Facile Synthesis, Characterization, and Photocatalytic Evaluation of In2O3/SnO2 Microsphere Photocatalyst for Efficient Degradation of Rhodamine B
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Photocatalysts
2.3. Characterization of Photocatalysts
2.4. Photocatalytic Activity Tests
2.5. Photocurrent Experiments
3. Results and Discussion
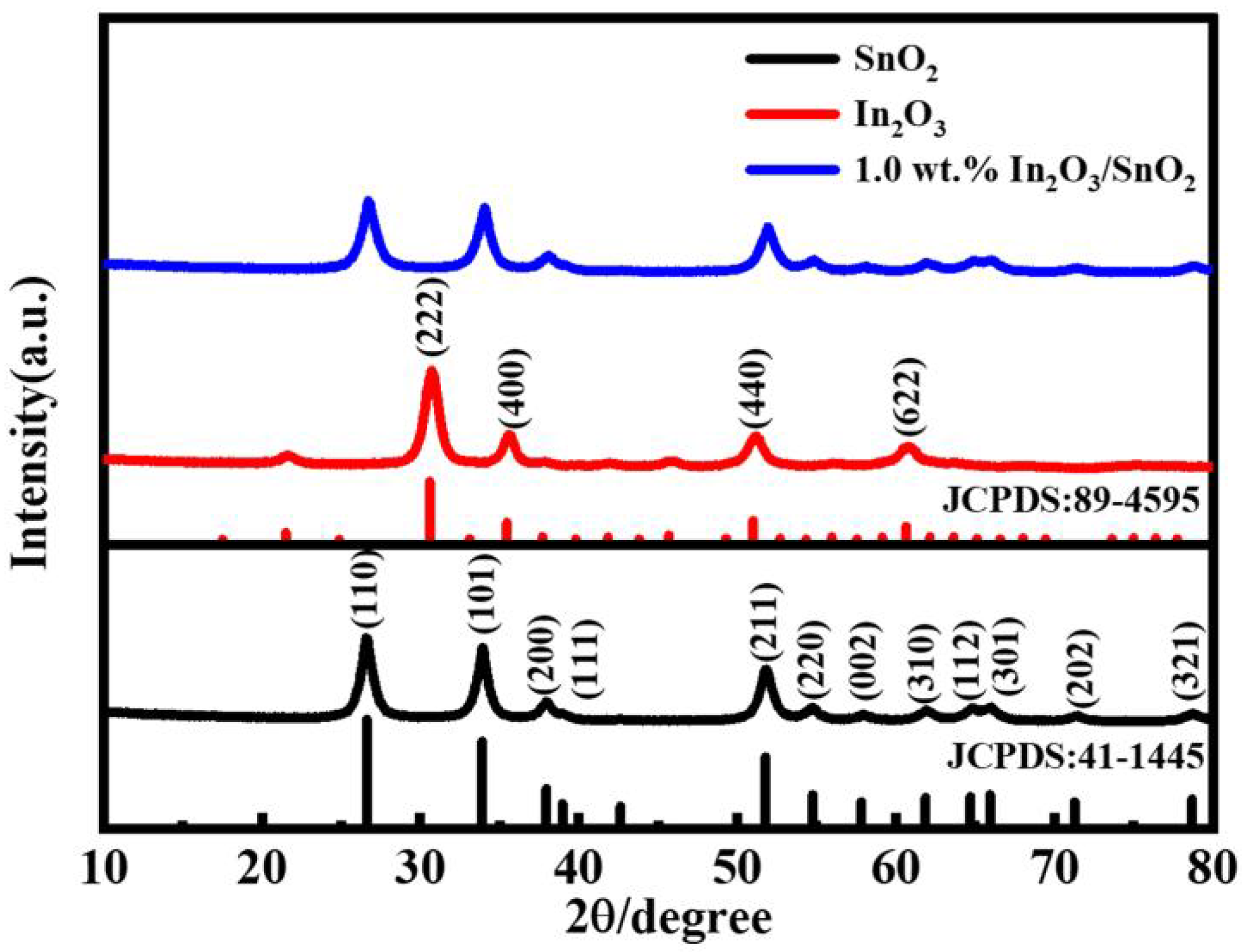
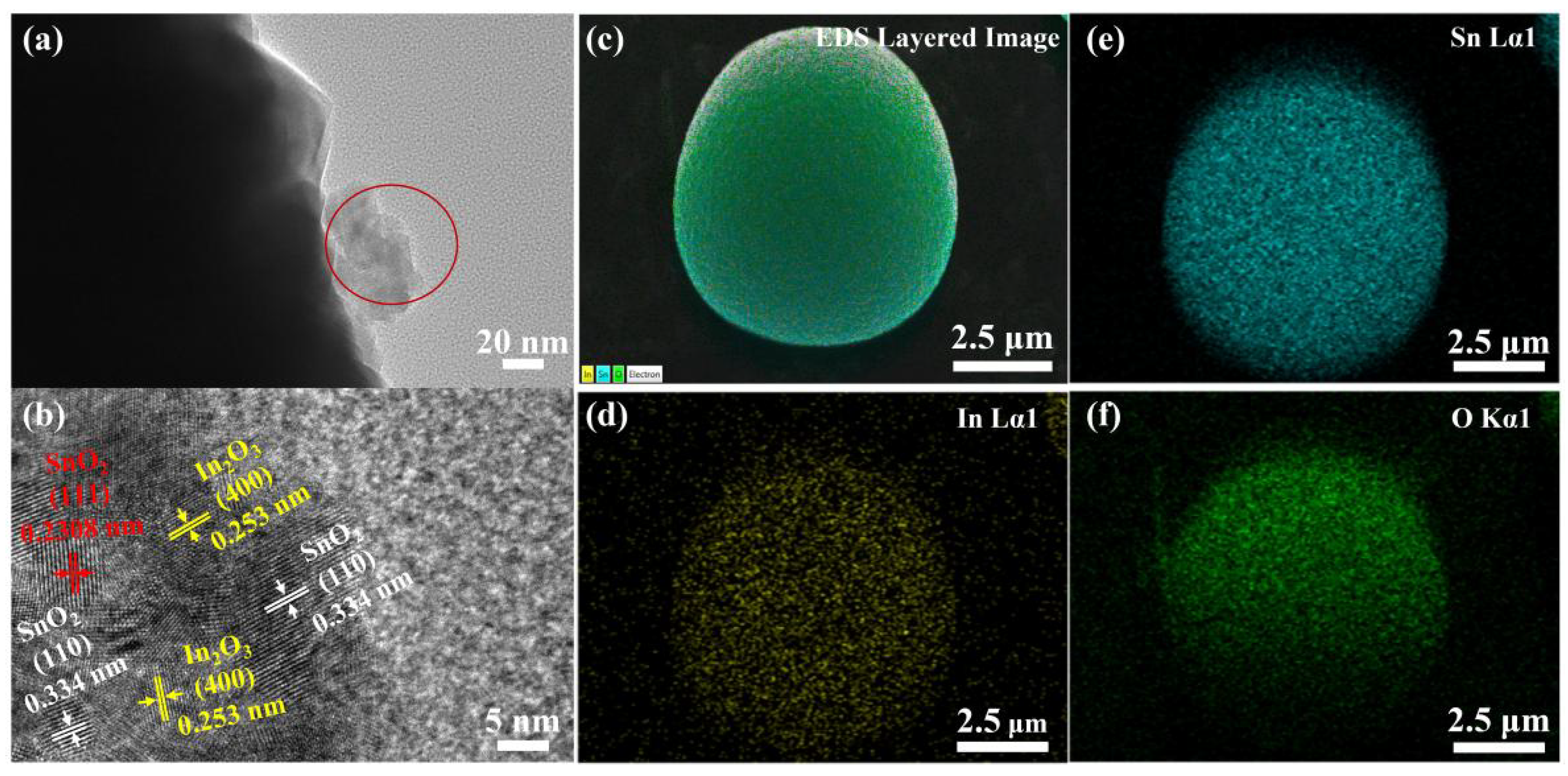
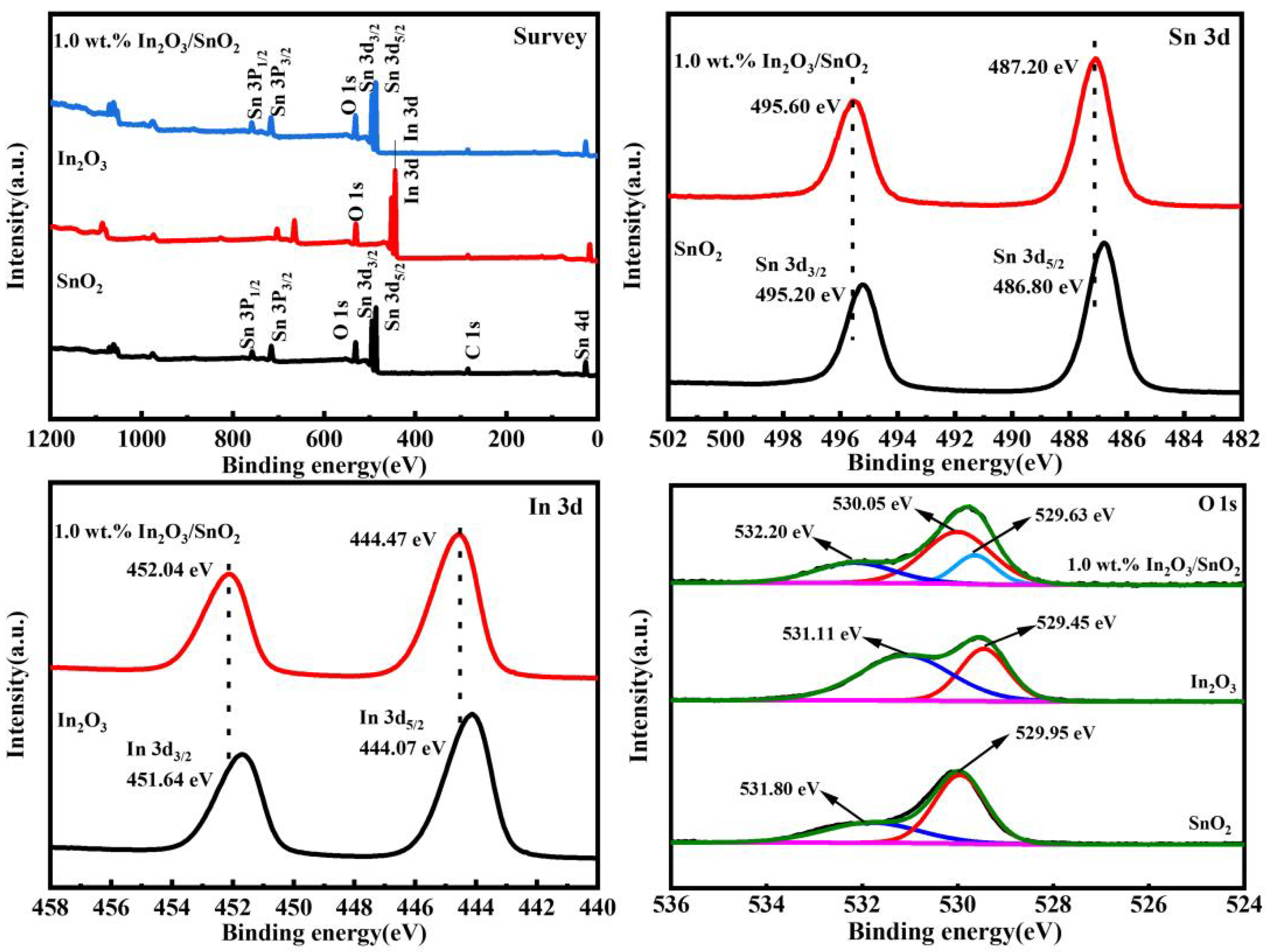
3.1. Morphology and Structure of the Catalyst
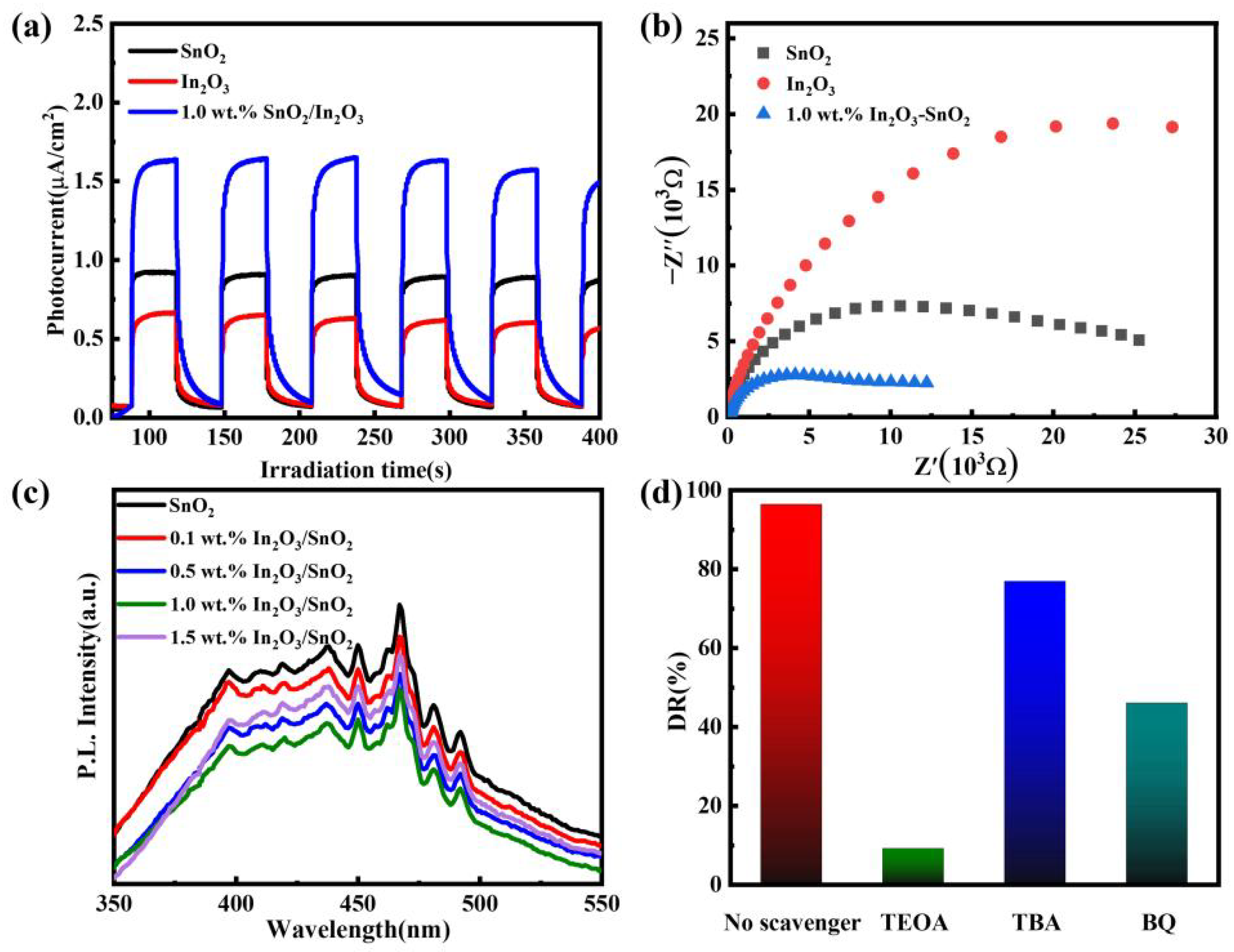
3.2. Photocatalytic Performance
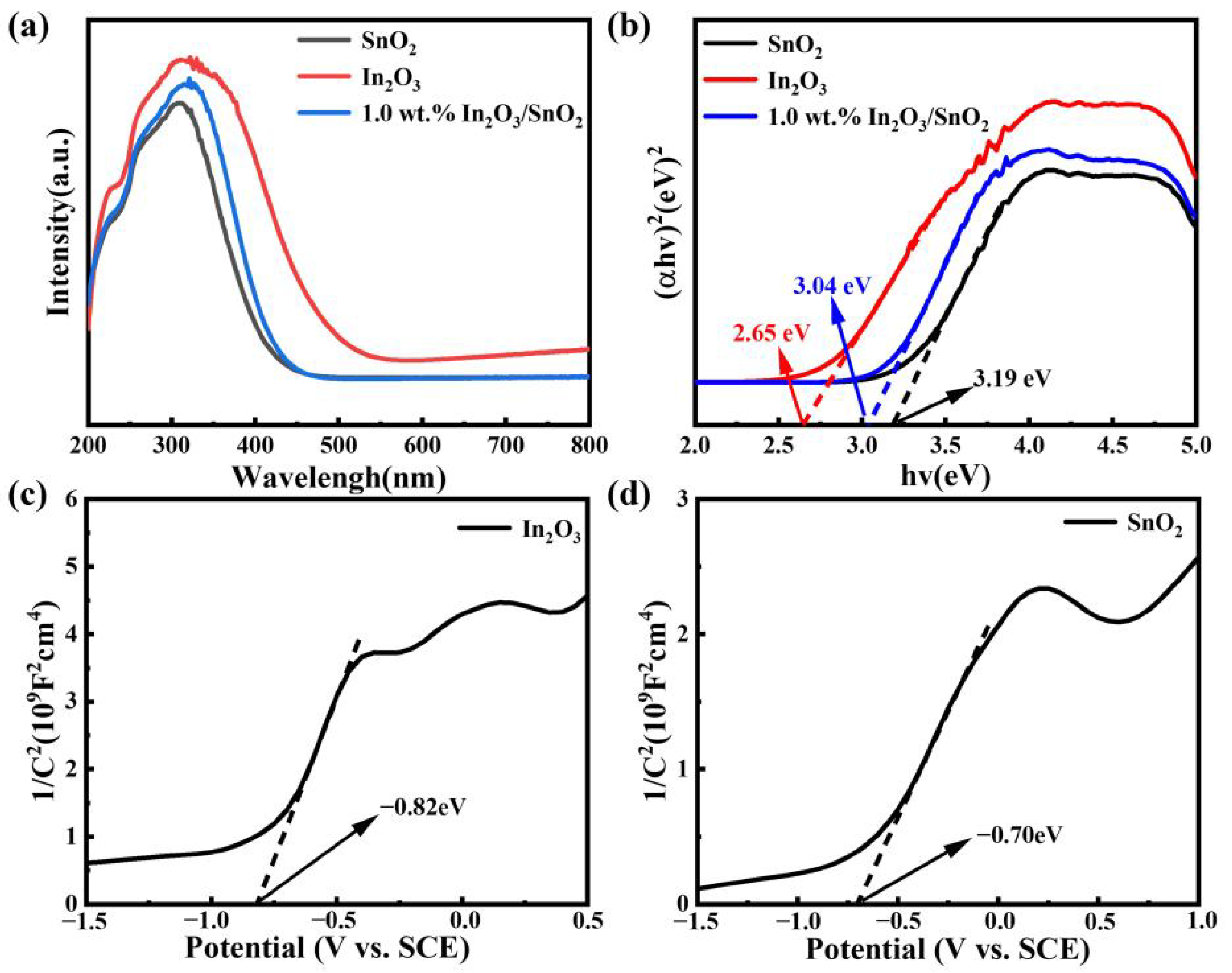
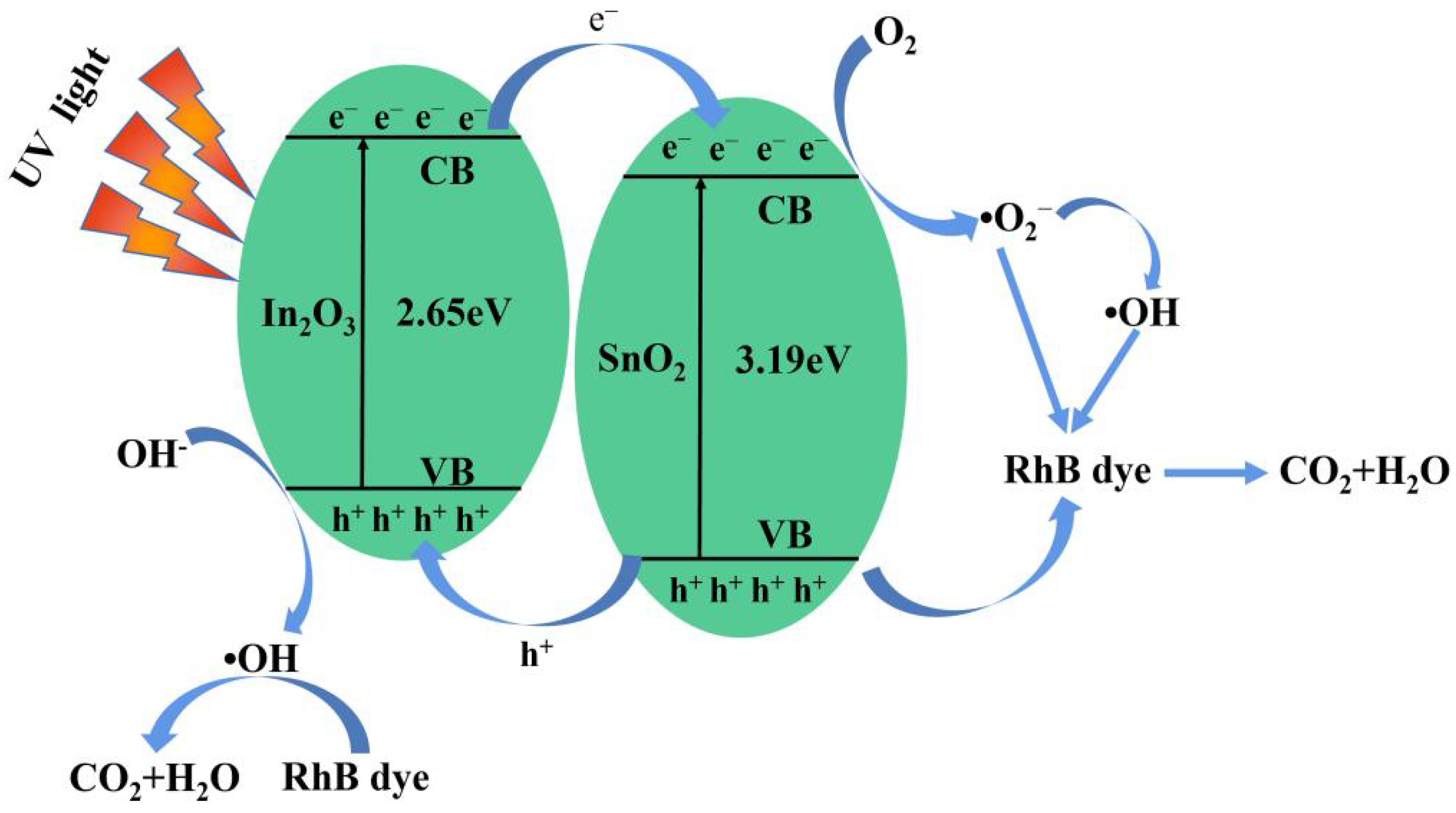
3.3. Catalytic Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yaseen, D.A.; Scholz, M. Textile dye wastewater characteristics and constituents of synthetic effluents: A critical review. Int. J. Environ. Sci. Technol. 2018, 16, 1193–1226. [Google Scholar] [CrossRef]
- Liu, H.; Chen, M.; Zhang, H.; Wang, B.; Peng, J.; Liu, G. One-Step Synthesis of Hierarchical Flower-like SnO2/BiOCOOH Microspheres with Enhanced Light Response for the Removal of Pollutants. Langmuir 2020, 36, 9005–9013. [Google Scholar] [CrossRef] [PubMed]
- Hegazey, R.M.; Abdelrahman, E.A.; Kotp, Y.H.; Hameed, A.M.; Subaihi, A. Facile fabrication of hematite nanoparticles from Egyptian insecticide cans for efficient photocatalytic degradation of rhodamine B dye. J. Mater. Res. Technol. 2020, 9, 1652–1661. [Google Scholar] [CrossRef]
- Hamdaoui, O. Intensification of the sorption of Rhodamine B from aqueous phase by loquat seeds using ultrasound. Desalination 2011, 271, 279–286. [Google Scholar] [CrossRef]
- Liu, S.; Kang, L.; Zhang, J.; Jung, E.; Lee, S.; Jun, S.C. Structural engineering and surface modification of MOF-derived cobalt-based hybrid nanosheets for flexible solid-state supercapacitors. Energy Storage Mater. 2020, 32, 167–177. [Google Scholar] [CrossRef]
- Kang, L.; Huang, C.; Zhang, J.; Zhang, M.; Zhang, N.; Liu, S.; Ye, Y.; Luo, C.; Gong, Z.; Wang, C.; et al. Effect of fluorine doping and sulfur vacancies of CuCo2S4 on its electrochemical performance in supercapacitors. Chem. Eng. J. 2020, 390, 124643. [Google Scholar] [CrossRef]
- Zhao, Y.; Deng, N.; Fan, Z.; Hu, Z.-T.; Fan, L.; Zhou, J.; Huang, X. On-site H2O2 electro-generation process combined with ultraviolet: A promising approach for odorous compounds purification in drinking water system. Chem. Eng. J. 2022, 430, 132829. [Google Scholar] [CrossRef]
- Rui, J.; Deng, N.; Zhao, Y.; Tao, C.; Zhou, J.; Zhao, Z.; Huang, X. Activation of persulfate via Mn doped Mg/Al layered double hydroxide for effective degradation of organics: Insights from chemical and structural variability of catalyst. Chemosphere 2022, 302, 134849. [Google Scholar] [CrossRef]
- Sridevi, A.; Ramji, B.R.; Venkatesan, G.K.D.P.; Sugumaran, V.; Selvakumar, P. A facile synthesis of TiO2/BiOCl and TiO2/BiOCl/La2O3 heterostructure photocatalyst for enhanced charge separation efficiency with improved UV-light catalytic activity towards Rhodamine B and Reactive Yellow 86. Inorg. Chem. Commun. 2021, 130, 108715. [Google Scholar] [CrossRef]
- Chen, N.; Liu, B.; Zhang, P.; Wang, C.; Du, Y.; Chang, W.; Hong, W. Enhanced photocatalytic performance of Ce-doped SnO2 hollow spheres by a one-pot hydrothermal method. Inorg. Chem. Commun. 2021, 132, 108848. [Google Scholar] [CrossRef]
- Nie, M.; Liao, J.; Cai, H.; Sun, H.; Xue, Z.; Guo, P.; Wu, M. Photocatalytic property of silver enhanced Ag/ZnO composite catalyst. Chem. Phys. Lett. 2021, 768, 138394. [Google Scholar] [CrossRef]
- Li, H.; Zhao, Y.; Yin, C.; Jiao, L.; Ding, L. WO3 nanocrystal prepared by self-assembly of phosphotungstic acid and dopamine for photocatalytic degradation of Congo red. Colloids Surf. A Physicochem. Eng. Asp. 2019, 572, 147–151. [Google Scholar] [CrossRef]
- Jabeen, S.; Iqbal, J.; Arshad, A.; Awan, M.S.; Warsi, M.F. (In1-xFex)2O3 nanostructures for photocatalytic degradation of various dyes. Mater. Chem. Phys. 2020, 243, 122516. [Google Scholar] [CrossRef]
- Ding, S.-S.; Huang, W.-Q.; Zhou, B.-X.; Peng, P.; Hu, W.-Y.; Long, M.-Q.; Huang, G.-F. The mechanism of enhanced photocatalytic activity of SnO2 through fullerene modification. Curr. Appl. Phys. 2017, 17, 1547–1556. [Google Scholar] [CrossRef]
- Ahn, K.; Pham-Cong, D.; Choi, H.S.; Jeong, S.-Y.; Cho, J.H.; Kim, J.; Kim, J.-P.; Bae, J.-S.; Cho, C.-R. Bandgap-designed TiO2/SnO2 hollow hierarchical nanofibers: Synthesis, properties, and their photocatalytic mechanism. Curr. Appl. Phys. 2016, 16, 251–260. [Google Scholar] [CrossRef]
- Srinivasan, N.R.; Bandyopadhyaya, R. SnxTi1-xO2 solid-solution-nanoparticle embedded mesoporous silica (SBA-15) hybrid as an engineered photocatalyst with enhanced activity. Faraday Discuss. 2016, 186, 353–370. [Google Scholar] [CrossRef]
- Zhou, B.; Li, Y.; Bai, J.; Li, X.; Li, F.; Liu, L. Controlled synthesis of rh-In2O3 nanostructures with different morphologies for efficient photocatalytic degradation of oxytetracycline. Appl. Surf. Sci. 2019, 464, 115–124. [Google Scholar] [CrossRef]
- Guan, Y.; Wang, D.; Zhou, X.; Sun, P.; Wang, H.; Ma, J.; Lu, G. Hydrothermal preparation and gas sensing properties of Zn-doped SnO2 hierarchical architectures. Sens. Actuators B Chem. 2014, 191, 45–52. [Google Scholar] [CrossRef]
- Qin, Y.; Li, H.; Lu, J.; Ding, Y.; Ma, C.; Liu, X.; Meng, M.; Yan, Y. Fabrication of Bi2WO6/In2O3 photocatalysts with efficient photocatalytic performance for the degradation of organic pollutants: Insight into the role of oxygen vacancy and heterojunction. Adv. Powder Technol. 2020, 31, 2890–2900. [Google Scholar] [CrossRef]
- Mohammad, A.; Khan, M.E.; Karim, M.R.; Cho, M.H. Synergistically effective and highly visible light responsive SnO2-g-C3N4 nanostructures for improved photocatalytic and photoelectrochemical performance. Appl. Surf. Sci. 2019, 495, 143432. [Google Scholar] [CrossRef]
- Jourshabani, M.; Shariatinia, Z.; Badiei, A. In Situ fabrication of SnO2/S-doped g-C3N4 nanocomposites and improved visible light driven photodegradation of methylene blue. J. Mol. Liq. 2017, 248, 688–702. [Google Scholar] [CrossRef]
- Shanmugasundaram, A.; Basak, P.; Manorama, S.V.; Krishna, B.; Sanyadanam, S. Hierarchical mesoporous In2O3 with enhanced CO sensing and photocatalytic performance: Distinct morphologies of In(OH)3 via self assembly coupled in situ solid-solid transformation. ACS Appl. Mater. Interfaces 2015, 7, 7679–7689. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yan, J.; Wang, R.; Li, S.; Brett, D.J.L.; Key, J.; Ji, S. Toward high practical capacitance of Ni(OH)2 using highly conductive CoB nanochain supports. J. Mater. Chem. A 2017, 5, 92–96. [Google Scholar] [CrossRef]
- Clark, A.H. Electrical and Optical Properties of Amorphous Germanium. Phys. Rev. 1967, 154, 750–757. [Google Scholar] [CrossRef]
- Li, Y.; Tian, X.; Wang, Y.; Yang, Q.; Diao, Y.; Zhang, B.; Yang, D. In Situ Construction of a MgSn(OH)6 Perovskite/SnO2 Type-II Heterojunction: A Highly Efficient Photocatalyst towards Photodegradation of Tetracycline. Nanomaterials 2019, 10, 53. [Google Scholar] [CrossRef]
- Soltanabadi, Y.; Jourshabani, M.; Shariatinia, Z. Synthesis of novel CuO/LaFeO3 nanocomposite photocatalysts with superior Fenton-like and visible light photocatalytic activities for degradation of aqueous organic contaminants. Sep. Purif. Technol. 2018, 202, 227–241. [Google Scholar] [CrossRef]
- Chen, Y.; Jiang, Y.; Chen, B.; Ye, F.; Duan, H.; Cui, H. Facile fabrication of N-doped carbon quantum dots modified SnO2 composites for improved visible light photocatalytic activity. Vacuum 2021, 191, 110371. [Google Scholar] [CrossRef]
- Zhu, Z.; Ma, C.; Yu, K.; Lu, Z.; Liu, Z.; Huo, P.; Tang, X.; Yan, Y. Synthesis Ce-doped biomass carbon-based g-C3N4 via plant growing guide and temperature-programmed technique for degrading 2-Mercaptobenzothiazole. Appl. Catal. B: Environ. 2020, 268, 118432. [Google Scholar] [CrossRef]
- Akhundi, A.; Habibi-Yangjeh, A.; Abitorabi, M.; Pouran, S.R. Review on photocatalytic conversion of carbon dioxide to value-added compounds and renewable fuels by graphitic carbon nitride-based photocatalysts. Catal. Rev. 2019, 61, 595–628. [Google Scholar] [CrossRef]
- Mousavi, M.; Habibi-Yangjeh, A.; Pouran, S.R. Review on magnetically separable graphitic carbon nitride-based nanocomposites as promising visible-light-driven photocatalysts. J. Mater. Sci. Mater. Electron. 2017, 29, 1719–1747. [Google Scholar] [CrossRef]
- Guo, M.-Z.; Poon, C.S. Superior photocatalytic NOx removal of cementitious materials prepared with white cement over ordinary Portland cement and the underlying mechanisms. Cem. Concr. Compos. 2018, 90, 42–49. [Google Scholar] [CrossRef]
- Li, Y.; Yang, Q.; Wang, Z.; Wang, G.; Zhang, B.; Zhang, Q.; Yang, D. Rapid fabrication of SnO2 nanoparticle photocatalyst: Computational understanding and photocatalytic degradation of organic dye. Inorg. Chem. Front. 2018, 5, 3005–3014. [Google Scholar] [CrossRef]
- Davis, M.; Hung-Low, F.; Hikal, W.M.; Hope-Weeks, L.J. Enhanced photocatalytic performance of Fe-doped SnO2 nanoarchitectures under UV irradiation: Synthesis and activity. J. Mater. Sci. 2013, 48, 6404–6409. [Google Scholar] [CrossRef]
- Chen, H.; Wang, Z.; Xue, Z.; Yu, C.; Pei, L.; Fan, C. Constructing Z-Scheme Bi2O3/In2O3 Heterojunction for Efficient Photocatalytic Degradation of Rhodamine B. Cryst. Res. Technol. 2020, 55, 2000093. [Google Scholar] [CrossRef]
- Jansanthea, P.; Kanthabangharn, J.; Chomkitichai, W.; Ketwaraporn, J.; Saovakon, C.; Wansao, C.; Wanaek, A.; Kraivuttinun, P.; Pookmanee, P.; Phanichphant, S. Temperature-controlled synthesis and photocatalytic properties of ZnO–SnO2 nanocomposites. J. Aust. Ceram. Soc. 2021, 57, 579–588. [Google Scholar] [CrossRef]

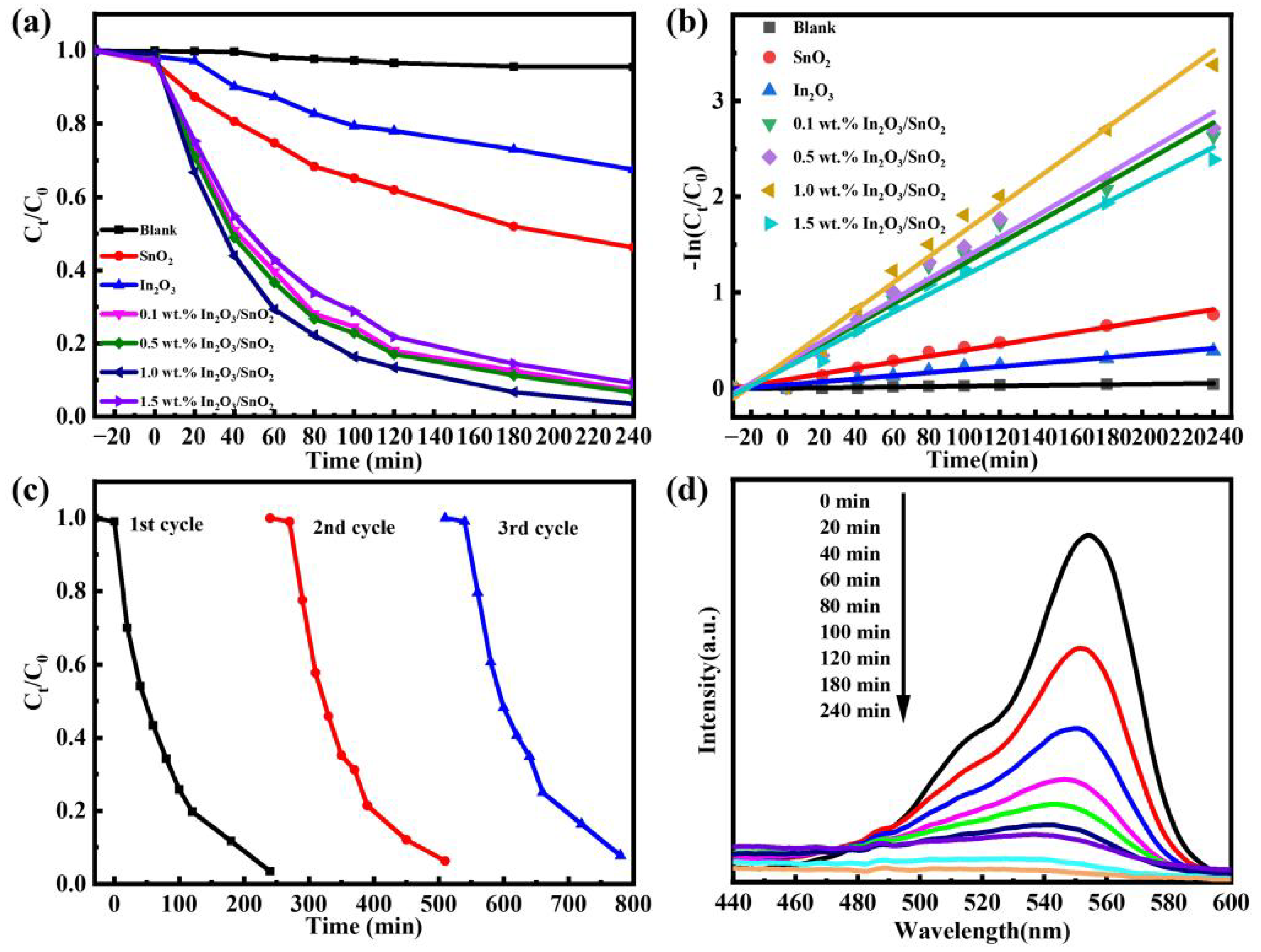
| Samples | Fitted Equation | ĸ (min−1) | Correlation Coefficient (R2) |
|---|---|---|---|
| Blank | ln(C0/C) = 0.0002056 t | 0.0002056 | 0.91181 |
| SnO2 | ln(C0/C) = 0.00305 t | 0.00305 | 0.98214 |
| In2O3 | ln(C0/C) = 0.00158 t | 0.00158 | 0.96773 |
| 0.1 wt.% In2O3/SnO2 | ln(C0/C) = 0.01049 t | 0.01049 | 0.97336 |
| 0.5 wt.% In2O3/SnO2 | ln(C0/C) = 0.01087 t | 0.01087 | 0.9719 |
| 1.0 wt.% In2O3/SnO2 | ln(C0/C) = 0.0002056 t | 0.01347 | 0.98141 |
| 1.5 wt.% In2O3/SnO2 | ln(C0/C) = 0.0002056 t | 0.00958 | 0.98119 |
| Catalyst | Pollutant Concentration | Catalyst Dosage (mg) | Light Source | Irradiation Time (min) | Activity (%) | Reference |
|---|---|---|---|---|---|---|
| SnO2 | a RhB (10 mg/L) | 45 mg | high-pressure Hg lamp (175 w) | 270 min | ~92% | [32] |
| Fe/SnO2 | RhB (10 mg/L) | 25 mg | UV light (250 w) | 120 min | ~55% | [33] |
| Bi2O3/In2O3 | RhB (10 mg/L) | 10 mg | Hg lamp (175 w) | 240 min | ~92% | [34] |
| ZnO/SnO2 | RhB (2 × 10−6 M) | 50 mg | two 6 W UV tube lamps | 120 min | ~80% | [35] |
| 1.0 wt.% In2O3/SnO2 | RhB (10 mg/L) | 50 mg | high-pressure Hg lamp (200 w) | 120 min 180 min 240 min | ~87% ~92% ~97% | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Guo, G.; Zhu, C.; Li, Y.; Jin, Y.; Zou, B.; He, H.; Wang, A. Facile Synthesis, Characterization, and Photocatalytic Evaluation of In2O3/SnO2 Microsphere Photocatalyst for Efficient Degradation of Rhodamine B. Nanomaterials 2022, 12, 3151. https://doi.org/10.3390/nano12183151
Wang C, Guo G, Zhu C, Li Y, Jin Y, Zou B, He H, Wang A. Facile Synthesis, Characterization, and Photocatalytic Evaluation of In2O3/SnO2 Microsphere Photocatalyst for Efficient Degradation of Rhodamine B. Nanomaterials. 2022; 12(18):3151. https://doi.org/10.3390/nano12183151
Chicago/Turabian StyleWang, Chunlan, Gangying Guo, Changjun Zhu, Yuqing Li, Yebo Jin, Bingsuo Zou, Han He, and Aolin Wang. 2022. "Facile Synthesis, Characterization, and Photocatalytic Evaluation of In2O3/SnO2 Microsphere Photocatalyst for Efficient Degradation of Rhodamine B" Nanomaterials 12, no. 18: 3151. https://doi.org/10.3390/nano12183151
APA StyleWang, C., Guo, G., Zhu, C., Li, Y., Jin, Y., Zou, B., He, H., & Wang, A. (2022). Facile Synthesis, Characterization, and Photocatalytic Evaluation of In2O3/SnO2 Microsphere Photocatalyst for Efficient Degradation of Rhodamine B. Nanomaterials, 12(18), 3151. https://doi.org/10.3390/nano12183151







