Platinum Nanoparticle Extraction, Quantification, and Characterization in Sediments by Single-Particle Inductively Coupled Plasma Time-of-Flight Mass Spectrometry
Abstract
1. Introduction
2. Materials and Methods
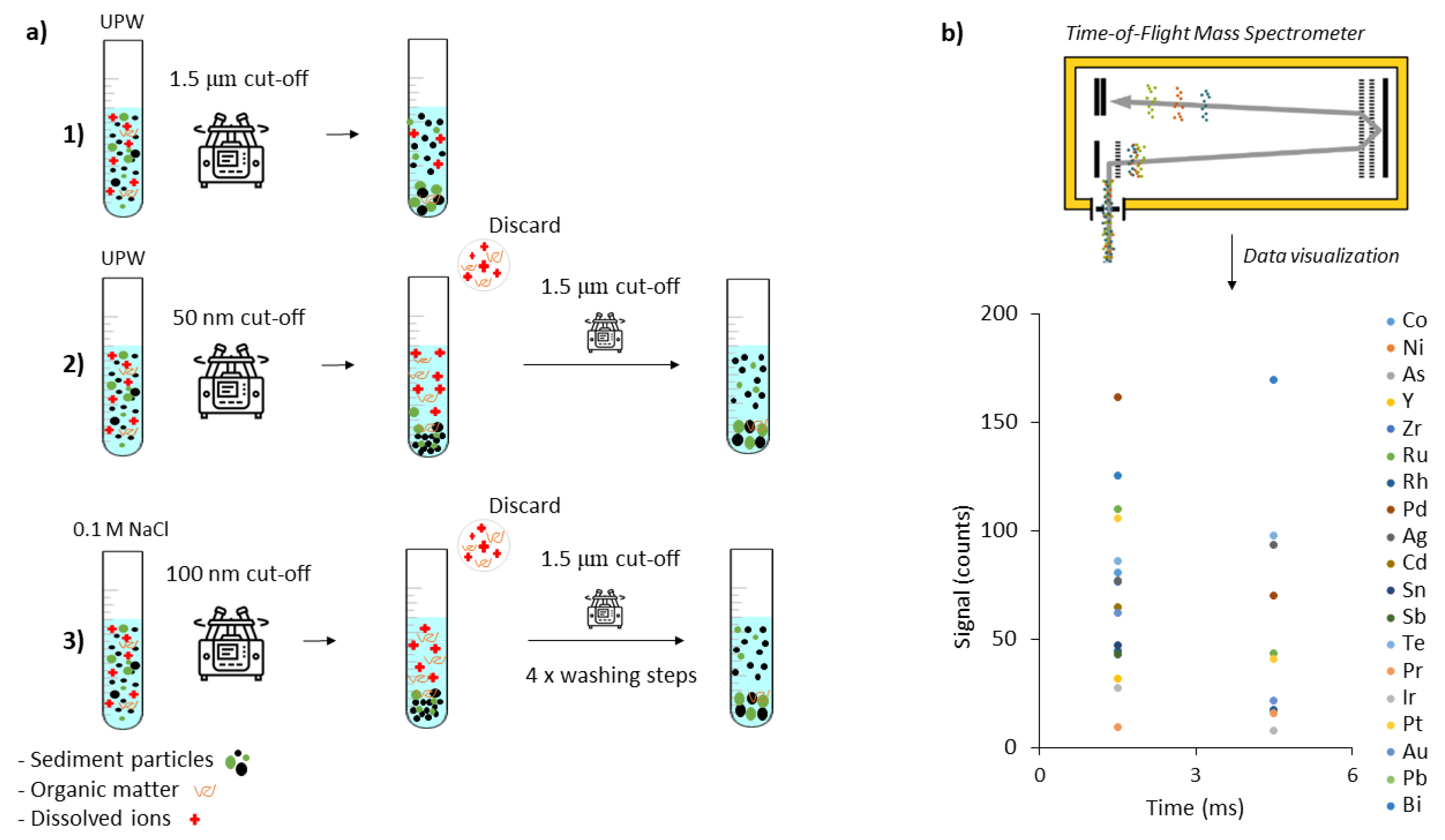
2.1. Nanoparticle Extraction Procedure
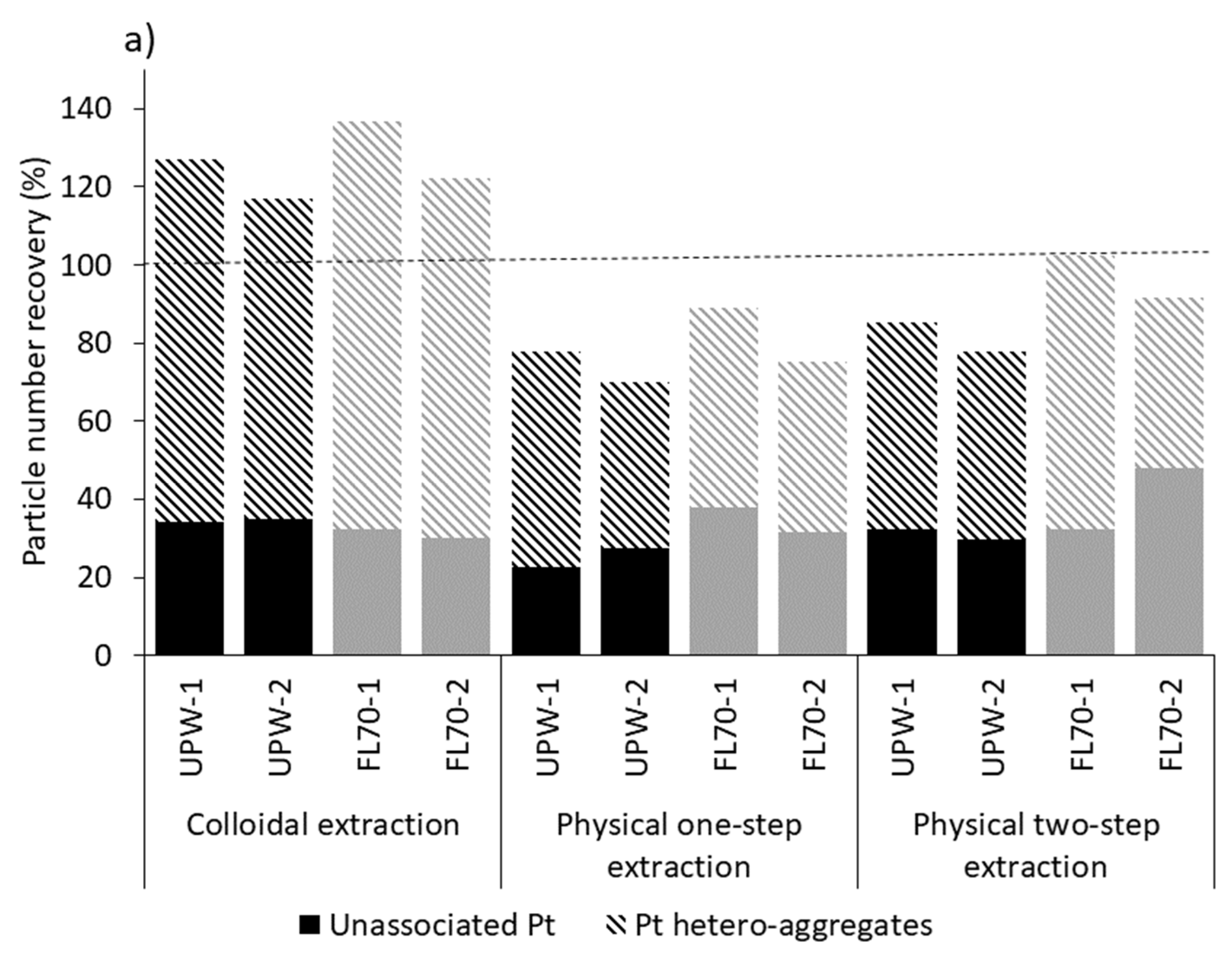
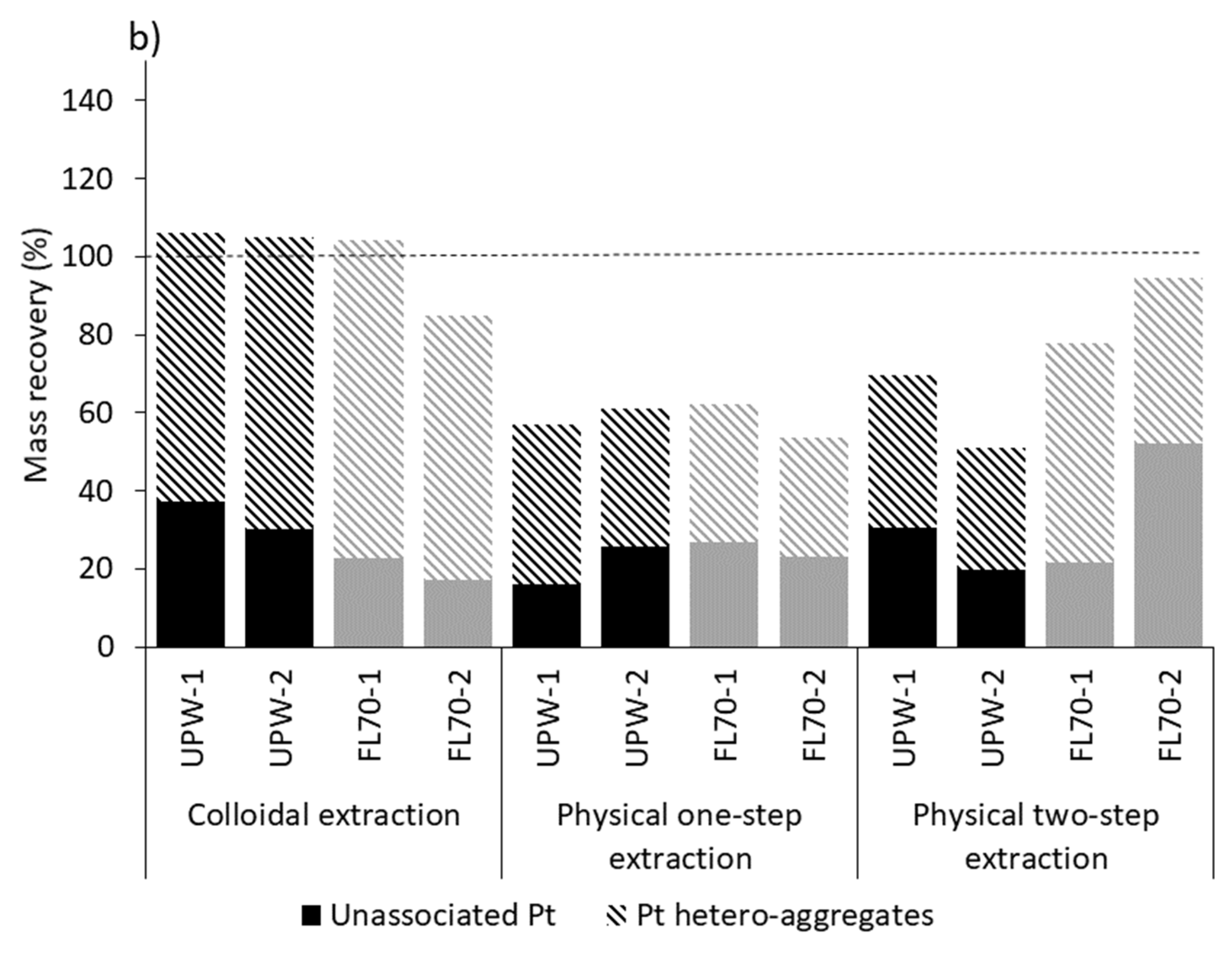
2.2. Pt Nanoparticle Extraction Efficiency
2.3. Single-Particle ICP-TOF-MS Analysis
2.4. Mineral and Carbon Analysis
3. Results and Discussion
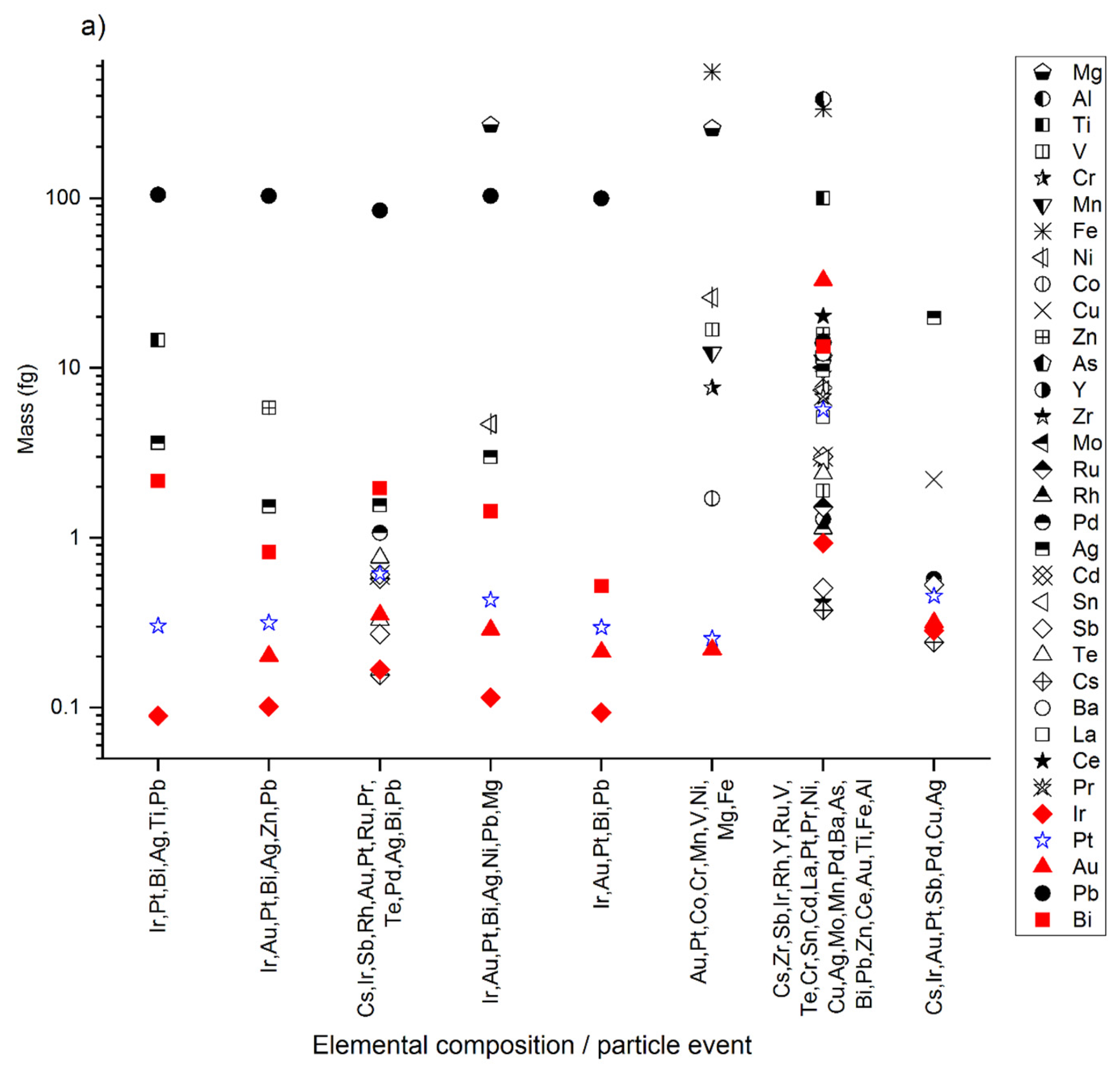
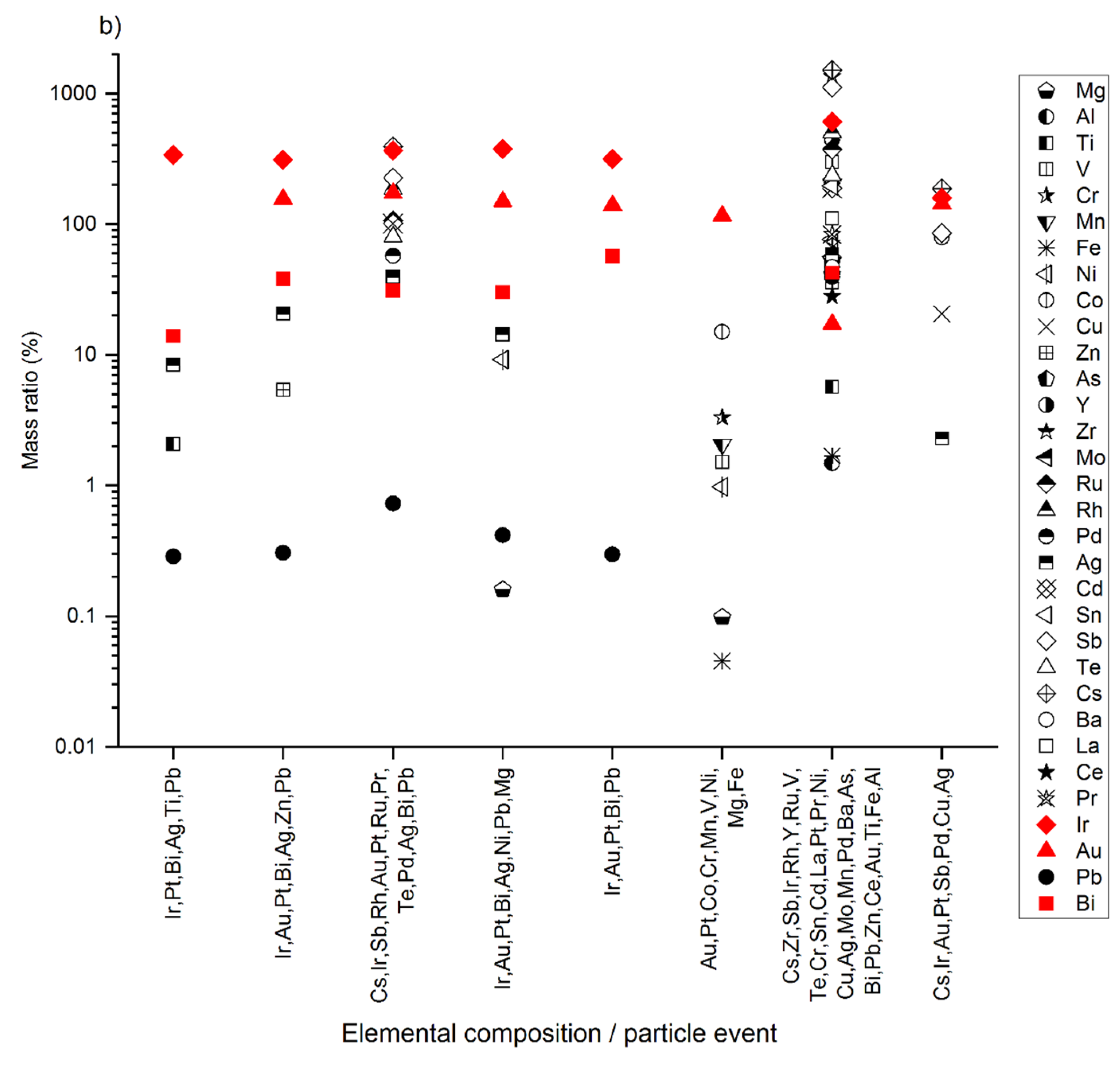
3.1. The Pristine Sediments and Natural Pt-Containing NPs
3.2. Extraction of Spiked Pure Pt NPs
3.3. Extracted Pure Pt NPs Appear as Unassociated and Hetero-Aggregated NPs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Heydari, S.; Tainio, M.; Woodcock, J.; de Nazelle, A. Estimating traffic contribution to particulate matter concentration in urban areas using a multilevel Bayesian meta-regression approach. Environ. Int. 2020, 141, 105800. [Google Scholar] [CrossRef]
- de Kok, T.M.C.M.; Driece, H.A.L.; Hogervorst, J.G.F.; Briedé, J.J. Toxicological assessment of ambient and traffic-related particulate matter: A review of recent studies. Mutat. Res.-Rev. Mutat. Res. 2006, 613, 103–122. [Google Scholar] [CrossRef]
- Li, N.; Sioutas, C.; Cho, A.; Schmitz, D.; Misra, C.; Sempf, J.; Wang, M.; Oberley, T.; Froines, J.; Nel, A. Ultrafine particulate pollutants induce oxidative stress and mitochondrial damage. Environ. Health Perspect. 2003, 111, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Holland, N.A.; Fraiser, C.R.; Sloan, R.C.; Devlin, R.B.; Brown, D.A.; Wingard, C.J. Ultrafine Particulate Matter Increases Cardiac Ischemia/Reperfusion Injury via Mitochondrial Permeability Transition Pore. Cardiovasc. Toxicol. 2017, 17, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.Y.; Chan, C.C.; Su, T.C. Particulate and gaseous pollutants on inflammation, thrombosis, and autonomic imbalance in subjects at risk for cardiovascular disease. Environ. Pollut. 2017, 223, 403–408. [Google Scholar] [CrossRef]
- Moreno-Ríos, A.L.; Tejeda-Benítez, L.P.; Bustillo-Lecompte, C.F. Sources, characteristics, toxicity, and control of ultrafine particles: An overview. Geosci. Front. 2022, 13, 101147. [Google Scholar] [CrossRef]
- Moldovan, M.; Palacios, M.A.; Gómez, M.M.; Morrison, G.; Rauch, S.; McLeod, C.; Ma, R.; Caroli, S.; Alimonti, A.; Petrucci, F.; et al. Environmental risk of particulate and soluble platinum group elements released from gasoline and diesel engine catalytic converters. Sci. Total Environ. 2002, 296, 199–208. [Google Scholar] [CrossRef]
- Helmers, E. Elements accompanying platinum emitted from automobile catalysts. Chemosphere 1996, 33, 405–419. [Google Scholar] [CrossRef]
- Schäfer, J.; Puchelt, H. Platinum-Group-Metals (PGM) emitted from automobile catalytic converters and their distribution in roadside soils. J. Geochem. Explor. 1998, 64, 307–314. [Google Scholar] [CrossRef]
- Palacios, M.A.; Gómez, M.M.; Moldovan, M.; Morrison, G.; Rauch, S.; McLeod, C.; Ma, R.; Laserna, J.; Lucena, P.; Caroli, S.; et al. Platinum-group elements: Quantification in collected exhaust fumes and studies of catalyst surfaces. Sci. Total Environ. 2000, 257, 1–15. [Google Scholar] [CrossRef]
- Cinti, D.; Angelone, M.; Masi, U.; Cremisini, C. Platinum levels in natural and urban soils from Rome and Latium (Italy): Significance for pollution by automobile catalytic converter. Sci. Total Environ. 2002, 293, 47–57. [Google Scholar] [CrossRef]
- Fritsche, J.; Meisel, T. Determination of anthropogenic input of Ru, Rh, Pd, Re, Os, Ir and Pt in soils along Austrian motorways by isotope dilution ICP-MS. Sci. Total Environ. 2004, 325, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Hooda, P.S.; Miller, A.; Edwards, A.C. The distribution of automobile catalysts-cast platinum, palladium and rhodium in soils adjacent to roads and their uptake by grass. Sci. Total Environ. 2007, 384, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Reith, F.; Campbell, S.G.; Ball, A.S.; Pring, A.; Southam, G. Platinum in Earth surface environments. Earth-Sci. Rev. 2014, 131, 1–21. [Google Scholar] [CrossRef]
- Rauch, S.; Fatoki, O.S. Impact of Platinum Group Element Emissions from Mining and Production Activities; Springer: Berlin/Heidelberg, Germany, 2015; pp. 19–29. [Google Scholar]
- Jackson, M.T.; Prichard, H.M.; Sampson, J. Platinum-group elements in sewage sludge and incinerator ash in the United Kingdom: Assessment of PGE sources and mobility in cities. Sci. Total Environ. 2010, 408, 1276–1285. [Google Scholar] [CrossRef]
- Laschka, D.; Nachtwey, M. Platinum in municipal sewage treatment plants. Chemosphere 1997, 34, 1803–1812. [Google Scholar] [CrossRef]
- Savignan, L.; Faucher, S.; Chéry, P.; Lespes, G. Platinum group elements contamination in soils: Review of the current state. Chemosphere 2021, 271, 129517. [Google Scholar] [CrossRef] [PubMed]
- Rauch, S.; Morrison, G.M. Environmental relevance of the platinum-group elements. Elements 2008, 4, 259–263. [Google Scholar] [CrossRef]
- Park, J.-W.; Hu, Z.; Gao, S.; Campbell, I.H.; Gong, H. Platinum group element abundances in the upper continental crust revisited–New constraints from analyses of Chinese loess. Geochim. Cosmochim. Acta 2012, 93, 63–76. [Google Scholar] [CrossRef]
- Montaño, M.D.; Lowry, G.V.; Von Der Kammer, F.; Blue, J.; Ranville, J.F. Current status and future direction for examining engineered nanoparticles in natural systems. Environ. Chem. 2014, 11, 351–366. [Google Scholar] [CrossRef]
- Borovinskaya, O.; Hattendorf, B.; Tanner, M.; Gschwind, S.; Günther, D. A prototype of a new inductively coupled plasma time-of-flight mass spectrometer providing temporally resolved, multi-element detection of short signals generated by single particles and droplets. J. Anal. At. Spectrom. 2013, 28, 226–233. [Google Scholar] [CrossRef]
- Praetorius, A.; Gundlach-Graham, A.; Goldberg, E.; Fabienke, W.; Navratilova, J.; Gondikas, A.; Kaegi, R.; Günther, D.; Hofmann, T.; Von Der Kammer, F. Single-particle multi-element fingerprinting (spMEF) using inductively-coupled plasma time-of-flight mass spectrometry (ICP-TOFMS) to identify engineered nanoparticles against the elevated natural background in soils. Environ. Sci. Nano 2017, 4, 307–314. [Google Scholar] [CrossRef]
- Bevers, S.; Montaño, M.D.; Rybicki, L.; Hofmann, T.; von der Kammer, F.; Ranville, J.F. Quantification and Characterization of Nanoparticulate Zinc in an Urban Watershed. Front. Environ. Sci. 2020, 8, 84. [Google Scholar] [CrossRef]
- Loosli, F.; Wang, J.; Rothenberg, S.; Bizimis, M.; Winkler, C.; Borovinskaya, O.; Flamigni, L.; Baalousha, M. Sewage spills are a major source of titanium dioxide engineered (nano)-particle release into the environment. Environ. Sci. Nano 2019, 6, 763–777. [Google Scholar] [CrossRef]
- von der Kammer, F. Characterization of Environmental Colloids Applying Field-Flow Fractionation–Multi Detection Analysis with Emphasis on Light Scattering Techniques. PhD Thesis, Hamburg University of Technology, Hamburg, Germany, 2005. [Google Scholar]
- Navratilova, J.; Praetorius, A.; Gondikas, A.; Fabienke, W.; von der Kammer, F.; Hofmann, T. Detection of engineered copper nanoparticles in soil using single particle ICP-MS. Int. J. Environ. Res. Public Health 2015, 12, 15756–15768. [Google Scholar] [CrossRef]
- Yi, Z.; Loosli, F.; Wang, J.; Berti, D.; Baalousha, M. How to distinguish natural versus engineered nanomaterials: Insights from the analysis of TiO2 and CeO2 in soils. Environ. Chem. Lett. 2020, 18, 215–227. [Google Scholar] [CrossRef]
- Loosli, F.; Yi, Z.; Wang, J.; Baalousha, M. Improved extraction efficiency of natural nanomaterials in soils to facilitate their characterization using a multimethod approach. Sci. Total Environ. 2019, 677, 34–46. [Google Scholar] [CrossRef]
- Plathe, K.L.; Von Der Kammer, F.; Hassellöv, M.; Moore, J.; Murayama, M.; Hofmann, T.; Hochella, M.F. Using FlFFF and aTEM to determine trace metalnanoparticle associations in riverbed sediment. Environ. Chem. 2010, 7, 82–93. [Google Scholar] [CrossRef]
- Bland, G.D.; Lowry, G.V. Multistep Method to Extract Moderately Soluble Copper Oxide Nanoparticles from Soil for Quantification and Characterization. Anal. Chem. 2020, 92, 9620–9628. [Google Scholar] [CrossRef]
- Dutschke, F.; Irrgeher, J.; Pröfrock, D. Optimisation of an extraction/leaching procedure for the characterisation and quantification of titanium dioxide (TiO2) nanoparticles in aquatic environments using SdFFF-ICP-MS and SEM-EDX analyses. Anal. Methods 2017, 9, 3626–3635. [Google Scholar] [CrossRef]
- Laborda, F.; Bolea, E.; Jiménez-Lamana, J. Single particle inductively coupled plasma mass spectrometry: A powerful tool for nanoanalysis. Anal. Chem. 2014, 86, 2270–2278. [Google Scholar] [CrossRef]
- Krystek, P.; Ulrich, A.; Garcia, C.C.; Manohar, S.; Ritsema, R. Application of plasma spectrometry for the analysis of engineered nanoparticles in suspensions and products. J. Anal. At. Spectrom. 2011, 26, 1701. [Google Scholar] [CrossRef]
- Meermann, B.; Nischwitz, V. ICP-MS for the analysis at the nanoscale-a tutorial review. J. Anal. At. Spectrom. 2018, 33, 1432–1468. [Google Scholar] [CrossRef]
- CCRMP, CANMET Mining and Mineral Sciences Laboratories. Lake Sediment Samples LKSD-1 to LKSD-4 Analysis Certificate. 2003, pp. 4–8. Available online: https://www.nrcan.gc.ca/sites/www.nrcan.gc.ca/files/mineralsmetals/pdf/mms-smm/tect-tech/ccrmp/cer-cer/lksd-1-eng.pdf (accessed on 1 July 2022).
- Lynch, J. Provisional elemental values for eight new geochemical lake sediment and stream sediment reference materials LKSD-1, LKSD-2, LKSD-3, LKSD-4, STSD-1, STSD-2, STSD-3 and STSD-4. Geostand. Newsl. 1990, 14, 153–167. [Google Scholar] [CrossRef]
- PubChem. PubChem Compound Summary for CID 23939, Platinum. Natl. Cent. Biotechnol. 2022. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Platinum (accessed on 1 July 2022).
- Hendriks, L.; Gundlach-Graham, A.; Hattendorf, B.; Günther, D. Characterization of a new ICP-TOFMS instrument with continuous and discrete introduction of solutions. J. Anal. At. Spectrom. 2017, 32, 548–561. [Google Scholar] [CrossRef]
- Hendriks, L.; Gundlach-Graham, A.; Günther, D. Analysis of Inorganic Nanoparticles by Single-particle Inductively Coupled Plasma Time-of-Flight Mass Spectrometry. Chim. Int. J. Chem. 2018, 72, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Pace, H.E.; Rogers, N.J.; Jarolimek, C.; Coleman, V.A.; Higgins, C.P.; Ranville, J.F. Determining transport efficiency for the purpose of counting and sizing nanoparticles via single particle inductively coupled plasma mass spectrometry. Anal. Chem. 2011, 83, 9361–9369. [Google Scholar] [CrossRef] [PubMed]
- Tanner, M. Shorter signals for improved signal to noise ratio, the influence of Poisson distribution. J. Anal. At. Spectrom. 2010, 25, 405–407. [Google Scholar] [CrossRef]
- Lee, S.; Bi, X.; Reed, R.B.; Ranville, J.F.; Herckes, P.; Westerhoff, P. Nanoparticle size detection limits by single particle ICP-MS for 40 elements. Environ. Sci. Technol. 2014, 48, 10291–10300. [Google Scholar] [CrossRef] [PubMed]
- Laborda, F.; Jiménez-Lamana, J.; Bolea, E.; Castillo, J.R. Critical considerations for the determination of nanoparticle number concentrations, size and number size distributions by single particle ICP-MS. J. Anal. At. Spectrom. 2013, 28, 1220–1232. [Google Scholar] [CrossRef]
- Mitrano, D.M.; Barber, A.; Bednar, A.; Westerhoff, P.; Higgins, C.P.; Ranville, J.F. Silver nanoparticle characterization using single particle ICP-MS (SP-ICP-MS) and asymmetrical flow field flow fractionation ICP-MS (AF4-ICP-MS). J. Anal. At. Spectrom. 2012, 27, 1131–1142. [Google Scholar] [CrossRef]
- Birke, M.; Rauch, U.; Stummeyer, J.; Lorenz, H.; Keilert, B. A review of platinum group element (PGE) geochemistry and a study of the changes of PGE contents in the topsoil of Berlin, Germany, between 1992 and 2013. J. Geochem. Explor. 2018, 187, 72–96. [Google Scholar] [CrossRef]
- Reith, F.; Cornelis, G. Effect of soil properties on gold- and platinum nanoparticle mobility. Chem. Geol. 2017, 466, 446–453. [Google Scholar] [CrossRef]
- Kim, S.T.; Kang, D.Y.; Lee, S.; Kim, W.S.; Lee, J.T.; Cho, H.S.; Kim, S.H. Separation and quantitation of silver nanoparticles using sedimentation field-flow fractionation. J. Liq. Chromatogr. Relat. Technol. 2007, 30, 2533–2544. [Google Scholar] [CrossRef]
- Saenmuangchin, R.; Siripinyanond, A. Flow field-flow fractionation for hydrodynamic diameter estimation of gold nanoparticles with various types of surface coatings. Anal. Bioanal. Chem. 2018, 410, 6845–6859. [Google Scholar] [CrossRef]
- Techarang, T.; Siripinyanond, A. Use of electrical field-flow fractionation for gold nanoparticles after improving separation efficiency by carrier liquid optimization. Anal. Chim. Acta 2021, 1144, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Rowley, M.C.; Grand, S.; Verrecchia, É.P. Calcium-mediated stabilisation of soil organic carbon. Biogeochemistry 2018, 137, 27–49. [Google Scholar] [CrossRef]
- Takahashi, Y.; Minai, Y.; Ambe, S.; Makide, Y.; Ambe, F. Comparison of adsorption behavior of multiple inorganic ions on kaolinite and silica in the presence of humic acid using the multitracer technique. Geochim. Cosmochim. Acta 1999, 63, 815–836. [Google Scholar] [CrossRef]
- Kubrakova, I.V.; Fortygin, A.V.; Lobov, S.G.; Koshcheeva, I.Y.; Tyutyunnik, O.A.; Mironenko, M.V. Migration of platinum, palladium, and gold in the water systems of platinum deposits. Geochem. Int. 2011, 49, 1072–1084. [Google Scholar] [CrossRef]
- Turner, A.; Crussell, M.; Millward, G.E.; Cobelo-Garcia, A.; Fisher, A.S. Adsorption kinetics of platinum group elements in river water. Environ. Sci. Technol. 2006, 40, 1524–1531. [Google Scholar] [CrossRef]
- Petosa, A.R.; Jaisi, D.P.; Quevedo, I.R.; Elimelech, M.; Tufenkji, N. Aggregation and deposition of engineered nanomaterials in aquatic environments: Role of physicochemical interactions. Environ. Sci. Technol. 2010, 44, 6532–6549. [Google Scholar] [CrossRef]
- Zhang, W.; Crittenden, J.; Li, K.; Chen, Y. Attachment efficiency of nanoparticle aggregation in aqueous dispersions: Modeling and experimental validation. Environ. Sci. Technol. 2012, 46, 7054–7062. [Google Scholar] [CrossRef] [PubMed]
- Labille, J.; Harns, C.; Bottero, J.Y.; Brant, J. Heteroaggregation of titanium dioxide nanoparticles with natural clay colloids. Environ. Sci. Technol. 2015, 49, 6608–6616. [Google Scholar] [CrossRef] [PubMed]
- Geitner, N.K.; O’Brien, N.J.; Turner, A.A.; Cummins, E.J.; Wiesner, M.R. Measuring Nanoparticle Attachment Efficiency in Complex Systems. Environ. Sci. Technol. 2017, 51, 13288–13294. [Google Scholar] [CrossRef] [PubMed]
- Hotze, E.M.; Phenrat, T.; Lowry, G.V. Nanoparticle Aggregation: Challenges to Understanding Transport and Reactivity in the Environment. J. Environ. Qual. 2010, 39, 1909–1924. [Google Scholar] [CrossRef]
- Praetorius, A.; Badetti, E.; Brunelli, A.; Clavier, A.; Gallego-Urrea, J.A.; Gondikas, A.; Hassellöv, M.; Hofmann, T.; Mackevica, A.; Marcomini, A.; et al. Strategies for determining heteroaggregation attachment efficiencies of engineered nanoparticles in aquatic environments. Environ. Sci. Nano 2020, 7, 351–367. [Google Scholar] [CrossRef]


| Elemental Association | Pt + Ir | Pt + Au | Pt + Bi | Pt + Ir + Au | Pt + Ir + Au + Bi | ||||
|---|---|---|---|---|---|---|---|---|---|
| Percent (%) | Pristine sediment | 92 ± 4 | 75 ± 2 | 81 ± 10 | 72 ± 2 | 66 ± 6 | |||
| Spiked sediment | 6.4 | 6.4 | 8.5 | 6.4 | 6.4 | ||||
| Pt/Ir | Pt/Au | Pt/Bi | Pt/Ir | Pt/Au | Pt/Ir | Pt/Au | Pt/Bi | ||
| Ratio | Pristine sediment | 3.38 ± 0.92 | 1.54 ± 0.21 | 0.91 ± 0.48 | 3.51 ± 0.78 | 1.54 ± 0.66 | 3.54 ± 0.78 | 1.56 ± 0.69 | 0.96 ± 1.40 |
| Spiked sediment | 4.24 ± 0.31 | 1.70 ± 0.13 | 1.77 ± 1.77 | 4.24 ± 0.31 | 1.70 ± 0.13 | 4.24 ± 0.31 | 1.70 ± 0.13 | 0.89 ± 0.34 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taskula, S.; Stetten, L.; von der Kammer, F.; Hofmann, T. Platinum Nanoparticle Extraction, Quantification, and Characterization in Sediments by Single-Particle Inductively Coupled Plasma Time-of-Flight Mass Spectrometry. Nanomaterials 2022, 12, 3307. https://doi.org/10.3390/nano12193307
Taskula S, Stetten L, von der Kammer F, Hofmann T. Platinum Nanoparticle Extraction, Quantification, and Characterization in Sediments by Single-Particle Inductively Coupled Plasma Time-of-Flight Mass Spectrometry. Nanomaterials. 2022; 12(19):3307. https://doi.org/10.3390/nano12193307
Chicago/Turabian StyleTaskula, Sara, Lucie Stetten, Frank von der Kammer, and Thilo Hofmann. 2022. "Platinum Nanoparticle Extraction, Quantification, and Characterization in Sediments by Single-Particle Inductively Coupled Plasma Time-of-Flight Mass Spectrometry" Nanomaterials 12, no. 19: 3307. https://doi.org/10.3390/nano12193307
APA StyleTaskula, S., Stetten, L., von der Kammer, F., & Hofmann, T. (2022). Platinum Nanoparticle Extraction, Quantification, and Characterization in Sediments by Single-Particle Inductively Coupled Plasma Time-of-Flight Mass Spectrometry. Nanomaterials, 12(19), 3307. https://doi.org/10.3390/nano12193307







