In Situ Control of the Eluted Ni Nanoparticles from Highly Doped Perovskite for Effective Methane Dry Reforming
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Ni Exsolved Perovskite Oxides
2.2. Characterization
2.3. Dry Reforming of Methane (DRM)
3. Results and Discussion
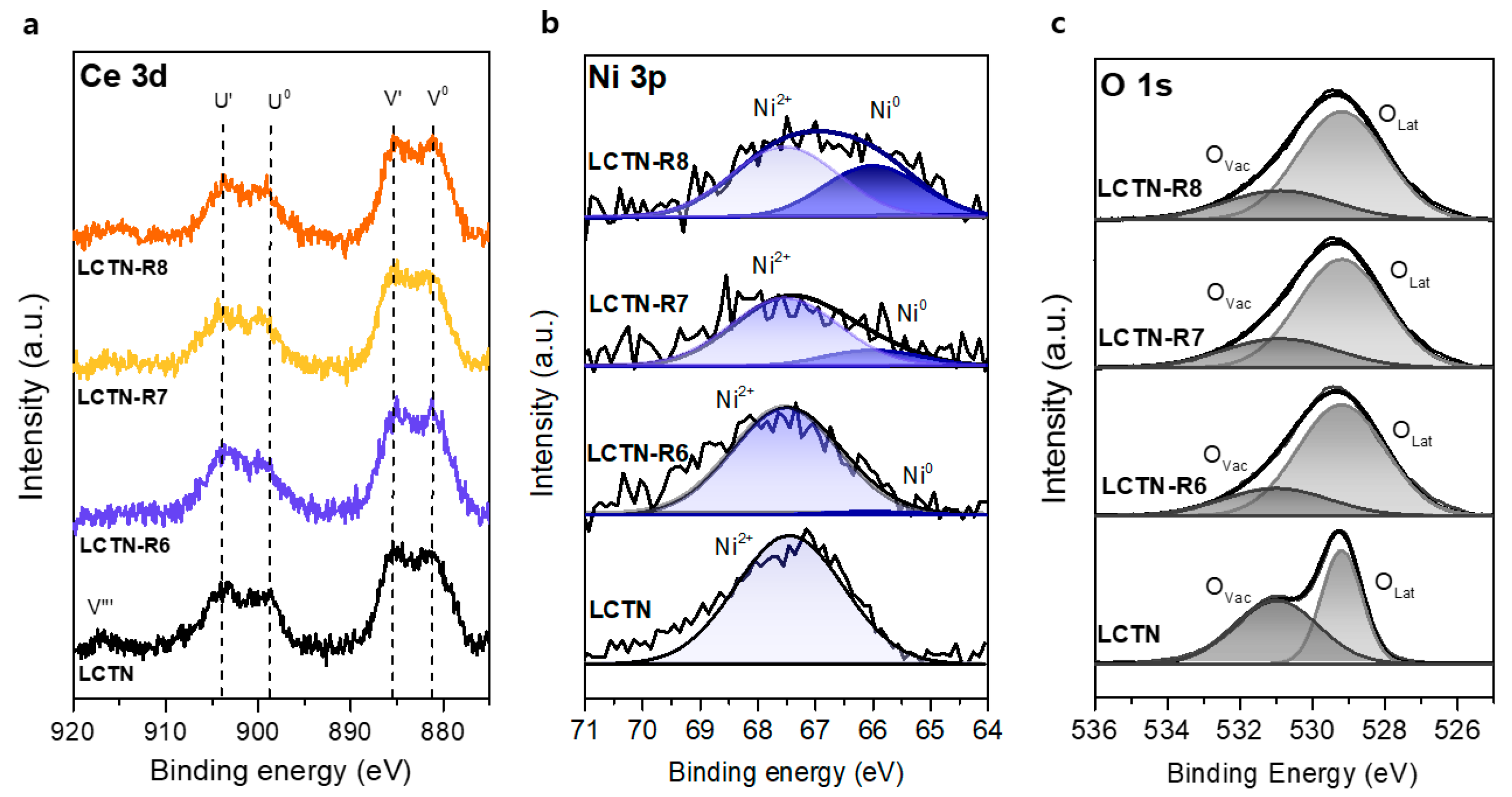
3.1. Synthesis and Characterization of Ni-Eluted LCTN
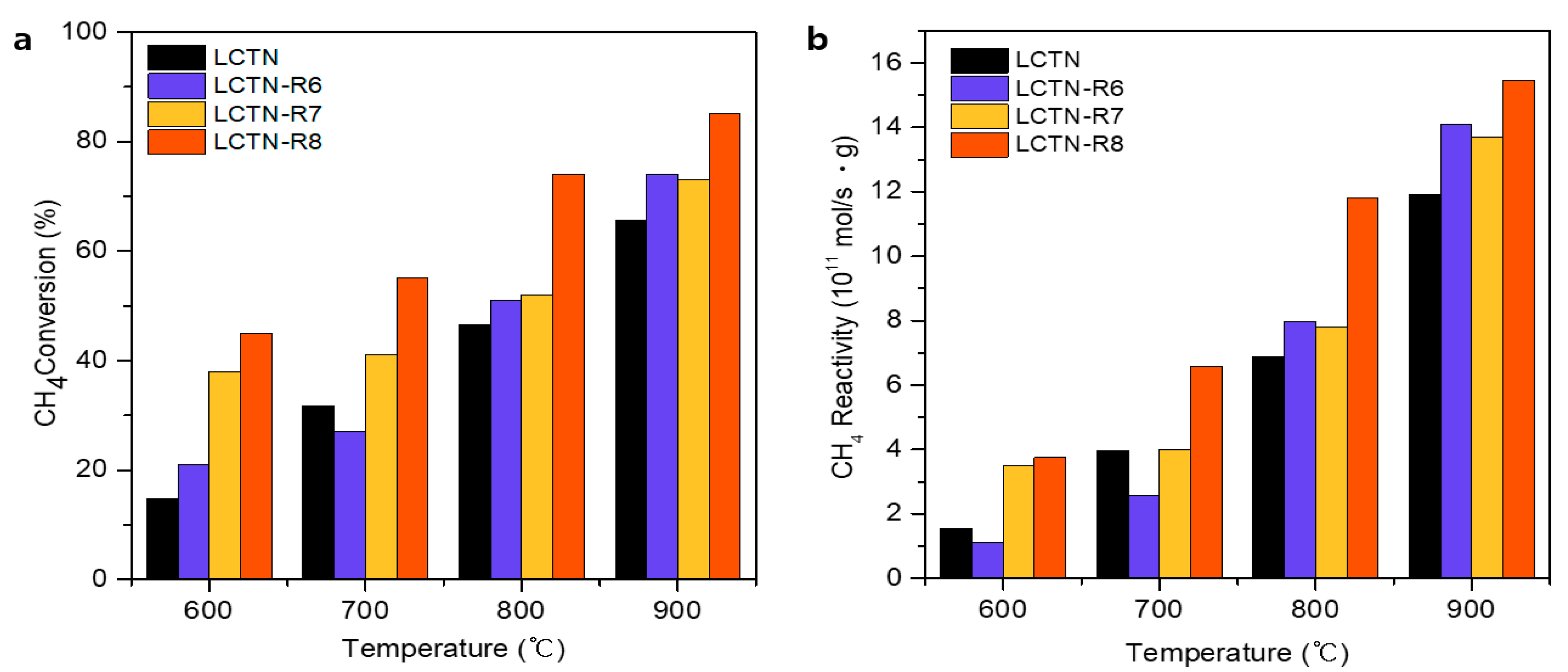
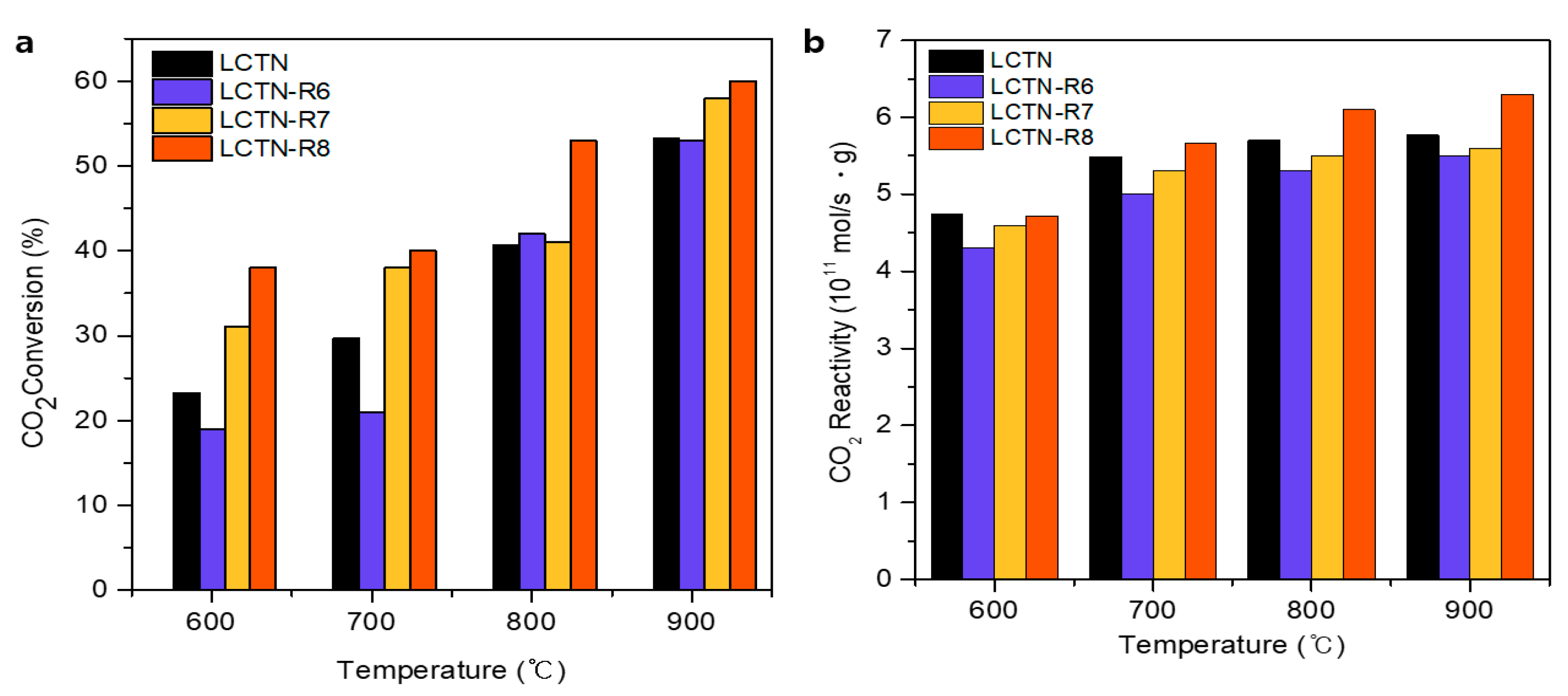
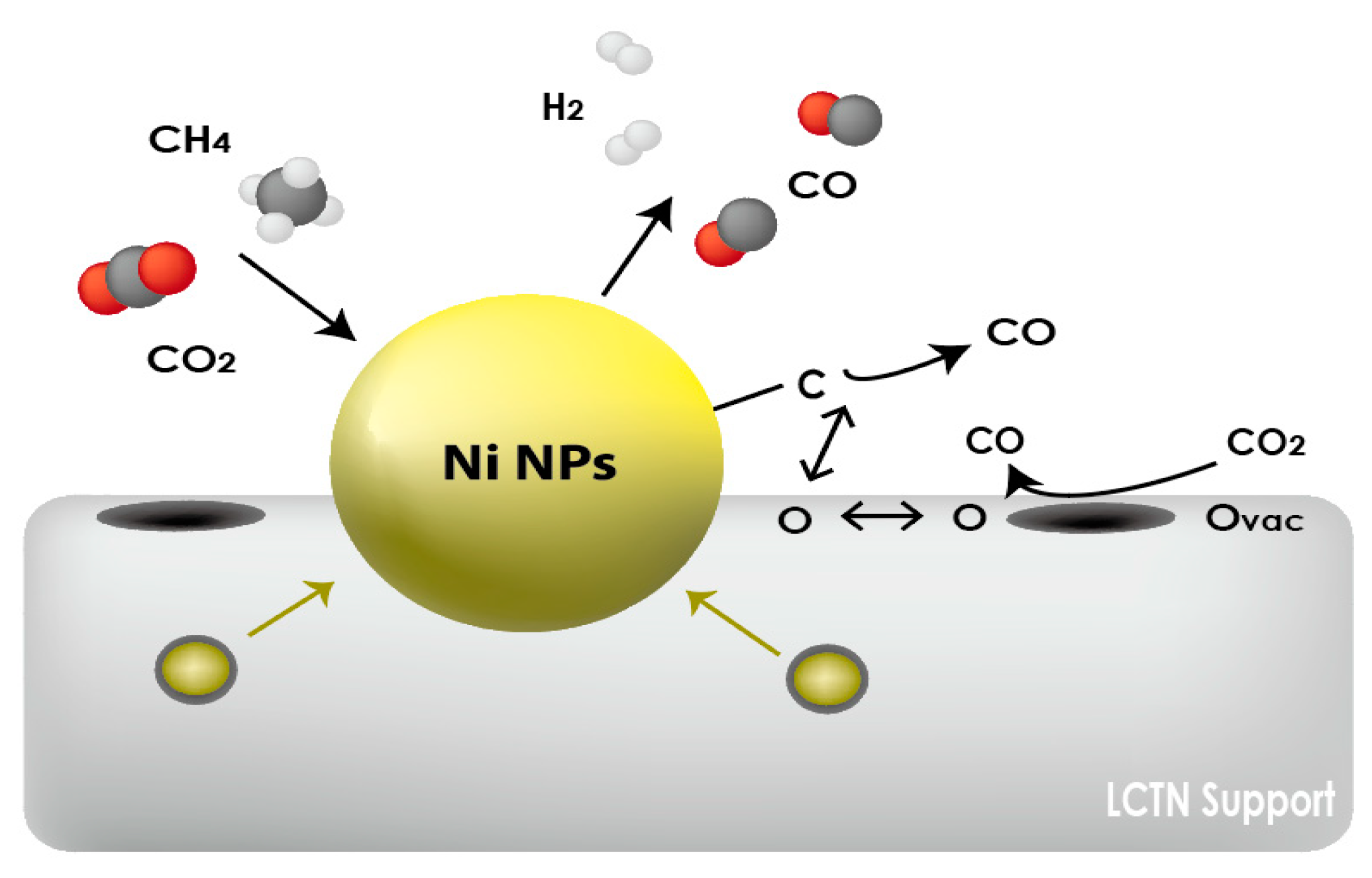
3.2. Catalytic Perperties for the DRM Reaction
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aramouni, N.A.K.; Touma, J.G.; Tarboush, B.A.; Zeaiter, J.; Ahmad, M.N. Catalyst design for dry reforming of methane: Analysis review. Renew. Sustain. Energy Rev. 2018, 82, 2570–2585. [Google Scholar] [CrossRef]
- Royer, S.; Duprez, D.; Can, F.; Courtois, X.; Batiot-Dupeyrat, C.; Laassiri, S. Perovskites as Substitutes of Noble Metals for Heterogeneous Catalysis: Dream or Reality. Chem. Rev. 2014, 114, 10292–10368. [Google Scholar] [CrossRef] [PubMed]
- Pena, M.A.; Fierro, J.L.G. Chemical Structures and Performance of Perovskite Oxides. Chem. Rev. 2001, 101, 1981–2017. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhao, T.; Dong, X.; Wang, M.H. Effects of Ce substitution at the A-site of LaNi0.5Fe0.5O3 perovskite on the enhanced catalytic activity for dry reforming of methane. Appl. Catal. B 2018, 224, 214–221. [Google Scholar] [CrossRef]
- Lee, C.; Jeon, Y.; Hata, S.; Park, J.-I.; Akiyoshi, R.; Saito, H. Three-dimensional arrangements of perovskite-type oxide nano-fiber webs for effective soot oxidation. Appl. Catal. B 2016, 191, 157–164. [Google Scholar] [CrossRef]
- Milt, V.G.; Ulla, M.A.; Miró, E.E. NOx trapping and soot combustion on BaCoO3−y perovskite: LRS and FTIR characterization. Appl. Catal. B 2005, 57, 13–21. [Google Scholar] [CrossRef]
- Kim, H.S.; Jeon, Y.; Kim, J.H.; Jang, G.Y.; Yoon, S.P.; Yun, J.W. Characteristics of Sr1−xYxTi1−yRuyO3+/−δ and Ru-impregnated Sr1−xYxTiO3+/−δ perovskite catalysts as SOFC anode for methane dry reforming. Appl. Surf. Sci. 2020, 510, 145450. [Google Scholar] [CrossRef]
- Eyssler, A.; Winkler, A.; Safonova, O.; Nachtegaal, M.; Matam, S.K.; Hug, P. On the State of Pd in Perovskite-Type Oxidation Catalysts of Composition A(B,Pd)O3±δ (A = La, Y; B = Mn, Fe, Co). Chem. Mater. 2012, 24, 1864–1875. [Google Scholar] [CrossRef]
- Kim, K.J.; Lim, C.; Bae, K.T.; Lee, J.J.; Oh, M.Y.; Kim, H.J.; Kim, H.; Kim, G.; Shin, T.H.; Han, J.W.; et al. Concurrent promotion of phase transition and bimetallic nanocatalyst exsolution in perovskite oxides driven by Pd doping to achieve highly active bifunctional fuel electrodes for reversible solid oxide electrochemical cells. Appl. Catal. B 2022, 314, 121517. [Google Scholar] [CrossRef]
- Crisafulli, C.; Scirè, S.; Minicò, S.; Solarino, L. Ni–Ru bimetallic catalysts for the CO2 reforming of methane. Appl. Catal. A: Gen 2002, 225, 1–9. [Google Scholar] [CrossRef]
- Neagu, D.; Tsekouras, G.; Miller, D.N.; Menard, H.; Irvine, J.T. In situ growth of nanoparticles through control of non-stoichiometry. Nat. Chem. 2013, 5, 916–923. [Google Scholar] [CrossRef]
- Chen, H.; Lim, C.; Zhou, M.; He, Z.; Sun, X.; Li, X. Activating Lattice Oxygen in Perovskite Oxide by B-Site Cation Doping for Modulated Stability and Activity at Elevated Temperatures. Adv. Sci. 2021, 8, 2102713. [Google Scholar] [CrossRef]
- Wei, T.; Jia, L.; Luo, J.-L.; Chi, B.; Pu, J.; Li, J. CO2 dry reforming of CH4 with Sr and Ni co-doped LaCrO3 perovskite catalysts. Appl. Surf. Sci. 2020, 506, 144699. [Google Scholar] [CrossRef]
- Neagu, D.; Kyriakou, V.; Roiban, I.L.; Aouine, M.; Tang, C.; Caravaca, A. In Situ Observation of Nanoparticle Exsolution from Perovskite Oxides: From Atomic Scale Mechanistic Insight to Nanostructure Tailoring. ACS Nano 2019, 13, 12996–13005. [Google Scholar] [CrossRef]
- Huang, R.; Han, J.W. Improved Catalytic Activity of the High-Temperature Water Gas Shift Reaction on Metal-Exsolved La0.9Ni0.05Fe0.95O3 by Controlling Reduction Time. ChemEngineering 2021, 5, 28. [Google Scholar] [CrossRef]
- Kothari, M.; Jeon, Y.; Miller, D.N.; Pascui, A.E.; Kilmartin, J.; Wails, D. Platinum incorporation into titanate perovskites to deliver emergent active and stable platinum nanoparticles. Nat. Chem. 2021, 13, 677–682. [Google Scholar] [CrossRef]
- Zubenko, D.; Singh, S.; Rosen, B.A. Exsolution of Re-alloy catalysts with enhanced stability for methane dry reforming. Appl. Catal. B 2017, 209, 711–719. [Google Scholar] [CrossRef]
- Oh, J.H.; Kwon, B.W.; Cho, J.; Lee, C.H.; Kim, M.K.; Choi, S.H.; Yoon, S.P.; Han, J.; Nam, S.W.; Kim, J.Y.; et al. Importance of Exsolution in Transition-Metal (Co, Rh, and Ir)-Doped LaCrO3 Perovskite Catalysts for Boosting Dry Reforming of CH4 Using CO2 for Hydrogen Production. Ind. Eng. Chem. Res. 2019, 58, 6385–6393. [Google Scholar] [CrossRef]
- Otto, S.-K.; Kousi, K.; Neagu, D.; Bekris, L.; Janek, J.; Metcalfe, I.S. Exsolved Nickel Nanoparticles Acting as Oxygen Storage Reservoirs and Active Sites for Redox CH4 Conversion. ACS Appl. Energy Mater. 2019, 2, 7288–7298. [Google Scholar] [CrossRef]
- Joo, S.; Seong, A.; Kwon, O.; Kim, K.; Lee, J.H.; Gorte, R.J. Highly active dry methane reforming catalysts with boosted in situ grown Ni-Fe nanoparticles on perovskite via atomic layer deposition. Sci. Adv. 2020, 6, 1573. [Google Scholar] [CrossRef]
- Arora, S.; Prasad, R. An overview on dry reforming of methane: Strategies to reduce carbonaceous deactivation of catalysts. RSC Adv. 2016, 6, 108668–108688. [Google Scholar] [CrossRef]
- Ji, D.H.; Wang, S.L.; Ge, X.Z.; Zhang, Q.Q.; Zhang, C.M.; Zeng, Z.W.; Bai, Y. The maximum predicted content of cation vacancies in inorganic and organic–inorganic perovskites: The role of the tolerance factor. Phys. Chem. Chem. Phys. 2017, 19, 17121. [Google Scholar] [CrossRef] [PubMed]
- Bartel, C.J.; Sutton, C.; Goldsmith, B.R.; Ouyang, R.; Musgrave, C.B.; Ghiringhelli, L.M.; Scheffler, M. New tolerance factor to predict the stability of perovskite oxides and halides. Sci. Adv. 2019, 5, 0693. [Google Scholar] [CrossRef] [PubMed]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryst. 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Kousi, K.; Neagu, D.; Bekris, L.; Papaioannou, E.I.; Metcalfe, I.S. Endogenous Nanoparticles Strain Perovskite Host Lattice Providing Oxygen Capacity and Driving Oxygen Exchange and CH4 Conversion to Syngas. Angew. Chem. Int. Ed. Engl. 2020, 59, 2510–2519. [Google Scholar] [CrossRef] [PubMed]
- Kousi, K.; Neagu, D.; Bekris, L.; Calì, E.; Kerherve, G.; Papaioannou, E.I. Low temperature methane conversion with perovskite-supported exo/endo-particles. J. Mater. Chem. 2020, 8, 12406–12417. [Google Scholar] [CrossRef]
- Marushina, E.V.; Kaczorowski, D.; Murashova, E.V.; Kurenbaeva, Z.M.; Gribanov, A.V. Crystal structure and unstable valence in a novel intermetallic phase Ce2Ru2Al. J. Alloys Compd. 2015, 650, 654–657. [Google Scholar] [CrossRef]
- Deka, D.J.; Kim, J.; Gunduz, S.; Aouine, M.; Millet, J.-M.M.; Co, A.C. Investigation of hetero-phases grown via in-situ exsolution on a Ni-doped (La,Sr)FeO3 cathode and the resultant activity enhancement in CO2 reduction. Appl. Catal. B 2021, 286, 119917. [Google Scholar] [CrossRef]
- Papargyriou, D.; Miller, D.N.; Irvine, J.T. Exsolution of Fe–Ni alloy nanoparticles from (La,Sr)(Cr,Fe,Ni)O3 perovskites as potential oxygen transport membrane catalysts for methane reforming. J. Mater. Chem. 2019, 7, 15812–15822. [Google Scholar] [CrossRef]
- Opitz, A.K.; Nenning, A.; Rameshan, C.; Kubicek, M.; Götsch, T.; Blume, R.; Hävecker, M.; Knop-Gericke, A.; Rupprechter, G.; Klötzer, B. Surface Chemistry of Perovskite-Type Electrodes during High Temperature CO2 Electrolysis Investigated by Operando Photoelectron Spectroscopy. ACS Appl. Mater. Interfaces 2017, 9, 35847–35860. [Google Scholar] [CrossRef]
- Chen, Z.; Kronawitter, C.X.; Yang, X.; Yeh, Y.W.; Yao, N.; Koel, B.E. The promoting effect of tetravalent cerium on the oxygen evolution activity of copper oxide catalysts. Phys. Chem. Chem. Phys. 2017, 19, 31545–31552. [Google Scholar] [CrossRef] [PubMed]
- Ganduglia-Pirovano, M.V.; Hofmann, A.; Sauer, J. Oxygen vacancies in transition metal and rare earth oxides: Current state of understanding and remaining challenges. Surf. Sci. Rep. 2007, 62, 219–270. [Google Scholar] [CrossRef]
- Kwon, O.; Sengodan, S.; Kim, K.; Kim, G.; Jeong, H.Y.; Shin, J. Exsolution trends and co-segregation aspects of self-grown catalyst nanoparticles in perovskites. Nat. Commun. 2017, 8, 15967. [Google Scholar] [CrossRef]
- Wang, L.; Al-Mamun, M.; Zhong, Y.L.; Liu, P.; Wang, Y.; Yang, H.G. Enhanced Thermochemical Water Splitting through Formation of Oxygen Vacancy in La0.6 Sr0.4 BO3-delta (B=Cr, Mn, Fe, Co, and Ni) Perovskites. Chempluschem 2018, 83, 924–928. [Google Scholar] [CrossRef]
- Zhang, S.; Ying, M.; Yu, J.; Zhan, W.; Wang, L.; Guo, Y. NixAl1O2-δ mesoporous catalysts for dry reforming of methane: The special role of NiAl2O4 spinel phase and its reaction mechanism. Appl. Catal. B 2021, 291, 120074. [Google Scholar] [CrossRef]
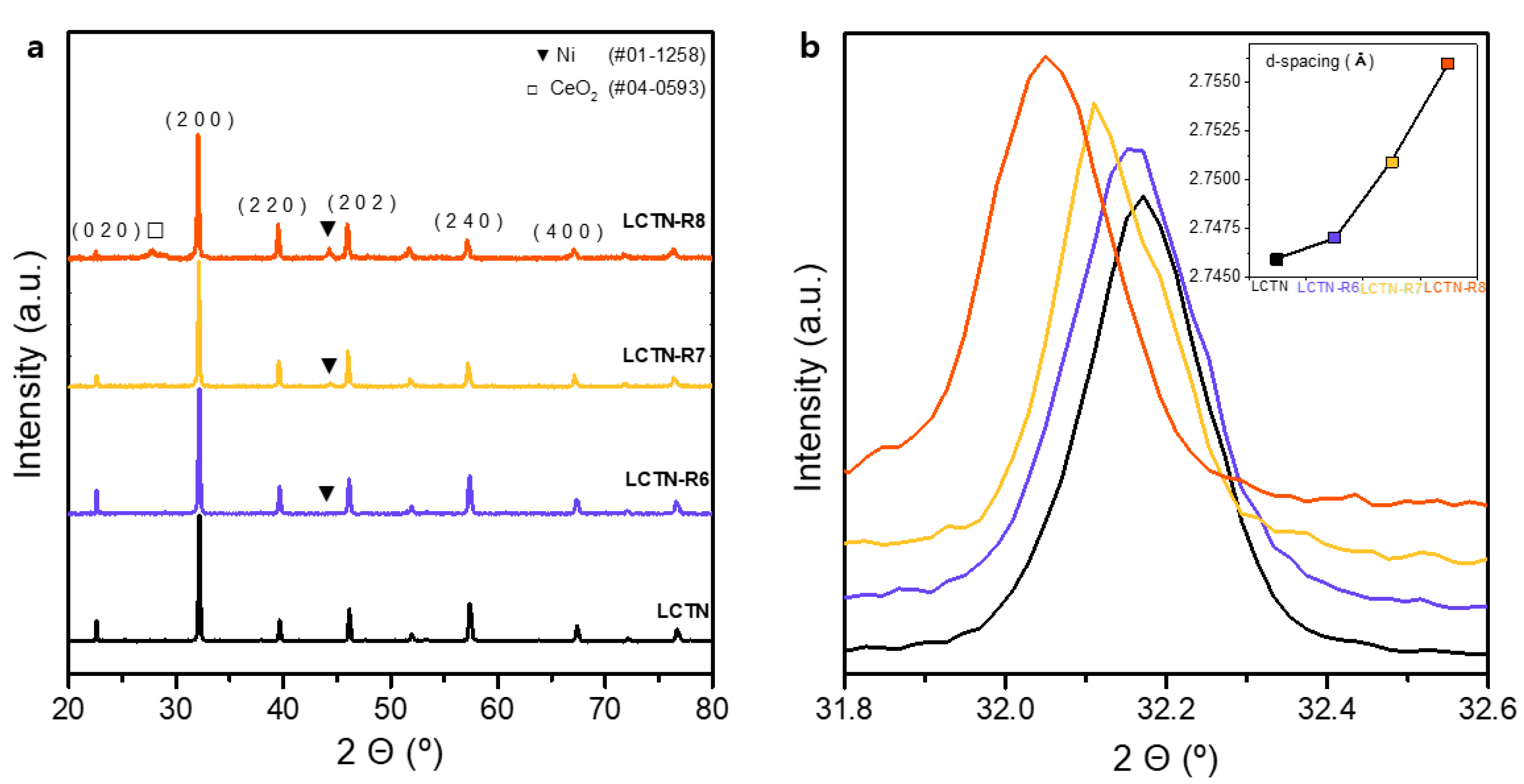
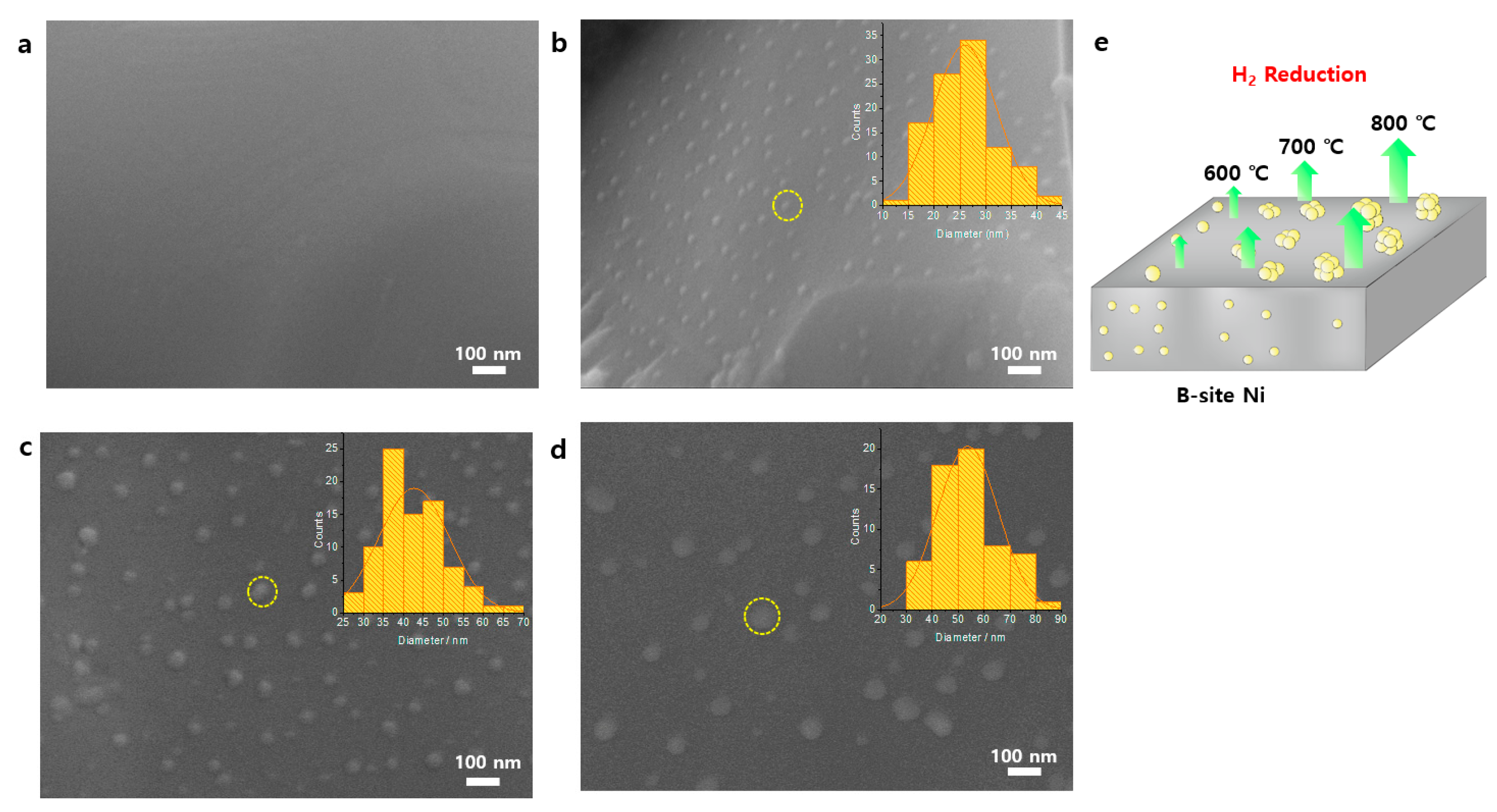
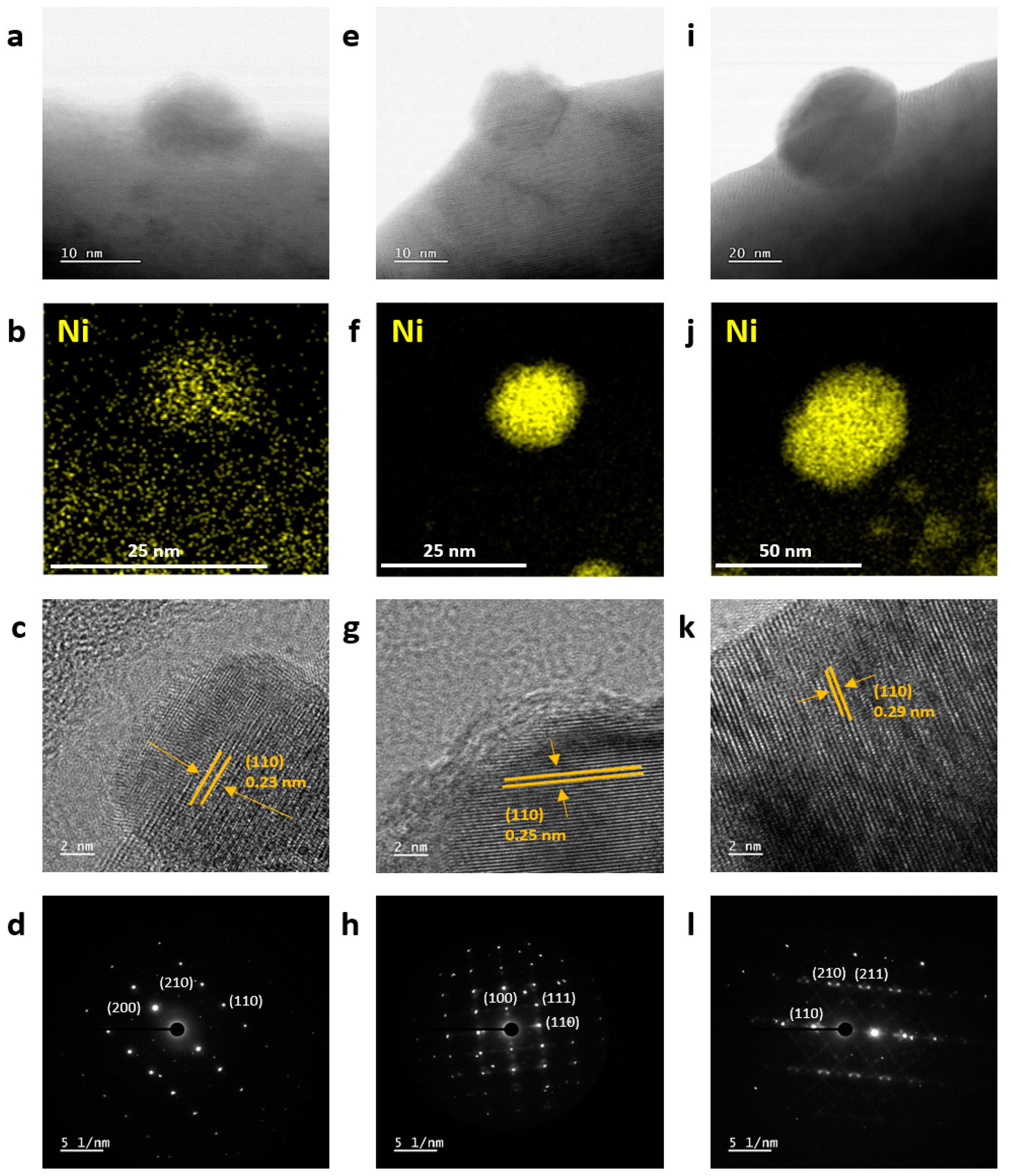
| Catalysts | (200) Peak (°) | d (Å) | Cell Volume (Å3) | Ni Metal Peak (°) | Ni Metal Crystallite Size 1 (Å) |
|---|---|---|---|---|---|
| LCTN | 32.17 | 2.746 | 242.18 | - | - |
| LCTN-R6 | 32.15 | 2.748 | 243.04 | 44.385 | 27.163 |
| LCTN-R7 | 32.11 | 2.751 | 244.42 | 44.364 | 30.752 |
| LCTN-R8 | 32.05 | 2.756 | 245.15 | 44.283 | 34.295 |
| Catalysts | Ni 2p | O 1s | ||
|---|---|---|---|---|
| BE (eV) 1 | Area (%) 2 | BE (eV) 1 | Area (%) 2 | |
| LCTN | 67.45 (Ni2+) | 100 | 529.2 (OLat) 531 (ODef) | 46.14 53.86 |
| LCTN-R6 | 67.5 (Ni2+) 66 (Ni0) | 94.94 5.06 | 529.2 (OLat) 531 (ODef) | 77.10 22.90 |
| LCTN-R7 | 67.5 (Ni2+) 66 (Ni0) | 78.54 21.46 | 529.2 (OLat) 530.9 (ODef) | 74.44 25.56 |
| LCTN-R8 | 67.5 (Ni2+) 66 (Ni0) | 69.91 30.09 | 529.15 (OLat) 531 (ODef) | 56.53 43.47 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.; Mane, R.; Han, K.; Kim, H.; Lee, C.; Jeon, Y. In Situ Control of the Eluted Ni Nanoparticles from Highly Doped Perovskite for Effective Methane Dry Reforming. Nanomaterials 2022, 12, 3325. https://doi.org/10.3390/nano12193325
Kim H, Mane R, Han K, Kim H, Lee C, Jeon Y. In Situ Control of the Eluted Ni Nanoparticles from Highly Doped Perovskite for Effective Methane Dry Reforming. Nanomaterials. 2022; 12(19):3325. https://doi.org/10.3390/nano12193325
Chicago/Turabian StyleKim, Heesu, Rasika Mane, Kyeongwon Han, Hyungjin Kim, Chanmin Lee, and Yukwon Jeon. 2022. "In Situ Control of the Eluted Ni Nanoparticles from Highly Doped Perovskite for Effective Methane Dry Reforming" Nanomaterials 12, no. 19: 3325. https://doi.org/10.3390/nano12193325
APA StyleKim, H., Mane, R., Han, K., Kim, H., Lee, C., & Jeon, Y. (2022). In Situ Control of the Eluted Ni Nanoparticles from Highly Doped Perovskite for Effective Methane Dry Reforming. Nanomaterials, 12(19), 3325. https://doi.org/10.3390/nano12193325






