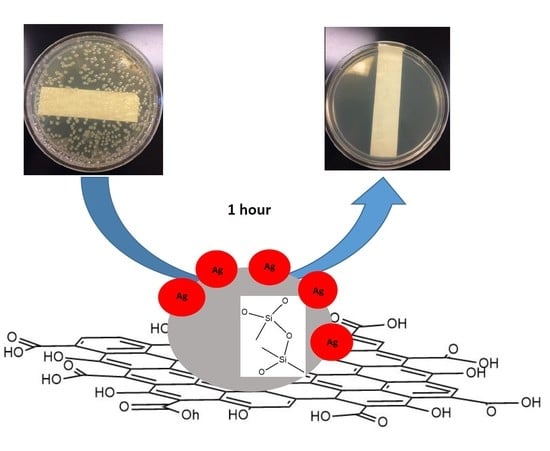
Silver Nanoparticles Functionalized Nanosilica Grown over Graphene Oxide for Enhancing Antibacterial Effect
Abstract
:1. Introduction
2. Experimental
2.1. Synthesis of Nanosilica Grown on Graphene Oxide
2.2. Synthesis of Silver Nanoparticles and Silver Nanoparticles Supported over Nanosilica/Graphene Oxide Composites
2.3. Characterization
2.4. Antibacterial Activity Assay
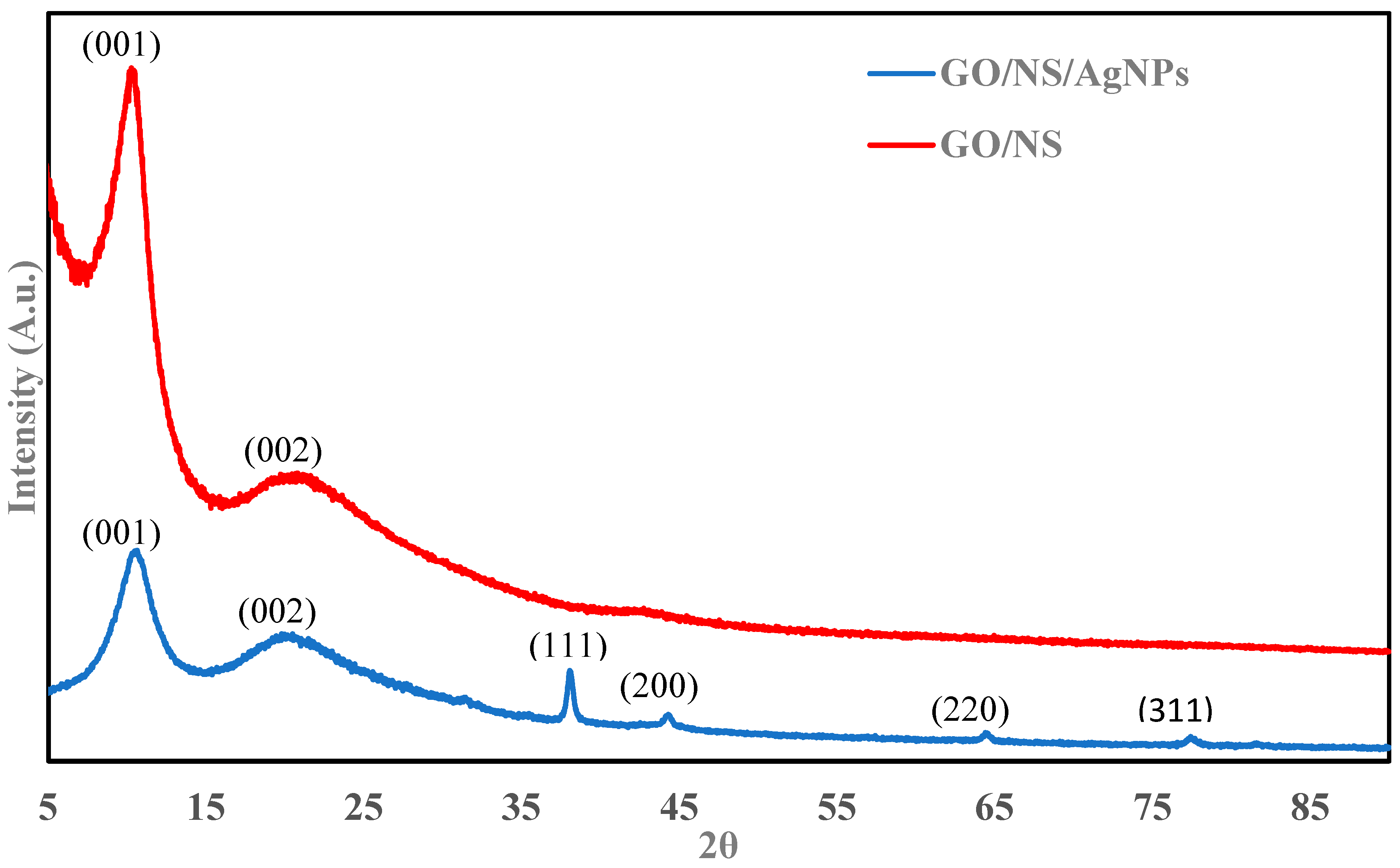
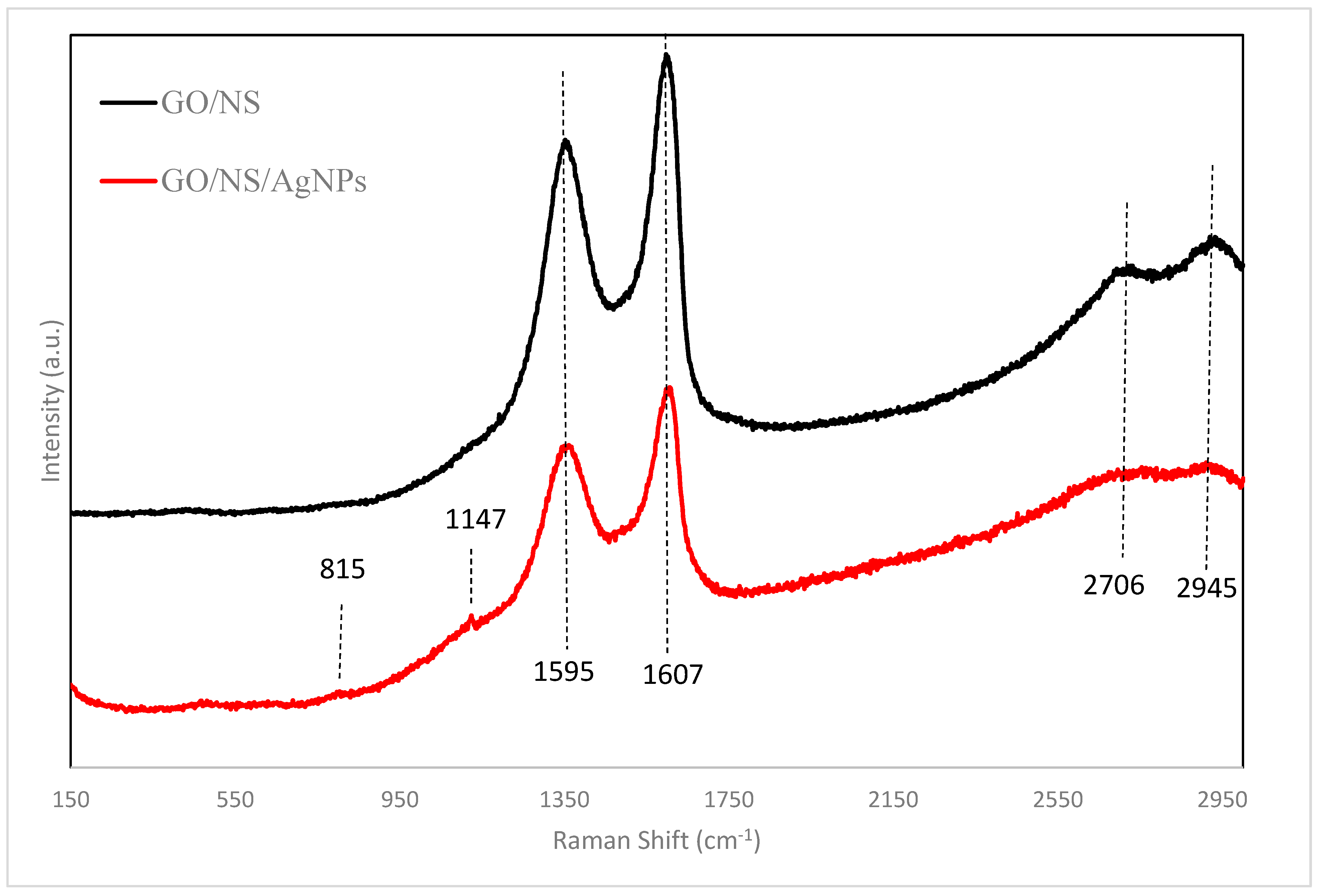
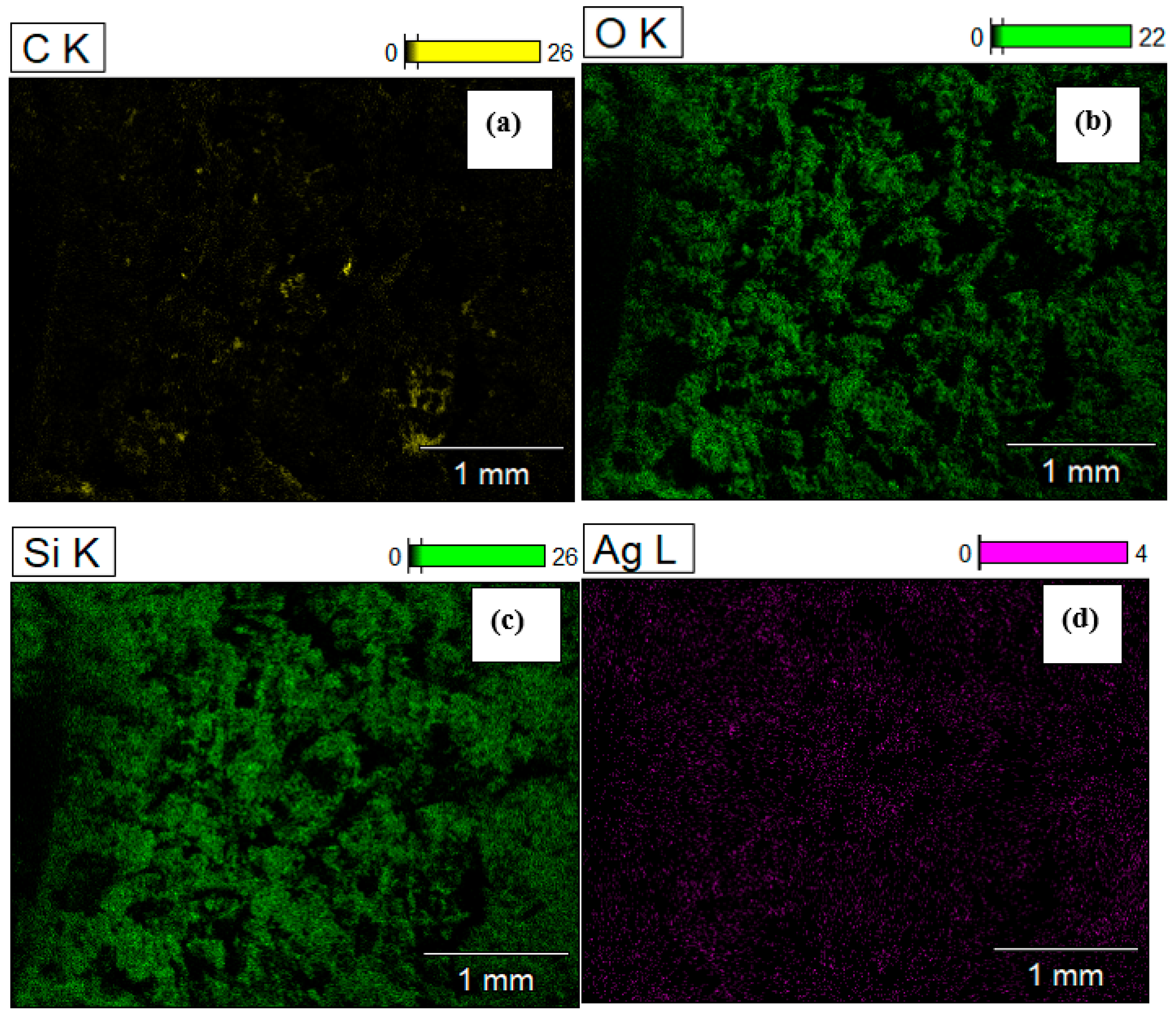
3. Result and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Prestinaci, F.; Pezzotti, P.; Pantosti, A. Antimicrobial resistance: A global multifaceted phenomenon. Pathog. Glob. Health 2015, 109, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Concillo, S.; Sessa, L.; Petrone, A.M.; Porta, A.; Diana, R.; Diana, R.; Lannell, P.; Piotto, S. Structure Modification of an Active Azo-Compound as a Route to New Antimicrobial Compounds. Molecules 2017, 22, 875. [Google Scholar] [CrossRef] [PubMed]
- Demir, B.; Broughton, R.M.; Qiao, M.; Huang, T.S.; Worley, S.D. N-Halamine Biocidal Materials with Superior Antimicrobial Efficacies for Wound Dressings. Molecules 2017, 22, 1582. [Google Scholar] [CrossRef]
- Ardila, N.; Daigle, F.; Heuzey, M.C.; Ajji, A. Antibacterial Activity of Neat Chitosan Powder and Flakes. Molecules 2017, 22, 100. [Google Scholar] [CrossRef]
- Lardani, L.; Derchi, G.; Marchio, V.; Carli, E. One-Year Clinical Performance of Activa™ Bioactive-Restorative Composite in Primary Molars. Children 2022, 9, 433. [Google Scholar] [CrossRef] [PubMed]
- Franci, G.; Falanga, A.; Galdiero, S.; Palomba, L.; Rai, M.; Morelli, G.; Galdiero, M. Silver Nanoparticles as Potential Antibacterial Agents. Molecules 2015, 20, 8856–8874. [Google Scholar] [CrossRef] [PubMed]
- Masri, A.; Anwar, A.; Ahmed, D.; Siddiqui, R.B.; Shah, M.S.; Khan, N.A. Silver Nanoparticle Conjugation-Enhanced Antibacterial Efficacy of Clinically Approved Drugs Cephradine and Vildagliptin. Antibiotics 2018, 7, 100. [Google Scholar] [CrossRef]
- Kvitek, A.P.L.; Smekalova, M.; Vecerova, R.; Kolar, M.; Roderova, M.; Dycka, F.; Sebela, M.; Prucek, R.; Tomanec, O.; Zboril, R. Bacterial resistance to silver nanoparticles and how to overcome it. Nature Nanotech. 2018, 13, 65–71. [Google Scholar] [CrossRef]
- McNeilly, O.; Mann, R.; Hamidian, M.; Gunawan, C. Emerging Concern for Silver Nanoparticle Resistance in Acinetobacter baumannii and Other Bacteria. Front. Microbiol. 2021, 12, 894. [Google Scholar] [CrossRef]
- Tejamaya, M.; Romer, I.; Merrifield, R.C.; Lead, J.R. Stability of Citrate, PVP, and PEG Coated Silver Nanoparticles in Ecotoxicology Media. Environ. Sci. Technol. 2012, 46, 7011–7017. [Google Scholar] [CrossRef]
- Dong, Y.; Wang, Q.; Wan, L.; Chi, Y. Carbon based dot capped silver nanoparticles for efficient surface-enhanced Raman scattering. J. Mater. Chem. C. 2016, 4, 7472–7477. [Google Scholar] [CrossRef]
- Quach, Q.; Briehler, E.; Elzamzami, A.; Huff, C.; Long, J.M.; Abdel-Fattah, T.M. Catalytic Activity of Beta-Cyclodextrin-Gold Nanoparticles Network in Hydrogen Evolution Reaction. Catalysts. 2021, 11, 118. [Google Scholar] [CrossRef]
- Huff, C.; Quach, Q.; Long, J.M.; Abdel-Fattah, T.M. Nanocomposite Catalyst Derived from Ultrafine Platinum Nanoparticles and Carbon Nanotubes for Hydrogen Generation. ECS J. Solid State Sci. Technol. 2020, 9, 101008. [Google Scholar] [CrossRef]
- Biehler, E.; Quach, Q.; Huff, C.; Abdel-Fattah, T.M. Organo-Nanocups Assist the Formation of Ultra-Small Palladium Nanoparticle Catalysts for Hydrogen Evolution Reaction. Materials. 2022, 15, 2692. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Fattah, T.M.; Loftis, D.; Biomed, A.M.J. Nanosized Controlled Surface Pretreatment of Biometallic Alloy 316L Stainless Steel. Nanotechnology 2011, 7, 794–800. [Google Scholar] [CrossRef]
- Mahapatro, A.; Negron, T.D.M.; Bonner, C.; Fattah, T.M.A. Nanolayers on Magnesium (Mg) Alloy for Metallic Bone Tissue Engineering Scaffolds. J. Biomater. Tissue Eng. 2013, 3, 196–204. [Google Scholar] [CrossRef]
- Mahapatro, R.B.A.; Bonner, C.; Abdel-Fattah, T.M. In vitro stability study of organophosphonic self assembled monolayers (SAMs) on cobalt chromium (Co–Cr) alloy. Mater. Sci. Eng. C 2013, 33, 2050–2058. [Google Scholar] [CrossRef]
- Kaya, S.; Cresswell, M.; Boccaccini, A.R. Mesoporous silica-based bioactive glasses for antibiotic-free antibacterial applications. Mater. Sci. Eng. C 2018, 83, 99–107. [Google Scholar] [CrossRef]
- Rojas-Andrade, M.D.; Chata, G.; Rouholiman, D.; Liu, J.; Saltikov, C.; Chen, S. Antibacterial mechanisms of graphene-based composite nanomaterials. Nanoscale 2017, 9, 994–1006. [Google Scholar] [CrossRef]
- Vallet-Regi, M.; Balas, F. Silica Materials for Medical Applications. Open Biomed. Eng. J. 2008, 2, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Campbell, E.; Hasan, M.T.; Pho, C.; Callaghan, K.; Akkaraju, G.R.; Naumov, A.V. Graphene Oxide as a Multifunctional Platform for Intracellular Delivery, Imaging, and Cancer Sensing. Sci. Rep. 2019, 9, 416. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Wang, X.; Ye, J.; Xue, X.; Zhang, F.; Zhang, H.; Hou, X.; Liu, X.; Zhang, Y. Enhanced antibacterial activity of silver-decorated sandwich-like mesoporous silica/reduced graphene oxide nanosheets through photothermal effect. Nanotechnology 2018, 29, 105704. [Google Scholar] [CrossRef]
- Liu, S.; Zheng, T.H.; Hofmann, M.; Burcombe, E.; Wei, J.; Jiang, R.; Kong, J.; Chen, Y. Antibacterial Activity of Graphite, Graphite Oxide, Graphene Oxide, and Reduced Graphene Oxide: Membrane and Oxidative Stress. ACS Nano. 2011, 5, 6971–6980. [Google Scholar] [CrossRef]
- Wang, Y.; Nor, Y.A.; Song, H.; Yang, Y.; Xu, C.; Yu, M.; Yu, C. Small-sized and large-pore dendritic mesoporous silica nanoparticles enhance antimicrobial enzyme delivery. J. Mater. Chem. B 2016, 4, 2646–2653. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Huo, P.; Zhang, R.; Liu, B. Antibacterial Properties of Graphene-Based Nanomaterials. Nanomaterials 2019, 9, 737. [Google Scholar] [CrossRef] [PubMed]
- Szunerits, S.; Boukherroub, R. Antibacterial activity of graphene-based materials. J. Mater. Chem. B 2016, 4, 6892–6912. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Chen, B.; Zhu, L. Graphene-coated materials using silica particles as a framework for highly efficient removal of aromatic pollutants in water. Sci. Rep. 2015, 5, 11641. [Google Scholar] [CrossRef]
- Kou, L.; Gao, C. Making silicananoparticle-covered graphene oxide nanohybrids as general building blocks for large-area superhydrophilic coatings. Nanoscale 2011, 3, 519–528. [Google Scholar] [CrossRef]
- Abdelkhalek, A.; El-Latif, M.A.; Ibrahim, H.; Hamad, H.; Showman, M. Controlled synthesis of graphene oxide/silica hybrid nanocomposites for removal of aromatic pollutants in water. Sci. Rep. 2022, 12, 7060. [Google Scholar] [CrossRef]
- Bhawal, P.; Ganguly, S.; Chaki, T.K.; Das, N.C. Synthesis and characterization of graphene oxide filled ethylene methyl acrylate hybrid nanocomposites. RSC Adv. 2016, 6, 20781. [Google Scholar] [CrossRef]
- Liu, X.; Wu, X.; Xing, Y.; Zhang, Y.; Zhang, X.; Pu, Q.; Wu, M.; Zhao, J.X. Reduced Graphene Oxide/Mesoporous Silica Nanocarriers for pH-Triggered Drug Release and Photothermal Therapy. ACS Appl. Bio Mater. 2020, 3, 2577–2587. [Google Scholar] [CrossRef]
- Rajapaksha, P.; Cheeseman, S.; Hombsch, S.; Murdoch, B.J.; Gangadoo, S.; Blanch, E.W.; Truong, Y.; Cozzolino, D.; McConville, C.F.; Crawford, E.J.; et al. Antibacterial Properties of Graphene Oxide-Copper Oxide Nanoparticle Nanocomposites. ACS Appl. Bio Mater. 2019, 2, 5687–5696. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.L.; Choi, H.J. Silica-Graphene Oxide Hybrid Composite Particles and Their Electroresponsive Characteristics. Langmuir. 2012, 28, 7055–7062. [Google Scholar] [CrossRef] [PubMed]
- Mehmood, Y.; Khan, I.U.; Shahzad, Y.; Khalid, S.H.; Asghar, S.; Irfan, M.; Asif, M.; Khalid, I.; Yousaf, A.M.; Hussain, T. Facile synthesis of mesoporous silica nanoparticles using modified sol-gel method: Optimization and in vitro cytotoxicity studies. Pak. J. Pharm. Sci. 2019, 32, 1805–1812. [Google Scholar] [PubMed]
- Meva, F.E.; Segnou, M.L.; Ebongue, C.O.; Ntoumba, A.A.; Kedi, P.B.E.; Deli, V.; Etoh, M.-A.; Mpondo, E.M. Spectroscopic synthetic optimizations monitoring of silver nanoparticles formation from Megaphrynium macrostachyum leaf extract. Rev. Bras. Farmacogn. 2016, 26, 640–646. [Google Scholar] [CrossRef]
- Kim, D.; Jeong, S.; Moon, J. Synthesis of silver nanoparticles using the polyol process and the influence of precursor injection. Nanotechnology 2006, 17, 4019–4024. [Google Scholar] [CrossRef]
- Ciplak, Z.; Yildiz, N.; Calimli, A. Investigation of Graphene/Ag Nanocomposites Synthesis Parameters for Two Different Synthesis Methods. Fuller. Nanotub. Carbon Nanostructures 2015, 23, 361–370. [Google Scholar] [CrossRef]
- He, D.; Peng, Z.; Gong, W.; Luo, Y.; Zhao, P.; Kong, L. Mechanism of a green graphene oxide reduction with reusable potassium carbonate. RSC Adv. 2015, 5, 11966–11972. [Google Scholar] [CrossRef]
- Nodeh, H.R.; Ibrahim, W.A.W.; Ali, I.; Sanagi, M.M. Development of magnetic graphene oxide adsorbent for the removal and preconcentration of As(III) and As(V) species from environmental water samples. Environ. Sci. Pollut. Res. 2016, 23, 9759–9773. [Google Scholar] [CrossRef]
- Su, G.; Yang, C.; Zhu, J.J. Fabrication of Gold Nanorods with Tunable Longitudinal Surface Plasmon Resonance Peaks by Reductive Dopamine. Langmuir 2015, 31, 817–823. [Google Scholar] [CrossRef]
- Perumbilavil, S.; Sankar, P.; Rose, T.P.; Philip, R. White light Z-scan measurements of ultrafast optical nonlinearity in reduced graphene oxide nanosheets in the 400–700 nm region. Appl. Phys. Lett. 2015, 107, 051104. [Google Scholar] [CrossRef]
- Carboni, D.; Lasio, B.; Alzari, V.; Mariani, A.; Loche, D.; Casula, M.F.; Malfatti, L.; Innocenzi, P. Graphene-mediated surface enhanced Raman scattering in silica mesoporous nanocomposite films. Phys. Chem. Chem. Phys. 2014, 16, 25809–25818. [Google Scholar] [CrossRef] [PubMed]
- Kora, A.J.; Arunachalam, J. Green Fabrication of Silver Nanoparticles by Gum Tragacanth (Astragalus gummifer): A Dual Functional Reductant and Stabilizer. J. Nanomater. 2012, 2012, 869765. [Google Scholar] [CrossRef]
- Setlow, P. Spores of Bacillus subtilis: Their resistance to and killing by radiation, heat and chemicals. J. Appl. Microbiol. 2006, 101, 514–525. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.H.; Kim, B.K.; Jo, Y.L.; Shim, J.J. Preparation and antibacterial activity of silver nanoparticles-decorated graphene composites. J. Supercrit. Fluids. 2012, 72, 28–35. [Google Scholar] [CrossRef]
- Prasad, K.; Lekshmi, G.S.; Ostrikov, K.; Lussini, V.; Blinco, J.; Mohandas, M.; Vasilev, K.; Bottle, S.; Bazaka, K.; Ostrikov, K. Synergic bactericidal effects of reduced graphene oxide and silver nanoparticles against Gram-positive and Gram-negative bacteria. Sci. Rep. 2017, 7, 1591. [Google Scholar] [CrossRef]
- Liu, J.; Li, S.; Fang, Y.; Zhu, Z. Boosting antibacterial activity with mesoporous silica nanoparticles supported silver nanoclusters. J. Colloid. Interface Sci. 2019, 555, 470–479. [Google Scholar] [CrossRef]
- Abdubabbus, M.M.; Jevremovic, I.; Jankovic, A.; Peric-Grujjic, A.; Matic, I.; Vukasinovic-Sekulic, M.; Hui, D.; Rhee, K.Y.; Miskovic-Stankovic, V. Biological activity of electrochemically synthesized silver doped polyvinyl alcohol/graphene composite hydrogel discs for biomedical applications. Compos. B Eng. 2016, 104, 26–34. [Google Scholar] [CrossRef]
- Luo, J.; Lai, J.; Zhang, N.; Liu, Y.; Liu, R.; Liu, X. Tannic Acid Induced Self-Assembly of Three-Dimensional Graphene with Good Adsorption and Antibacterial Properties. ACS Sustain. Chem. Eng. 2016, 4, 1404–1413. [Google Scholar] [CrossRef]
- Montes-Duarte, G.G.; Tostado-Blazquez, G.; Castro, K.L.S.; Araujo, J.R.; Achete, C.A.; Sanchez-Salas, J.L.; Campos-Delgado, J. Key parameters to enhance the antibacterial effect of graphene oxide in solution. RSC Adv. 2021, 11, 6509–6516. [Google Scholar] [CrossRef]
- Dutta, D.; Goswami, S.; Dubey, R.; Dwivedi, S.K.; Puzari, A. Antimicrobial activity of silver-coated hollow poly(methylmethacrylate) microspheres for water decontamination. Environ. Sci. Eur. 2021, 33, 22. [Google Scholar] [CrossRef]
- Bhargav, H.S.; Shastri, S.D.; Poornav, S.P.; Darshan, K.M.; Nayak, M.M. Measurement of the Zone of Inhibition of an Antibiotic. IEEE 2016, 5, 409–414. [Google Scholar] [CrossRef]
- Chamidah, A.; Hardoko, H.; Prihanto, A.A. Antibacterial activities of β-glucan (laminaran) against gram-negative and gram-positive bacteria. AIP Conf. Proc. 2017, 1884, 020011. [Google Scholar] [CrossRef]
- Arkowski, J.; Obremska, M.; Kedzierski, K.; Slawuta, A.; Wawrzynska, M. Applications for graphene and its derivatives in medical devices: Current knowledge and future applications. Adv. Clin. Exp. Med. 2020, 12, 1497–1504. [Google Scholar] [CrossRef]
- Pagano, S.; Costanzi, G.L.E.; Balloni, S.; Bruscoli, S.; Flamini, S.; Coniglio, M.; Valenti, C.; Cianetti, S.; Marinucci, L. Morpho-functional effects of different universal dental adhesives on human gingival fibroblasts: An in vitro study. Odontology 2021, 109, 524–539. [Google Scholar] [CrossRef]



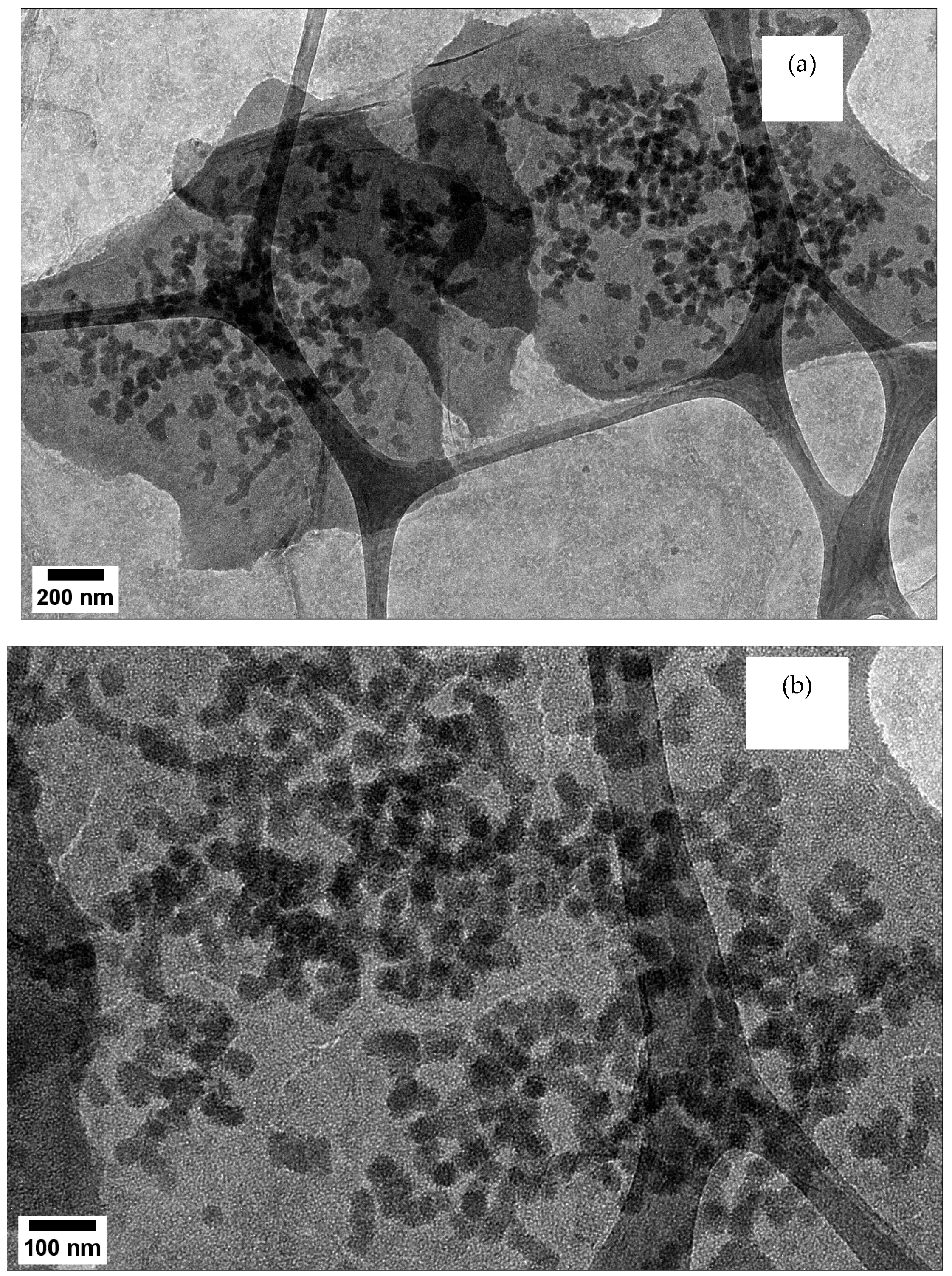
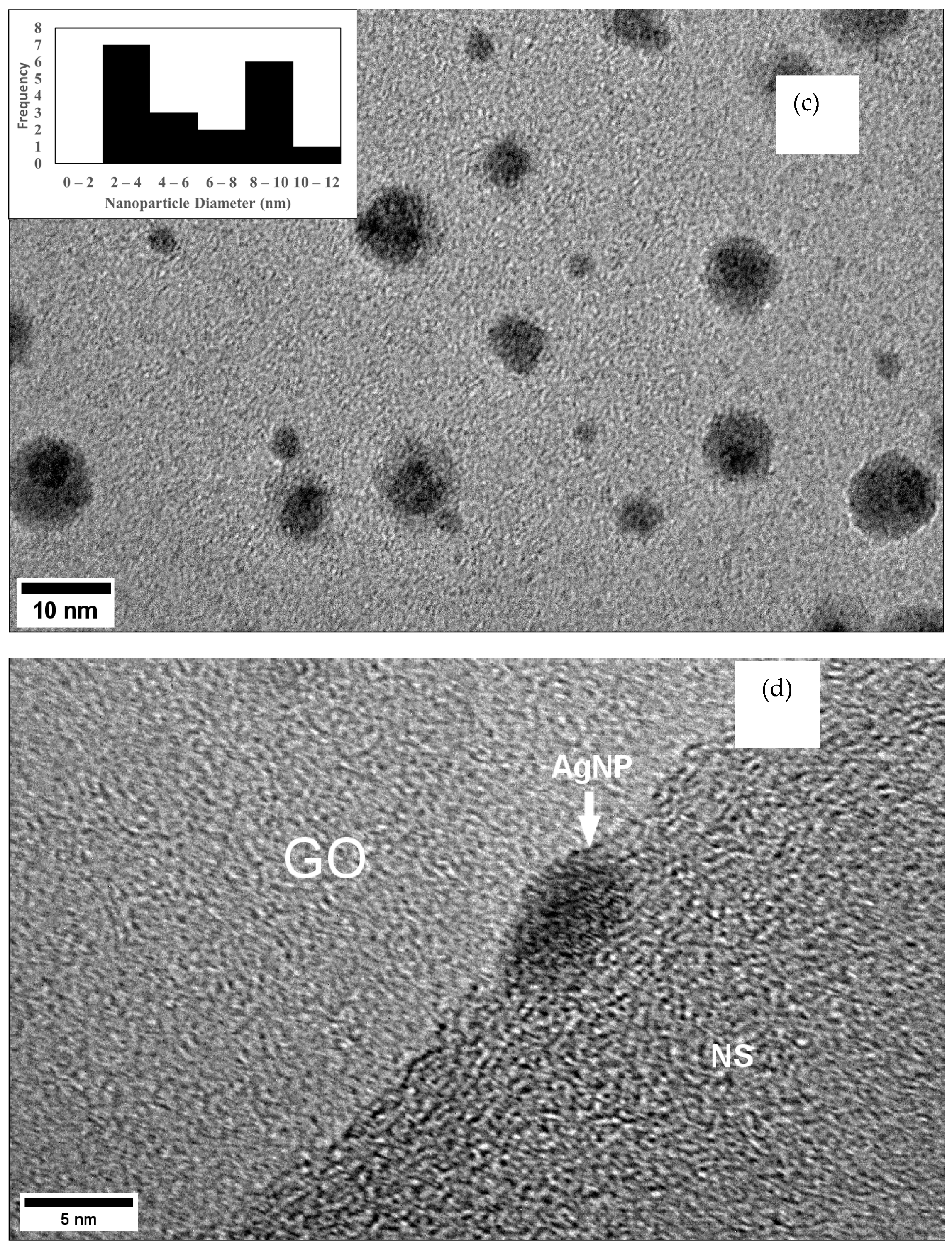


| Composite | BET Surface Area (m2/g) | BJH Adsorption Pore Volume (cm3/g) | BJH Adsorption Pore Size (nm) |
|---|---|---|---|
| GO/NS | 62.09 | 0.11 | 7.77 |
| GO/NS/AgNPs | 25.83 | 0.04 | 7.08 |
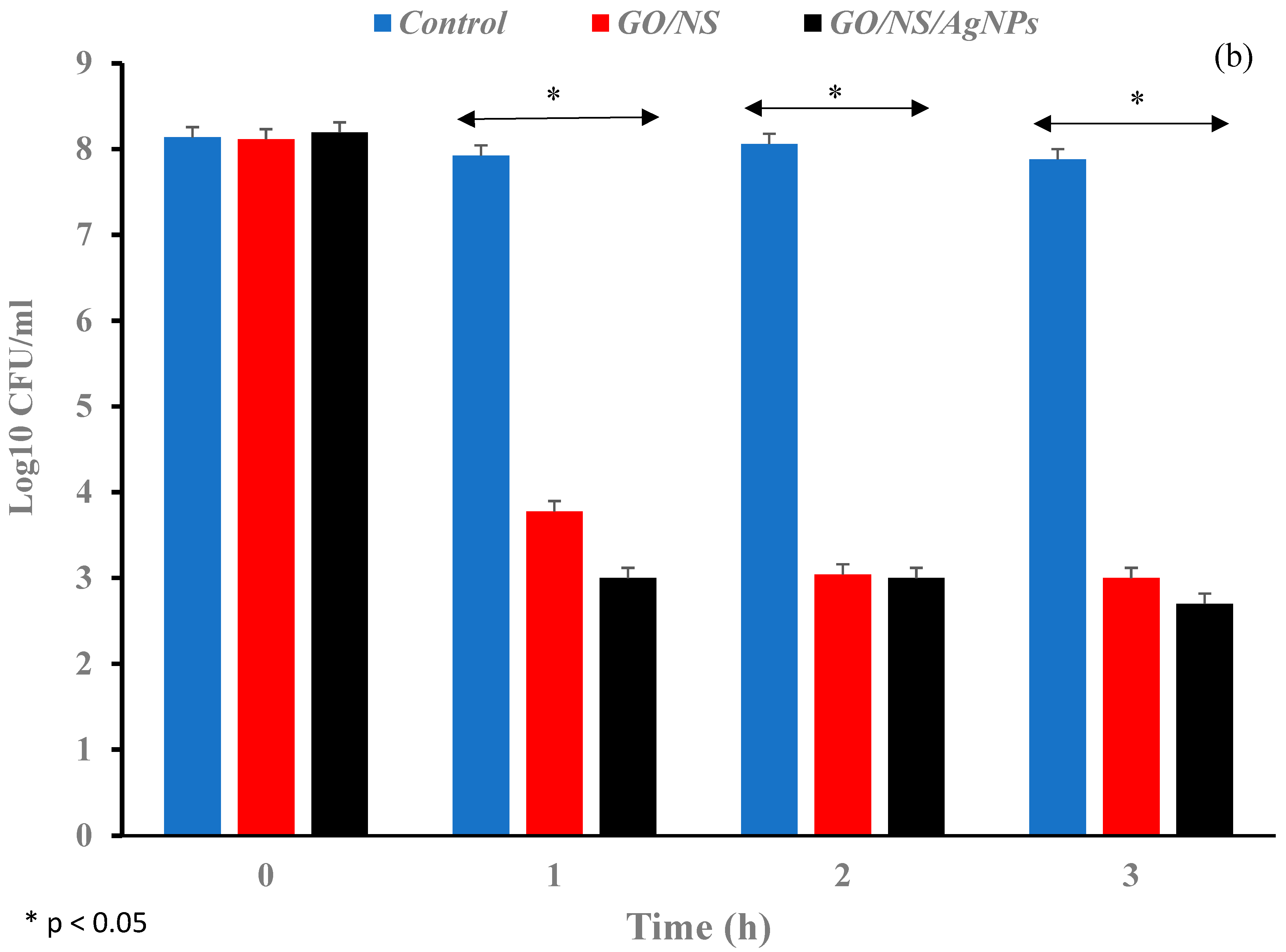
| Materials | Time Length | Log Reduction (Gram-Negative Bacteria) | Log Reduction (Gram-Positive Bacteria) | Reference |
|---|---|---|---|---|
| rGO-Ag 1 | 24 h | 5 | 5 | [45] |
| rGO-nAg 1 | 2–2.5 h | 1.31 | 1 | [46] |
| rGO 2 | 2–2.5 h | 0.4 | 0.4 | [46] |
| nAg | 2–2.5 h | 0.4 | 0.4 | [46] |
| AgNC-MSNs 3 | 12 h | 5.2 | 3.5 | [47] |
| Ag/PVAGr 4 | 1 h | 5 | ~3 | [48] |
| TA-GA 5 | 4 h | Less than 0.5 | 4 | [49] |
| GO 6 | 3 h | 0.5–1 | NA | [50] |
| GO/CeO2 7 | 3 h | 6 | NA | [50] |
| Silver coated PMMA 8 microsphere | 24 h | 4 | 4 | [51] |
| GO/NS | 1 h | ~7 | 4.3 | This work |
| GO/NS/AgNPs | 1 h | ~7 | 5.2 | This work |
| Species | Zone of Inhibiton Diameter (mm) | |
|---|---|---|
| GO/NS | GO/NS/AgNPs | |
| E. coli | 70 ± 1.0 | 90 ± 1.0 |
| B. subtilis | 80 ± 1.0 | 100 ± 1.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quach, Q.; Abdel-Fattah, T.M. Silver Nanoparticles Functionalized Nanosilica Grown over Graphene Oxide for Enhancing Antibacterial Effect. Nanomaterials 2022, 12, 3341. https://doi.org/10.3390/nano12193341
Quach Q, Abdel-Fattah TM. Silver Nanoparticles Functionalized Nanosilica Grown over Graphene Oxide for Enhancing Antibacterial Effect. Nanomaterials. 2022; 12(19):3341. https://doi.org/10.3390/nano12193341
Chicago/Turabian StyleQuach, Qui, and Tarek M. Abdel-Fattah. 2022. "Silver Nanoparticles Functionalized Nanosilica Grown over Graphene Oxide for Enhancing Antibacterial Effect" Nanomaterials 12, no. 19: 3341. https://doi.org/10.3390/nano12193341
APA StyleQuach, Q., & Abdel-Fattah, T. M. (2022). Silver Nanoparticles Functionalized Nanosilica Grown over Graphene Oxide for Enhancing Antibacterial Effect. Nanomaterials, 12(19), 3341. https://doi.org/10.3390/nano12193341