Synergy of Au–Pt for Enhancing Ethylene Photodegradation Performance of Flower-like TiO2
Abstract
:1. Introduction
2. Experimental Section
2.1. Chemicals
2.2. Synthesis of TiO2 Microspheres (TiO2 MSs)
2.3. Synthesis of Au or Pt NPs Loaded on TiO2 Microspheres
2.4. Synthesis of Au–Pt NPs Loaded on TiO2 Microspheres
2.5. Characterizations
2.6. Photocatalytic Experiments
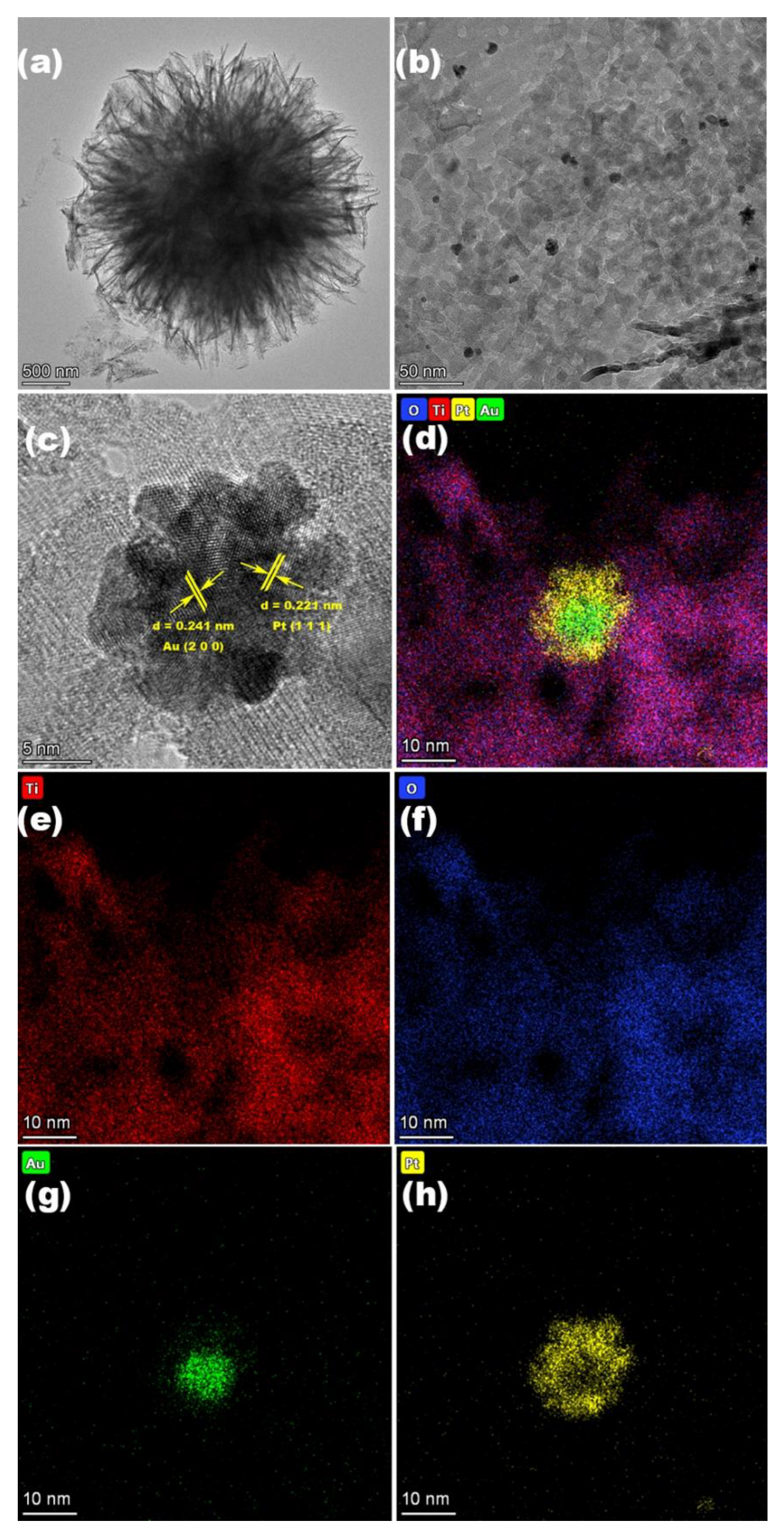
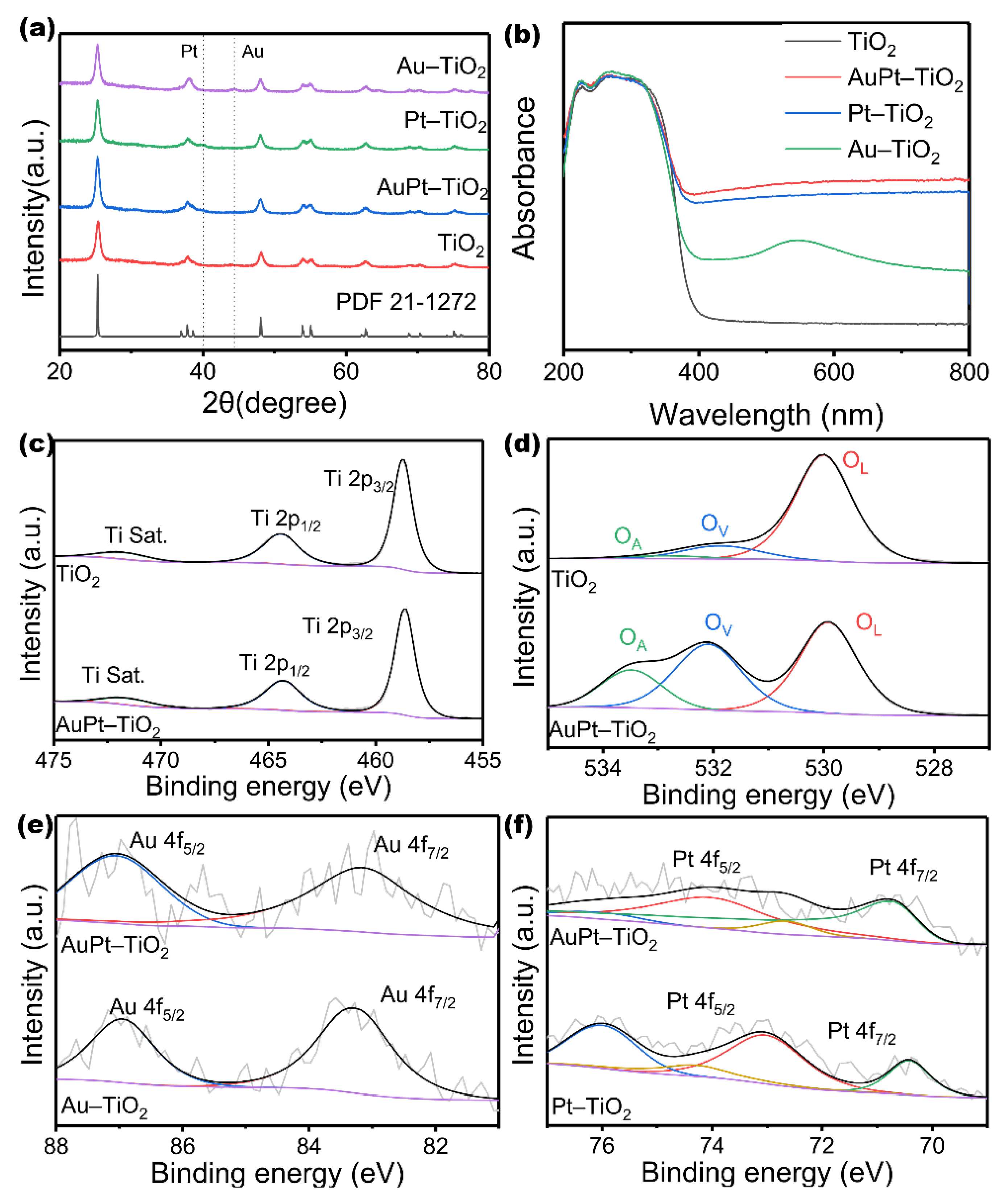
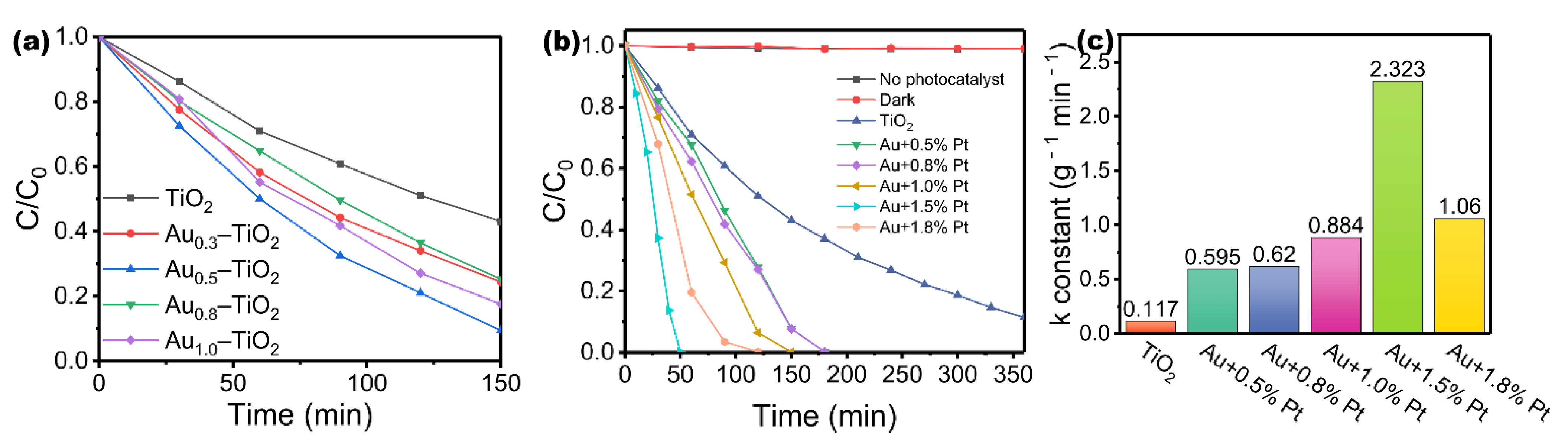
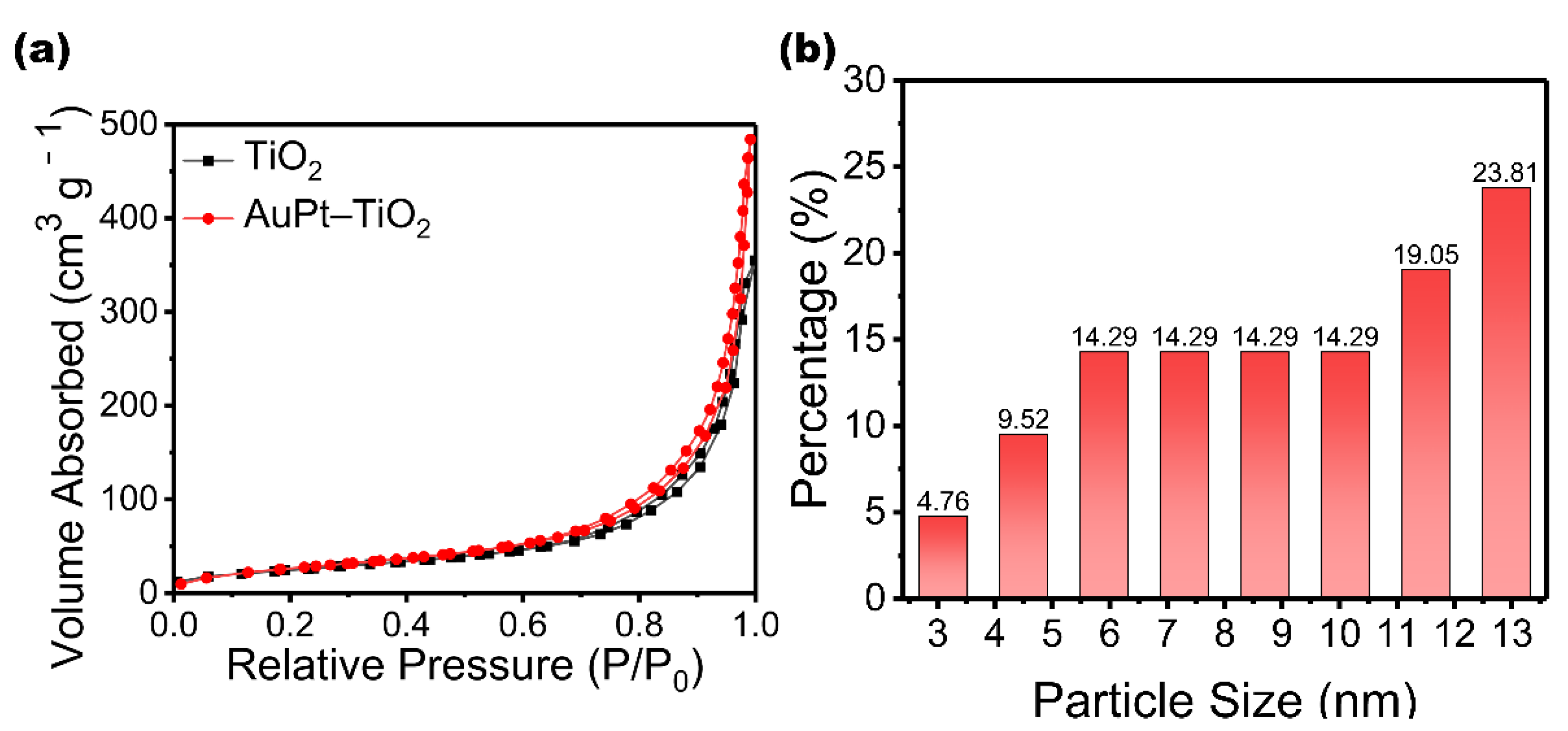
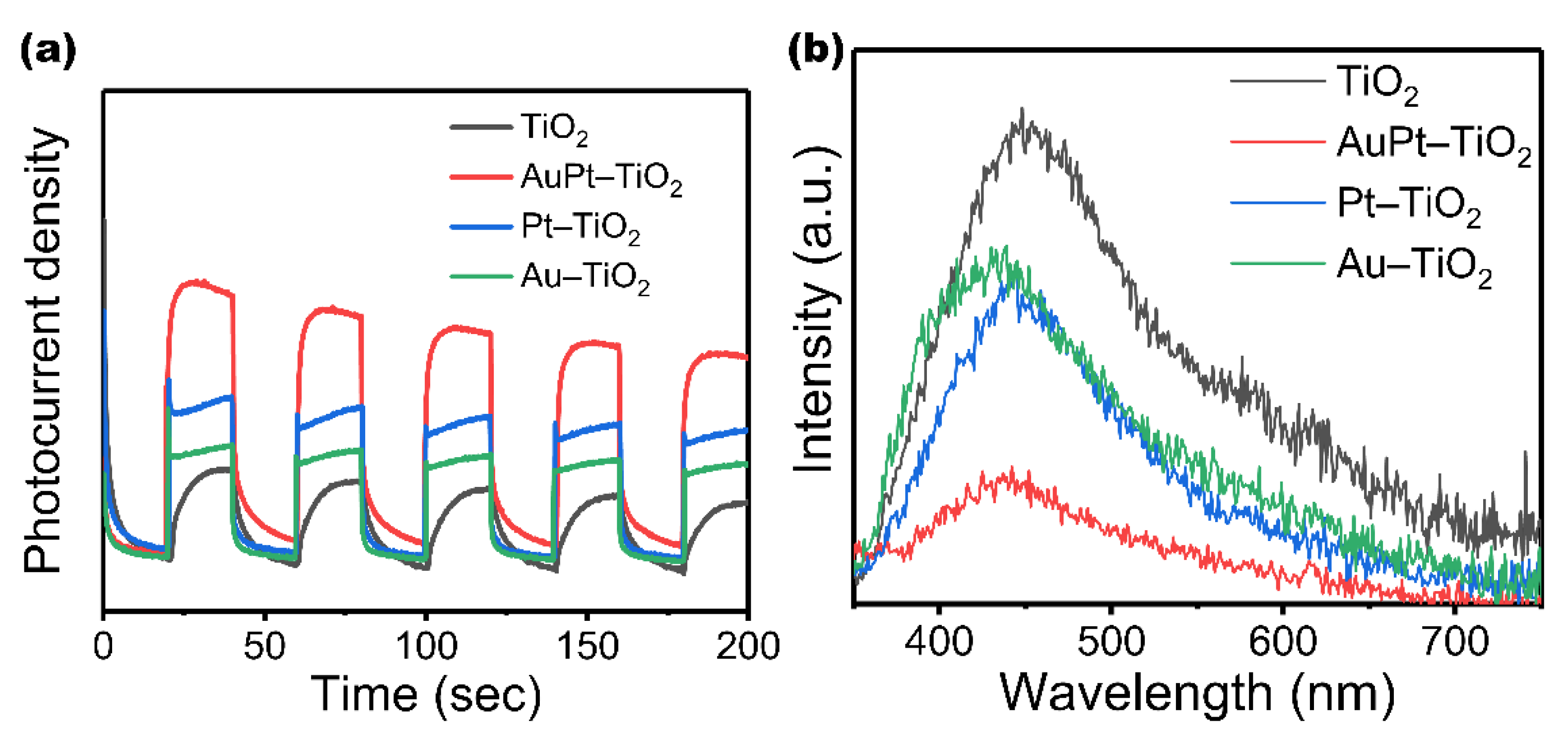
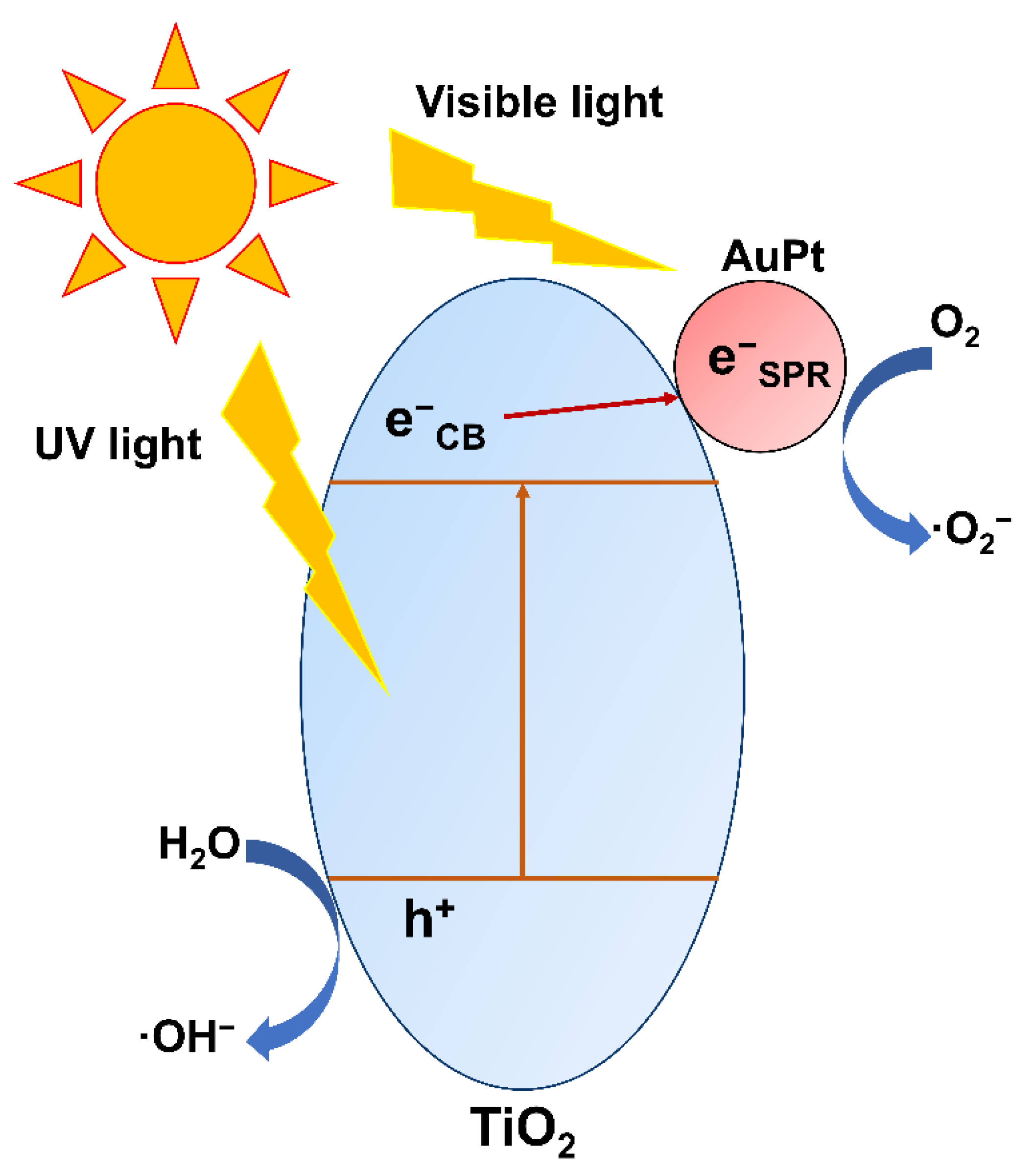
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saltveit, M.E. Effect of ethylene on quality of fresh fruits and vegetables. Postharvest Biol. Tec. 1999, 15, 279–292. [Google Scholar] [CrossRef]
- Keller, N.; Ducamp, M.; Robert, D.; Keller, V. Ethylene Removal and Fresh Product Storage: A Challenge at the Frontiers of Chemistry. Toward an Approach by Photocatalytic Oxidation. Chem. Rev. 2013, 113, 5029–5070. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, S.; Tanaka, S.; Tsukamoto, H. Kinetic studies of oxidation of ethylene over a TiO2 photocatalyst. J. Photochem. Photobiol. A 1999, 121, 55–61. [Google Scholar] [CrossRef]
- Thalluri, S.M.; Hussain, M.; Saracco, G.; Barber, J.; Russo, N. Green-Synthesized BiVO4 Oriented along {040} Facets for Visible-Light-Driven Ethylene Degradation. Ind. Eng. Chem. Res. 2014, 53, 2640–2646. [Google Scholar] [CrossRef]
- Cui, Z.; Liu, X.; Liang, X.; Wang, P.; Zhang, Q.; Wang, Z.; Zheng, Z.; Liu, Y.; Dai, Y.; Huang, B. ZnO nanorods modified with noble metal-free Co3O4 nanoparticles as a photocatalyst for efficient ethylene degradation under light irradiation. Catal. Sci. Technol. 2019, 9, 6191–6198. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, P.; Zhu, X.; Zhang, Q.; Wang, Z.; Liu, Y.; Zou, G.; Dai, Y.; Whangbo, M.; Huang, B. Composite of CH3NH3PbI3 with Reduced Graphene Oxide as a Highly Efficient and Stable Visible-Light Photocatalyst for Hydrogen Evolution in Aqueous HI Solution. Adv. Mater. 2018, 30, 1704342. [Google Scholar] [CrossRef]
- Chen, G.; Wang, P.; Wu, Y.; Zhang, Q.; Wu, Q.; Wang, Z.; Zheng, Z.; Liu, Y.; Dai, Y.; Huang, B. Lead-Free Halide Perovskite Cs3Bi2xSb2–2xI9 (x ≈ 0.3) Possessing the Photocatalytic Activity for Hydrogen Evolution Comparable to that of (CH3NH3)PbI3. Adv. Mater. 2020, 32, 2001344. [Google Scholar] [CrossRef]
- Li, S.; Liang, J.; Wei, P.; Liu, Q.; Xie, L.; Luo, Y.; Sun, X. ITO@TiO2 nanoarray: An efficient and robust nitrite reduction reaction electrocatalyst toward NH3 production under ambient conditions. eScience 2022, 2, 382–388. [Google Scholar] [CrossRef]
- Belapurkar, A.D.; Kamble, V.S.; Dey, G.R. Photo-oxidation of ethylene in gas phase and methanol and formic acid in liquid phase on synthesized TiO2 and Au/TiO2 catalysts. Mater. Chem. Phys. 2010, 123, 801–805. [Google Scholar] [CrossRef]
- Kumar, S.; Fedorov, A.G.; Gole, J.L. Photodegradation of ethylene using visible light responsive surfaces prepared from titania nanoparticle slurries. Appl. Catal. B Environ. 2005, 57, 93–107. [Google Scholar] [CrossRef]
- Liang, X.; Wang, P.; Li, M.; Zhang, Q.; Wang, Z.; Dai, Y.; Zhang, X.; Liu, Y.; Whangbo, M.; Huang, B. Adsorption of gaseous ethylene via induced polarization on plasmonic photocatalyst Ag/AgCl/TiO2 and subsequent photodegradation. Appl. Catal. B Environ. 2018, 220, 356–361. [Google Scholar] [CrossRef]
- Chen, X.; Li, R.; Pan, X.; Huang, X.; Yi, Z. Fabrication of In2O3-Ag-Ag3PO4 composites with Z-scheme configuration for photocatalytic ethylene degradation under visible light irradiation. Chem. Eng. J. 2017, 320, 644–652. [Google Scholar] [CrossRef]
- Park, D.; Zhang, J.; Ikeue, K.; Yamashita, H.; Anpo, M. Photocatalytic Oxidation of Ethylene to CO2 and H2O on Ultrafine Powdered TiO2 Photocatalysts in the Presence of O2 and H2O. J. Catal. 1999, 185, 114–119. [Google Scholar] [CrossRef]
- Liu, H.; Wang, Z.; Li, H.; Zhang, X.; Qin, X.; Dai, Y.; Wang, P.; Liu, Y.; Huang, B. Photocatalytic degradation of ethylene by Ga2O3 polymorphs. RSC Adv. 2018, 8, 14328–14334. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zheng, L.; Bao, X.; Wang, Z.; Wang, P.; Liu, Y.; Cheng, H.; Dai, Y.; Huang, B.; Zheng, Z. Substrate-dependent ALD of Cux on TiO2 and its performance in photocatalytic CO2 reduction. Chem. Eng. J. 2021, 405, 126654. [Google Scholar] [CrossRef]
- Bao, X.; Li, H.; Wang, Z.; Tong, F.; Liu, M.; Zheng, Z.; Wang, P.; Cheng, H.; Liu, Y.; Dai, Y.; et al. TiO2/Ti3C2 as an efficient photocatalyst for selective oxidation of benzyl alcohol to benzaldehyde. Appl. Catal. B: Environ. 2021, 286, 119885. [Google Scholar] [CrossRef]
- Liu, M.; Bao, X.; Ma, F.; Wang, M.; Zheng, L.; Wang, Z.; Wang, P.; Liu, Y.; Cheng, H.; Dai, Y.; et al. Enhanced stability and activity towards photocatalytic CO2 reduction via supercycle ALD of Cu and TiO2. Chem. Eng. J. 2022, 429, 132022. [Google Scholar] [CrossRef]
- Bao, X.; Zhang, M.; Wang, Z.; Dai, D.; Wang, P.; Cheng, H.; Liu, Y.; Zheng, Z.; Dai, Y.; Huang, B. Molten-salt assisted synthesis of Cu clusters modified TiO2 with oxygen vacancies for efficient photocatalytic reduction of CO2 to CO. Chem. Eng. J. 2022, 445, 136718. [Google Scholar] [CrossRef]
- Zhou, M.; Wang, H. Insight into the photoexcitation effect on the catalytic activation of H2 and C-H bonds on TiO2(110) surface. Chin. Chem. Lett. 2022, 33, 4705–4709. [Google Scholar] [CrossRef]
- Xiong, J.; Zhang, M.; Lu, M.; Zhao, K.; Han, C.; Cheng, G.; Wen, Z. Achieving simultaneous Cu particles anchoring in meso-porous TiO2 nanofabrication for enhancing photo-catalytic CO2 reduction through rapid charge separation. Chin. Chem. Lett. 2022, 33, 1313–1316. [Google Scholar] [CrossRef]
- Zhang, Q.; Xiao, Y.; Yang, L.; Wen, Y.; Xiong, Z.; Lei, L.; Wang, L.; Zeng, Q. Branched core-shell a-TiO2@N-TiO2 nanospheres with gradient-doped N for highly efficient photocatalytic applications. Chin. Chem. Lett. 2022. [CrossRef]
- Wu, Y.; Wu, Q.; Zhang, Q.; Lou, Z.; Liu, K.; Ma, Y.; Wang, Z.; Zheng, Z.; Cheng, H.; Liu, Y.; et al. An organometal halide perovskite supported Pt single-atom photocatalyst for H2 evolution. Energy Environ. Sci. 2022, 15, 1271–1281. [Google Scholar] [CrossRef]
- Su, H.; Soldatov, M.A.; Roldugin, V.; Liu, Q. Platinum single-atom catalyst with self-adjustable valence state for large-current-density acidic water oxidation. eScience 2022, 2, 102–109. [Google Scholar] [CrossRef]
- Jiang, Z.; Zhang, X.; Sun, W.; Yang, D.; Duchesne, P.N.; Gao, Y.; Wang, Z.; Yan, T.; Yuan, Z.; Yang, G.; et al. Building a Bridge from Papermaking to Solar Fuels. Angew. Chem. Int. Ed. 2019, 58, 14850–14854. [Google Scholar] [CrossRef]
- Jiang, Z.; Miao, W.; Zhu, X.; Yang, G.; Yuan, Z.; Chen, J.; Ji, X.; Kong, F.; Huang, B. Modifying lewis base on TiO2 nanosheets for enhancing CO2 adsorption and the separation of photogenerated charge carriers. Appl. Catal. B Environ. 2019, 256, 117881. [Google Scholar] [CrossRef]
- Zhu, X.; Liang, X.; Wang, P.; Dai, Y.; Huang, B. Porous Ag-ZnO microspheres as efficient photocatalyst for methane and ethylene oxidation: Insight into the role of Ag particles. Appl. Surf. Sci. 2018, 456, 493–500. [Google Scholar] [CrossRef]
- Li, M.; Zhang, N.; Long, R.; Ye, W.; Wang, C.; Xiong, Y. PdPt Alloy Nanocatalysts Supported on TiO2: Maneuvering Metal-Hydrogen Interactions for Light-Driven and Water-Donating Selective Alkyne Semihydrogenation. Small 2017, 13, 1604173. [Google Scholar] [CrossRef]
- Ye, W.; Chen, S.; Ye, M.; Ren, C.; Ma, J.; Long, R.; Wang, C.; Yang, J.; Song, L.; Xiong, Y. Pt4PdCu0.4 alloy nanoframes as highly efficient and robust bifunctional electrocatalysts for oxygen reduction reaction and formic acid oxidation. Nano. Energy 2017, 39, 532–538. [Google Scholar] [CrossRef]
- Li, Y.; Wang, L.; Low, J.; Wu, D.; Hu, C.; Jiang, W.; Ma, J.; Wang, C.; Long, R.; Song, L.; et al. Integrating bimetallic AuPd nanocatalysts with a 2D aza-fused π-conjugated microporous polymer for light-driven benzyl alcohol oxidation. Chin. Chem. Lett. 2020, 31, 231–234. [Google Scholar] [CrossRef]
- Hu, C.; Chen, X.; Jin, J.; Han, Y.; Chen, S.; Ju, H.; Cai, J.; Qiu, Y.; Gao, C.; Wang, C.; et al. Surface Plasmon Enabling Nitrogen Fixation in Pure Water through a Dissociative Mechanism under Mild Conditions. J. Am. Chem. Soc. 2019, 141, 7807–7814. [Google Scholar] [CrossRef]
- Zhai, H.; Liu, X.; Wang, Z.; Liu, Y.; Zheng, Z.; Qin, X.; Zhang, X.; Wang, P.; Huang, B. ZnO nanorod decorated by Au-Ag alloy with greatly increased activity for photocatalytic ethylene oxidation. Chin. J. Catal. 2020, 41, 1613–1621. [Google Scholar] [CrossRef]
- Liao, J.; He, J.; Xu, H.; Kuang, D.; Su, C. Effect of TiO2 morphology on photovoltaic performance of dye-sensitized solar cells: Nanoparticles, nanofibers, hierarchical spheres and ellipsoid spheres. J. Mater. Chem. 2012, 22, 7910. [Google Scholar] [CrossRef]
- Hao, Q.; Juluri, B.K.; Zheng, Y.B.; Wang, B.; Chiang, I.; Jensen, L.; Crespi, V.; Eklund, P.C.; Huang, T.J. Effects of Intrinsic Fano Interference on Surface Enhanced Raman Spectroscopy: Comparison between Platinum and Gold. J. Phys. Chem. C 2010, 114, 18059–18066. [Google Scholar] [CrossRef]
- Mahmood, A.; Wang, X.; Xie, X.; Sun, J. Atomically Dispersed Pt on TiO2 Nanosheets for Catalytic Gaseous Acetaldehyde Abatement. ACS Appl. Nano Mater. 2021, 4, 3799–3810. [Google Scholar] [CrossRef]
- Cao, B.; Li, G.; Li, H. Hollow spherical RuO2@TiO2@Pt bifunctional photocatalyst for coupled H2 production and pollutant degradation. Appl. Catal. B Environ. 2016, 194, 42–49. [Google Scholar] [CrossRef]
- Su, N.; Hu, X.; Zhang, J.; Huang, H.; Cheng, J.; Yu, J.; Ge, C. Plasma-induced synthesis of Pt nanoparticles supported on TiO2 nanotubes for enhanced methanol electro-oxidation. Appl. Surf. Sci. 2017, 399, 403–410. [Google Scholar] [CrossRef]
- Wang, Y.; Cheng, P.; Li, X.; Wang, C.; Feng, C.; Lu, G. Revealing the relationship between the Au decoration method and the enhanced acetone sensing performance of a mesoporous In2O3-based gas sensor. J. Mater. Chem. C 2020, 8, 78–88. [Google Scholar] [CrossRef]
- Serafin, J.; Ouzzine, M.; Sreńscek-Nazzal, J.; Llorca, J. Photocatalytic hydrogen production from alcohol aqueous solutions over TiO2-activated carbon composites decorated with Au and Pt. J. Photochem. 2022, 425, 113726. [Google Scholar] [CrossRef]
- Bai, Y.; Ye, L.; Wang, L.; Shi, X.; Wang, P.; Bai, W. A dual-cocatalyst-loaded Au/BiOI/MnOx system for enhanced photocatalytic greenhouse gas conversion into solar fuels. Environ. Sci-Nano. 2016, 3, 902–909. [Google Scholar] [CrossRef]
- Shang, Y.; Shi, R.; Cui, Y.; Che, Q.; Wang, J.; Yang, P. Urchin-Like WO2.72 Microspheres Decorated with Au and PdO Nanoparticles for the Selective Detection of Trimethylamine. Acs. Appl. Nano. Mater. 2020, 3, 5554–5564. [Google Scholar] [CrossRef]
- Shang, Y.; Cui, Y.; Shi, R.; Zhang, A.; Wang, Y.; Yang, P. Self-reduction combined with photo-deposition decorating Au nanoparticles on urchin-like WO2.72 for enhancement of trimethylamine-sensing performance. Mat. Sci. Semicon. Proc. 2019, 101, 131–138. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, W.; Zhao, Y.; Dai, D.; Zhang, Q.; Wang, Z.; Liu, Y.; Zheng, Z.; Cheng, H.; Dai, Y.; Huang, B.; et al. Synergy of Au–Pt for Enhancing Ethylene Photodegradation Performance of Flower-like TiO2. Nanomaterials 2022, 12, 3221. https://doi.org/10.3390/nano12183221
Meng W, Zhao Y, Dai D, Zhang Q, Wang Z, Liu Y, Zheng Z, Cheng H, Dai Y, Huang B, et al. Synergy of Au–Pt for Enhancing Ethylene Photodegradation Performance of Flower-like TiO2. Nanomaterials. 2022; 12(18):3221. https://doi.org/10.3390/nano12183221
Chicago/Turabian StyleMeng, Wanzhen, Yunrui Zhao, Dujuan Dai, Qianqian Zhang, Zeyan Wang, Yuanyuan Liu, Zhaoke Zheng, Hefeng Cheng, Ying Dai, Baibiao Huang, and et al. 2022. "Synergy of Au–Pt for Enhancing Ethylene Photodegradation Performance of Flower-like TiO2" Nanomaterials 12, no. 18: 3221. https://doi.org/10.3390/nano12183221
APA StyleMeng, W., Zhao, Y., Dai, D., Zhang, Q., Wang, Z., Liu, Y., Zheng, Z., Cheng, H., Dai, Y., Huang, B., & Wang, P. (2022). Synergy of Au–Pt for Enhancing Ethylene Photodegradation Performance of Flower-like TiO2. Nanomaterials, 12(18), 3221. https://doi.org/10.3390/nano12183221








