Dual-Purpose Sensing Nanoprobe Based on Carbon Dots from o-Phenylenediamine: pH and Solvent Polarity Measurement
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Carbon Dot (CD) Synthesis
2.3. Experimental Setup
3. Results and Discussion
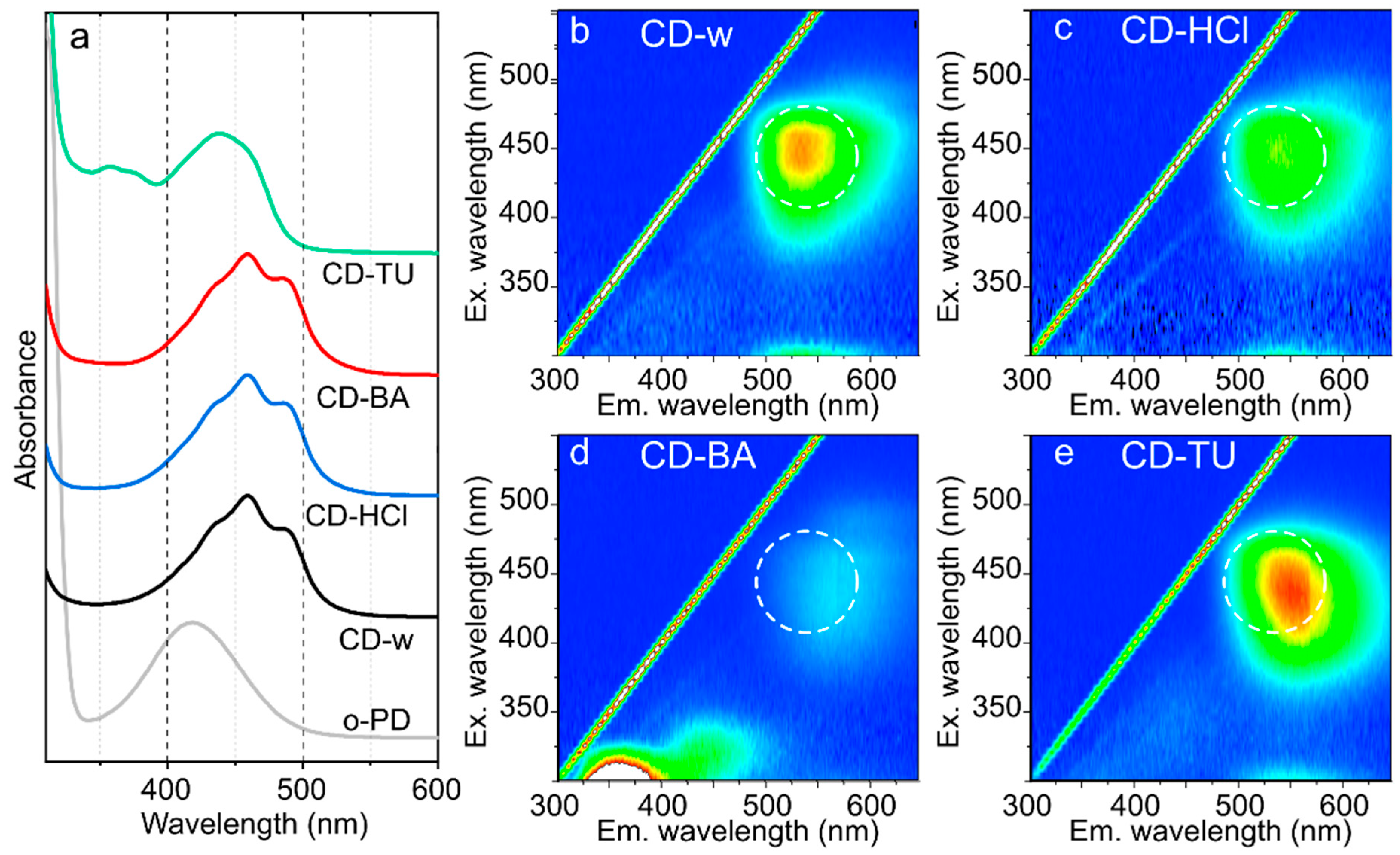
3.1. Chemical Reactions during CDs Synthesis
3.2. Morphology and Optical Centers
3.3. Optical Properties Depending on CDs Morphology
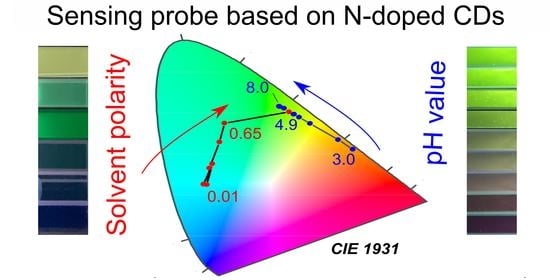
3.4. Solvent Polarity Probe
3.5. pH Probe
3.6. Dual-Purpose Sensing Probe and Sensing Test Strip
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Gloag, L.; Mehdipour, M.; Chen, D.; Tilley, R.D.; Gooding, J.J. Advances in the Application of Magnetic Nanoparticles for Sensing. Adv. Mater. 2019, 31, 1904385. [Google Scholar] [CrossRef]
- Vu, C.C.; Kim, S.J.; Kim, J. Flexible Wearable Sensors—An Update in View of Touch-Sensing. Sci. Technol. Adv. Mater. 2021, 22, 26–36. [Google Scholar] [CrossRef]
- Morawska, L.; Thai, P.K.; Liu, X.; Asumadu-Sakyi, A.; Ayoko, G.; Bartonova, A.; Bedini, A.; Chai, F.; Christensen, B.; Dunbabin, M.; et al. Applications of Low-Cost Sensing Technologies for Air Quality Monitoring and Exposure Assessment: How Far Have They Gone? Environ. Int. 2018, 116, 286–299. [Google Scholar] [CrossRef]
- Alam, A.U.; Qin, Y.; Nambiar, S.; Yeow, J.T.W.; Howlader, M.M.R.; Hu, N.-X.; Deen, M.J. Polymers and Organic Materials-Based PH Sensors for Healthcare Applications. Prog. Mater. Sci. 2018, 96, 174–216. [Google Scholar] [CrossRef]
- Yousefi, H.; Su, H.-M.; Imani, S.M.; Alkhaldi, K.; Filipe, C.D.M.; Didar, T.F. Intelligent Food Packaging: A Review of Smart Sensing Technologies for Monitoring Food Quality. ACS Sens. 2019, 4, 808–821. [Google Scholar] [CrossRef] [PubMed]
- Foorginezhad, S.; Mohseni-Dargah, M.; Firoozirad, K.; Aryai, V.; Razmjou, A.; Abbassi, R.; Garaniya, V.; Beheshti, A.; Asadnia, M. Recent Advances in Sensing and Assessment of Corrosion in Sewage Pipelines. Process Saf. Environ. Prot. 2021, 147, 192–213. [Google Scholar] [CrossRef]
- Steinegger, A.; Wolfbeis, O.S.; Borisov, S.M. Optical Sensing and Imaging of PH Values: Spectroscopies, Materials, and Applications. Chem. Rev. 2020, 120, 12357–12489. [Google Scholar] [CrossRef]
- Deng, Y.; Zhou, Y.; Li, Q.; Qian, J. One-Step Hydrothermal Synthesis of Nitrogen-Doped Carbon Dots for High-Sensitivity Visual Detection of Nitrite and Ascorbic Acid. Anal. Methods 2021, 13, 3685–3692. [Google Scholar] [CrossRef]
- Fathi, P.; Moitra, P.; McDonald, M.M.; Esch, M.B.; Pan, D. Near-Infrared Emitting Dual-Stimuli-Responsive Carbon Dots from Endogenous Bile Pigments. Nanoscale 2021, 13, 13487–13496. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Zhuang, S.; Yu, Y.; Li, H.; Liu, Y. Ratiometric Dual-Emission of Rhodamine-B Grafted Carbon Dots for Full-Range Solvent Components Detection. Anal. Chim. Acta 2021, 1174, 338743. [Google Scholar] [CrossRef] [PubMed]
- Alaş, M.Ö.; Genç, R. Solvatochromic Surface-Passivated Carbon Dots for Fluorometric Moisture Sensing in Organic Solvents. ACS Appl. Nano Mater. 2021, 4, 7974–7987. [Google Scholar] [CrossRef]
- Wang, W.; Wu, J.; Xing, Y.; Wang, Z. Solvent-Dependent Red Emissive Carbon Dots and Their Applications in Sensing and Solid-State Luminescence. Sens. Actuators B Chem. 2022, 360, 131645. [Google Scholar] [CrossRef]
- Wang, B.; Yuan, X.; Lv, X.; Mei, Y.; Peng, H.; Li, L.; Guo, Y.; Du, J.; Zheng, B.; Xiao, D. Carbon Dots-Based Room-Temperature Phosphorescent Test Strip: Visual and Convenient Water Detection in Organic Solvents. Dyes Pigment. 2021, 189, 109226. [Google Scholar] [CrossRef]
- Yu, C.; Zhang, D.; Zhu, Q.; Chao, D.; Liu, H.; Zhou, L. Preparation and Characterisation of Dual Sensing Carbon Dots for Water and Cu2+ Detection. Dyes Pigment. 2022, 198, 110008. [Google Scholar] [CrossRef]
- Madhu, M.; Tseng, W.-L. NaCl Nanocrystal-Encapsulated Carbon Dots as a Solution-Based Sensor for Phosphorescent Sensing of Trace Amounts of Water in Organic Solvents. Anal. Methods 2021, 13, 4949–4954. [Google Scholar] [CrossRef] [PubMed]
- Barati, A.; Shamsipur, M.; Abdollahi, H. Carbon Dots with Strong Excitation-Dependent Fluorescence Changes towards PH. Application as Nanosensors for a Broad Range of PH. Anal. Chim. Acta 2016, 931, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Chang, D.; Zhao, Z.; Shi, H.; Feng, J.; Yang, Y.; Shi, L. Ratiometric Fluorescent Carbon Dots for Enantioselective Sensing of L-Lysine and PH Discrimination in Vivo and in Vitro. Sens. Actuators B Chem. 2022, 362, 131792. [Google Scholar] [CrossRef]
- Li, S.; Song, X.; Wang, Y.; Hu, Z.; Yan, F.; Feng, G. Developed a Ratiometric Fluorescence PH Nanosensor Based on Label-Free Carbon Dots for Intracellular Lysosome Imaging and Water PH Monitoring with a Smartphone. Dyes Pigment. 2021, 193, 109490. [Google Scholar] [CrossRef]
- Zhu, P.; Li, J.; Gao, L.; Xiong, J.; Tan, K. Strategy to Synthesize Tunable Multiemission Carbon Dots and Their Multicolor Visualization Application. ACS Appl. Mater. Interfaces 2021, 13, 33354–33362. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Wang, M.; Liu, M.; Zhang, J.; Zhao, Y. A Portable Optical Fiber Sensing Platform Based on Fluorescent Carbon Dots for Real-Time PH Detection. Adv. Mater. Interfaces 2022, 9, 2101633. [Google Scholar] [CrossRef]
- Afonso, A.C.P.; Correia, A.S.; Duarte, D.; Brandão, A.T.S.C.; de Yuso, M.d.V.M.; Jiménez-Jiménez, J.; Vale, N.; Pereira, C.M.; Algarra, M.; Pinto da Silva, L. An Active Surface Preservation Strategy for the Rational Development of Carbon Dots as PH-Responsive Fluorescent Nanosensors. Chemosensors 2021, 9, 191. [Google Scholar] [CrossRef]
- Li, D.; Ushakova, E.v.; Rogach, A.L.; Qu, S. Optical Properties of Carbon Dots in the Deep-Red to Near-Infrared Region Are Attractive for Biomedical Applications. Small 2021, 17, 2102325. [Google Scholar] [CrossRef]
- Vedamalai, M.; Periasamy, A.P.; Wang, C.W.; Tseng, Y.T.; Ho, L.C.; Shih, C.C.; Chang, H.T. Carbon Nanodots Prepared from O-Phenylenediamine for Sensing of Cu2+ Ions in Cells. Nanoscale 2014, 6, 13119–13125. [Google Scholar] [CrossRef] [PubMed]
- Casey, J.R.; Grinstein, S.; Orlowski, J. Sensors and Regulators of Intracellular PH. Nat. Rev. Mol. Cell Biol. 2010, 11, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Shi, B.; Zhang, L.; Lan, C.; Zhao, J.; Su, Y.; Zhao, S. One-Pot Green Synthesis of Oxygen-Rich Nitrogen-Doped Graphene Quantum Dots and Their Potential Application in PH-Sensitive Photoluminescence and Detection of Mercury(II) Ions. Talanta 2015, 142, 131–139. [Google Scholar] [CrossRef]
- Sun, Y.P.; Zhou, B.; Lin, Y.; Wang, W.; Fernando, K.A.S.; Pathak, P.; Meziani, M.J.; Harruff, B.A.; Wang, X.; Wang, H.; et al. Quantum-Sized Carbon Dots for Bright and Colorful Photoluminescence. J. Am. Chem. Soc. 2006, 128, 7756–7757. [Google Scholar] [CrossRef]
- Zhang, M.; Su, R.; Zhong, J.; Fei, L.; Cai, W.; Guan, Q.; Li, W.; Li, N.; Chen, Y.; Cai, L.; et al. Red/Orange Dual-Emissive Carbon Dots for PH Sensing and Cell Imaging. Nano Res. 2019, 12, 815–821. [Google Scholar] [CrossRef]
- Ye, X.; Xiang, Y.; Wang, Q.; Li, Z.; Liu, Z. A Red Emissive Two-Photon Fluorescence Probe Based on Carbon Dots for Intracellular PH Detection. Small 2019, 15, 1901673. [Google Scholar] [CrossRef] [PubMed]
- Chao, D.; Lyu, W.; Liu, Y.; Zhou, L.; Zhang, Q.; Deng, R.; Zhang, H. Solvent-Dependent Carbon Dots and Their Applications in the Detection of Water in Organic Solvents. J. Mater. Chem. C Mater. 2018, 6, 7527–7532. [Google Scholar] [CrossRef]
- Liu, J.H.; Li, D.Y.; He, J.H.; Yuan, D.; Li, R.S.; Zhen, S.J.; Li, Y.F.; Huang, C.Z. Polarity-Sensitive Polymer Carbon Dots Prepared at Room-Temperature for Monitoring the Cell Polarity Dynamics during Autophagy. ACS Appl. Mater. Interfaces 2020, 12, 4815–4820. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, R.; Feng, B.; Zhong, X.; Ostrikov, K. Photoluminescence Mechanism of Carbon Dots: Triggering High-Color-Purity Red Fluorescence Emission through Edge Amino Protonation. Nat. Commun. 2021, 12, 6856. [Google Scholar] [CrossRef] [PubMed]
- Bratsch, S.G. Standard Electrode Potentials and Temperature Coefficients in Water at 298.15 K. J. Phys. Chem. Ref. Data 1989, 18, 1–21. [Google Scholar] [CrossRef]
- Wang, B.; Wei, Z.; Sui, L.; Yu, J.; Zhang, B.; Wang, X.; Feng, S.; Song, H.; Yong, X.; Tian, Y.; et al. Electron–Phonon Coupling-Assisted Universal Red Luminescence of o-Phenylenediamine-Based Carbon Dots. Light Sci. Appl. 2022, 11, 172. [Google Scholar] [CrossRef]
- Jiang, K.; Ma, S.; Bi, H.; Chen, D.; Han, X. Morphology Controllable Fabrication of Poly-o-Phenylenediamine Microstructures Tuned by the Ionic Strength and Their Applications in PH Sensors. J. Mater. Chem. A 2014, 2, 19208–19213. [Google Scholar] [CrossRef]
- Pretsch, E.; Bühlmann, P.; Affolter, C.; Pretsch, E.; Bhuhlmann, P.; Affolter, C. Structure Determination of Organic Compounds; Springer: Berlin/Heidelberg, Germany, 2009; ISBN 978-3-540-93809-5. [Google Scholar]
- Lakowicz, J.R. (Ed.) Principles of Fluorescence Spectroscopy; Springer: Boston, MA, USA, 2006; ISBN 978-0-387-31278-1. [Google Scholar]
- Valeur, B.; Berberan-Santos, M.N. Molecular Fluorescence: Principles and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2012; ISBN 3527328378. [Google Scholar]
- Pedro, S.G.; Salinas-Castillo, A.; Ariza-Avidad, M.; Lapresta-Fernández, A.; Sánchez-González, C.; Martínez-Cisneros, C.S.; Puyol, M.; Capitan-Vallvey, L.F.; Alonso-Chamarro, J. Microsystem-Assisted Synthesis of Carbon Dots with Fluorescent and Colorimetric Properties for PH Detection. Nanoscale 2014, 6, 6018–6024. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Tang, B.; Wang, L.; Bao, H.; Lu, Y.; Guan, C.; Zhang, L.; Le, M.; Liu, Z.; Wu, M. Machine-Learning-Driven Synthesis of Carbon Dots with Enhanced Quantum Yields. ACS Nano 2020, 14, 14761–14768. [Google Scholar] [CrossRef]
- Guo, H.; Zhang, X.; Chen, Z.; Zhang, L.; Wang, L.; Xu, J.; Wu, M. High-Energy Short-Wave Blue Light Conversion Films via Carbon Quantum Dots for Preventing Retinal Photochemical Damage. Carbon 2022, 199, 431–438. [Google Scholar] [CrossRef]
- Stepanidenko, E.; Khavlyuk, P.; Arefina, I.; Cherevkov, S.; Xiong, Y.; Döring, A.; Varygin, G.; Kurdyukov, D.; Eurov, D.; Golubev, V.; et al. Strongly Luminescent Composites Based on Carbon Dots Embedded in a Nanoporous Silicate Glass. Nanomaterials 2020, 10, 1063. [Google Scholar] [CrossRef]




| Solvent a | pH (Measured) | Average pH | Color c | |||||
|---|---|---|---|---|---|---|---|---|
| W | 1.0 | - | - | - | - | - | - |  |
| W/E | 0.85 ± 0.05 b | 0.70 ± 0.15 | 1.45 ± 0.10 | 4.10 ± 0.05 | 4.4 ± 0.3 | 4.4 ± 0.2 | 4.4 ± 0.1 |  |
| W/E | 0.85 ± 0.05 | 0.70 ± 0.15 | 0.80 ± 0.10 | 4.70 ± 0.05 | 5.1 ± 0.3 | 4.8 ± 0.2 | 5.0 ± 0.1 |  |
| W/E | 0.85 ± 0.05 | 0.65 ± 0.15 | 0.75 ± 0.10 | 5.90 ± 0.05 | 7.7 ± 0.4 | 6.2 ± 0.2 | 7.0 ± 0.1 |  |
| E | 0.654 | - | - | - | - | - | - |  |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vedernikova, A.A.; Miruschenko, M.D.; Arefina, I.A.; Babaev, A.A.; Stepanidenko, E.A.; Cherevkov, S.A.; Spiridonov, I.G.; Danilov, D.V.; Koroleva, A.V.; Zhizhin, E.V.; et al. Dual-Purpose Sensing Nanoprobe Based on Carbon Dots from o-Phenylenediamine: pH and Solvent Polarity Measurement. Nanomaterials 2022, 12, 3314. https://doi.org/10.3390/nano12193314
Vedernikova AA, Miruschenko MD, Arefina IA, Babaev AA, Stepanidenko EA, Cherevkov SA, Spiridonov IG, Danilov DV, Koroleva AV, Zhizhin EV, et al. Dual-Purpose Sensing Nanoprobe Based on Carbon Dots from o-Phenylenediamine: pH and Solvent Polarity Measurement. Nanomaterials. 2022; 12(19):3314. https://doi.org/10.3390/nano12193314
Chicago/Turabian StyleVedernikova, Anna A., Mikhail D. Miruschenko, Irina A. Arefina, Anton A. Babaev, Evgeniia A. Stepanidenko, Sergei A. Cherevkov, Igor G. Spiridonov, Denis V. Danilov, Aleksandra V. Koroleva, Evgeniy V. Zhizhin, and et al. 2022. "Dual-Purpose Sensing Nanoprobe Based on Carbon Dots from o-Phenylenediamine: pH and Solvent Polarity Measurement" Nanomaterials 12, no. 19: 3314. https://doi.org/10.3390/nano12193314
APA StyleVedernikova, A. A., Miruschenko, M. D., Arefina, I. A., Babaev, A. A., Stepanidenko, E. A., Cherevkov, S. A., Spiridonov, I. G., Danilov, D. V., Koroleva, A. V., Zhizhin, E. V., & Ushakova, E. V. (2022). Dual-Purpose Sensing Nanoprobe Based on Carbon Dots from o-Phenylenediamine: pH and Solvent Polarity Measurement. Nanomaterials, 12(19), 3314. https://doi.org/10.3390/nano12193314