The Lattice Distortion, Defect Evolution and Electrochemical Performance Improvement in Zn-VO2(B) Nanorods
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. XRD
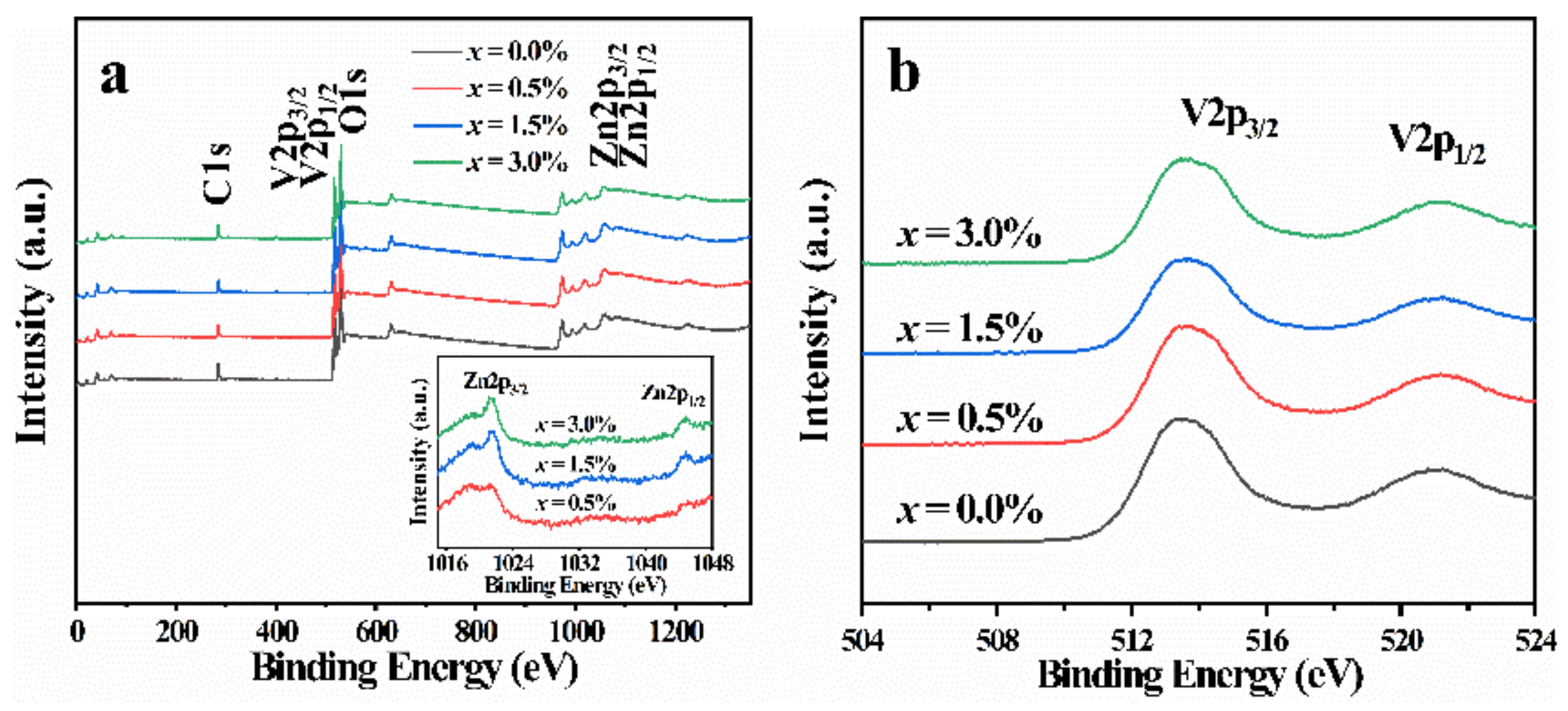
3.2. XPS
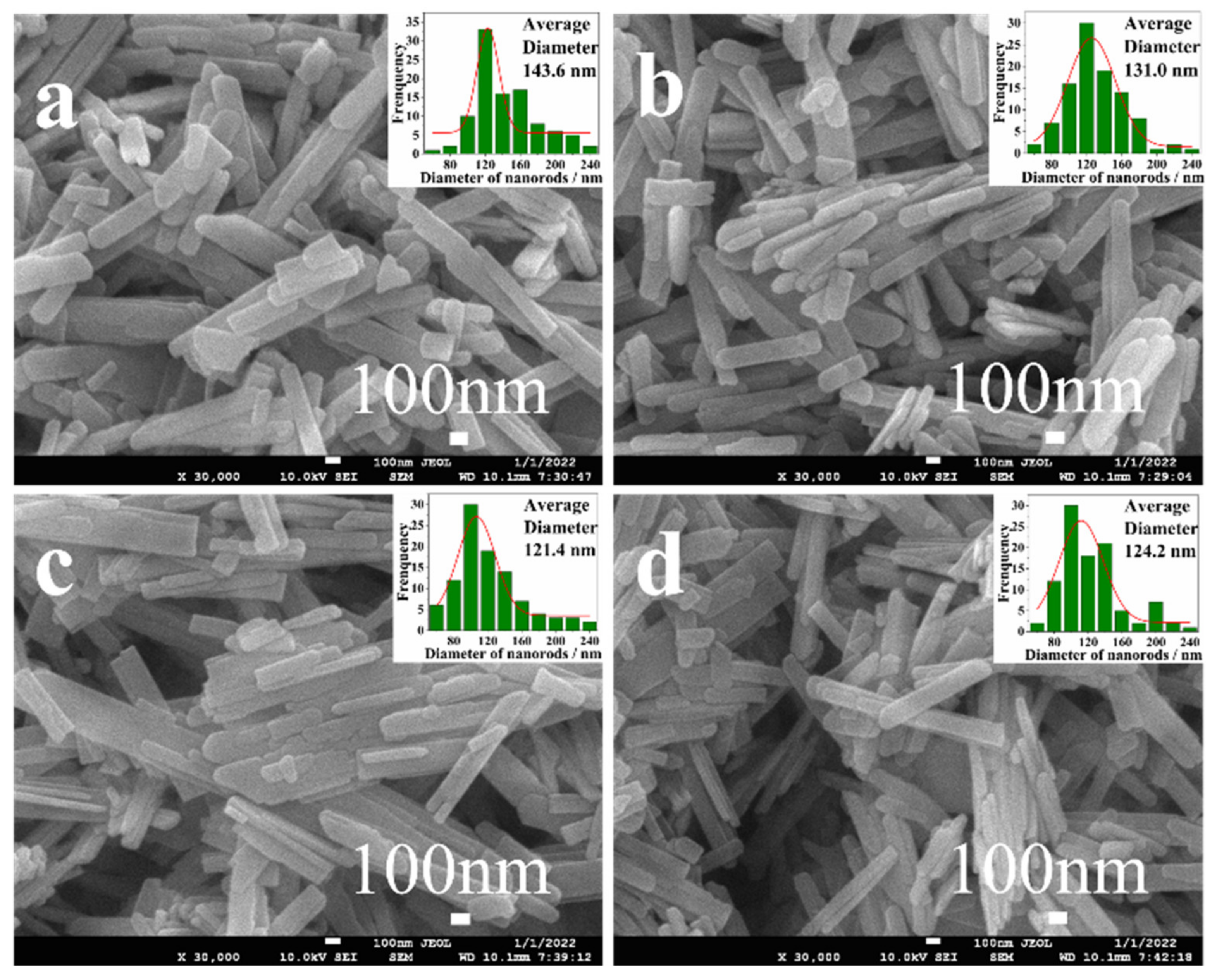
3.3. SEM
3.4. Positron Annihilation Analysis
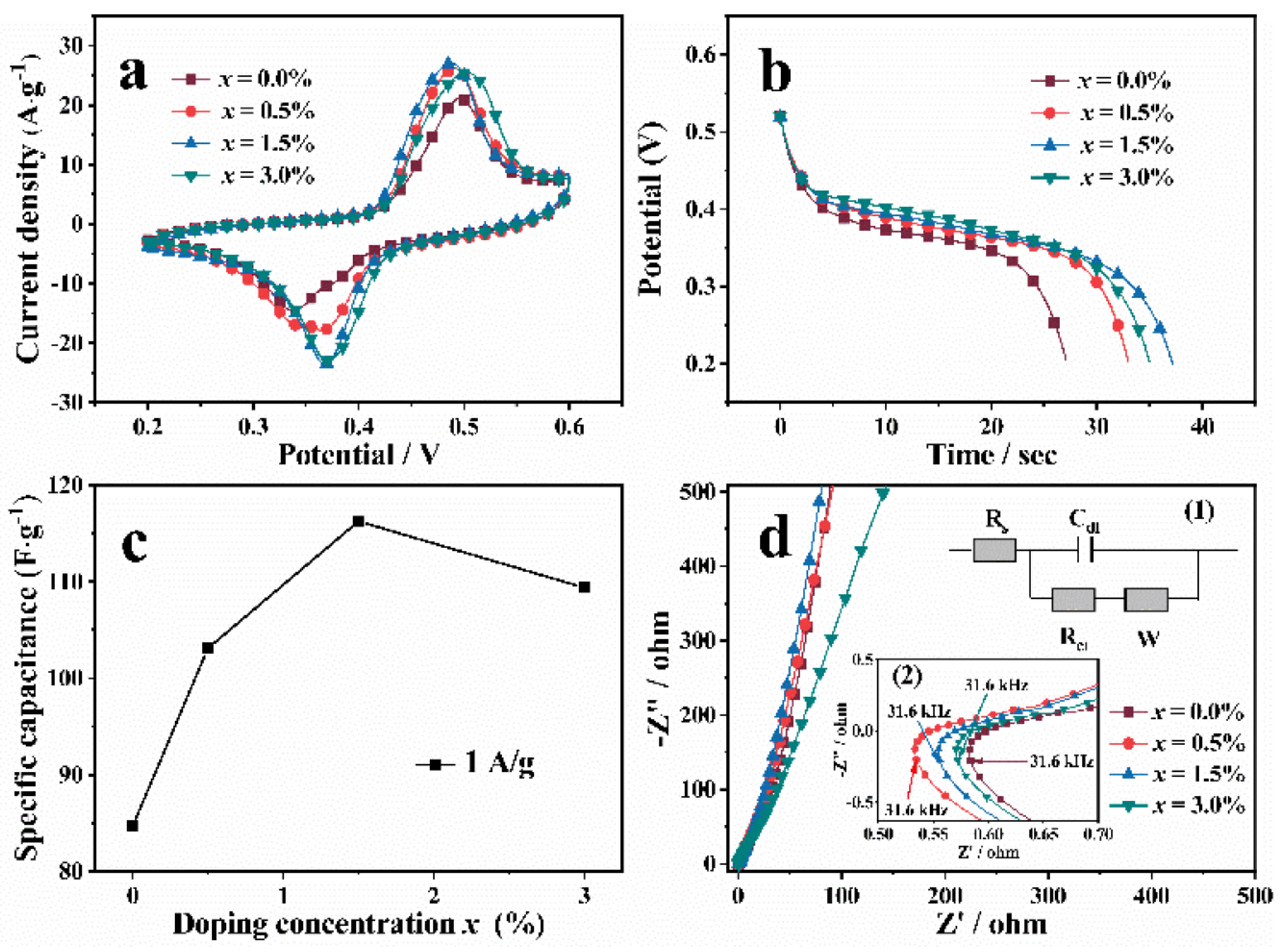
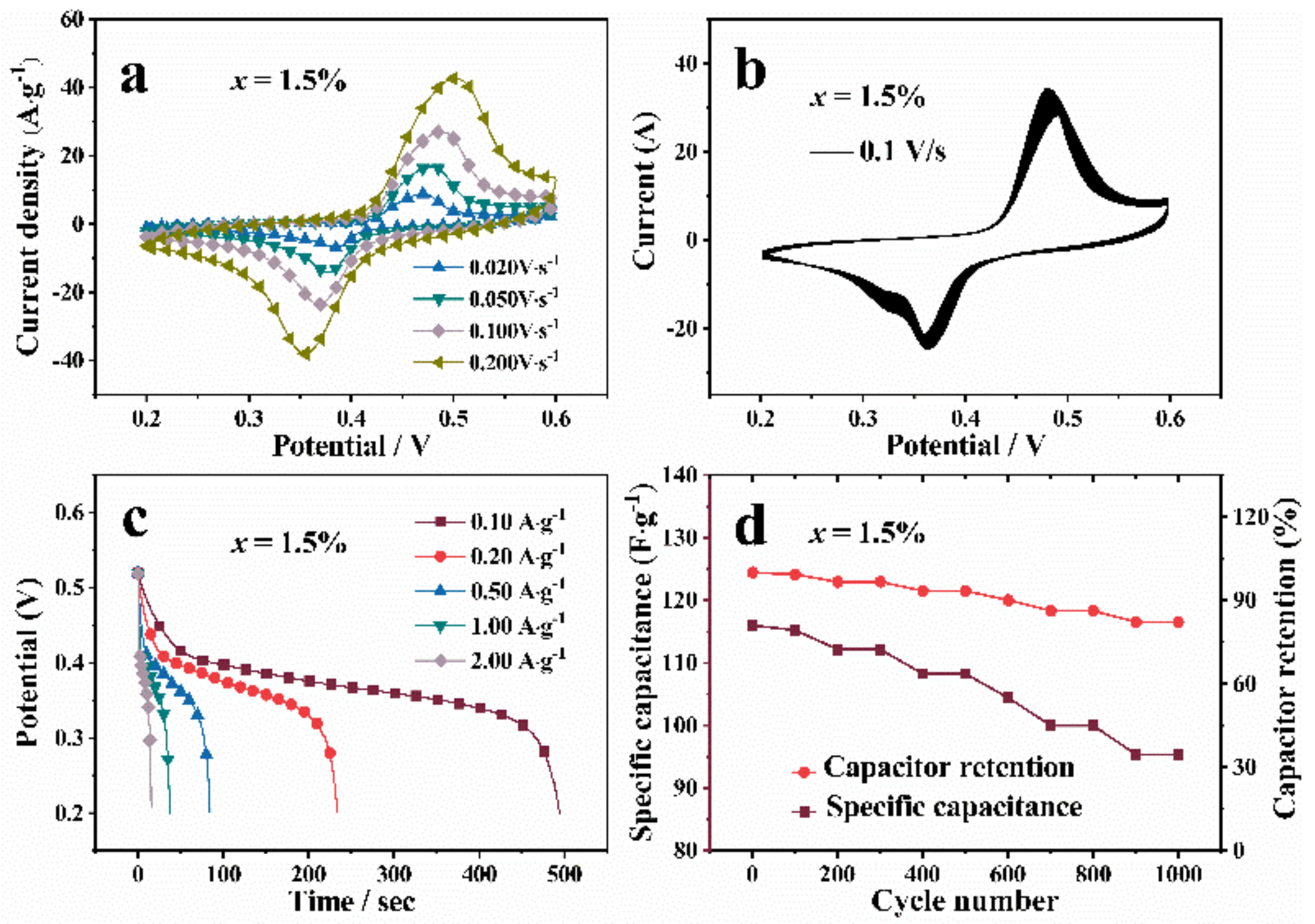
3.5. Electrochemical Performance Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lopez, J.; Mackanic, D.G.; Cui, Y.; Bao, Z. Designing polymers for advanced battery chemistries. Nat. Rev. Mater. 2019, 4, 312–330. [Google Scholar] [CrossRef]
- Hou, H.; Qiu, X.; Wei, W.; Zhang, Y.; Ji, X. Carbon anode materials for advanced sodium-ion batteries. Adv. Energy Mater. 2017, 7, 1602898. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Kim, Y. Challenges for rechargeable li batteries. Chem. Mater. 2010, 22, 587. [Google Scholar] [CrossRef]
- Cakan, R.D.; Palacin, M.R.; Croguennec, L. Rechargeable aqueous electrolyte batteries, from univalent to multivalent cation chemistry. J. Mater. Chem. A 2019, 7, 20519–20539. [Google Scholar] [CrossRef]
- Rashad, M.; Asif, M.; Ahmed, I.; He, Z.; Yin, L.; Wei, Z.X.; Wang, Y. Quest for carbon and vanadium oxide based rechargeable magnesium ion batteries. J. Magnes. Alloy 2020, 8, 364–373. [Google Scholar] [CrossRef]
- Zhuang, H.; Yang, F. Vanadium metal-organic framework derived 2D hierarchical VO2 nanosheets grown on carbon cloth for advanced flexible energy storage devices. Surf. Interfaces 2021, 25, 101232. [Google Scholar] [CrossRef]
- Wang, L.; Huang, K.W.; Chen, J.; Zheng, J. Ultralong cycle stability of aqueous zinc-ion batteries with zinc vanadium oxide cathodes. Sci. Adv. 2019, 5, 4279. [Google Scholar] [CrossRef]
- Luo, H.; Wang, B.; Wang, C.; Wu, F.; Jin, F.; Cong, B.; Dou, S. Synergistic deficiency and heterojunction engineering boosted VO2 redox kinetics for aqueous zinc–ion batteries with superior comprehensive performance. Energy Storage Mater. 2020, 33, 390–398. [Google Scholar] [CrossRef]
- Cen, Y.; Li, S.; Zhou, Y.; Cai, X.; Wang, X.; Xiang, Q.; Hu, B.; Yu, D.; Liu, Y.; Chen, C. Ultrathin VO2(B) nanosheets as cathode material for high-performance hybrid magnesium-lithium ion batteries. J. Electrochem. Soc. 2019, 166, A1660. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Lv, T.T.; Wang, H.; Guo, X.T.; Liu, C.S.; Pang, H. Nsutite-type VO2 microcrystals as highly durable cathode materials for aqueous zinc-ion batteries. Chem. Eng. J. 2021, 417, 128408. [Google Scholar] [CrossRef]
- Khan, Z.; Singh, P.; Ansari, S.A.; Manippady, S.R.; Jaiswal, A.; Saxena, M. VO2 nanostructures for batteries and supercapacitors: A review. Small 2021, 17, 2006651. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Yan, Y.; Cai, Y.; Cao, J.; Feng, J. Nanoarchitectured design of vertical-standing arrays for supercapacitors: Progress, challenges, and perspectives. Adv. Funct. Mater. 2021, 31, 2006030. [Google Scholar] [CrossRef]
- Yan, Y.; Lin, J.; Xu, T.; Liu, B.; Huang, K.; Qiao, L.; Qi, J. Atomic-level platinum filling into Ni-vacancies of dual-deficient NiO for boosting electrocatalytic hydrogen evolution. Adv. Funct. Mater. 2022, 13, 2200434. [Google Scholar] [CrossRef]
- Li, M.; Mou, J.; Zhong, L.; Liu, T.; Xu, Y.; Pan, W.; Liu, M. Porous ultrathin W-doped VO2 nanosheets enable boosted Zn2+ (De) intercalation kinetics in VO2 for high-performance aqueous Zn-ion batteries. ACS Sustain. Chem. Eng. 2021, 9, 14193–14201. [Google Scholar] [CrossRef]
- Liu, Y.; Zou, Y.; Guo, M.; Hui, Z.; Zhao, L. Boosting the active sites and kinetics of VO2 by Mn pre-intercalated and PVP modified nanostructure to improve the cycle stability for aqueous zinc batteries. Chem. Eng. J. 2022, 433, 133528. [Google Scholar] [CrossRef]
- Lv, T.T.; Luo, X.; Yuan, G.Q.; Yang, S.Y.; Pang, H. Layered VO2 @ N–doped carbon composites for high-performance rechargeable aqueous zinc–ion batteries. Chem. Eng. J. 2022, 428, 131211. [Google Scholar] [CrossRef]
- Kang, M.S.; Park, S.K.; Nakhanivej, P.; Shin, K.H.; Yeon, J.S.; Park, H.S. Mesoporous VO2(B) nanorods deposited onto graphene architectures for enhanced rate capability and cycle life of Li ion battery cathodes. J. Alloys Compd. 2021, 855, 157361. [Google Scholar] [CrossRef]
- Li, Z.; Ren, Y.; Mo, L.; Liu, C.; Hsu, K.; Ding, Y.; Cao, G. Impacts of oxygen vacancies on zinc ion intercalation in VO2. ACS Nano 2020, 14, 5581–5589. [Google Scholar] [CrossRef]
- Zhang, W.; Xiao, Y.; Zuo, C.; Tang, W.; Liu, G.; Wang, S.; Luo, P. Adjusting the valence state of vanadium in VO2(B) by extracting oxygen anions for high–performance aqueous zinc–ion batteries. ChemSusChem 2021, 14, 971–978. [Google Scholar] [CrossRef]
- Zhang, Z.; Xi, B.; Wang, X.; Ma, X.; Chen, W.; Feng, J.; Xiong, S. Oxygen defects engineering of VO2·xH2O nanosheets via in situ polypyrrole polymerization for efficient aqueous zinc ion storage. Adv. Funct. Mater. 2021, 31, 2103070. [Google Scholar] [CrossRef]
- Liu, D.W.; Zhang, Q.J.; Ding, S.P.; Yan, F.F.; Dai, H.Y.; Li, T.; Xue, R.Z.; Chen, J.; Gong, G.S.; Shang, C.; et al. Microdefects evolution and electrochemical performance modulation of Mn doped VO2(B) nanorods. J. Alloys Compd. 2022, 911, 164975. [Google Scholar] [CrossRef]
- Liu, D.W.; Yang, P.; Dai, H.Y.; Li, T.; Xue, R.Z.; Chen, J.; Chen, Z.P. Effects of oxalic acid concentration on the microstructures and properties of nano–VO2(B). J. Solid State Electrochem. 2019, 23, 2951–2959. [Google Scholar] [CrossRef]
- Kang, J.; Liu, J.; Shi, F.; Dong, Y.; Jiang, S. The thermochromic characteristics of Zn-doped VO2 that were prepared by the hydrothermal and post-annealing process and their polyurethane composite films. Ceram. Int. 2021, 47, 15631–15638. [Google Scholar] [CrossRef]
- Zhai, X.Z.; Deng, H.M.; Zhou, W.L.; Yang, P.X.; Chu, J.H. Strain-induced structural phase transition, ferromagnetic and optical properties of Bi1−xTbxFeO3 thin films. J. Phys. D. 2015, 48, 385002. [Google Scholar] [CrossRef]
- Puska, M.; Nieminen, R. Theory of positrons in solids and on solid surfaces. Rev. Mod. Phys. 1994, 66, 841. [Google Scholar] [CrossRef]
- Wang, M.; Dai, H.; Li, T.; Chen, J.; Yan, F.; Xue, R.; Xing, X.; Chen, D.; Ping, T.; He, J. The evolution of structure and properties in GdMn(1-x)TixO3 ceramics. J. Mater. Sci. Mater. Electron. 2021, 32, 27348–27361. [Google Scholar] [CrossRef]
- Li, Z.; Qin, Z.; Zhang, W.; Li, Z. Controlled synthesis of Ni(OH)2/MoS2 nanohybrids for high-performance supercapacitors. Mater. Chem. Phys. 2018, 209, 291–297. [Google Scholar] [CrossRef]
- Encinas-Sánchez, V.; de Miguel, M.; Lasanta, M.; García-Martín, G.; Pérez, F. Electrochemical impedance spectroscopy (EIS): An efficient technique for monitoring corrosion processes in molten salt environments in CSP applications. Sol. Energy Mater. Sol. Cells 2019, 191, 157–163. [Google Scholar] [CrossRef]
- Brug, G.J.; van den Eeden, A.L.; Sluyters-Rehbach, M.; Sluyters, J.H. The analysis of electrode impedances complicated by the presence of a constant phase element. J. Electroanal. Chem. 1984, 176, 275–295. [Google Scholar] [CrossRef]
- Yan, Y.T.; Lin, J.H.; Liu, T.; Liu, B.S.; Wang, B.; Qiao, L.; Tu, J.C.; Cao, J.; Qi, J.L. Corrosion behavior of stainless steel-tungsten carbide joints brazed with AgCuX (X = In, Ti) alloys. Corros. Sci. 2022, 200, 110231. [Google Scholar] [CrossRef]



| Doping Concentration | Lattice Parameters | β | |||
|---|---|---|---|---|---|
| x | a(Å) | b(Å) | c(Å) | V(Å3) | |
| 0.0% | 12.085076 | 3.692486 | 6.435457 | 274.704 | 106.948 |
| 0.5% | 12.107583 | 3.699608 | 6.451428 | 276.424 | 106.952 |
| 1.5% | 12.103891 | 3.697008 | 6.451905 | 276.200 | 106.929 |
| 3.0% | 12.109881 | 3.699087 | 6.456372 | 276.705 | 106.936 |
| Sample | Positron Lifetime/ps | Intensity/% | Bulk Lifetime/ps | ||||
|---|---|---|---|---|---|---|---|
| x | τ1 | τ2 | τ3 | I1 | I2 | I3 | τb |
| 0.0% | 256.0 | 433.1 | 1149.3 | 58.7545 | 39.8729 | 1.3726 | 310.9707 |
| 0.5% | 248.9 | 388.7 | 1315.7 | 48.4924 | 51.1953 | 0.3123 | 306.2451 |
| 1.5% | 222.2 | 356.7 | 1277.8 | 23.2777 | 75.9608 | 0.7615 | 314.7481 |
| 3.0% | 228.3 | 361.7 | 1479.5 | 20.4139 | 79.0256 | 0.5605 | 324.7799 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, D.; Zhang, Q.; Chen, X.; Zhu, P.; Yan, F.; Wang, X.; Dai, H.; Chen, J.; Gong, G.; Shang, C.; et al. The Lattice Distortion, Defect Evolution and Electrochemical Performance Improvement in Zn-VO2(B) Nanorods. Nanomaterials 2022, 12, 3196. https://doi.org/10.3390/nano12183196
Liu D, Zhang Q, Chen X, Zhu P, Yan F, Wang X, Dai H, Chen J, Gong G, Shang C, et al. The Lattice Distortion, Defect Evolution and Electrochemical Performance Improvement in Zn-VO2(B) Nanorods. Nanomaterials. 2022; 12(18):3196. https://doi.org/10.3390/nano12183196
Chicago/Turabian StyleLiu, Dewei, Qijie Zhang, Xiaohong Chen, Penggang Zhu, Fufeng Yan, Xuzhe Wang, Haiyang Dai, Jing Chen, Gaoshang Gong, Cui Shang, and et al. 2022. "The Lattice Distortion, Defect Evolution and Electrochemical Performance Improvement in Zn-VO2(B) Nanorods" Nanomaterials 12, no. 18: 3196. https://doi.org/10.3390/nano12183196
APA StyleLiu, D., Zhang, Q., Chen, X., Zhu, P., Yan, F., Wang, X., Dai, H., Chen, J., Gong, G., Shang, C., Xie, L., & Zhai, X. (2022). The Lattice Distortion, Defect Evolution and Electrochemical Performance Improvement in Zn-VO2(B) Nanorods. Nanomaterials, 12(18), 3196. https://doi.org/10.3390/nano12183196





