Low Toxicity of Metal-Organic Framework MOF-74(Co) Nano-Particles In Vitro and In Vivo
Abstract
1. Introduction
2. Materials and Methods
3. Results
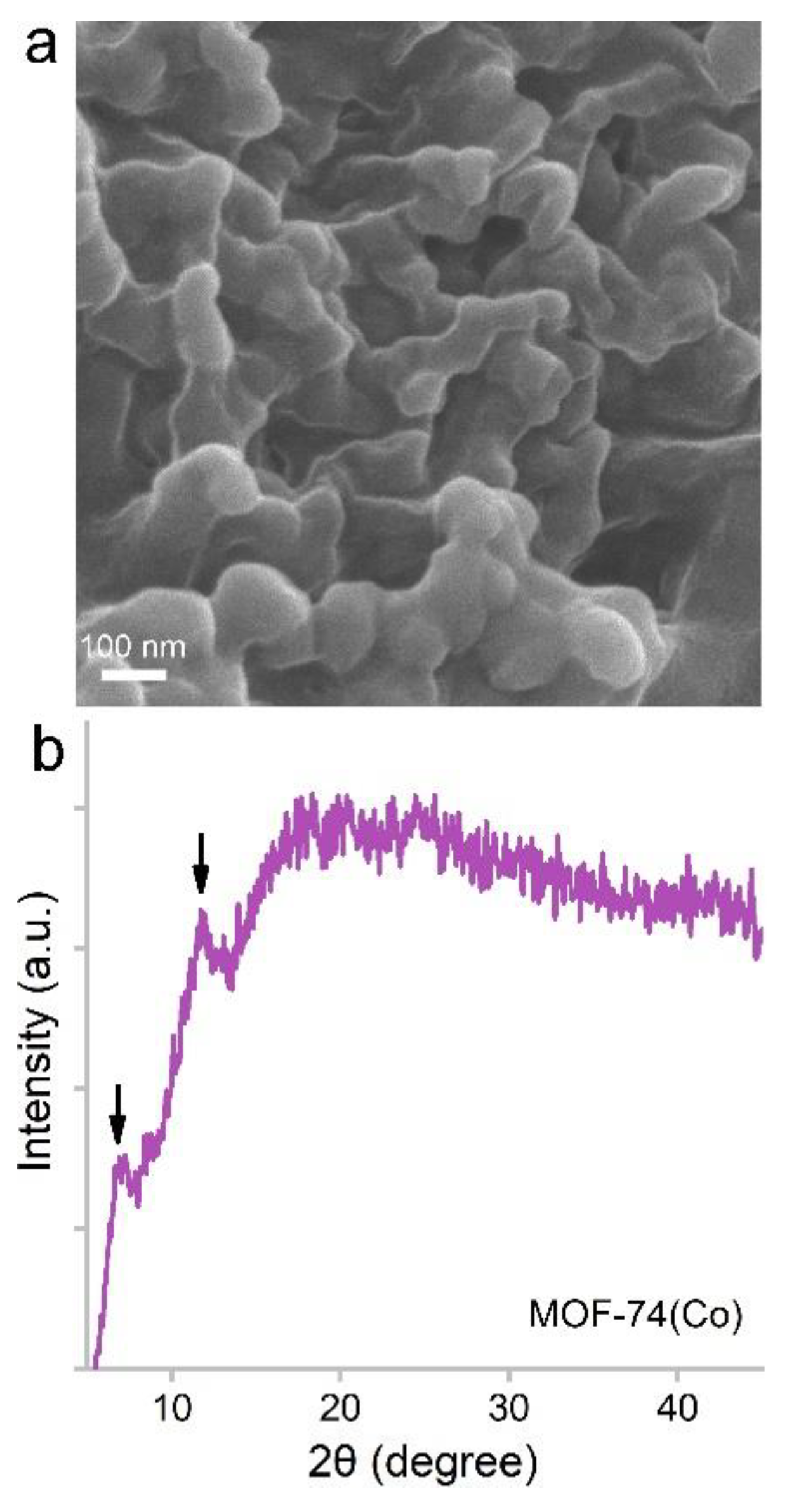
3.1. Characterization of MOF-74(Co) NPs
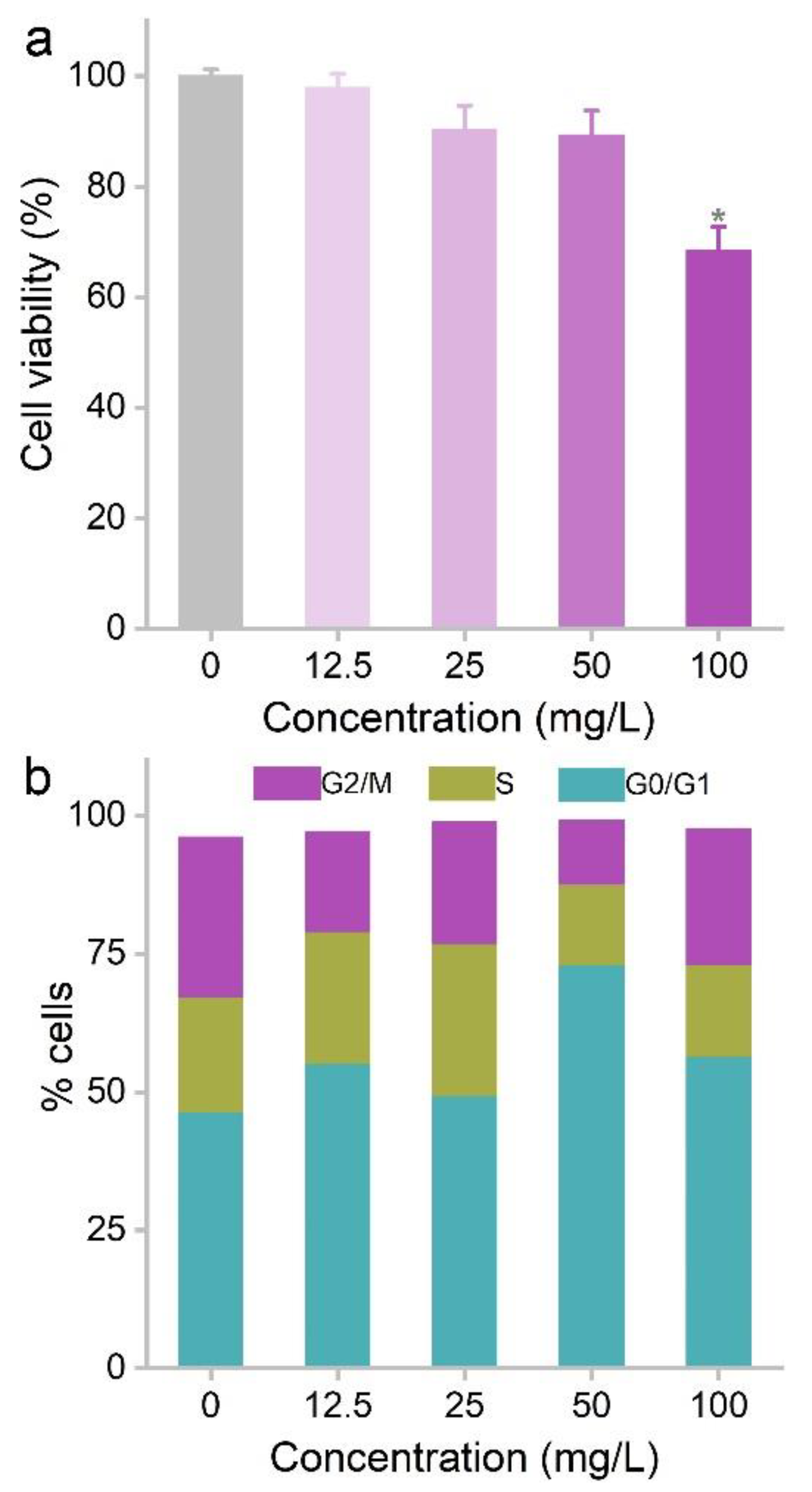
3.2. Cytotoxicity of MOF-74(Co) NPs
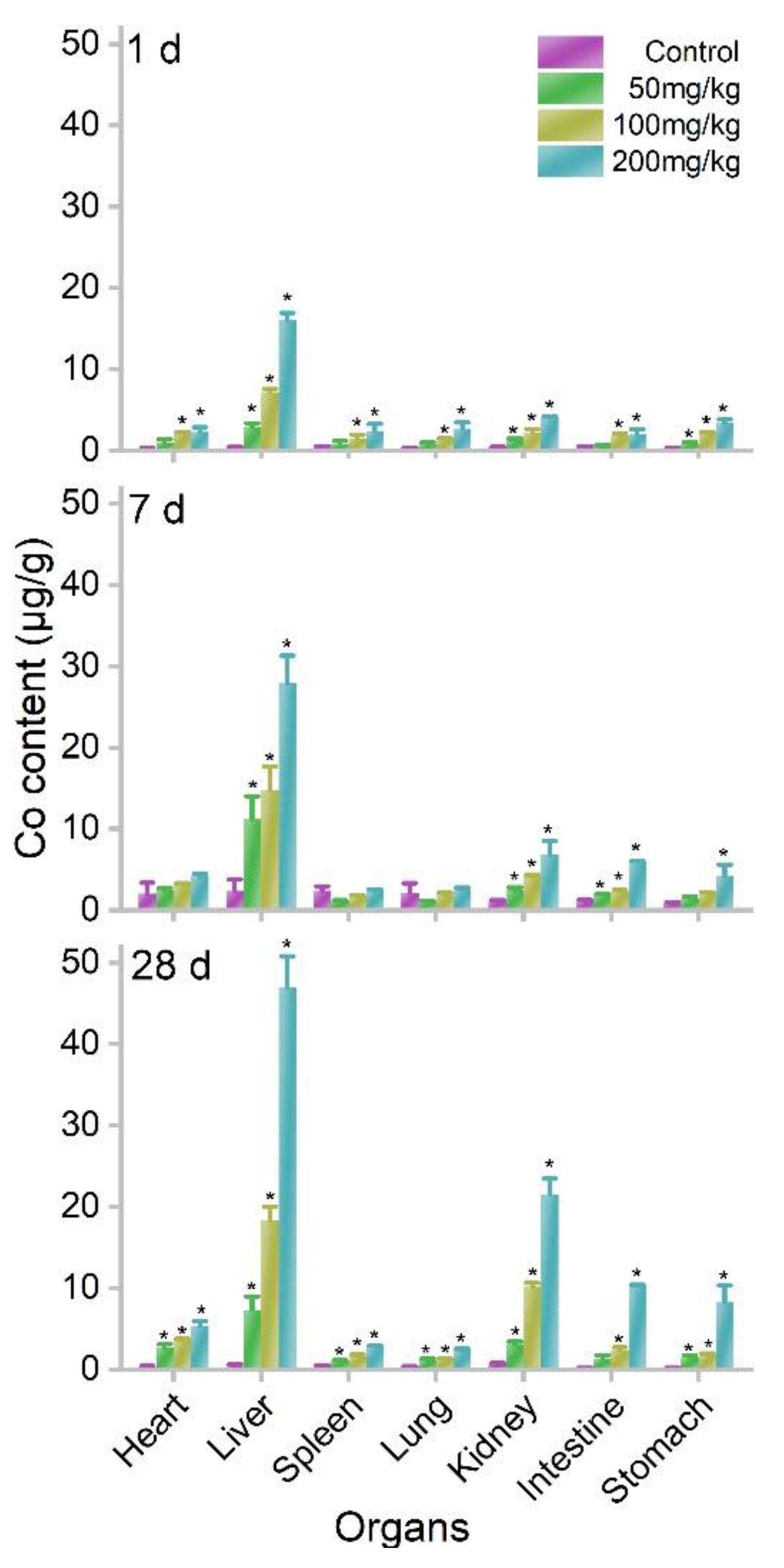
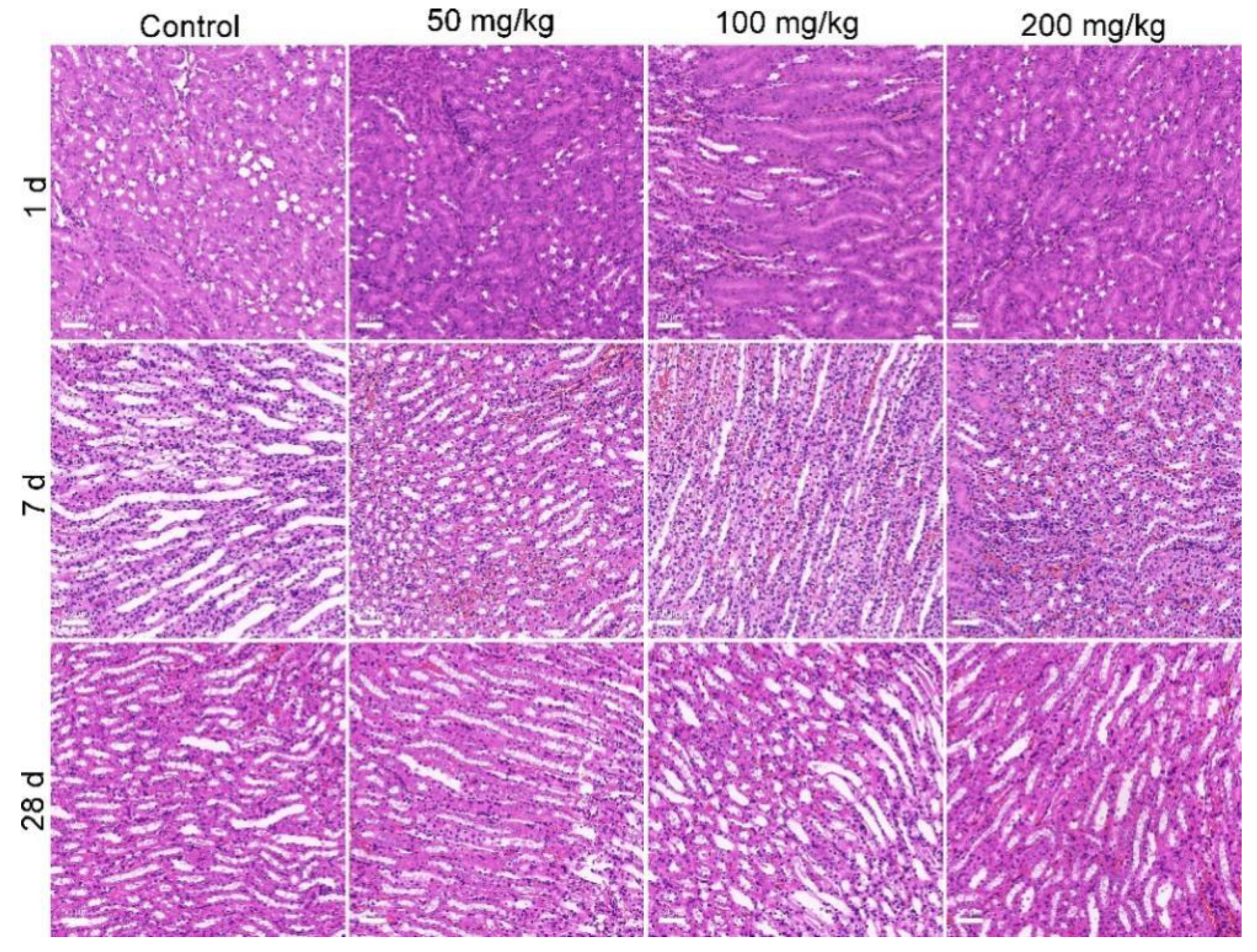
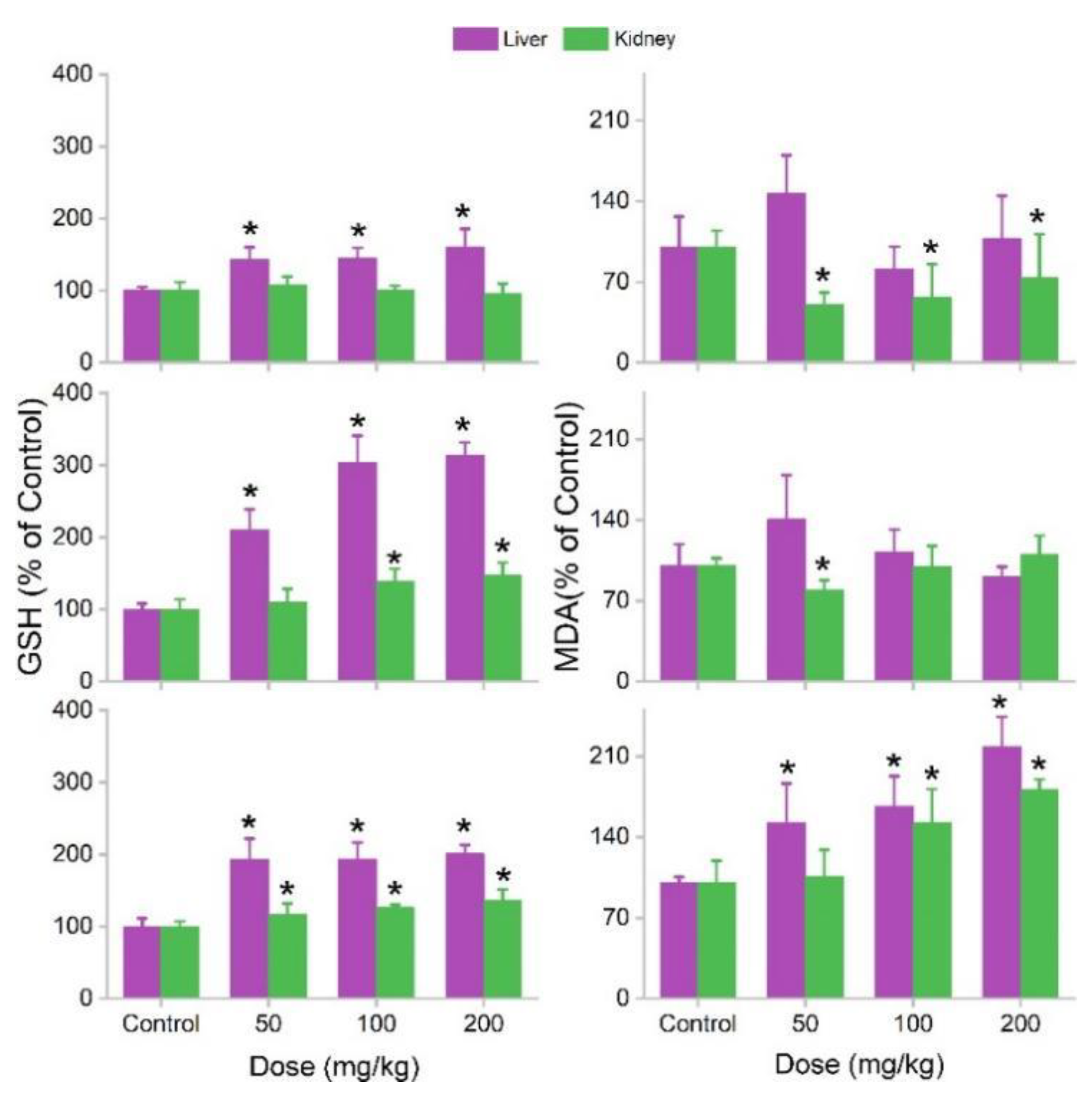
3.3. Biodistribution and Oral Toxicity of MOF-74(Co) NPs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zurrer, T.; Wong, K.; Horlyck, J.; Lovell, E.C.; Wright, J.; Bedford, N.M.; Han, Z.; Liang, K.; Scott, J.; Amal, R. Mixed-metal MOF-74 templated catalysts for efficient carbon dioxide capture and methanation. Adv. Funct. Mater. 2021, 31, 2007624. [Google Scholar] [CrossRef]
- Ding, S.; Wan, J.Q.; Ma, Y.W.; Wang, Y.; Li, X.T.; Sun, J.; Pu, M.J. Targeted degradation of dimethyl phthalate by activating persulfate using molecularly imprinted Fe-MOF-74. Chemosphere 2021, 270, 128620. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.Y.; Liang, C.H.; Qin, X.P.; Gu, Y.J.; Gao, P.; Shao, M.H.; Wong, W.-T. Zeolitic imidazolate framework cores decorated with Pd nanoparticles and coated further with metal-organic framework shells (ZIF-8@Pd@MOF-74) as nanocatalysts for chemoselective hydrogenation reactions. ACS Appl. Nano Mater. 2020, 3, 7242–7251. [Google Scholar] [CrossRef]
- Wang, C.L.; Li, X.R.; Yang, W.P.; Xu, Y.X.; Pang, H. Solvent regulation strategy of Co-MOF-74 microflower for supercapacitors. Chin. Chem. Lett. 2021, 32, 2909–2913. [Google Scholar] [CrossRef]
- Ge, Y.M.; Wang, K.; Li, H.L.; Tian, Y.; Wu, Y.T.; Lin, Z.W.; Lin, Y.Y.; Wang, S.W.; Zhang, J.R.; Tang, B. An Mg-MOFs based multifunctional medicine for the treatment of osteoporotic pain. Mater. Sci. Eng. C 2021, 129, 112386. [Google Scholar] [CrossRef]
- Guan, X.; Li, Q.; Maimaiti, T.; Lan, S.K.; Ouyang, P.; Ouyang, B.W.; Wu, X.; Yang, S.T. Toxicity and photosynthetic inhibition of metal-organic framework MOF-199 to pea seedlings. J. Hazard. Mater. 2021, 409, 124521. [Google Scholar] [CrossRef]
- Ouyang, B.W.; Yilihamu, A.; Liu, D.; Ouyang, P.; Zhang, D.; Wu, X.; Yang, S.T. Toxicity and environmental impact of multi-walled carbon nanotubes to nitrogen-fixing bacterium Azotobacter chroococcum. J. Environ. Chem. Eng. 2021, 9, 10529. [Google Scholar] [CrossRef]
- Ouyang, B.W.; Liu, F.S.; Liang, C.Z.; Zhang, J.H.; Hu, R.N.; Yuan, H.H.; Hai, R.D.; Yuan, Y.; Wu, X.; Yang, S.T. Toxicity and activity inhibition of metal-organic framework MOF-199 to nitrogen-fixing bacterium Azotobacter vinelandii. Sci. Total Environ. 2022, 813, 151912. [Google Scholar] [CrossRef]
- Ma, Q.; Zhang, Q.Q.; Maimaiti, T.; Lan, S.K.; Liu, X.W.; Wang, Y.Q.; Li, Q.; Luo, H.Z.; Yu, B.W.; Yang, S.T. Carbonization reduces the toxicity of metal-organic framework MOF-199 to white-rot fungus Phanerochaete chrysosporium. J. Environ. Chem. Eng. 2021, 9, 106705. [Google Scholar] [CrossRef]
- Hao, F.; Yan, X.P. Nano-sized zeolite-like metal-organic frameworks induced hematological effects on red blood cell. J. Hazard. Mater. 2022, 424, 127353. [Google Scholar] [CrossRef]
- Deng, S.X.; Yan, X.T.; Xiong, P.; Li, G.L.; Ku, T.T.; Liu, N.; Liao, C.Y.; Jiang, G.B. Nanoscale cobalt-based metal-organic framework impairs learning and memory ability without noticeable general toxicity: First in vivo evidence. Sci. Total Environ. 2021, 771, 145063. [Google Scholar] [CrossRef] [PubMed]
- Li, D.D.; Zhou, Q.X.; Hu, X.G.; Mu, L.; Zeng, H.; Luo, J.W. Environmental decomposition and remodeled phytotoxicity of framework-based nanomaterials. J. Hazard. Mater. 2022, 422, 126846. [Google Scholar] [CrossRef] [PubMed]
- Wagner, A.; Liu, Q.; Rose, O.L.; Eden, A.; Vijay, A.; Rojanasakul, Y.; Dinu, C.Z. Toxicity screening of two prevalent metal organic frameworks for therapeutic use in human lung epithelial cells. Int. J. Nanomed. 2019, 14, 7583–7591. [Google Scholar] [CrossRef] [PubMed]
- Ren, F.; Yang, B.C.; Cai, J.; Jiang, Y.D.; Xu, J.; Wang, S. Toxic effect of zinc nanoscale metal-organic frameworks on rat pheochromocytoma (PC12) cells in vitro. J. Hazard. Mater. 2014, 271, 283–291. [Google Scholar] [CrossRef]
- Zhu, Z.; Jiang, S.K.; Liu, Y.H.; Gao, X.M.; Hu, S.S.; Zhang, X.; Huang, C.; Wan, Q.B.; Wang, J.; Pei, X. Micro or nano: Evaluation of biosafety and biopotency of magnesium metal organic framework-74 with different particle sizes. Nano Res. 2020, 13, 511–526. [Google Scholar] [CrossRef]
- Fan, G.D.; Bao, M.C.; Zheng, X.M.; Hong, L.; Zhan, J.J.; Chen, Z.; Qu, F.S. Growth inhibition of harmful cyanobacteria by nanocrystalline Cu-MOF-74: Efficiency and its mechanisms. J. Hazard. Mater. 2019, 367, 529–538. [Google Scholar] [CrossRef]
- Javanbakht, S.; Hemmati, A.; Namazi, H.; Heydari, A. Carboxymethylcellulose-coated 5-fluorouracil@MOF-5 nano-hybrid as a bio-nanocomposite carrier for the anticancer oral delivery. Int. J. Biol. Macromol. 2020, 155, 876–882. [Google Scholar] [CrossRef]
- Zou, J.J.; Wei, G.H.; Xiong, G.X.; Yu, Y.H.; Li, S.H.; Hu, L.F.; Ma, S.Q.; Tian, J. Efficient oral insulin delivery enabled by transferrin-coated acid-resistant metal-organic framework nanoparticles. Sci. Adv. 2022, 8, eabm4677. [Google Scholar] [CrossRef]
- Liu, C.H.; Chiu, H.C.; Sung, H.L.; Yeh, J.Y.; Wu, K.C.W.; Liu, S.H. Acute oral toxicity and repeated dose 28-day oral toxicity studies of MIL-101 nanoparticles. Regul. Toxicol. Pharmacol. 2019, 104, 104426. [Google Scholar] [CrossRef]
- Manuel, D.G.; Lvaro, M.; Isabel, D.; Manuel, S.S. Nanoscaled M-MOF-74 materials prepared at room temperature. Cryst. Growth Des. 2014, 14, 2479–2487. [Google Scholar] [CrossRef]
- Leporatti, S. Thinking about Enhanced Permeability and Retention Effect (EPR). J. Pers. Med. 2022, 12, 1259. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.S.; Leng, X.; Luo, J.Y.; You, L.T.; Qu, C.H.; Dong, X.X.; Huang, H.L.; Yin, X.B.; Ni, J. In vitro toxicity study of a porous iron(III) metal-organic framework. Molecules 2019, 24, 1211. [Google Scholar] [CrossRef] [PubMed]
- Yen, C.I.; Liu, S.M.; Lo, W.S.; Wu, J.W.; Liu, Y.H.; Chein, R.J.; Yang, R.Q.; Wu, K.C.W.; Hwu, J.R.; Ma, N.H.; et al. Cytotoxicity of postmodified zeolitic imidazolate framework-90 (ZIF-90) nanocrystals: Correlation between functionality and toxicity. Chem. Eur. J. 2016, 32, 2925–2929. [Google Scholar] [CrossRef]
- Tamames, T.C.; Denise, C.; Edurne, I.; Ragon, F.; Serre, C.; Prieto, M.J.B.; Horcajada, P. Cytotoxicity of nanoscaled metal-organic frameworks. J. Mater. Chem. B 2014, 2, 262–271. [Google Scholar] [CrossRef]
- Jafari, S.; Zhila, I.; Loghman, A.; Jaymand, M.; Samadian, H.; Kashani, V.O.; Derakhshankhah, H.; Hayati, P.; Noori, F.; Mansouri, K.; et al. Human plasma protein corona decreases the toxicity of pillar-layer metal organic framework. Sci. Rep. 2020, 10, 14569. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.Q.; Chen, D.; He, J. Fe-MIL-101 exhibits selective cytotoxicity and inhibition of angiogenesis in ovarian cancer cells via downregulation of MMP. Sci. Rep. 2016, 6, 26126. [Google Scholar] [CrossRef]
- Motakef, K.N.; Shojaosadati, S.A.; Morsali, A. In situ synthesis of a drug-loaded MOF at room temperature. Microporous Mesoporous Mater. 2014, 186, 73–79. [Google Scholar] [CrossRef]
- Han, C.C.; Yang, J.; Gu, J.L. Immobilization of silver nanoparticles in Zr-based MOFs: Induction of apoptosis in cancer cells. J. Nanoparticle Res. 2018, 20, 77–88. [Google Scholar] [CrossRef]
- Hadi, R.B.; Fatemeh, K.; Xie, K. MOF Mediated Destruction of Cancer Using the Cell’s Own Hydrogen Peroxide. ACS Appl. Mater. Interfaces 2017, 9, 33599–33608. [Google Scholar] [CrossRef]
- Flavia, R.S.; Larissa, C.C.; Maria, R. Induction of cancer cell death by apoptosis and slow release of 5-fluoracil from metal-organic frameworks Cu-BTC. Biomed. Pharmacother. 2013, 67, 707–713. [Google Scholar] [CrossRef]
- Zeraati, M.; Moghaddam, M.M.; Hosseinzadegan, S. Porous Cu-MOF nanostructures with anticancer properties prepared by a controllable ultrasound-assisted reverse micelle synthesis of Cu-MOF. BMC Chem. 2022, 16, 10. [Google Scholar] [CrossRef]
- Lin, W.X.; Hu, Q.; Yu, J.C.; Jiang, K.; Yang, Y.Y.; Xiang, S.C.; Cui, Y.J.; Yang, Y.; Wang, Z.Y.; Qian, G.D. Low cytotoxic metal-organic frameworks as temperature-responsive drug carriers. Chem. Eur. J. 2016, 81, 804–810. [Google Scholar] [CrossRef]
- Arcuri, C.; Monarca, L.; Ragonese, F.; Mecca, C.; Bruscoli, S.; Giovagnoli, S.; Donato, R.; Bereshchenko, O.; Fiorett, B.; Costantino, F. Probing internalization effects and biocompatibility of ultrasmall zirconium metal-organic frameworks UiO-66 NP in U251 glioblastoma cancer cells. Nanomaterials 2018, 8, 867. [Google Scholar] [CrossRef]
- Hoop, M.; Walde, C.F.; Riccò, R.; Mushtaq, F.; Terzopoulou, A.; Chen, X.Z.; Demello, A.J.; Doonan, C.J.; Falcaro, P.; Nelson, B.J. Biocompatibility characteristics of the metal organic framework ZIF-8 for therapeutical applications. Appl. Mater. Today 2018, 11, 13–21. [Google Scholar] [CrossRef]
- Smith, T.; Thompson, B.D.; Barnaby, C.F. Measurements of 60Co organ burdens in rats and their use in calculations of equilibrium dose-rates to various organs of man. Health Phys. 1971, 20, 195–204. [Google Scholar] [CrossRef]
- Smith, T.; Edmonds, C.J.; Barnaby, C.F. Absorption and Retention of Cobalt in Man by Whole-body Counting. Health Phys. 1972, 22, 359–367. [Google Scholar] [CrossRef]
- Simon-Yarza, T.; Baati, T.; Neffati, F.; Njim, L.; Couvreur, P.; Serre, C.; Gref, R.; Najjar, M.F.; Zakhama, A.; Horcajada, P. In vivo behavior of MIL-100 nanoparticles at early times after intravenous administration. Int. J. Pharm. 2016, 511, 1042–1047. [Google Scholar] [CrossRef]
- Baati, T.; Njim, L.; Neffati, F.; Kerkeni, A.; Bouttemi, M.; Gref, R.; Najjar, M.F.; Zakhama, A.; Couvreur, P.; Serre, C.; et al. In depth analysis of the in vivo toxicity of nanoparticles of porous iron(iii) metal-organic frameworks. Chem. Sci. 2013, 4, 1597–1607. [Google Scholar] [CrossRef]
- Chang, X.L.; Chen, L.Y.; Liu, B.N.; Yang, S.T.; Wang, H.F.; Cao, A.N.; Chen, C.Y. Stable isotope labeling of nanomaterials for biosafety evaluation and drug development. Chin. Chem. Lett. 2022, 33, 3303–3314. [Google Scholar] [CrossRef]
- Domingo, J.L.; Llobet, J.M.; Bernat, R. A study of the effects of cobalt administered orally to rats. Arch. Farmacol. Toxicol. 1984, 10, 13–20. Available online: https://pubmed.ncbi.nlm.nih.gov/6508364 (accessed on 21 May 2020).
- Roy, G.; Holly, M.D. Studies on iron and cobalt metabolism. JAMA 1955, 158, 1349–1352. [Google Scholar] [CrossRef]
- Mao, Y. Generation of reactive oxygen species by Co(II) from H2O2 in the presence of chelators in relation to DNA damage and 2′-deoxyguanosine hydroxylation. J. Toxicol. Environ. Health 1996, 47, 61–75. [Google Scholar] [CrossRef] [PubMed]
- Christova, T.; Duridanova, D.; Braykova, A.; Setchenska, M.; Bolton, T. Heme oxygenase is the main protective enzyme in rat liver upon 6-day administration of cobalt chloride. Arch. Toxicol. 2001, 75, 445–451. [Google Scholar] [CrossRef] [PubMed]


| MOF Material | Cell Line | Dose Range | IC50 | Ref. |
|---|---|---|---|---|
| MIL-160 | BEAS-2B | 1–750 mg/L | 421 mg/L | [13] |
| Fe-MIL-101 | SKOV3 | 1.56–50 mg/L | 23.6 mg/L | [26] |
| MOF-Zn2(1,4-bdc)2(dabco) | HuH7 | 0.01–10,000 mg/L | 1000 mg/L | [27] |
| UiO-66 | HeLa | 10–100 mg/L | >100 mg/L | [28] |
| IRMOF-3 | PC12 | 25–400 mg/L | 100 mg/L | [14] |
| rMOF-FA | HeLa | 0–120 mg/L | 43 mg/L | [29] |
| Cu-BTC | MCF7 | 0–75 mg/L | 3.49 mg/L | [30] |
| HT-29 | >25 mg/L | |||
| HL-60 | >25 mg/L | |||
| NCI-H292 | >25 mg/L | |||
| Cu-MOF | MCF-7 | 0–200 mg/L | 109 mg/L | [31] |
| MOF-74(Co) | LO2 cells | 0–100 mg/L | >100 mg/L | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lan, S.; Zhang, J.; Li, X.; Pan, L.; Li, J.; Wu, X.; Yang, S.-T. Low Toxicity of Metal-Organic Framework MOF-74(Co) Nano-Particles In Vitro and In Vivo. Nanomaterials 2022, 12, 3398. https://doi.org/10.3390/nano12193398
Lan S, Zhang J, Li X, Pan L, Li J, Wu X, Yang S-T. Low Toxicity of Metal-Organic Framework MOF-74(Co) Nano-Particles In Vitro and In Vivo. Nanomaterials. 2022; 12(19):3398. https://doi.org/10.3390/nano12193398
Chicago/Turabian StyleLan, Suke, Jiahao Zhang, Xin Li, Lejie Pan, Juncheng Li, Xian Wu, and Sheng-Tao Yang. 2022. "Low Toxicity of Metal-Organic Framework MOF-74(Co) Nano-Particles In Vitro and In Vivo" Nanomaterials 12, no. 19: 3398. https://doi.org/10.3390/nano12193398
APA StyleLan, S., Zhang, J., Li, X., Pan, L., Li, J., Wu, X., & Yang, S.-T. (2022). Low Toxicity of Metal-Organic Framework MOF-74(Co) Nano-Particles In Vitro and In Vivo. Nanomaterials, 12(19), 3398. https://doi.org/10.3390/nano12193398







