Uniaxial and Coaxial Nanofibers PCL/Alginate or PCL/Gelatine Transport and Release Tamoxifen and Curcumin Affecting the Viability of MCF7 Cell Line
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of PCL Nanofiber Solutions and Electrospinning
2.3. Physicochemical Characterization
2.4. In Vitro Release Profile
2.5. In Vitro Cytotoxicity Tests
2.6. Statistical Analysis
3. Results
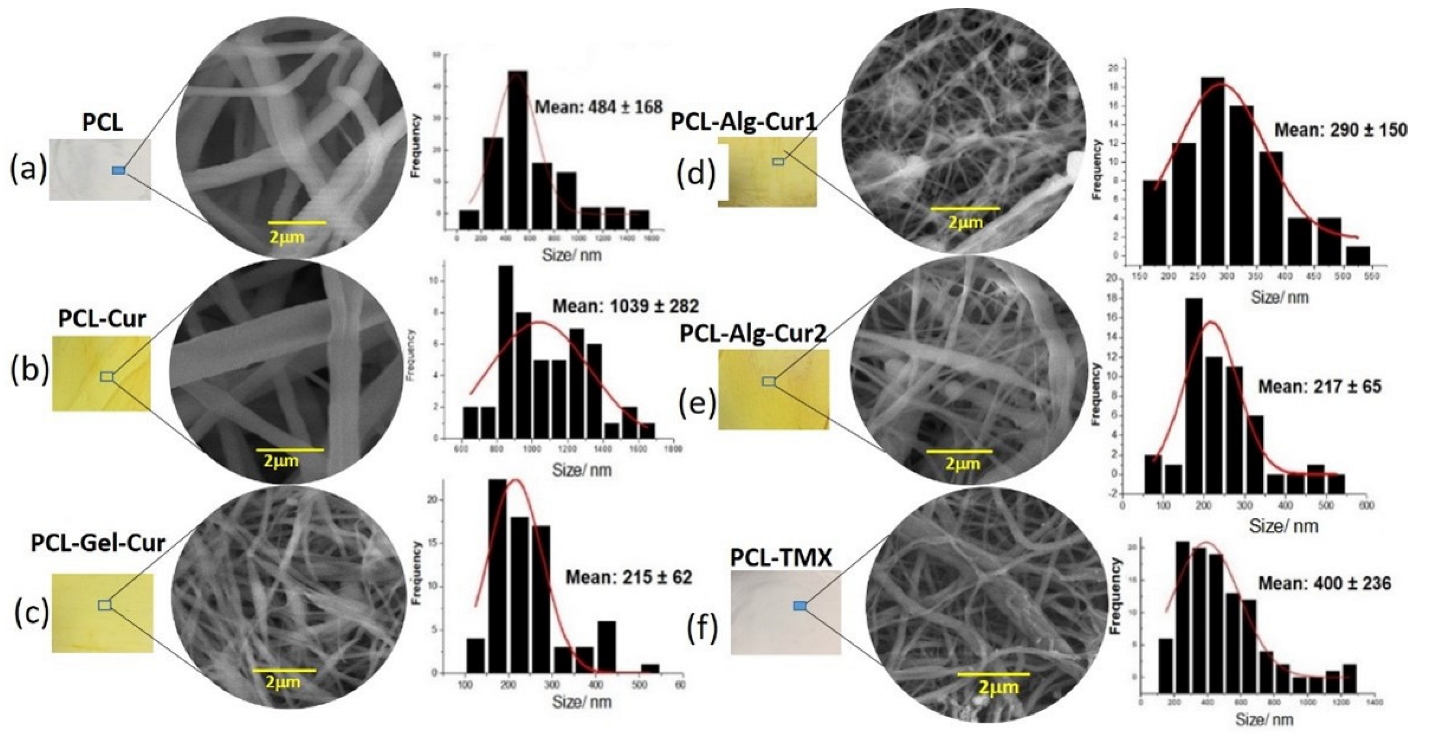
3.1. Morphological Characterization
3.2. Physicochemical Characterization of the Starting Materials and Nanofibers
3.3. In Vitro Release Profile
3.4. In Vitro Cytotoxicity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Paul, D. The Systemic Hallmarks of Cancer. J. Cancer Metastasis Treat. 2020, 2020, 29. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.-M. Genetic and Hormonal Risk Factors in Breast Cancer. J. Natl. Cancer Inst. 2000, 92, 1126–1135. [Google Scholar] [CrossRef] [PubMed]
- Hilakivi-Clarke, L.; Cabanes, A.; Olivo, S.; Kerr, L.; Bouker, K.B.; Clarke, R. Do Estrogens Always Increase Breast Cancer Risk? J. Steroid Biochem. Mol. Biol. 2002, 80, 163–174. [Google Scholar] [CrossRef]
- Karimi, Z.; Jessri, M.; Houshiar-Rad, A.; Mirzaei, H.-R.; Rashidkhani, B. Dietary Patterns and Breast Cancer Risk among Women. Public Health Nutr. 2014, 17, 1098–1106. [Google Scholar] [CrossRef] [PubMed]
- Dierssen-Sotos, T.; Palazuelos-Calderón, C.; Jiménez-Moleón, J.-J.; Aragonés, N.; Altzibar, J.M.; Castaño-Vinyals, G.; Martín-Sanchez, V.; Gómez-Acebo, I.; Guevara, M.; Tardón, A.; et al. Reproductive Risk Factors in Breast Cancer and Genetic Hormonal Pathways: A Gene-Environment Interaction in the MCC-Spain Project. BMC Cancer 2018, 18, 280. [Google Scholar] [CrossRef] [PubMed]
- Harvie, M.; Hooper, L.; Howell, A.H. Central Obesity and Breast Cancer Risk: A Systematic Review. Obes. Rev. 2003, 4, 157–173. [Google Scholar] [CrossRef]
- Krauss, K.; Stickeler, E. Endocrine Therapy in Early Breast Cancer. Breast Care 2020, 15, 337–346. [Google Scholar] [CrossRef]
- Burstein, H.J.; Lacchetti, C.; Anderson, H.; Buchholz, T.A.; Davidson, N.E.; Gelmon, K.A.; Giordano, S.H.; Hudis, C.A.; Solky, A.J.; Stearns, V.; et al. Adjuvant Endocrine Therapy for Women With Hormone Receptor–Positive Breast Cancer: ASCO Clinical Practice Guideline Focused Update. J. Clin. Oncol. 2019, 37, 423–438. [Google Scholar] [CrossRef]
- Huang, B.; Warner, M.; Gustafsson, J.-Å. Estrogen Receptors in Breast Carcinogenesis and Endocrine Therapy. Mol. Cell. Endocrinol. 2015, 418, 240–244. [Google Scholar] [CrossRef] [PubMed]
- Waks, A.G.; Winer, E.P. Breast Cancer Treatment. JAMA 2019, 321, 316. [Google Scholar] [CrossRef]
- Arpino, G.; Wiechmann, L.; Osborne, C.K.; Schiff, R. Crosstalk between the Estrogen Receptor and the HER Tyrosine Kinase Receptor Family: Molecular Mechanism and Clinical Implications for Endocrine Therapy Resistance. Endocr. Rev. 2008, 29, 217–233. [Google Scholar] [CrossRef]
- Salami, S.; Karami-Tehrani, F. Biochemical Studies of Apoptosis Induced by Tamoxifen in Estrogen Receptor Positive and Negative Breast Cancer Cell Lines. Clin. Biochem. 2003, 36, 247–253. [Google Scholar] [CrossRef]
- Mandlekar, S.; Kong, A.-N.T. Mechanisms of Tamoxifen-Induced Apoptosis. Apoptosis 2001, 6, 469–477. [Google Scholar] [CrossRef]
- Cuzick, J.; Powles, T.; Veronesi, U.; Forbes, J.; Edwards, R.; Ashley, S.; Boyle, P. Overview of the Main Outcomes in Breast-Cancer Prevention Trials. Lancet 2003, 361, 296–300. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Rayess, Y.E.; Rizk, A.A.; Sadaka, C.; Zgheib, R.; Zam, W.; Sestito, S.; Rapposelli, S.; Neffe-Skocińska, K.; Zielińska, D.; et al. Turmeric and Its Major Compound Curcumin on Health: Bioactive Effects and Safety Profiles for Food, Pharmaceutical, Biotechnological and Medicinal Applications. Front. Pharmacol. 2020, 11, 01021. [Google Scholar] [CrossRef]
- Shukla, S.; Penta, D.; Mondal, P.; Meeran, S.M. Epigenetics of Breast Cancer: Clinical Status of Epi-Drugs and Phytochemicals. In Breast Cancer Metastasis and Drug Resistance; Advances in Experimental Medicine and Biology; Ahmad, A., Ed.; Springer International Publishing: Cham, Switzerland, 2019; Volume 1152, pp. 293–310. ISBN 978-3-030-20300-9. [Google Scholar]
- Thresiamma, K.C.; George, J.; Kuttan, R. Protective Effect of Curcumin, Ellagic Acid and Bixin on Radiation Induced Toxicity. Indian J. Exp. Biol. 1996, 34, 845–847. [Google Scholar] [PubMed]
- Das, R.K.; Kasoju, N.; Bora, U. Encapsulation of Curcumin in Alginate-Chitosan-Pluronic Composite Nanoparticles for Delivery to Cancer Cells. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Ali, B.H.; Marrif, H.; Noureldayem, S.A.; Bakheit, A.O.; Blunden, G. Some Biological Properties of Curcumin: A Review. Nat. Prod. Commun. 2006, 1, 1934578X0600100. [Google Scholar] [CrossRef] [Green Version]
- Gangwar, R.K.; Tomar, G.B.; Dhumale, V.A.; Zinjarde, S.; Sharma, R.B.; Datar, S. Curcumin Conjugated Silica Nanoparticles for Improving Bioavailability and Its Anticancer Applications. J. Agric. Food Chem. 2013, 61, 130926133947000. [Google Scholar] [CrossRef] [PubMed]
- Banik, U.; Parasuraman, S.; Adhikary, A.K.; Othman, N.H. Curcumin: The Spicy Modulator of Breast Carcinogenesis. J. Exp. Clin. Cancer Res. 2017, 36, 98. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Chen, Z. The Effect of Curcumin on Breast Cancer Cells. J. Breast Cancer 2013, 16, 133. [Google Scholar] [CrossRef] [PubMed]
- Cardona-Echeverry, A.; Prada-Arismendy, J. Deciphering the Role of Wnt Signaling in Acute Myeloid Leukemia Prognosis: How Alterations in DNA Methylation Come into Play in Patients’ Prognosis. J. Cancer Res. Clin. Oncol. 2020, 146, 3097–3109. [Google Scholar] [CrossRef]
- Yallapu, M.M.; Othman, S.F.; Curtis, E.T.; Bauer, N.A.; Chauhan, N.; Kumar, D.; Jaggi, M.; Chauhan, S.C. Curcumin-Loaded Magnetic Nanoparticles for Breast Cancer Therapeutics and Imaging Applications. Int. J. Nanomed. 2012, 7, 1761. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, H.; Makabel, B.; Cui, Q.; Li, J.; Su, C.; Ashby, C.R., Jr.; Chen, Z.; Zhang, J. The Targeting of Non-coding RNAs by Curcumin: Facts and Hopes for Cancer Therapy (Review). Oncol. Rep. 2019, 42, 20–34. [Google Scholar] [CrossRef]
- Mirzaei, H.; Masoudifar, A.; Sahebkar, A.; Zare, N.; Sadri Nahand, J.; Rashidi, B.; Mehrabian, E.; Mohammadi, M.; Mirzaei, H.R.; Jaafari, M.R. MicroRNA: A Novel Target of Curcumin in Cancer Therapy. J. Cell. Physiol. 2018, 233, 3004–3015. [Google Scholar] [CrossRef]
- Priyadarsini, K. The Chemistry of Curcumin: From Extraction to Therapeutic Agent. Molecules 2014, 19, 20091–20112. [Google Scholar] [CrossRef]
- Muniyappan, N.; Pandeeswaran, M.; Amalraj, A. Green Synthesis of Gold Nanoparticles Using Curcuma Pseudomontana Isolated Curcumin: Its Characterization, Antimicrobial, Antioxidant and Anti- Inflammatory Activities. Environ. Chem. Ecotoxicol. 2021, 3, 117–124. [Google Scholar] [CrossRef]
- Peer, D.; Karp, J.M.; Hong, S.; Farokhzad, O.C.; Margalit, R.; Langer, R. Nanocarriers as an Emerging Platform for Cancer Therapy. Nat. Nanotechnol. 2007, 2, 751–760. [Google Scholar] [CrossRef]
- Ranjbar-Mohammadi, M.; Rabbani, S.; Bahrami, S.H.; Joghataei, M.T.; Moayer, F. Antibacterial Performance and in Vivo Diabetic Wound Healing of Curcumin Loaded Gum Tragacanth/Poly(ε-Caprolactone) Electrospun Nanofibers. Mater. Sci. Eng. C 2016, 69, 1183–1191. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.-Q.; Ba, K. Construction a Long-Circulating Delivery System of Liposomal Curcumin by Coating Albumin. ACS Omega 2020, 5, 16502–16509. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Wei, Q.; Zhou, Y.; Wang, J.; Liu, Q.; Xu, H. A Systematic Analysis of FDA-Approved Anticancer Drugs. BMC Syst. Biol. 2017, 11, 87. [Google Scholar] [CrossRef] [PubMed]
- Mansoori, B.; Mohammadi, A.; Davudian, S.; Shirjang, S.; Baradaran, B. The Different Mechanisms of Cancer Drug Resistance: A Brief Review. Adv. Pharm. Bull. 2017, 7, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Vinogradov, S.; Wei, X. Cancer Stem Cells and Drug Resistance: The Potential of Nanomedicine. Nanomedicine 2012, 7, 597–615. [Google Scholar] [CrossRef] [PubMed]
- Dash, T.K.; Konkimalla, V.B. Poly-є-Caprolactone Based Formulations for Drug Delivery and Tissue Engineering: A Review. J. Control. Release 2012, 158, 15–33. [Google Scholar] [CrossRef] [PubMed]
- Adamu, B.F.; Gao, J.; Jhatial, A.K.; Kumelachew, D.M. A Review of Medicinal Plant-Based Bioactive Electrospun Nano Fibrous Wound Dressings. Mater. Des. 2021, 209, 109942. [Google Scholar] [CrossRef]
- Kumari, A.; Yadav, S.K.; Yadav, S.C. Biodegradable Polymeric Nanoparticles Based Drug Delivery Systems. Colloids Surf. B Biointerfaces 2010, 75, 1–18. [Google Scholar] [CrossRef]
- Reneker, D.H.; Yarin, A.L. Electrospinning Jets and Polymer Nanofibers. Polymer 2008, 49, 2387–2425. [Google Scholar] [CrossRef]
- Rogina, A. Electrospinning Process: Versatile Preparation Method for Biodegradable and Natural Polymers and Biocomposite Systems Applied in Tissue Engineering and Drug Delivery. Appl. Surf. Sci. 2014, 296, 221–230. [Google Scholar] [CrossRef]
- Pelipenko, J.; Kocbek, P.; Kristl, J. Critical Attributes of Nanofibers: Preparation, Drug Loading, and Tissue Regeneration. Int. J. Pharm. 2015, 484, 57–74. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, P.; Oliveira, S.; de Castro Rodrigues, G.; Gontijo, S.; Lula, I.; Cortés, M.; Denadai, Â.; Sinisterra, R. Development of Sulfadiazine-Decorated PLGA Nanoparticles Loaded with 5-Fluorouracil and Cell Viability. Molecules 2015, 20, 879–899. [Google Scholar] [CrossRef] [PubMed]
- Alven, S.; Aderibigbe, B.A. Fabrication of Hybrid Nanofibers from Biopolymers and Poly (Vinyl Alcohol)/Poly (ε-Caprolactone) for Wound Dressing Applications. Polymers 2021, 13, 2104. [Google Scholar] [CrossRef] [PubMed]
- Ghaee, A.; Bagheri-Khoulenjani, S.; Amir Afshar, H.; Bogheiri, H. Biomimetic Nanocomposite Scaffolds Based on Surface Modified PCL-Nanofibers Containing Curcumin Embedded in Chitosan/Gelatin for Skin Regeneration. Compos. Part B Eng. 2019, 177, 107339. [Google Scholar] [CrossRef]
- Escobar, M.L.; Rivera, A.; Aristizábal, G.F.A. Comparison of Resazurin and MTT Methods on Studies of Citotoxicity in Human Tumor Cell Lines. VITAE Rev. Fac. Quím. Farm. 2010, 17, 67–74. [Google Scholar]
- Uzarski, J.S.; DiVito, M.D.; Wertheim, J.A.; Miller, W.M. Essential Design Considerations for the Resazurin Reduction Assay to Noninvasively Quantify Cell Expansion within Perfused Extracellular Matrix Scaffolds. Biomaterials 2017, 129, 163–175. [Google Scholar] [CrossRef]
- Borra, R.C.; Lotufo, M.A.; Gagioti, S.M.; de Mesquita Barros, F.; Andrade, P.M. A Simple Method to Measure Cell Viability in Proliferation and Cytotoxicity Assays. Braz. Oral Res. 2009, 23, 255–262. [Google Scholar] [CrossRef]
- Basak, V.; Bahar, T.E.; Emine, K.; Yelda, K.; Mine, K.; Figen, S.; Rustem, N. Evaluation of Cytotoxicity and Gelatinases Activity in 3T3 Fibroblast Cell by Root Repair Materials. Biotechnol. Biotechnol. Equip. 2016, 30, 984–990. [Google Scholar] [CrossRef]
- Guarino, V.; Cirillo, V.; Taddei, P.; Alvarez-Perez, M.A.; Ambrosio, L. Tuning Size Scale and Crystallinity of PCL Electrospun Fibres via Solvent Permittivity to Address HMSC Response. Macromol. Biosci. 2011, 11, 1694–1705. [Google Scholar] [CrossRef]
- Zong, X.; Kim, K.; Fang, D.; Ran, S.; Hsiao, B.S.; Chu, B. Structure and Process Relationship of Electrospun Bioabsorbable Nanofiber Membranes. Polymer 2002, 43, 4403–4412. [Google Scholar] [CrossRef]
- Haider, A.; Haider, S.; Kang, I.-K. A Comprehensive Review Summarizing the Effect of Electrospinning Parameters and Potential Applications of Nanofibers in Biomedical and Biotechnology. Arab. J. Chem. 2018, 11, 1165–1188. [Google Scholar] [CrossRef]
- Mackie, W.; Perez, S.; Rizzo, R.; Taravel, F.; Vignon, M. Aspects of the Conformation of Polyguluronate in the Solid State and in Solution. Int. J. Biol. Macromol. 1983, 5, 329–341. [Google Scholar] [CrossRef]
- Nie, H.; He, A.; Zheng, J.; Xu, S.; Li, J.; Han, C.C. Effects of Chain Conformation and Entanglement on the Electrospinning of Pure Alginate. Biomacromolecules 2008, 9, 1362–1365. [Google Scholar] [CrossRef]
- Shalumon, K.T.; Anulekha, K.H.; Girish, C.M.; Prasanth, R.; Nair, S.V.; Jayakumar, R. Single Step Electrospinning of Chitosan/Poly(Caprolactone) Nanofibers Using Formic Acid/Acetone Solvent Mixture. Carbohydr. Polym. 2010, 80, 413–419. [Google Scholar] [CrossRef]
- Gautam, S.; Dinda, A.K.; Mishra, N.C. Fabrication and Characterization of PCL/Gelatin Composite Nanofibrous Scaffold for Tissue Engineering Applications by Electrospinning Method. Mater. Sci. Eng. C 2013, 33, 1228–1235. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ouyang, H.; Lim, C.T.; Ramakrishna, S.; Huang, Z.-M. Electrospinning of Gelatin Fibers and Gelatin/PCL Composite Fibrous Scaffolds. J. Biomed. Mater. Res. 2005, 72B, 156–165. [Google Scholar] [CrossRef]
- Kolev, T.M.; Velcheva, E.A.; Stamboliyska, B.A.; Spiteller, M. DFT and Experimental Studies of the Structure and Vibrational Spectra of Curcumin. Int. J. Quantum Chem. 2005, 102, 1069–1079. [Google Scholar] [CrossRef]
- Mohan, P.R.K.; Sreelakshmi, G.; Muraleedharan, C.V.; Joseph, R. Water Soluble Complexes of Curcumin with Cyclodextrins: Characterization by FT-Raman Spectroscopy. Vib. Spectrosc. 2012, 62, 77–84. [Google Scholar] [CrossRef]
- Yallapu, M.M.; Jaggi, M.; Chauhan, S.C. β-Cyclodextrin-Curcumin Self-Assembly Enhances Curcumin Delivery in Prostate Cancer Cells. Colloids Surf. B Biointerfaces 2010, 79, 113–125. [Google Scholar] [CrossRef]
- van Hoogmoed, C.G.; Busscher, H.J.; de Vos, P. Fourier Transform Infrared Spectroscopy Studies of Alginate-PLL Capsules with Varying Compositions. J. Biomed. Mater. Res. 2003, 67A, 172–178. [Google Scholar] [CrossRef]
- Ki, C.S.; Baek, D.H.; Gang, K.D.; Lee, K.H.; Um, I.C.; Park, Y.H. Characterization of Gelatin Nanofiber Prepared from Gelatin–Formic Acid Solution. Polymer 2005, 46, 5094–5102. [Google Scholar] [CrossRef]
- Elzein, T.; Nasser-Eddine, M.; Delaite, C.; Bistac, S.; Dumas, P. FTIR Study of Polycaprolactone Chain Organization at Interfaces. J. Colloid Interface Sci. 2004, 273, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Gorodzha, S.N.; Surmeneva, M.A.; Surmenev, R.A. Fabrication and Characterization of Polycaprolactone Cross- Linked and Highly-Aligned 3-D Artificial Scaffolds for Bone Tissue Regeneration via Electrospinning Technology. IOP Conf. Ser. Mater. Sci. Eng. 2015, 98, 012024. [Google Scholar] [CrossRef]
- Li, L.; Fang, Y.; Vreeker, R.; Appelqvist, I.; Mendes, E. Reexamining the Egg-Box Model in Calcium−Alginate Gels with X-Ray Diffraction. Biomacromolecules 2007, 8, 464–468. [Google Scholar] [CrossRef]
- Abdelrazek, E.M.; Hezma, A.M.; El-khodary, A.; Elzayat, A.M. Spectroscopic Studies and Thermal Properties of PCL/PMMA Biopolymer Blend. Egypt. J. Basic Appl. Sci. 2016, 3, 10–15. [Google Scholar] [CrossRef]
- Thangadurai, S.; Abraham, J.T.; Srivastava, A.K.; Nataraja Moorthy, M.; Shukla, S.K.; Anjaneyulu, Y. X-Ray Powder Diffraction Patterns for Certain.BETA.-Lactam, Tetracycline and Macrolide Antibiotic Drugs. Anal. Sci. 2005, 21, 833–838. [Google Scholar] [CrossRef] [PubMed]
- Toro, R.; Díaz de Delgado, G.; Bahsas, A.; Delgado, J.M. The Presence of Polymorphism in Oxytetracycline Hydrochloride Shown by X-Ray Powder Diffraction Techniques. Z. für Krist. Suppl. 2007, 2007, 563–568. [Google Scholar] [CrossRef]
- SreeHarsha, N.; Hiremath, J.G.; Chilukuri, S.; Aitha, R.K.; Al-Dhubiab, B.E.; Venugopala, K.N.; Alzahrani, A.M.; Meravanige, G. An Approach to Enhance Dissolution Rate of Tamoxifen Citrate. BioMed Res. Int. 2019, 2019, 2161348. [Google Scholar] [CrossRef]
- Kaloustian, J.; Pauli, A.M.; Pastor, J. DTA Identification of Polycaprolactone. J. Therm. Anal. 1991, 37, 1767–1773. [Google Scholar] [CrossRef]
- Chen, Z.; Xia, Y.; Liao, S.; Huang, Y.; Li, Y.; He, Y.; Tong, Z.; Li, B. Thermal Degradation Kinetics Study of Curcumin with Nonlinear Methods. Food Chem. 2014, 155, 81–86. [Google Scholar] [CrossRef]
- Mishra, R.K.; Majeed, A.B.A.; Banthia, A.K. Development and Characterization of Pectin/Gelatin Hydrogel Membranes for Wound Dressing. Int. J. Plast. Technol. 2011, 15, 82–95. [Google Scholar] [CrossRef]
- Soares, J.P.; Santos, J.E.; Chierice, G.O.; Cavalheiro, E.T.G. Thermal Behavior of Alginic Acid and Its Sodium Salt. Eclética Quím. 2004, 29, 57–64. [Google Scholar] [CrossRef]
- Sampath, M.; Lakra, R.; Korrapati, P.; Sengottuvelan, B. Curcumin Loaded Poly (Lactic-Co-Glycolic) Acid Nanofiber for the Treatment of Carcinoma. Colloids Surf. B Biointerfaces 2014, 117, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Shababdoust, A.; Ehsani, M.; Shokrollahi, P.; Zandi, M. Fabrication of Curcumin-Loaded Electrospun Nanofiberous Polyurethanes with Anti-Bacterial Activity. Prog. Biomater. 2018, 7, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.; McClements, D.J. Formulation of More Efficacious Curcumin Delivery Systems Using Colloid Science: Enhanced Solubility, Stability, and Bioavailability. Molecules 2020, 25, 2791. [Google Scholar] [CrossRef]
- Govindaraju, R.; Karki, R.; Chandrashekarappa, J.; Santhanam, M.; Shankar, A.K.K.; Joshi, H.K.; Divakar, G. Enhanced Water Dispersibility of Curcumin Encapsulated in Alginate-Polysorbate 80 Nano Particles and Bioavailability in Healthy Human Volunteers. Pharm. Nanotechnol. 2019, 7, 39–56. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, S.; Bansal, M.; Khan, G.; Yadav, S.K.; Singh, A.K.; Prakash, P.; Mishra, B. Development, Optimization and Evaluation of Curcumin Loaded Biodegradable Crosslinked Gelatin Film for the Effective Treatment of Periodontitis. Drug Dev. Ind. Pharm. 2018, 44, 1212–1221. [Google Scholar] [CrossRef] [PubMed]
- Merrell, J.G.; McLaughlin, S.W.; Tie, L.; Laurencin, C.T.; Chen, A.F.; Nair, L.S. Curcumin-Loaded Poly(ε-Caprolactone) Nanofibres: Diabetic Wound Dressing with Anti-Oxidant and Anti-Inflammatory Properties. Clin. Exp. Pharmacol. Physiol. 2009, 36, 1149–1156. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Park, M.R.; Kim, M.S.; Kwon, O.H. Polyphenol-Loaded Polycaprolactone Nanofibers for Effective Growth Inhibition of Human Cancer Cells. Mater. Chem. Phys. 2012, 133, 674–680. [Google Scholar] [CrossRef]
- Venugopal, J.; Zhang, Y.Z.; Ramakrishna, S. Fabrication of Modified and Functionalized Polycaprolactone Nanofibre Scaffolds for Vascular Tissue Engineering. Nanotechnology 2005, 16, 2138–2142. [Google Scholar] [CrossRef]
- Mochane, M.J.; Motsoeneng, T.S.; Sadiku, E.R.; Mokhena, T.C.; Sefadi, J.S. Morphology and Properties of Electrospun PCL and Its Composites for Medical Applications: A Mini Review. Appl. Sci. 2019, 9, 2205. [Google Scholar] [CrossRef] [Green Version]
- Joshi, D.; Garg, T.; Goyal, A.K.; Rath, G. Advanced Drug Delivery Approaches against Periodontitis. Drug Deliv. 2016, 23, 363–377. [Google Scholar] [CrossRef] [PubMed]











| NANOFIBER | Abbreviation | Type | Solution PCL (Final %) | Solution Alginate (Final %) | Solution Gelatine (Final %) | CUR | TMX |
|---|---|---|---|---|---|---|---|
| (mg) | (mg) | ||||||
| PCL | PCL | Uniaxial | 100 | - | - | - | - |
| PCL–Curcumin | PCL–Cur | Uniaxial | 100 | - | - | 100 | - |
| PCL–Tamoxifen | PCL–TMX | Uniaxial | 100 | - | - | - | 15 |
| PCL–Alginate–Curcumin | PCL–Alg–Cur1 | Uniaxial | 80 | 20 | - | 100 | - |
| PCL–Alginate-Curcumin | PCL–Alg–Cur2 | Coaxial | 80 | 20 | - | 100 | - |
| PCL–Gelatine–Curcumin | PCL–Gel–Cur | Coaxial | 50 | - | 50 | 100 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suárez, D.F.; Pinzón-García, A.D.; Sinisterra, R.D.; Dussan, A.; Mesa, F.; Ramírez-Clavijo, S. Uniaxial and Coaxial Nanofibers PCL/Alginate or PCL/Gelatine Transport and Release Tamoxifen and Curcumin Affecting the Viability of MCF7 Cell Line. Nanomaterials 2022, 12, 3348. https://doi.org/10.3390/nano12193348
Suárez DF, Pinzón-García AD, Sinisterra RD, Dussan A, Mesa F, Ramírez-Clavijo S. Uniaxial and Coaxial Nanofibers PCL/Alginate or PCL/Gelatine Transport and Release Tamoxifen and Curcumin Affecting the Viability of MCF7 Cell Line. Nanomaterials. 2022; 12(19):3348. https://doi.org/10.3390/nano12193348
Chicago/Turabian StyleSuárez, Diego Fernando, Ana Delia Pinzón-García, Rubén Darío Sinisterra, Anderson Dussan, Fredy Mesa, and Sandra Ramírez-Clavijo. 2022. "Uniaxial and Coaxial Nanofibers PCL/Alginate or PCL/Gelatine Transport and Release Tamoxifen and Curcumin Affecting the Viability of MCF7 Cell Line" Nanomaterials 12, no. 19: 3348. https://doi.org/10.3390/nano12193348






