Fabrication and Development of Binder-Free Mn–Fe–S Mixed Metal Sulfide Loaded Ni-Foam as Electrode for the Asymmetric Coin Cell Supercapacitor Device
Abstract
:1. Introduction
2. Experimental Methods
3. Characterization Techniques
4. Results and Discussions
4.1. X-ray Diffraction (XRD)
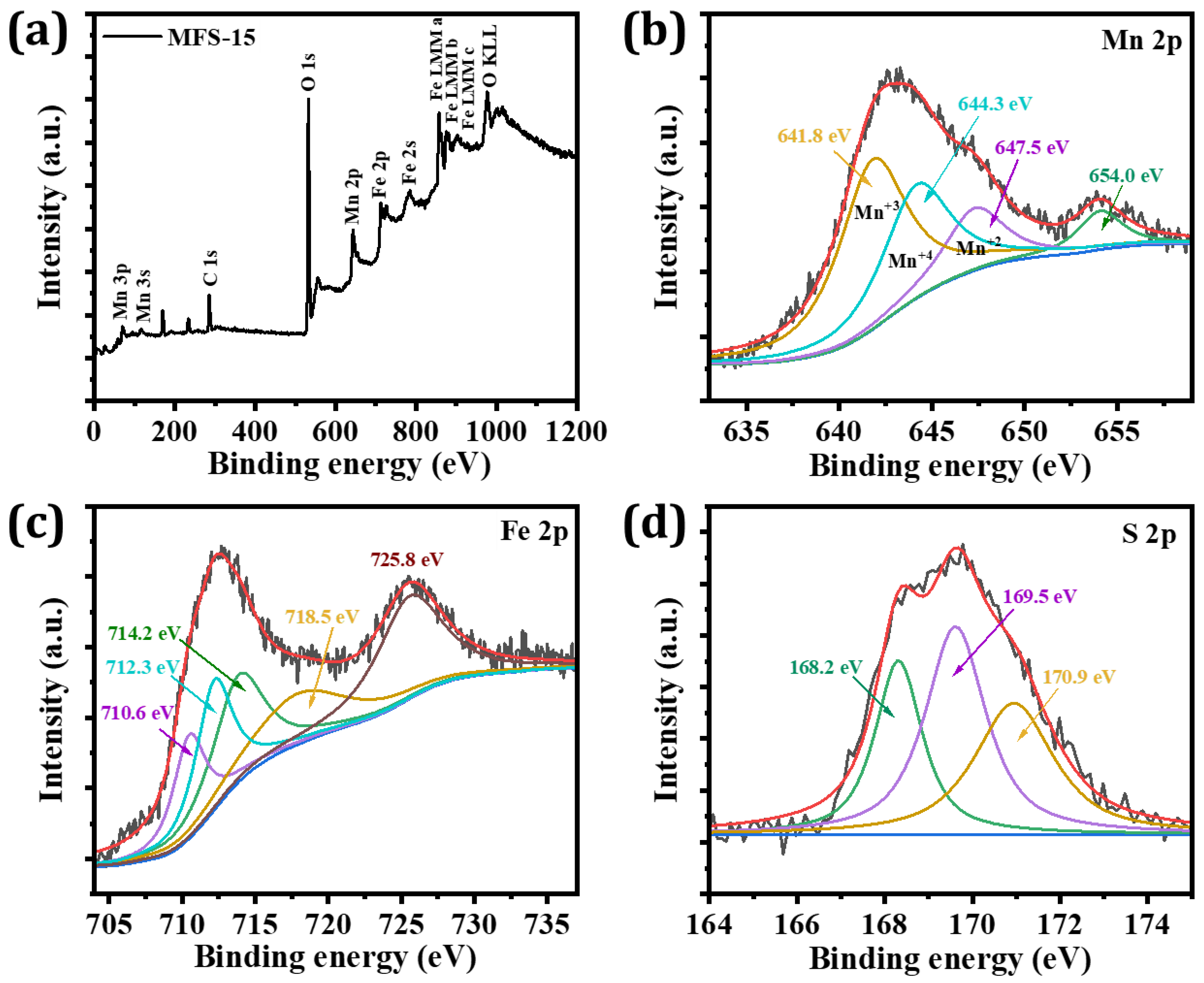
4.2. X-ray Photoelectron Spectroscopy (XPS)
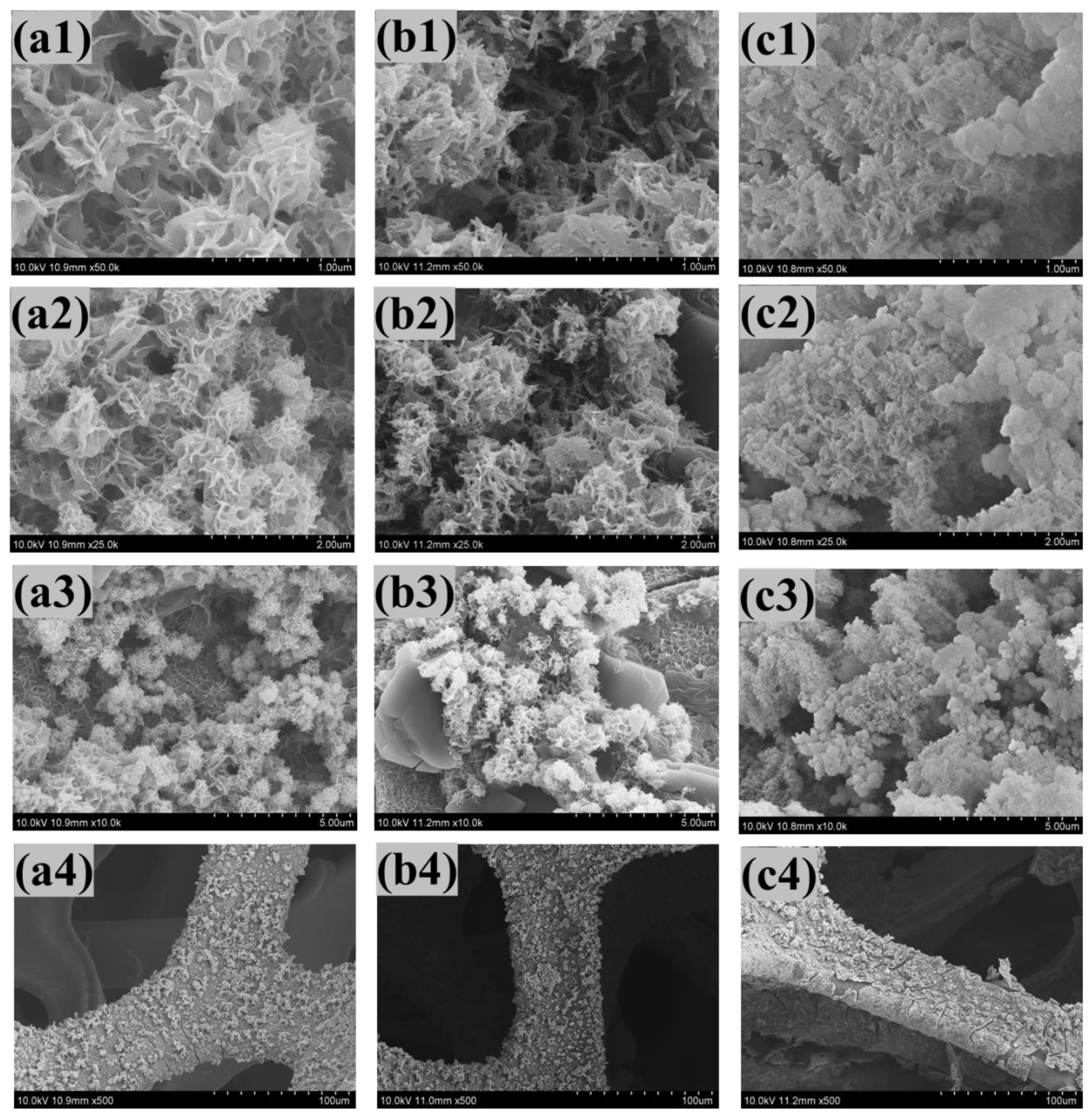
4.3. Field Emission Scanning Electron Microscopy (FE−SEM)
4.4. Energy-Dispersive X-ray Spectroscopy (EDS)
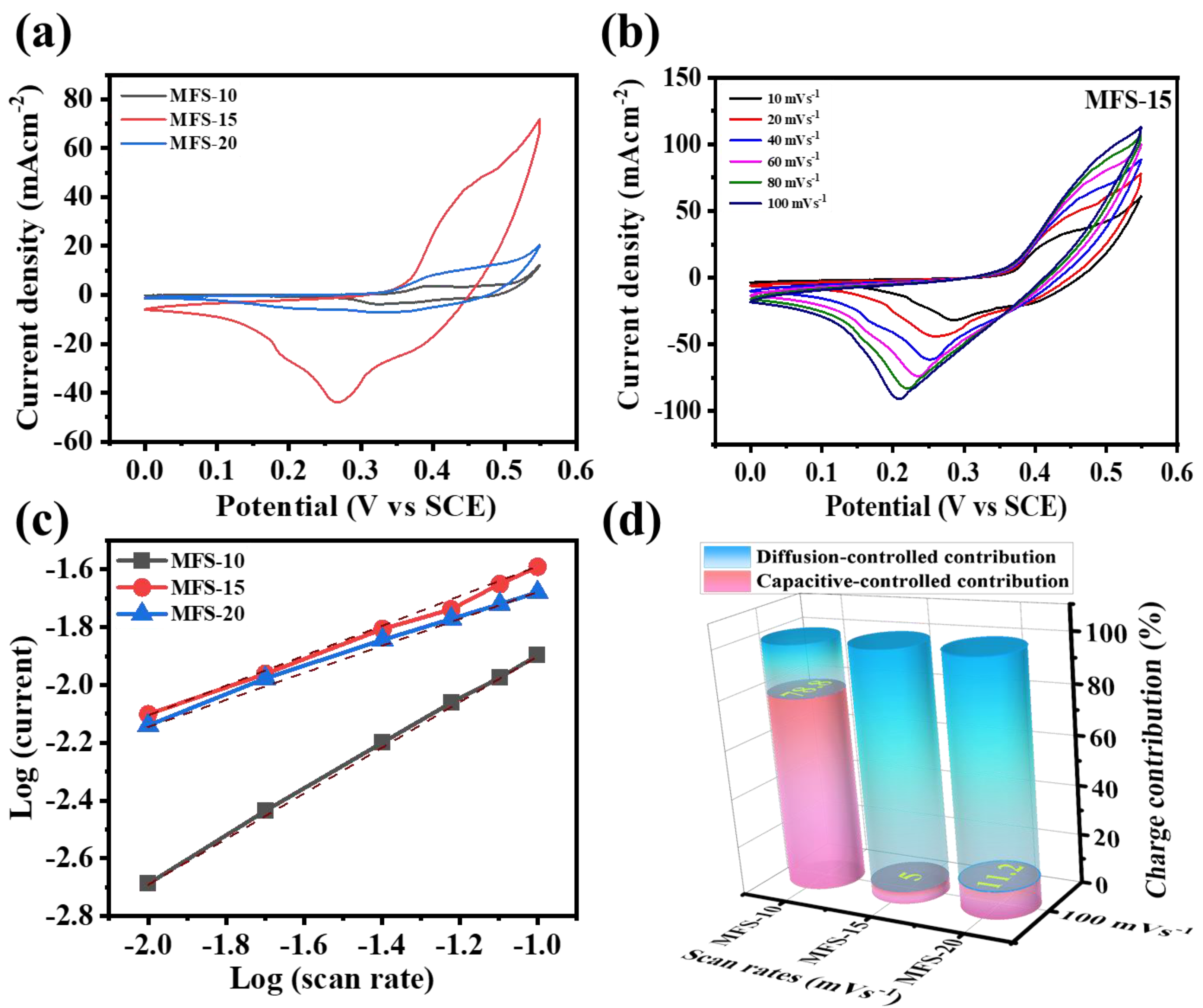
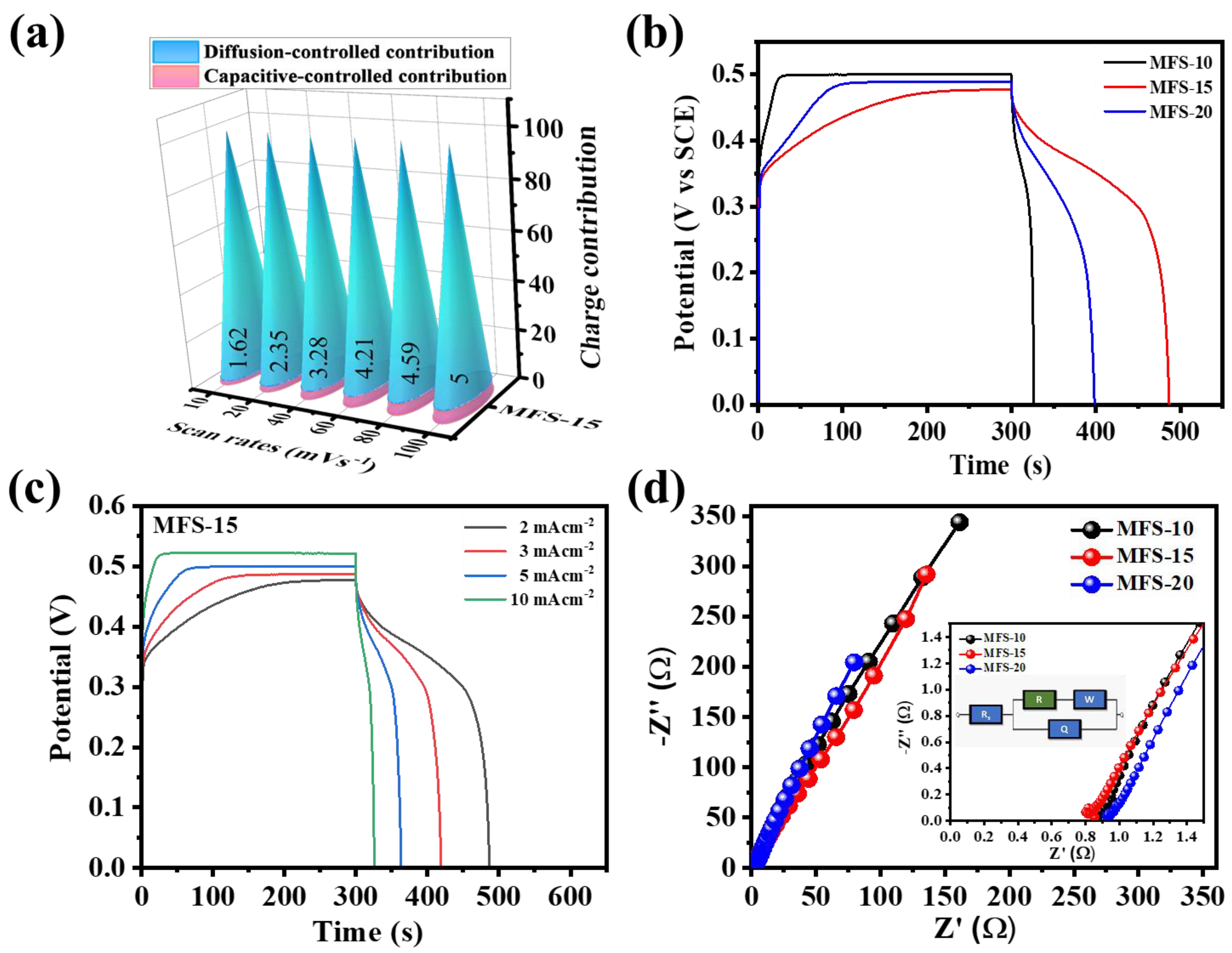
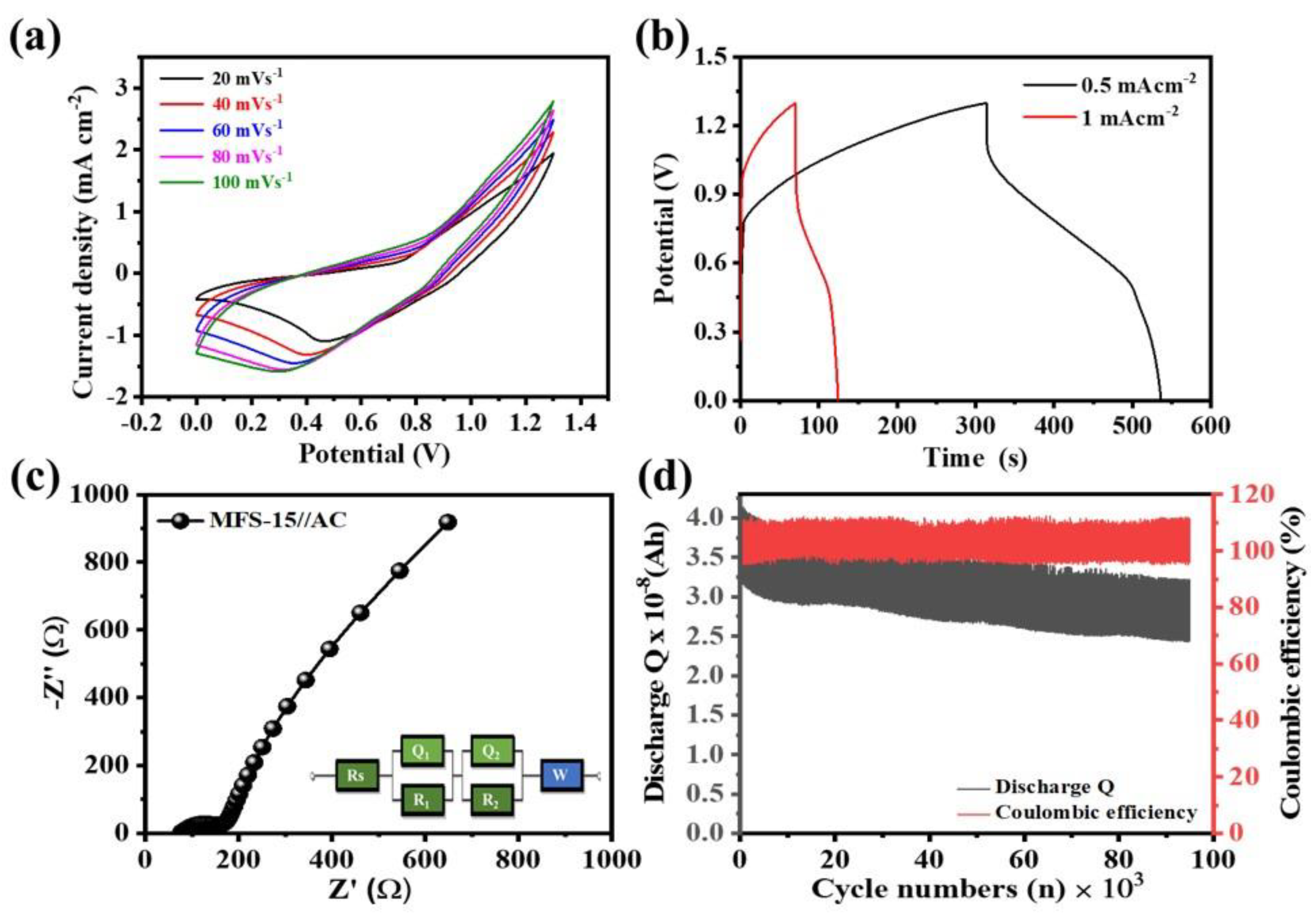
4.5. Electrochemical Measurements
4.6. Asymmetric Coin Cell Device
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sahoo, R.; Lee, T.H.; Pham, D.T.; Luu, T.H.T.; Lee, Y.H. Fast-Charging High-Energy Battery-Supercapacitor Hybrid: Anodic Reduced Graphene Oxide-Vanadium(IV) Oxide Sheet-on-Sheet Heterostructure. ACS Nano 2019, 13, 10776. [Google Scholar] [CrossRef]
- Liu, C.; Neale, Z.G.; Cao, G. Understanding electrochemical potentials of cathode materials in rechargeable batteries. Mater. Today 2016, 19, 109. [Google Scholar] [CrossRef]
- Conway, B.E. Electrochemical Supercapacitors Scientific Fundamentals and Technological Applications. In Advances in Lithium-Ion Batteries; Springer: New York, NY, USA, 1999; pp. 481–505. [Google Scholar]
- Gogotsi, Y.; Simon, P. Materials for electrochemical capacitors. Nat. Mater. 2008, 7, 845. [Google Scholar]
- Fan, H.; Niu, R.; Duan, J.; Liu, W.; Shen, W. Fe3O4@Carbon Nanosheets for All-Solid-State Supercapacitor Electrodes. ACS Appl. Mater. Interfaces 2016, 8, 19475. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Xia, X.; Shi, F.; Zhan, J.; Tu, J.; Fan, H. Transition metal carbides and nitrides in energy storage and conversion. Adv. Sci. 2015, 3, 1500286. [Google Scholar] [CrossRef] [PubMed]
- Rehman, J.; Eid, K.; Ali, R.; Fan, X.; Murtaza, G.; Faizan, M.; Laref, A.; Zheng, W.; Varma, R. Engineering of Transition Metal Sulfide Nanostructures as Efficient Electrodes for High-Performance Supercapacitors. ACS Appl. Energy Mater. 2022, 5, 6481. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, D.; Shen, G. Flexible Supercapacitors Based on Ternary Metal Oxide (Sulfide, Selenide) Nanostructures. Flex. Supercapacitors 2022, 121–156. [Google Scholar] [CrossRef]
- Balamurugan, J.; Li, C.; Thanh, T.; Park, O.; Kim, N.; Lee, J. Hierarchical design of Cu1−xNixS nanosheets for high-performance asymmetric solid-state supercapacitors. J. Mater. Chem. A 2017, 5, 19760. [Google Scholar] [CrossRef]
- Soonmin, H. Deposition of metal sulphide thin films by chemical bath deposition technique: Review. Int. J. Thin Film Sci. Technol. 2021, 10, 45. [Google Scholar]
- Gao, Y.; Zhao, L. Review on recent advances in nanostructured transition-metal-sulfide-based electrode materials for cathode materials of asymmetric supercapacitors. Chem. Eng. J. 2022, 430, 132745. [Google Scholar] [CrossRef]
- Cui, M.; Meng, X. Overview of transition metal-based composite materials for supercapacitor electrodes. Nanoscale Adv. 2020, 2, 5516. [Google Scholar] [CrossRef]
- Lin, L.; Li, X.; Huang, Y.; Sun, H. Synthesizing Ni-based ternary metal compounds for battery-supercapacitor hybrid devices with and without using nickel precursors. Mater. Sci. Semicond. Process. 2019, 98, 81. [Google Scholar] [CrossRef]
- Sun, H.; Lin, L.; Huang, Y.; Hong, W. Nickel precursor-free synthesis of nickel cobalt-based ternary metal oxides for asymmetric supercapacitors. Electrochim. Acta 2018, 281, 692. [Google Scholar] [CrossRef]
- Chou, S.; Lin, L.; Chiu, Y. Pulse reverse electrodeposited nickel cobalt sulfide nanosheets on Ni foam as battery-type electrode for battery supercapacitor hybrids. J. Energy Storage 2019, 25, 100903. [Google Scholar] [CrossRef]
- Zhang, K.; Park, M.; Zhou, L.; Lee, G.; Shin, J.; Hu, Z.; Chou, S.; Chen, J.; Kang, Y. Cobalt-Doped FeS2 Nanospheres with Complete Solid Solubility as a High-Performance Anode Material for Sodium-Ion Batteries. Angew. Chem. Int. Ed. 2016, 55, 12822. [Google Scholar] [CrossRef]
- Wang, D.; Gong, M.; Chou, H.; Pan, C.; Chen, H.; Wu, Y.; Lin, M.; Guan, M.; Yang, J.; Chen, C.; et al. Highly active and stable hybrid catalyst of cobalt-doped FeS2 nanosheets-carbon nanotubes for hydrogen evolution reaction. J. Am. Chem. Soc. 2015, 137, 1587. [Google Scholar] [CrossRef]
- Jiang, J.; Lu, S.; Gao, H.; Zhang, X.; Yu, H.Q. Ternary FeNiS2 ultrathin nanosheets as an electrocatalyst for both oxygen evolution and reduction reactions. Nano Energy 2016, 27, 526. [Google Scholar] [CrossRef]
- Chaki, S.; Chauhan, S.; Tailor, J.; Deshpande, M. Synthesis of manganese sulfide (MnS) thin films by chemical bath deposition and their characterization. J. Mater. Res. Technol. 2017, 6, 123. [Google Scholar] [CrossRef]
- Khani, H.; Wipf, D. Iron Oxide Nanosheets and Pulse-Electrodeposited Ni-Co-S Nanoflake Arrays for High-Performance Charge Storage. ACS Appl. Mater. Interfaces 2017, 9, 6967. [Google Scholar] [CrossRef]
- Yu, M.; Li, X.; Ma, Y.; Liu, R.; Liu, J.; Li, S. Nanohoneycomb-like manganese cobalt sulfide/three dimensional graphene-nickel foam hybid electrodes for high-rate capability supercapacitors. Appl. Surf. Sci. 2017, 396, 1816. [Google Scholar] [CrossRef]
- Kulkarni, P.; Nataraj, S.; Balakrishna, R.; Nagaraju, D.; Reddy, M. Nanostructured binary and ternary metal sulfides: Synthesis methods and their application in energy conversion and storage devices. J. Mater. Chem. A 2017, 5, 22040. [Google Scholar] [CrossRef]
- Marimuthu, T.; Anandhan, N.; Thangamuthu, R.; Surya, S. Effect of hexamethylenetetramine on the properties of electrodeposited ZnO thin films for dye sensitized solar cell applications. J. Mater. Sci. Mater. Electron. 2018, 29, 12830. [Google Scholar] [CrossRef]
- Marimuthu, T.; Anandhan, N.; Thangamuthu, R. Electrochemical synthesis of one-dimensional ZnO nanostructures on ZnO seed layer for DSSC applications. Appl. Surf. Sci. 2018, 428, 385. [Google Scholar] [CrossRef]
- Marimuthu, T.; Anandhan, N.; Thangamuthu, R. A facile electrochemical–hydrothermal synthesis and characterization of zinc oxide hierarchical structure for dye sensitized solar cell applications. J. Mater. Sci. 2018, 53, 12441. [Google Scholar] [CrossRef]
- Chen, Y.; Jing, C.; Fu, X.; Shen, M.; Li, K.; Liu, X.; Yao, H.; Zhang, Y.; Yao, K. Synthesis of porous NiCoS nanosheets with Al leaching on ordered mesoporous carbon for high-performance supercapacitors. Chem. Eng. J. 2020, 384, 123367. [Google Scholar] [CrossRef]
- Zhang, C.; Cai, X.; Qian, Y.; Jiang, H.; Zhou, L.; Li, B.; Lai, L.; Shen, Z.; Huang, W. Electrochemically Synthesis of Nickel Cobalt Sulfide for High-Performance Flexible Asymmetric Supercapacitors. Adv. Sci. 2018, 5, 1700375. [Google Scholar] [CrossRef]
- Bahaa, A.; Balamurugan, J.; Kim, N.H.; Lee, J.H. Metal-organic framework derived hierarchical copper cobalt sulfide nanosheet arrays for high-performance solid-state asymmetric supercapacitors. J. Mater. Chem. A 2019, 7, 8620. [Google Scholar] [CrossRef]
- Moosavifard, S.; Fani, S.; Rahmanian, M. Hierarchical CuCo2S4 hollow nanoneedle arrays as novel binder-free electrodes for high-performance asymmetric supercapacitors. Chem. Commun. 2016, 52, 4517. [Google Scholar] [CrossRef]
- Mohammadi, A.; Moosavifard, S.; Tabrizi, A.G.; Abdi, M.; Karimi, G. Nanoporous CuCo2S4 microspheres: A novel positive electrode for high-performance hybrid energy storage devices. ACS Appl. Energy Mater. 2019, 2, 627. [Google Scholar] [CrossRef]
- Chen, Y.; Li, Z.; Lou, X. General Formation of MxCo3−xS4 (M = Ni, Mn, Zn) Hollow Tubular Structures for Hybrid Supercapacitors. Angew. Chem. Int. Ed. 2015, 54, 10521. [Google Scholar] [CrossRef]
- Marsudi, M.; Ma, Y.; Prakoso, B.; Hutani, J.; Wibowo, A.; Zong, Y.; Liu, Z.; Sumboja, A. Manganese oxide nanorods decorated table sugar derived carbon as efficient bifunctional catalyst in rechargeable Zn-air batteries. Catalysts 2020, 10, 64. [Google Scholar] [CrossRef]
- Wang, M.; Chen, K.; Liu, J.; He, Q.; Li, G.; Li, F. Efficiently enhancing electrocatalytic activity of α-MnO2 nanorods/N-doped ketjenblack carbon for oxygen reduction reaction and oxygen evolution reaction using facile regulated hydrothermal treatment. Catalysts 2018, 8, 138. [Google Scholar] [CrossRef] [Green Version]
- Serhan, M.; Sprowls, M.; Jackemeyer, D.; Long, M.; Perez, I.; Maret, W.; Tao, N.; Forzani, E. Total iron measurement in human serum with a smartphone. AIChE Annu. Meet. Conf. Proc. 2019, 2, 303. [Google Scholar]
- Tresintsi, S.; Simeonidis, K.; Pliatsikas, N.; Vourlias, G.; Patsalas, P.; Mitrakas, M. The role of SO42− Surface distribution in arsenic removal by iron oxy-hydroxides. J. Solid State Chem. 2014, 213, 145. [Google Scholar] [CrossRef]
- Alam, M.; Karmakar, K.; Pal, M.; Mandal, K. Electrochemical supercapacitor based on double perovskite Y2NiMnO6 nanowires. RSC Adv. 2016, 6, 114722. [Google Scholar] [CrossRef]
- Tabrizi, A.; Arsalani, N.; Mohammadi, A.; Namazi, H.; Ghadimi, L.S.; Ahadzadeh, I. Facile synthesis of a MnFe2O4/rGO nanocomposite for an ultra-stable symmetric supercapacitor. New J. Chem. 2017, 41, 4974. [Google Scholar] [CrossRef]
- Lindström, H.; Södergren, S.; Solbrand, A.; Rensmo, H.; Hjelm, J.; Hagfeldt, A.; Lindquist, S. Li+ ion insertion in TiO2 (anatase). 1. Chronoamperometry on CVD films and nanoporous films. J. Phys. Chem. B 1997, 101, 7710. [Google Scholar] [CrossRef]
- Simon, P.; Gogotsi, Y.; Dunn, B. Where do batteries end and supercapacitors begin? Science 2014, 343, 1210. [Google Scholar] [CrossRef]
- Wang, Q.; Yang, H.; Meng, T.; Yang, J.; Huang, B.; Gu, F.; Zhang, S.; Meng, C.; Tong, Y. Boosting Electron Transfer with Heterointerface Effect for High-Performance Lithium-Ion Storage. Energy Storage Mater. 2021, 36, 365. [Google Scholar] [CrossRef]
- Brezesinski, T.; Wang, J.; Tolbert, S.H.; Dunn, B. Ordered mesoporous α-MoO3 with iso-oriented nanocrystalline walls for thin-film pseudocapacitors. Nat. Mater. 2010, 9, 146. [Google Scholar] [CrossRef]
- Liu, T.; Pell, W.; Conway, B.; Roberson, S. Behavior of Molybdenum Nitrides as Materials for Electrochemical Capacitors: Comparison with Ruthenium Oxide. J. Electrochem. Soc. 1998, 145, 1882. [Google Scholar] [CrossRef]
- MacArthur, D. The Proton Diffusion Coefficient for the Nickel Hydroxide Electrode. J. Electrochem. Soc. 1970, 117, 729. [Google Scholar] [CrossRef]
- Teli, A.; Pawar, S.; Dubal, D. Effect of Concentration on the Charge Storage Kinetics of Nanostructured MnO2 Thin-Film Supercapacitors Synthesized by the Hydrothermal Method. Energies 2020, 13, 6124. [Google Scholar] [CrossRef]
- Teli, A.; Bhat, T.; Beknalkar, S.; Mane, S.; Chaudhary, L.; Patil, D.; Pawar, S.; Efstathiadis, H.; Shin, J. Bismuth manganese oxide based electrodes for asymmetric coin cell supercapacitor. Chem. Eng. J. 2022, 430, 133138. [Google Scholar] [CrossRef]
- Sun, D.; Yan, X.; Lang, J.; Xue, Q. High performance supercapacitor electrode based on graphene paper via flame-induced reduction of graphene oxide paper. J. Power Sources 2013, 222, 52. [Google Scholar] [CrossRef]
- Viand, A.; Mahjani, M.; Jafarian, M. Investigation of anomalous diffusion and multifractal dimensions in polypyrrole film. J. Electroanal. Chem. 2012, 671, 51. [Google Scholar] [CrossRef]
- Samuel, E.; Aldalbahi, A.; El-Newehy, M.; El-Hamshary, H.; Yoon, S. Flexible and freestanding manganese/iron oxide carbon nanofibers for supercapacitor electrodes. Ceram. Int. 2022, 48, 18374. [Google Scholar] [CrossRef]
- Lee, M.; Chang, J.; Hsieh, Y.; Tsai, W. Annealed Mn–Fe binary oxides for supercapacitor applications. J. Power Sources 2008, 185, 1550. [Google Scholar] [CrossRef]
- Makkar, P.; Ghosh, N. Facile Synthesis of MnFe2O4 Hollow Sphere-Reduced Graphene Oxide Nanocomposites as Electrode Materials for All-Solid-State. ACS Appl. Energy Mater. 2020, 3, 2653. [Google Scholar] [CrossRef]
- Yavuz, A.; Bedir, M.; Tunc, A. Manganese–iron coated flexible graphite sheet having wide potential window for energy storage devices. J. Energy Storage 2022, 50, 104253. [Google Scholar] [CrossRef]
- Ahmed, N.; Ali, B.; Ramadan, M.; Allam, N. Three-Dimensional Interconnected Binder-Free Mn−Ni−S Nanosheets for High Performance Asymmetric Supercapacitor Devices with Exceptional Cyclic Stability. ACS Appl. Energy Mater. 2019, 2, 3717. [Google Scholar] [CrossRef]
- Zhu, G.; Yang, J.; Liu, Y.; Xie, X.; Ji, Z.; Yin, J.; Shen, X. Porous Fe-Mn-O nanocomposites: Synthesis and supercapacitor electrode application. Prog. Nat. Sci. 2016, 26, 264. [Google Scholar] [CrossRef]
- Ray, A.; Roy, A.; Ghosh, M.; Ramos-Ramónc, J.A.; Saha, S.; Pal, U.; Bhattacharya, S.K.; Das, S. Study on charge storage mechanism in working electrodes fabricated by sol-gel derived spinel NiMn2O4 nanoparticles for supercapacitor application. Appl. Surf. Sci. 2019, 463, 513. [Google Scholar] [CrossRef]
- Pender, J.; Jha, G.; Youn, D.; Ziegler, J.; Andoni, I.; Choi, E.; Heller, A.; Dunn, B.; Weiss, P.; Penner, R.; et al. Electrode Degradation in Lithium-Ion Batteries. ACS Nano 2020, 14, 1243. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Chen, G. Ionic liquid-based electrolytes for supercapacitor and supercapattery. Front. Chem. 2019, 7, 272. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Sample Code | Potential Window (V) | Areal Capacitance (mF cm−2) | Energy Density (mWh cm−2) | Power Density (mW cm−2) | Series Resistance Rs (Ω) |
|---|---|---|---|---|---|
| MFS-10 | 0.50 | 108.0 | 0.0037 | 0.50 | 0.96 |
| MFS-15 | 0.47 | 795.7 | 0.0244 | 0.47 | 0.85 |
| MFS-20 | 0.49 | 400.0 | 0.0134 | 0.49 | 0.93 |
| Current Density (mA cm−2) | Potential Window (V) | Areal Capacitance (mF cm−2) | Energy Density (mWh cm−2) | Power Density (mW cm−2) |
|---|---|---|---|---|
| 2 | 0.47 | 795.7 | 0.0244 | 0.47 |
| 3 | 0.48 | 725.0 | 0.0232 | 0.72 |
| 5 | 0.50 | 630.1 | 0.0218 | 1.25 |
| 10 | 0.52 | 480.7 | 0.0181 | 2.6 |
| Sr. No. | Material | Method | Nanostructure | Specific Capacitance | Stability | Ref. |
|---|---|---|---|---|---|---|
| 1 | Manganese-iron oxide | Electrospinning | Nanofibers | 467 Fg−1 at 1 Ag−1 | 10,000 cycles—94% | [48] |
| 2 | Mn–Fe binary oxide | anodic deposition | Nano-spheres | 280 Fg−1 at 5 mV s−1 | - | [49] |
| 3 | MnFe2O4 RGO | Hydrothermal | Hollow sphere | 768 F g−1 at 8 Ag−1 | 4000 cycles—95% | [50] |
| 4 | Mn–Fe-based alloys | electrodeposition | Nano-flakes | 1.66 F cm−2 at 5 mV s−1 | - | [51] |
| 5 | Mn−Ni−S | Electrodeposition | 3D interconnected nanosheets | 2849 Fg−2 at 1 Ag−1 | 11,000 cycles—90% | [52] |
| 6 | Fe–Mn–O nanocomposites | Chemical precipitation | Irregular spherical particles | 125.2 Fg−1 at 0.2 Ag−1 | 1000 cycles—100% | [53] |
| 7 | Mn–Fe–S | electrodeposition | fragmented porous nanofibrous | 795.7 mF cm−2 at 2 mA cm−2 | 95,000 cycles—78.7% | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, J.C.; Yang, H.K.; Lee, J.S.; Lee, J.H.; Kang, M.G.; Kwon, E. Fabrication and Development of Binder-Free Mn–Fe–S Mixed Metal Sulfide Loaded Ni-Foam as Electrode for the Asymmetric Coin Cell Supercapacitor Device. Nanomaterials 2022, 12, 3193. https://doi.org/10.3390/nano12183193
Shin JC, Yang HK, Lee JS, Lee JH, Kang MG, Kwon E. Fabrication and Development of Binder-Free Mn–Fe–S Mixed Metal Sulfide Loaded Ni-Foam as Electrode for the Asymmetric Coin Cell Supercapacitor Device. Nanomaterials. 2022; 12(18):3193. https://doi.org/10.3390/nano12183193
Chicago/Turabian StyleShin, Jae Cheol, Hee Kwon Yang, Jeong Seok Lee, Jong Hyuk Lee, Min Gyu Kang, and Ein Kwon. 2022. "Fabrication and Development of Binder-Free Mn–Fe–S Mixed Metal Sulfide Loaded Ni-Foam as Electrode for the Asymmetric Coin Cell Supercapacitor Device" Nanomaterials 12, no. 18: 3193. https://doi.org/10.3390/nano12183193






