A Flexible and Robust Structural Color Film Obtained by Assembly of Surface-Modified Melanin Particles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Measurements
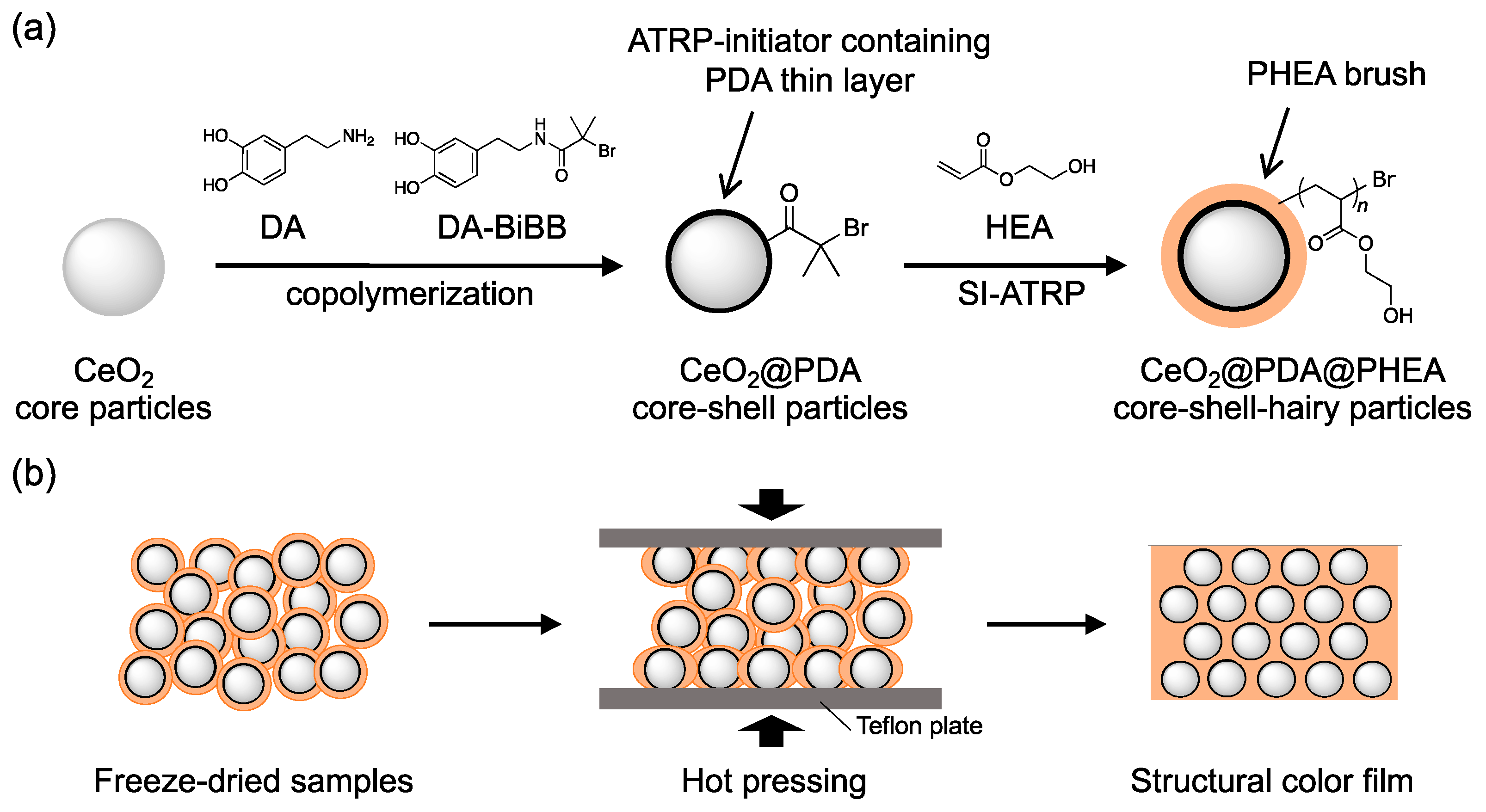
2.3. Preparation of the CeO2@PDA Core–Shell Particles
2.4. Preparation of the CeO2@PDA@PHEA(X) Core-Shell-Hairy Particles
2.5. Preparation of the Pellet Samples
2.6. Preparation of Structural Color Films
3. Results and Discussion
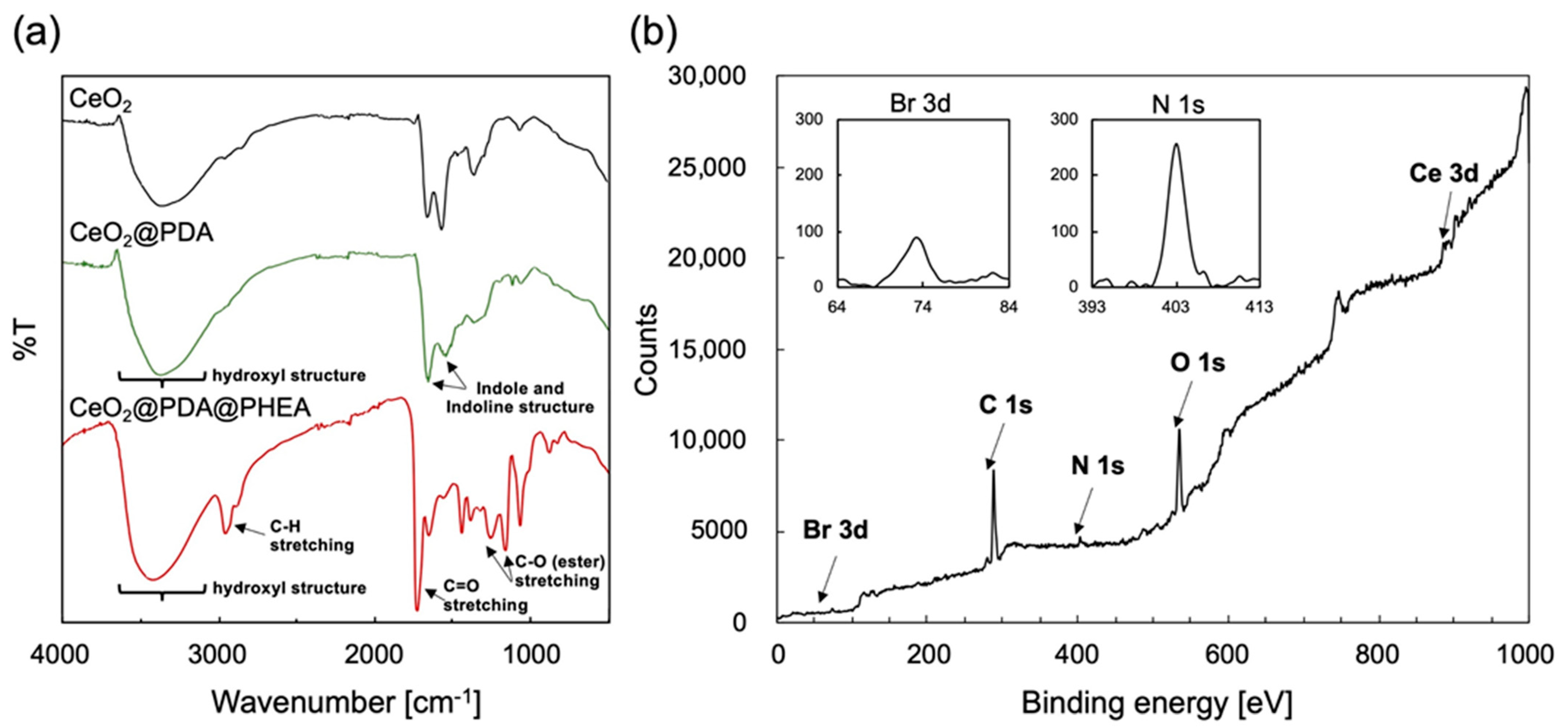
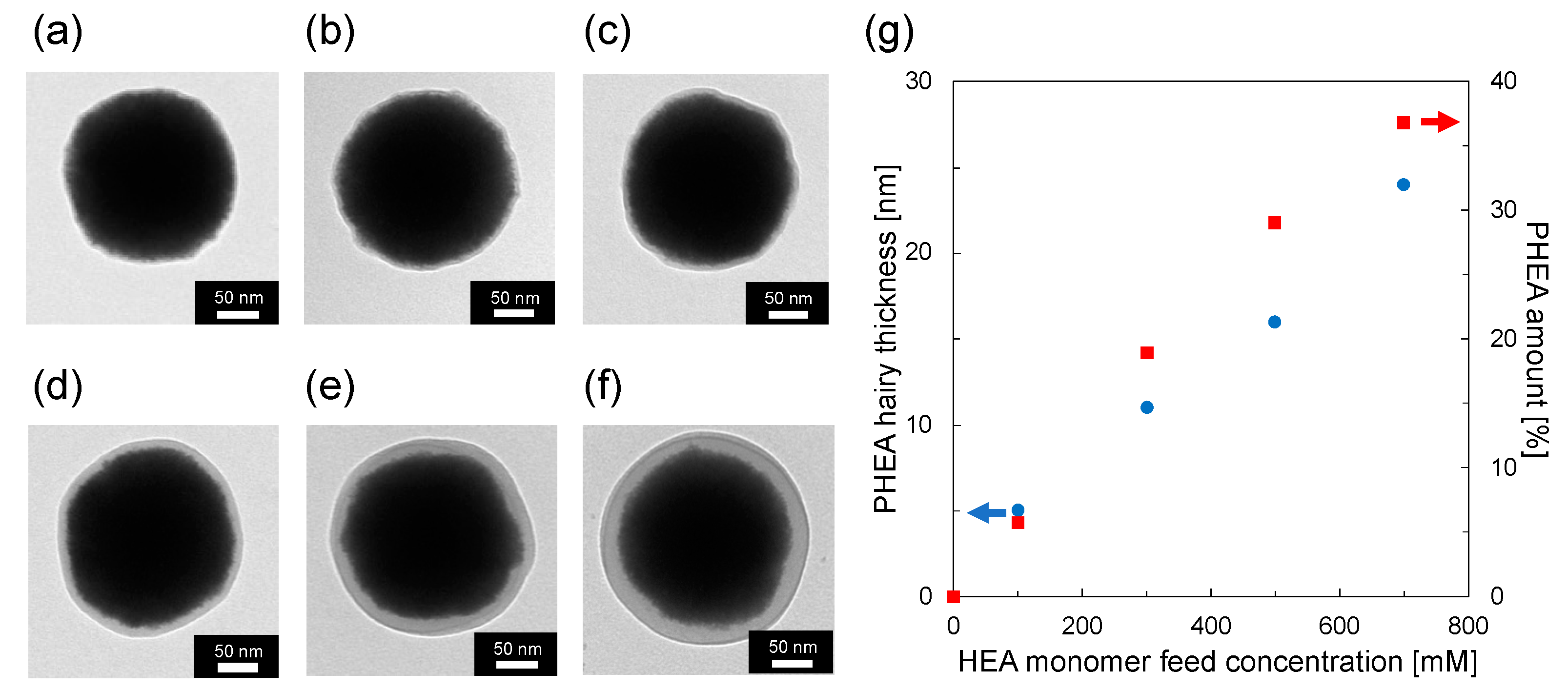
3.1. Preparation of CeO2@PDA@PHEA Core–Shell-Hairy Particles
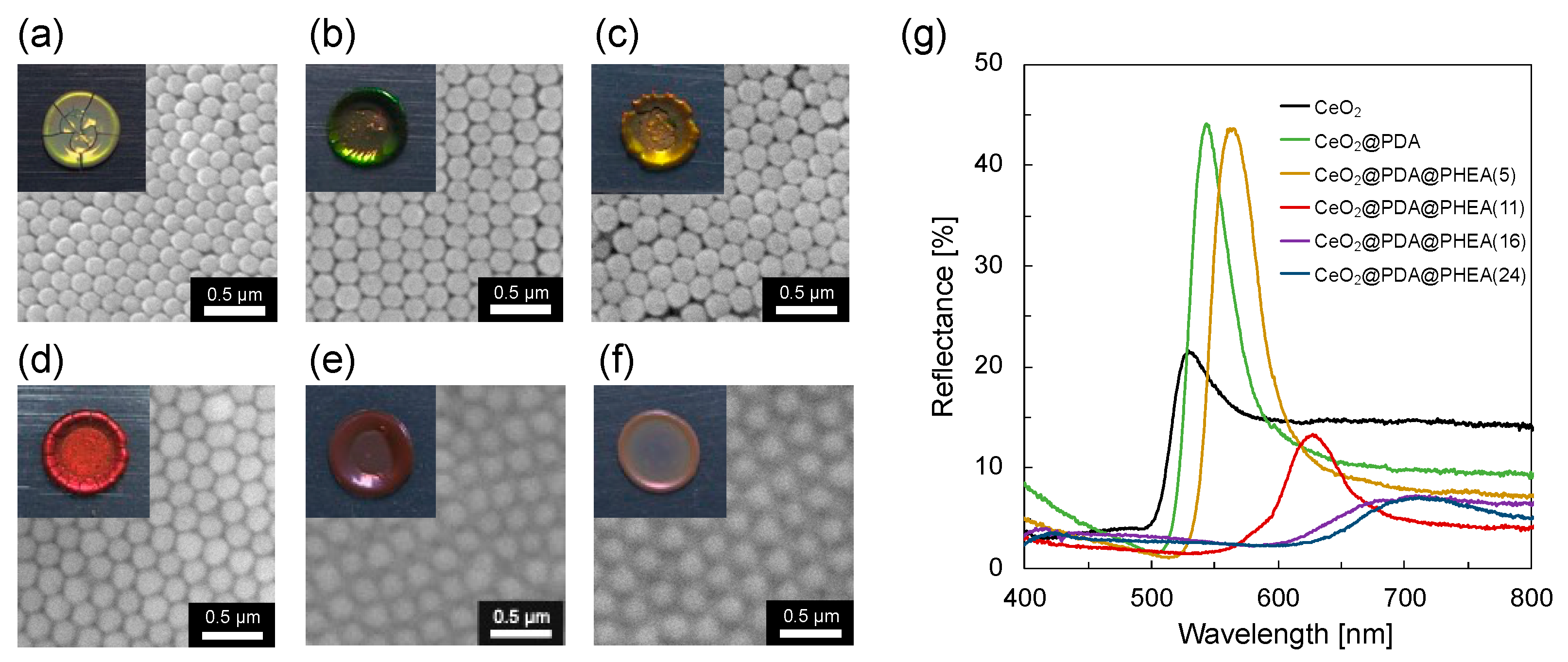
3.2. Effect of PHEA Hairy Layer Thickness on the Coloration of Pellet Samples
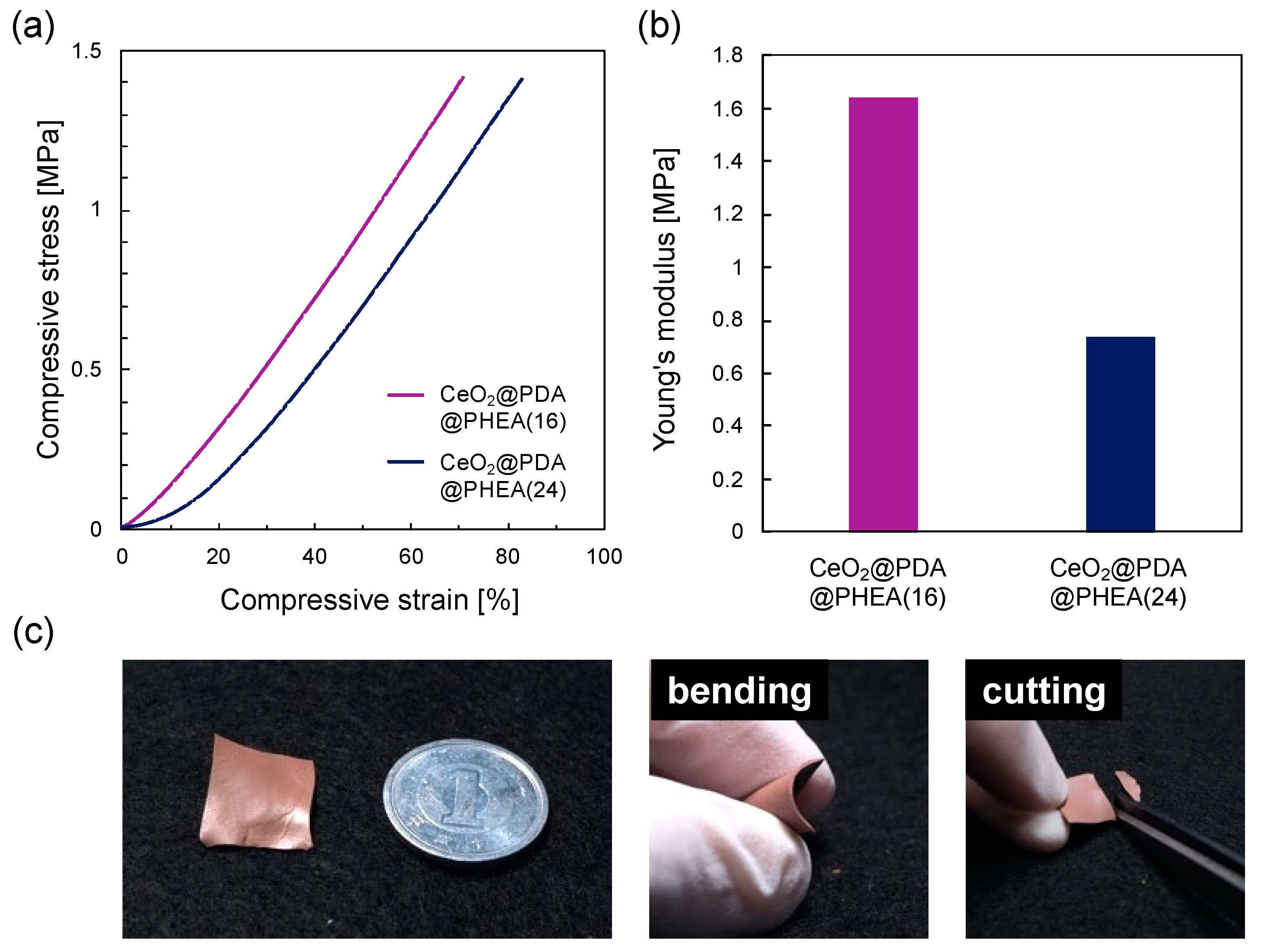
3.3. Preparation and Characterization of Structural Color Films
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mba, J.C.; Mitomo, H.; Yonamine, Y.; Wang, G.; Matsuo, Y.; Ijiro, K. Hysteresis in the thermo-responsive assembly of hexa (ethylene glycol) derivative-modified gold nanodiscs as an effect of shape. Nanomaterials 2022, 12, 1421. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Mitra, A.; Aïssa, B. Metamaterials and metasurfaces: A review from the perspectives of materials, mechanisms and advanced metadevices. Nanomaterials 2022, 12, 1027. [Google Scholar] [CrossRef] [PubMed]
- Alamer, F.A.; Beyari, R.F. Overview of the influence of silver, gold, and titanium nanoparticles on the physical properties of PEDOT: PSS-coated cotton fabrics. Nanomaterials 2022, 12, 1609. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, K.; Hayakawa, T.; Fudouzi, H. Introduction of planar defect into colloidal photonic-crystal films and their optical properties. Adv. Powder. Technol. 2022, 33, 103594. [Google Scholar] [CrossRef]
- Yu, J.; Lee, C.H.; Kan, C.W.; Jin, S. Fabrication of structural-coloured carbon fabrics by thermal assisted gravity sedimentation method. Nanomaterials 2020, 10, 1133. [Google Scholar] [CrossRef]
- Neterebskaia, V.O.; Goncharenko, A.O.; Morozova, S.M.; Kolchanov, D.S.; Vinogradov, A.V. Inkjet printing humidity sensing pattern based on self-organizing polystyrene spheres. Nanomaterials 2020, 10, 1358. [Google Scholar] [CrossRef] [PubMed]
- Hong, R.; Shi, Y.; Wang, X.Q.; Peng, L.; Wu, X.; Cheng, H.; Chen, S. Highly sensitive mechanochromic photonic gel towards fast- responsive fingerprinting. RSC Adv. 2017, 7, 33258. [Google Scholar] [CrossRef]
- Dong, Y.; Bazrafshan, A.; Pokutta, A.; Sulejmani, F.; Sun, W.; Combs, J.D.; Clarke, K.C.; Salaita, K. Chameleon-inspired strain-accommodating smart skin. ACS Nano 2019, 13, 9918. [Google Scholar] [CrossRef]
- Hatton, B.; Michchenko, L.; Davis, S.; Sandhage, K.H.; Aizenberg, J. Assembly of large-area, highly ordered, crack-free inverse opal films. Proc. Natl. Acad. Sci. USA 2010, 107, 10354. [Google Scholar] [CrossRef]
- Chiappini, A.; Chiasera, A.; Berneschi, S.; Aremellini, C.; Carpentiero, A.; Mazzola, M.; Moser, E.; Varas, S.; Righini, G.C.; Ferrari, M. Sol–gel-derived photonic structures: Fabrication, assessment, and application. J. Sol Gel Sci. Technol. 2011, 60, 408. [Google Scholar] [CrossRef]
- Ding, H.; Liu, C.; Gu, H.; Zhao, Y.; Wang, B.; Gu, Z. Responsive colloidal crystal for spectrometer grating. ACS Photonics 2014, 1, 121. [Google Scholar] [CrossRef]
- Lee, G.H.; Han, S.H.; Kim, J.H.; Lee, J.M.; Kim, S.H. Colloidal photonic inks for mechanochromic films and patterns with structural colors of high saturation. Chem. Mater. 2019, 31, 8154. [Google Scholar] [CrossRef]
- Schroden, R.C.; Daous, M.A.; Blanford, C.F.; Stein, A. Optical properties of inverse opal photonic crystals. Chem. Mater. 2002, 14, 3305. [Google Scholar] [CrossRef]
- Armstrong, E.; O’Dwyer, C. Artificial opal photonic crystals and inverse opal structures-fundamentals and applications from optics to energy storage. J. Mater. Chem. C 2015, 3, 6109. [Google Scholar] [CrossRef]
- Shimamoto, H.; Cheng, C.H.; Kamitani, K.; Kojio, K.; Higaki, Y.; Takahara, A. Nanocomposite elastomers composed of silica nanoparticles grafted with a comb-shaped copolymer brush. Macromolecules 2019, 52, 5963. [Google Scholar] [CrossRef]
- Cheng, C.H.; Masuda, S.; Nozaki, S.; Nagano, C.; Hirai, T.; Kojio, K.; Takahara, A. Fabrication and deformation of mechanochromic nanocomposite elastomers based on rubbery and glassy block copolymer-grafted silica nanoparticles. Macromolecules 2020, 53, 4541. [Google Scholar] [CrossRef]
- Ohno, K.; Mizuta, Y. Structural color materials using polymer-brush-decorated hybrid particles. ACS Appl. Polym. Mater. 2020, 2, 368. [Google Scholar] [CrossRef]
- Iwasaki, T.; Harada, S.; Okoshi, T.; Moriya, M.; Kojima, T.; Kishikawa, K.; Kohri, M. Effect of the polydopamine composite method on structural coloration: Comparison of binary and unary assembly of colloidal particles. Langmuir 2020, 36, 11880. [Google Scholar] [CrossRef] [PubMed]
- Forster, J.D.; Noh, H.; Liew, S.F.; Saranathan, V.; Schreck, C.F.; Yang, L.; Park, J.G.; Prum, R.O.; Mochrie, S.G.J.; O’Hern, C.S.; et al. Biomimetic isotropic nanostructures for structural coloration. Adv. Mater. 2010, 22, 2939. [Google Scholar] [CrossRef]
- Takeoka, Y.; Yoshioka, S.; Takano, A.; Arai, S.; Nueangnoraj, K.; Nishihara, H.; Teshima, M.; Ohtsuka, Y.; Seki, T. Production of colored pigments with amorphous arrays of black and white colloidal particles. Angew. Chem. Int. Ed. 2013, 52, 7261. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Han, P.; Zhou, H.; Wu, N.; Wei, Y.; Yao, X.; Zhou, J.; Song, Y. Highly brilliant noniridescent structural colors enabled by graphene nanosheets containing graphene quantum dots. Adv. Funct. Mater. 2018, 28, 1802585. [Google Scholar] [CrossRef]
- Fu, F.; Chen, Z.; Wang, H.; Liu, C.; Liu, Y.; Zhao, Y. Graphene hybrid colloidal crystal arrays with photo-controllable structural colors. Nanoscale 2019, 11, 10846. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, S.; Kinoshita, S. Effect of macroscopic structure in iridescent color of the peacock feathers. Forma 2002, 17, 169. [Google Scholar]
- Kinoshita, S.; Yoshioka, S.; Fujii, Y.; Okamoto, N. Photophysics of structural color in the Morpho butterflies. Forma 2002, 17, 103. [Google Scholar]
- d’Ischia, M.; Napolitano, A.; Ball, V.; Chen, C.T.; Buehler, M.J. Polydopamine and eumelanin: From structure-property relationships to a unified tailoring strategy. Acc. Chem. Res. 2014, 47, 3541. [Google Scholar] [CrossRef]
- Bao, X.; Zhao, J.; Sun, J.; Hu, M.; Yang, X. Polydopamine nanoparticles as efficient scavengers for reactive oxygen species in periodontal disease. ACS Nano 2018, 12, 8882. [Google Scholar] [CrossRef] [PubMed]
- Farnad, N.; Farhadi, K.; Voelcker, N.H. Polydopamine nanoparticles as a new and highly selective biosorbent for the removal of copper (II) ions from aqueous solutions. Water Air Soil Pollut. 2012, 223, 3535. [Google Scholar] [CrossRef]
- Kohri, M.; Nannichi, Y.; Taniguchi, T.; Kishikawa, K. Biomimetic non-iridescent structural color materials from polydopamine black particles that mimic melanin granules. J. Mater. Chem. C 2015, 3, 720. [Google Scholar] [CrossRef]
- Kawamura, A.; Kohri, M.; Morimoto, G.; Nannichi, Y.; Taniguchi, T.; Kishikawa, K. Full-color biomimetic photonic materials with iridescent and non-iridescent structural colors. Sci. Rep. 2016, 6, 33984. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, T.; Tamai, Y.; Yamamoto, M.; Taniguchi, T.; Kishikawa, K.; Kohri, M. Melanin precursor influence on structural colors from artificial melanin particles: PolyDOPA, polydopamine, and polynorepinephrine. Langmuir 2018, 34, 11814. [Google Scholar] [CrossRef] [PubMed]
- Kohri, M.; Tamai, Y.; Kawamura, A.; Jido, K.; Yamamoto, M.; Taniguchi, T.; Kishikawa, K.; Fujii, S.; Teramoto, N.; Ishii, H.; et al. Ellipsoidal artificial melanin particles as building blocks for biomimetic structural coloration. Langmuir 2019, 35, 5574. [Google Scholar] [CrossRef] [PubMed]
- Kohri, M. Artificial melanin particles: New building blocks for biomimetic structural coloration. Polym. J. 2019, 51, 1127. [Google Scholar] [CrossRef]
- Kohri, M. Progress in polydopamine-based melanin mimetic materials for structural color generation. Sci. Technol. Adv. Mater. 2020, 21, 833. [Google Scholar] [CrossRef] [PubMed]
- Kohri, M.; Yanagimoto, K.; Kawamura, A.; Hamada, K.; Imai, Y.; Watanabe, T.; Ono, T.; Taniguchi, T.; Kishikawa, K. Polydopamine-based 3D colloidal photonic materials: Structural color balls and fibers from melanin-like particles with polydopamine shell layers. ACS Appl. Mater. Interfaces 2018, 10, 7640. [Google Scholar] [CrossRef] [PubMed]
- Kohri, M.; Kobayashi, A.; Okoshi, T.; Shirasaka, H.; Hirai, K.; Ujiie, K.; Kojima, T.; Kishikawa, K. Bright solvent sensor using an inverse opal structure containing melanin-mimicking polydopamine. Chem. Lett. 2021, 50, 106. [Google Scholar] [CrossRef]
- Okoshi, T.; Iwasaki, T.; Takahashi, S.; Iwasaki, Y.; Kishikawa, K.; Kohri, M. Control of structural coloration by natural sunlight irradiation on a melanin precursor polymer inspired by skin tanning. Biomacromolecules 2021, 22, 1730. [Google Scholar] [CrossRef]
- Takahashi, S.; Kishikawa, K.; Kohri, M. External stimulus control of structural color visibility using colloidal particles covered with a catecholic polymer shell layer. Polym. J. 2022, 54, 1039. [Google Scholar] [CrossRef]
- Yamanlar, S.; Sant, S.; Boudou, T.; Picart, C.; Khademhosseini, A. Surface functionalization of hyaluronic acid hydrogels by polyelectrolyte multilayer films. Biomaterials 2011, 32, 5590. [Google Scholar] [CrossRef]
- Kohri, M.; Kohma, H.; Shinoda, Y.; Yamauchi, M.; Yagai, S.; Kojima, T.; Taniguchi, T.; Kishikawa, K. A colorless functional polydopamine thin layer as a basis for polymer capsules. Polym. Chem. 2013, 4, 2696. [Google Scholar] [CrossRef]
- Nishizawa, N.; Kawamura, A.; Kohri, M.; Nakamura, Y.; Fujii, S. Polydopamine particle as a particulate emulsifier. Polymers 2016, 8, 62. [Google Scholar] [CrossRef]
- Zhu, B.; Edmondson, S. Polydopamine-melanin initiators for surface-initiated ATRP. Polymer 2011, 52, 2141. [Google Scholar] [CrossRef]
- Ge, J.; Yin, Y. Responsive photonic crystals. Angew. Chem. Int. Ed. 2011, 50, 1492. [Google Scholar] [CrossRef]
- Yi, B.; Shen, H. Liquid-immune structural colors with angle-independence inspired from hollow melanosomes. Chem. Commun. 2017, 53, 9234. [Google Scholar] [CrossRef]
- Hernández, J.C.R.; Pradas, M.M.; Ribelles, J.L.G. Properties of poly(2-hydroxyethyl acrylate)-silica nanocomposites obtained by the sol-gel process. J. Non. Cryst. Solids 2008, 354, 1900. [Google Scholar] [CrossRef]
- Xiao, M.; Li, Y.; Allen, M.; Deheyn, D.; Yue, X.; Zhao, J.; Gianneschi, N.; Shawkey, M.; Dhinojwala, A. Bio-inspired structural colors produced via self-assembly of synthetic melanin nanoparticles. ACS Nano. 2015, 9, 5454. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, S.; Yoshioka, S.; Miyazaki, J. Physics of structural colors. Rep. Prog. Phys. 2008, 71, 076401. [Google Scholar] [CrossRef]
- Fitzpatrick, S.; McCabe, J.F.; Petts, C.R.; Booth, S.W. Effect of moisture on polyvinylpyrrolidone in accelerated stability testing. Int. J. Pharm. 2002, 246, 143. [Google Scholar] [CrossRef]
- Chaudhuri, T.K.; Patel, M.G. High refractive index films of ZnS/PVP nanocomposite by in situ thermolysis. J. Exp. Nanosci. 2015, 10, 135. [Google Scholar] [CrossRef]
- Krishna, M.G.; Hartridge, A.; Bhattacharya, A.K. Temperature and ionic size dependence of the properties of ceria based optionic thin films. Mater. Sci. Eng. C 1998, B55, 14. [Google Scholar] [CrossRef]
- Zhao, Y.; Xie, Z.; Gu, H.; Zhu, C.; Gu, Z. Bio-inspired variable structural color materials. Chem. Soc. Rev. 2012, 41, 3297. [Google Scholar] [CrossRef]
- Fan, W.; Zeng, J.; Gan, Q.; Ji, D.; Song, H.; Liu, W.; Wu, L. Iridescence-controlled and flexibly tunable retroreflective structural color film for smart displays. Sci. Adv. 2019, 5, 8775. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Koyama, M.; Fudouzi, H.; Hojo, T.; Akiyama, E. Availability of opal photonic crystal films for visualizing heterogeneous strain evolution in steels: Example of lüders deformation. ISIJ Int. 2020, 60, 2604. [Google Scholar] [CrossRef]
- Fudouzi, H.; Sawada, T. Photonic rubber sheets with tunable color by elastic deformation. Langmuir 2006, 22, 1365. [Google Scholar] [CrossRef]
- Schäfer, C.G.; Winter, T.; Heidt, S.; Dietz, C.; Ding, T.; Baumberg, J.J.; Gallei, M. Smart polymer inverse-opal photonic crystal films by melt-shear organization for hybrid core–shell architectures. J. Mater. Chem. C 2015, 3, 2204. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Zheng, J.; Li, X.; Liu, G.; Zhou, L.; Zhou, W.; Shao, J. Polystyrene@poly(methyl methacrylate-butyl acrylate) core-shell nanoparticles for fabricating multifunctional photonic crystal films as mechanochromic and solvatochromic sensors. ACS Appl. Nano Mater. 2022, 5, 729. [Google Scholar] [CrossRef]
- Schäfer, C.G.; Gallei, M.; Zahn, J.T.; Engelhardt, J.; Hellmann, G.P.; Rehahn, M. Reversible light-, thermo-, and mechano-responsive elastomeric polymer opal films. Chem. Mater. 2013, 25, 2309. [Google Scholar] [CrossRef]
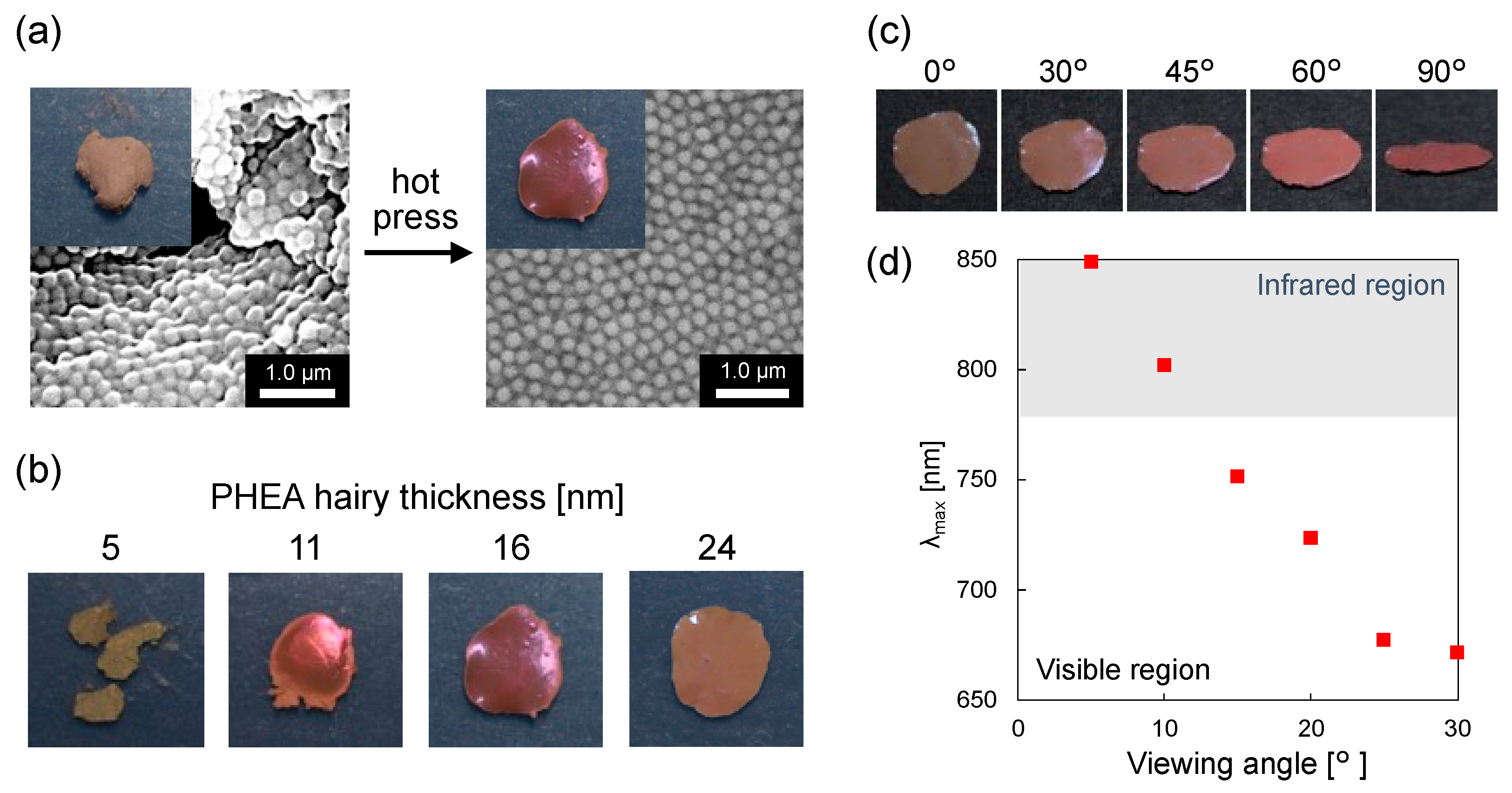
| CeO2 | CeO2@PDA | CeO2@PDA@PHEA(X) | |||||
|---|---|---|---|---|---|---|---|
| X = 5 | X = 11 | X = 16 | X = 24 | ||||
| nparticle | 1.93 * | 1.92 | 1.87 | 1.81 | 1.77 | 1.72 | |
| Center-to-center distance (d) (nm) | 190 ± 6 | 204 ± 4 | 208 ± 4 | 219 ± 5 | 236 ± 8 | 258 ± 17 | |
| Diameter of the particles (nm) | 198 ± 5 | 202 ± 7 | 211 ± 8 | 223 ± 9 | 233 ± 7 | 249 ± 9 | |
| λmax [nm] | Experimental values | 529 | 544 | 565 | 628 | 709 | 714 |
| Calculated values_gap: air | 538 | 575 | 572 | 586 | 619 | 660 | |
| Calculated values_gap: PHEA | - | - | 604 | 620 | 657 | 702 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoshioka, D.; Kishikawa, K.; Kohri, M. A Flexible and Robust Structural Color Film Obtained by Assembly of Surface-Modified Melanin Particles. Nanomaterials 2022, 12, 3338. https://doi.org/10.3390/nano12193338
Yoshioka D, Kishikawa K, Kohri M. A Flexible and Robust Structural Color Film Obtained by Assembly of Surface-Modified Melanin Particles. Nanomaterials. 2022; 12(19):3338. https://doi.org/10.3390/nano12193338
Chicago/Turabian StyleYoshioka, Daiki, Keiki Kishikawa, and Michinari Kohri. 2022. "A Flexible and Robust Structural Color Film Obtained by Assembly of Surface-Modified Melanin Particles" Nanomaterials 12, no. 19: 3338. https://doi.org/10.3390/nano12193338





