–131. [Google Scholar] [CrossRef]


| Samples | Elements (Atomic %) | |
|---|---|---|
| Si | Ti | |
| Mica | 100.0 | - |
| white mica/TiO2 | 79.0 | 21.0 |
| yellow mica/TiO2 | 68.5 | 31.5 |
| red mica/TiO2 | 59.0 | 41.0 |
| violet mica/TiO2 | 50.8 | 49.2 |
| blue mica/TiO2 | 44.3 | 55.7 |
| green mica/TiO2 | 38.1 | 61.9 |
| Sample | Ion Concentration (ppm) | |
|---|---|---|
| Na+ | Ca2+ | |
| white mica/TiO2 | 21.8 | 13.2 |
| yellow mica/TiO2 | 16.5 | 8.2 |
| red mica/TiO2 | 12.1 | 5.8 |
| violet mica/TiO2 | 11.0 | 2.7 |
| blue mica/TiO2 | 9.0 | 1.3 |
| green mica/TiO2 | 7.6 | 0.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jekal, S.; Kim, J.; Lu, Q.; Kim, D.-H.; Noh, J.; Kim, H.-Y.; Kim, M.-J.; Kim, M.-S.; Oh, W.-C.; Choi, H.-J.; et al. Development of Novel Colorful Electrorheological Fluids. Nanomaterials 2022, 12, 3113. https://doi.org/10.3390/nano12183113
Jekal S, Kim J, Lu Q, Kim D-H, Noh J, Kim H-Y, Kim M-J, Kim M-S, Oh W-C, Choi H-J, et al. Development of Novel Colorful Electrorheological Fluids. Nanomaterials. 2022; 12(18):3113. https://doi.org/10.3390/nano12183113
Chicago/Turabian StyleJekal, Suk, Jiwon Kim, Qi Lu, Dong-Hyun Kim, Jungchul Noh, Ha-Yeong Kim, Min-Jeong Kim, Min-Sang Kim, Won-Chun Oh, Hyoung-Jin Choi, and et al. 2022. "Development of Novel Colorful Electrorheological Fluids" Nanomaterials 12, no. 18: 3113. https://doi.org/10.3390/nano12183113
APA StyleJekal, S., Kim, J., Lu, Q., Kim, D.-H., Noh, J., Kim, H.-Y., Kim, M.-J., Kim, M.-S., Oh, W.-C., Choi, H.-J., & Yoon, C.-M. (2022). Development of Novel Colorful Electrorheological Fluids. Nanomaterials, 12(18), 3113. https://doi.org/10.3390/nano12183113