Stabilizing Li-Rich Layered Cathode Materials Using a LiCoMnO4 Spinel Nanolayer for Li-Ion Batteries
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cathode Preparation
2.2. Characterization
2.3. Electrochemical Measurements
3. Results
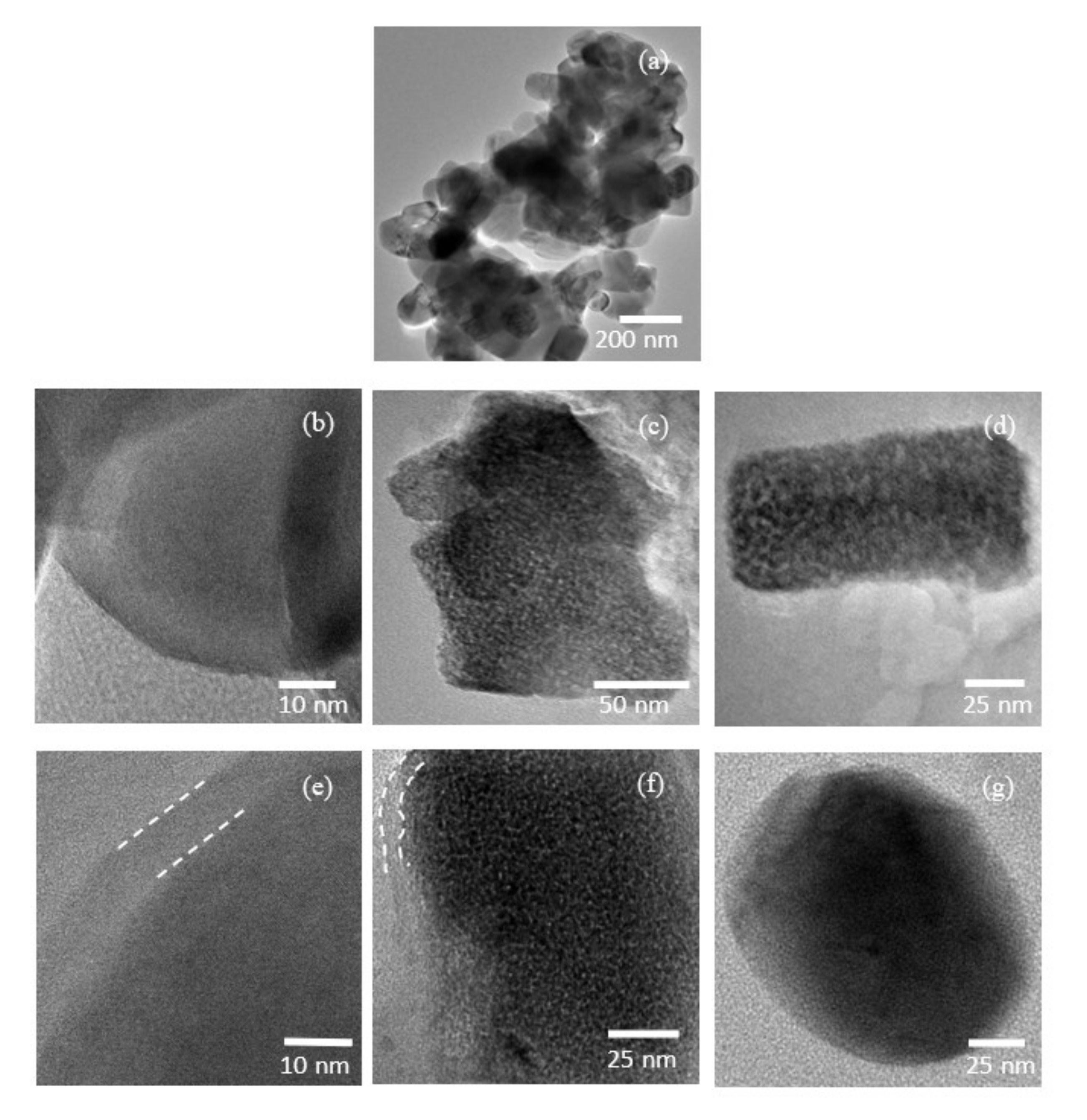
3.1. Morphological Characterization
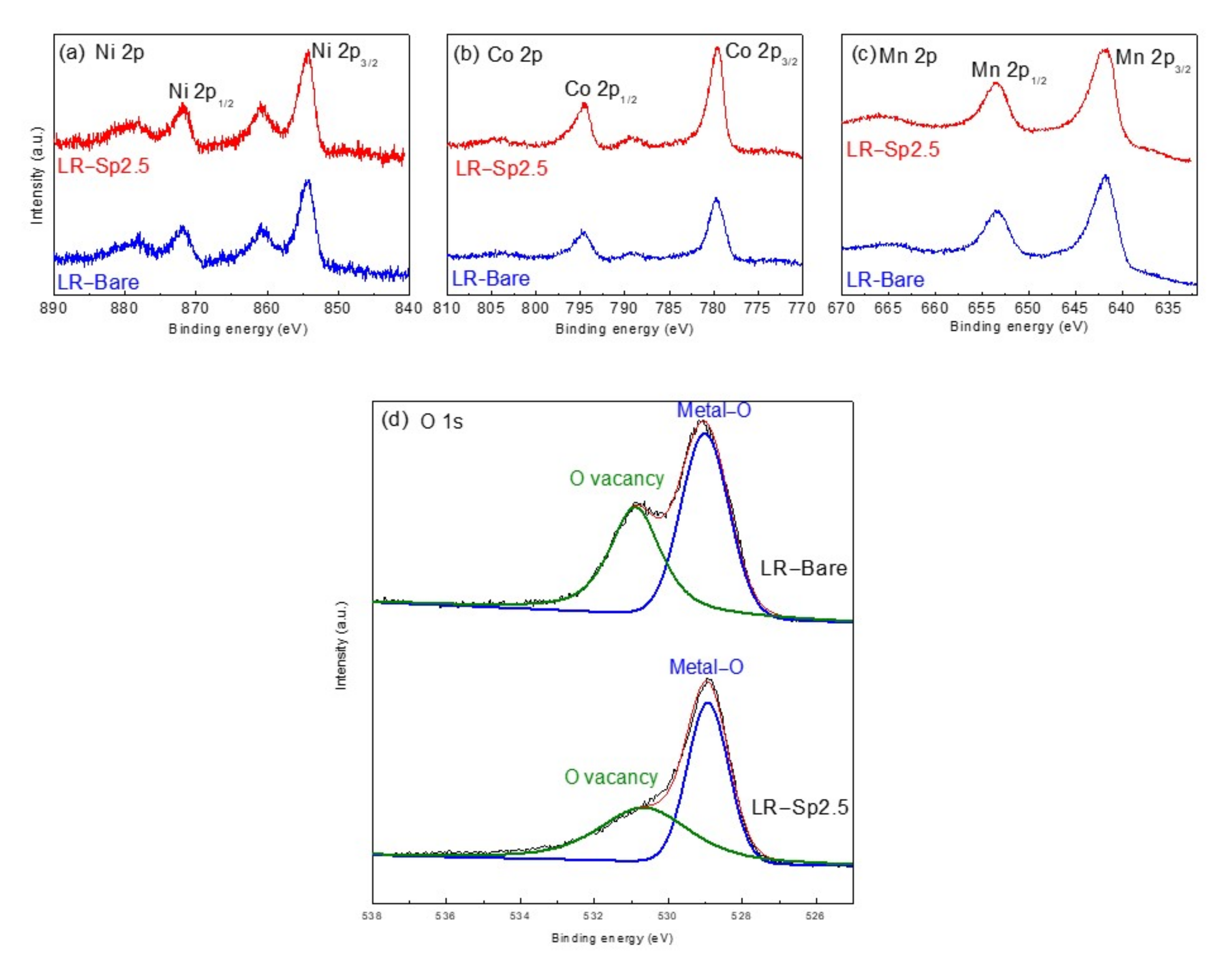
3.2. Structural Characterization
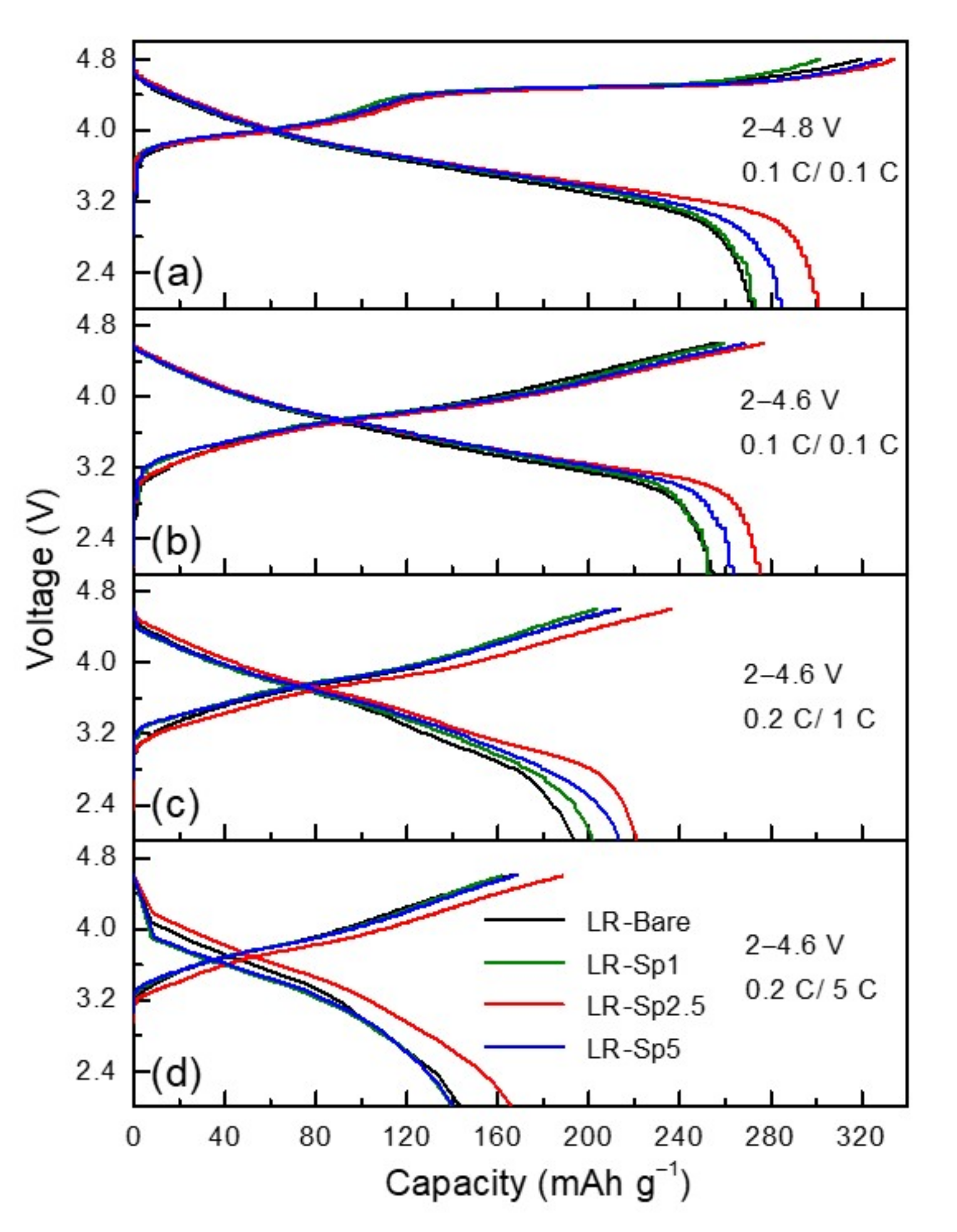
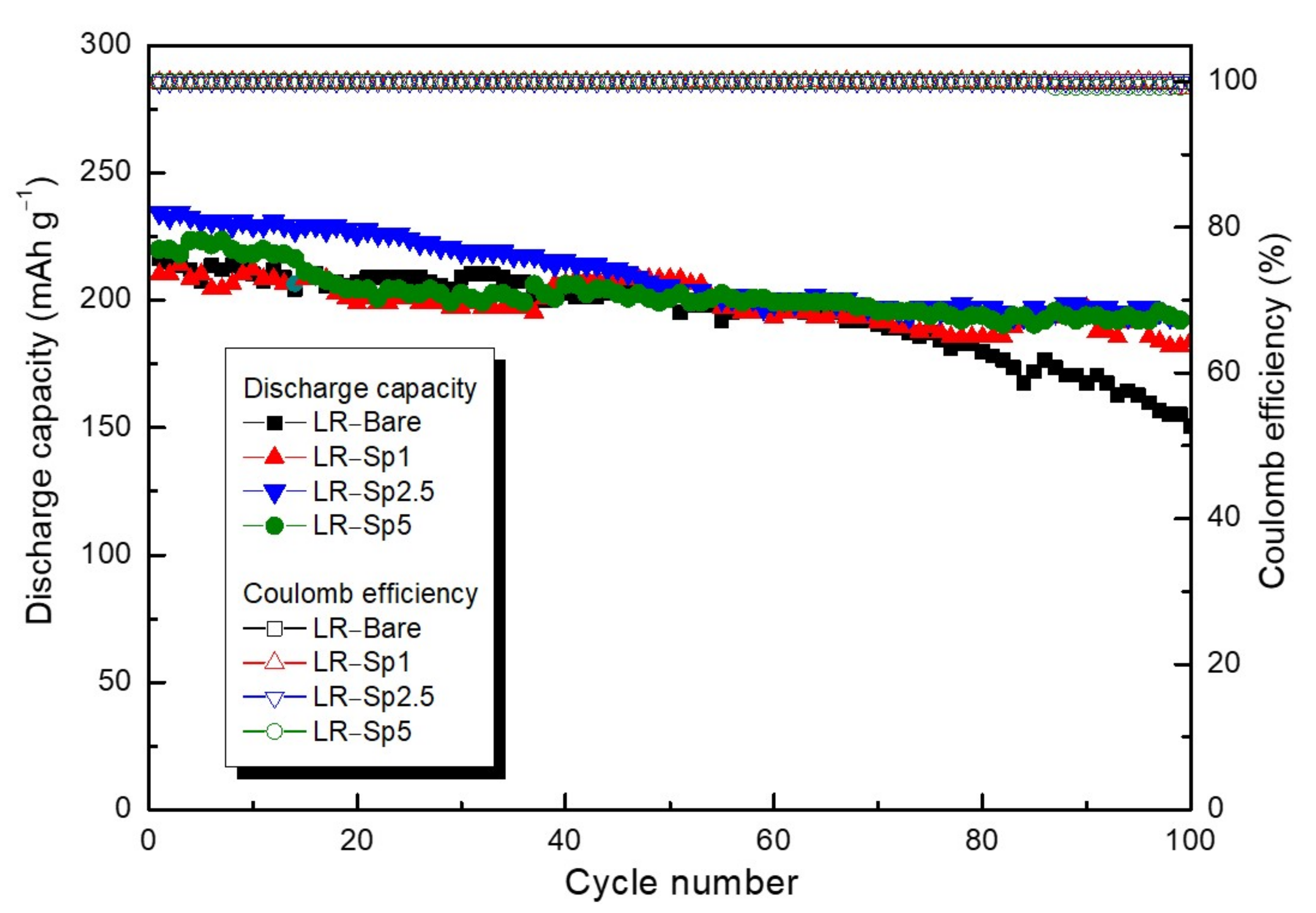
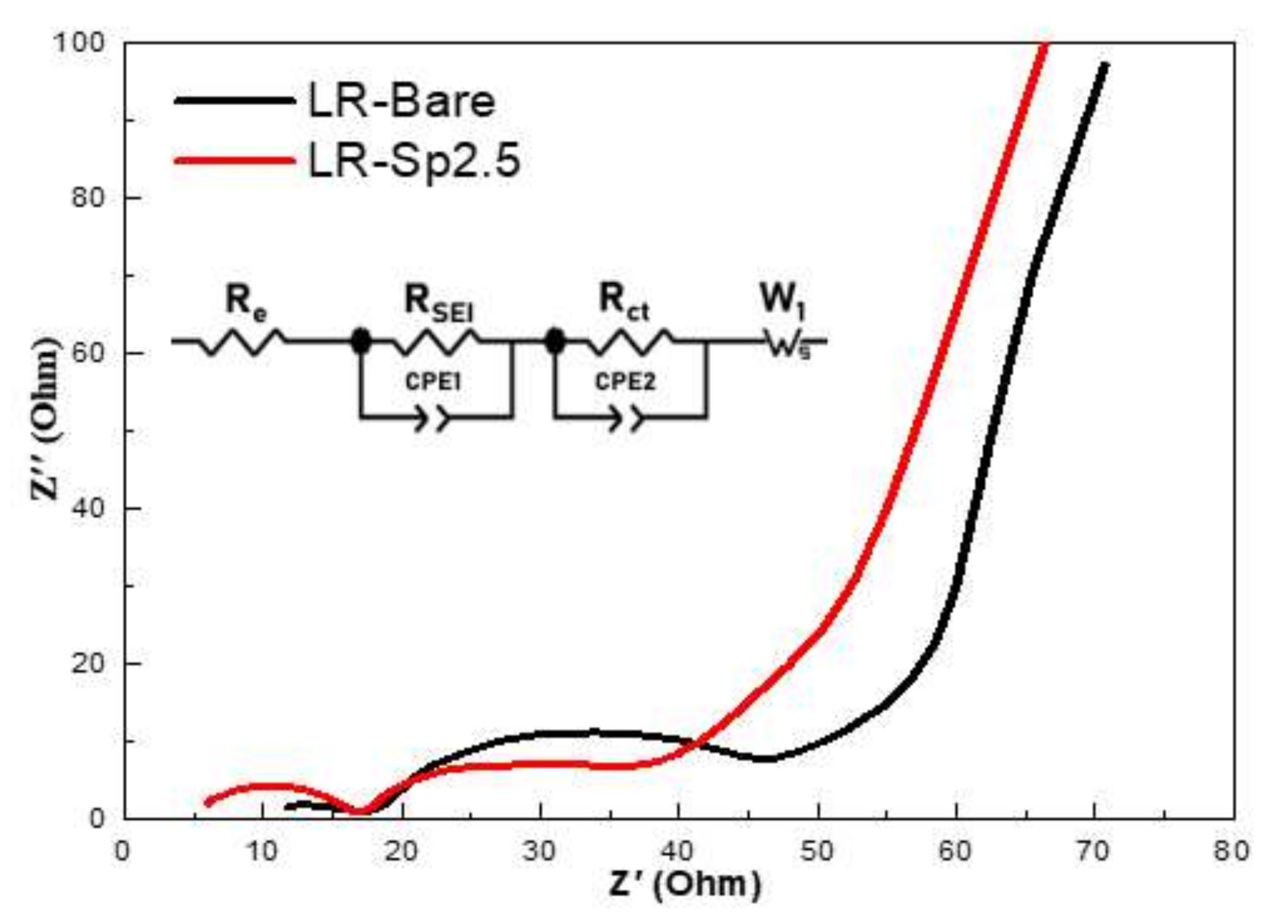
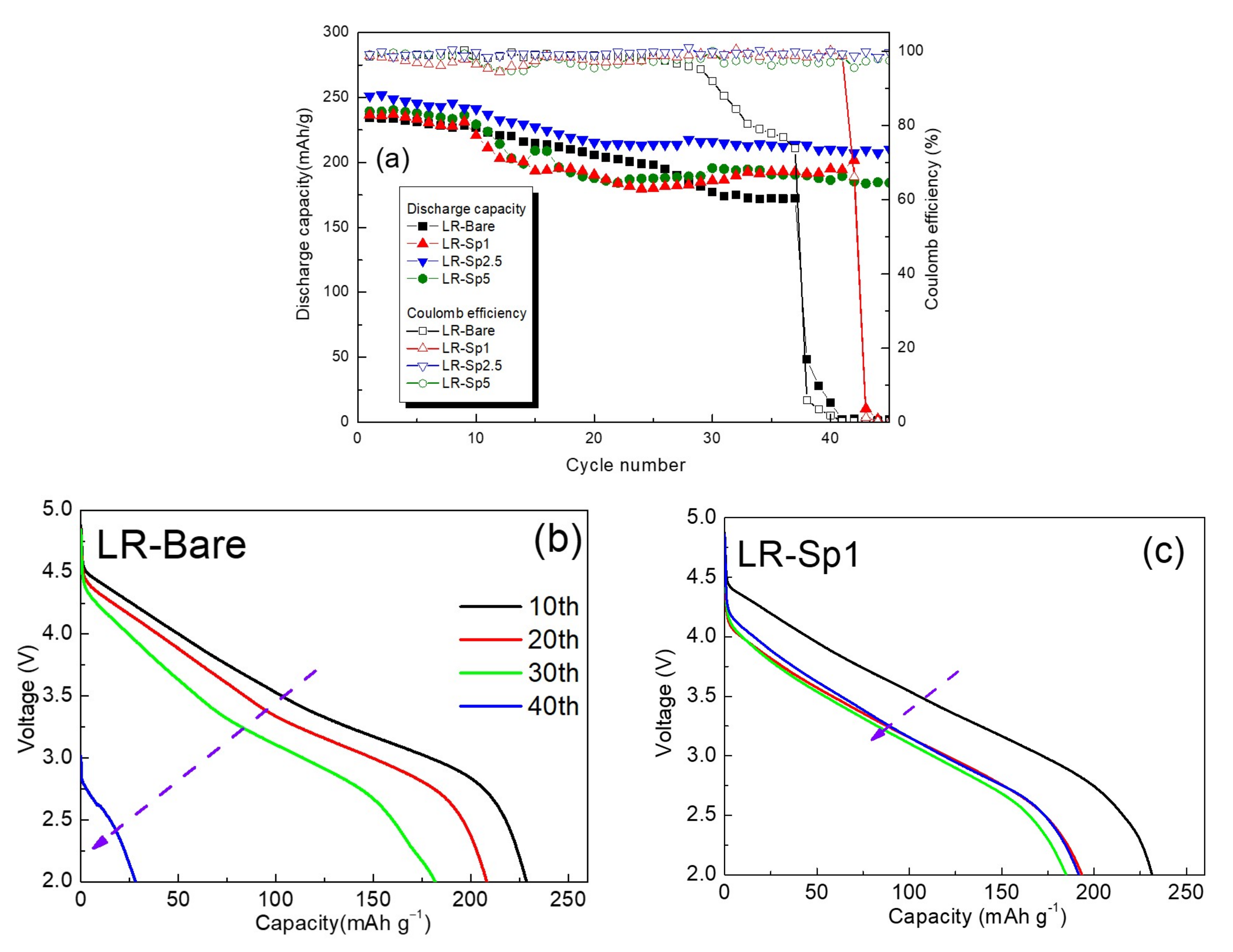
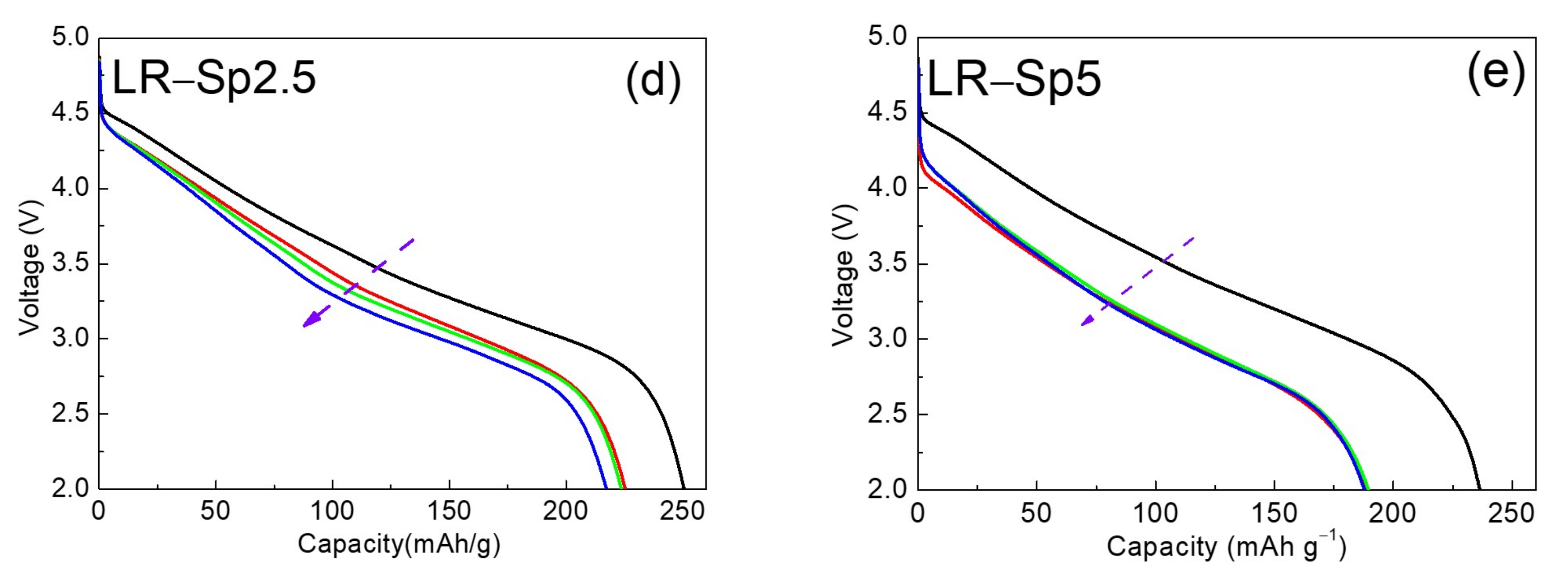
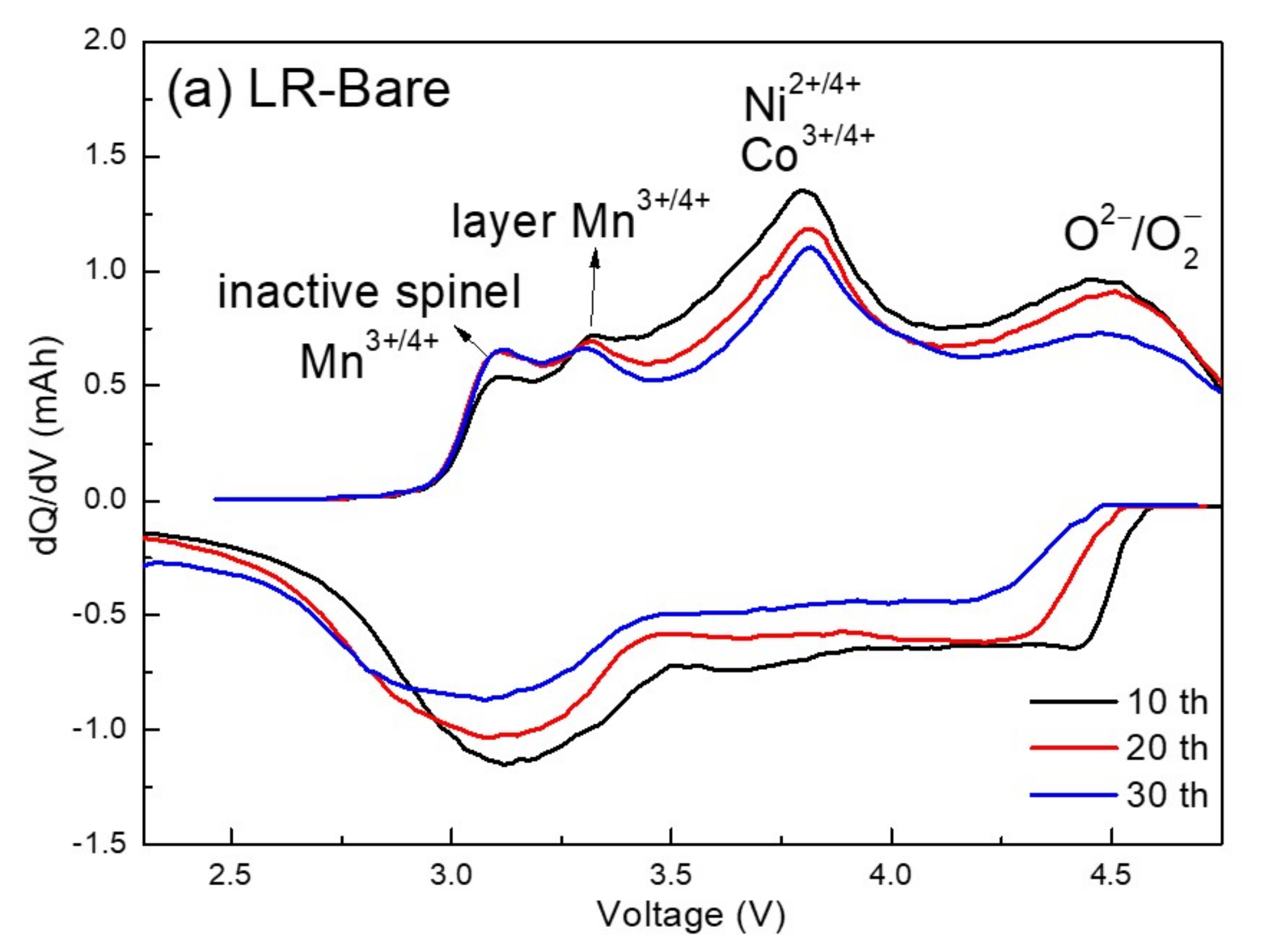
3.3. Electrochemical Characterization
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Goodenough, J.B.; Park, K. −S. The Li−ion rechargeable battery: A perspective. J. Am. Chem. Soc. 2013, 135, 1167–1176. [Google Scholar] [CrossRef] [PubMed]
- Thackeray, M.M.; Wolverton, C.; Isaacs, E.D. Electrical energy storage for transportation−approaching the limits of, and going beyond, lithium−ion batteries. Energy Environ. Sci. 2012, 5, 7854–7863. [Google Scholar] [CrossRef]
- Yu, R.; Wang, X.; Fu, Y.; Wang, L.; Cai, S. Effect of magnesium doping on properties of lithium−rich layered oxide cathodes based on a one−step co−precipitation strategy. J. Mater. Chem. A 2016, 4, 4941–4951. [Google Scholar] [CrossRef]
- Pang, W.K.; Lin, H.-F.; Peterson, V.K.; Lu, C.-Z.; Liu, C.-E.; Liao, S.-C.; Chen, J.M. Effects of Fluorine and Chromium Doping on the Performance of Lithium−Rich Li1+xMO2 (M = Ni, Mn, Co) Positive Electrode. Chem. Mater. 2017, 29, 10299–10311. [Google Scholar] [CrossRef]
- Lin, H.-F.; Guo, H.-L.; Hsiao, S.-C. Electrochemical performances of lithium rich cathodes prepared via co−precipitation method with different precipitator and atmosphere. J. Mater. Sci. Mater. Electron. 2018, 29, 10427–10436. [Google Scholar] [CrossRef]
- Yang, X.; Wang, D.; Yu, R.; Bai, Y.; Shu, H.; Ge, L.; Guo, H.; Wei, Q.; Liu, L.; Wang, X. Suppressed capacity/voltage fading of high−capacity lithium−rich layered materials via the design of heterogeneous distribution in the composition. J. Mater. Chem. A 2014, 2, 3899–3911. [Google Scholar] [CrossRef]
- Wang, G.; Wang, X.; Yi, L.; Yu, R.; Liu, M.; Yang, X. Preparation and performance of 0.5Li2MnO3·0.5LiNi1/3Co1/3Mn1/3O2 with a fusiform porous micro−nano structure. J. Mater. Chem. A 2016, 4, 15929–15939. [Google Scholar] [CrossRef]
- Li, J.; Camardese, J.; Glazier, S.; Dahn, J.R. Structural and Electrochemical Study of the Li–Mn–Ni Oxide System within the Layered Single Phase Region. Chem. Mater. 2014, 26, 7059–7066. [Google Scholar] [CrossRef]
- Martha, S.K.; Nanda, J.; Veith, G.M.; Dudney, N.J. Electrochemical and rate performance study of high−voltage lithium−rich composition: Li1.2Mn0.525Ni0.175Co0.1O2. J. Power Source 2012, 199, 220–226. [Google Scholar] [CrossRef]
- Thackeray, M.M.; Kang, S.-H.; Johnson, C.S.; Vaughey, J.T.; Benedek, R.; Hackney, S.A. Li2MnO3–stabilized LiMO2 (M= Mn, Ni, Co) electrodes for lithium–ion batteries. J. Mater. Chem. 2007, 17, 3112. [Google Scholar] [CrossRef]
- Wang, D.; Belharouak, I.; Zhou, G.K. Amine Nanoarchitecture Multi–Structural Cathode Materials for High Capacity Lithium Batteries. Adv. Funct. Mater. 2012, 23, 1070. [Google Scholar] [CrossRef]
- Xu, B.; Fell, C.R.; Chi, M.; Meng, Y.S. Identifying surface structural changes in layered Li–excess nickel manganeseoxides in high voltage lithium ion batteries: A joint experimental and theoretical study. Energy Environ. Sci. 2011, 4, 2223. [Google Scholar] [CrossRef]
- Kottegoda, I.R.M.; Kadoma, Y.; Ikuta, H.; Uchimoto, Y.; Wakihara, M. Enhancement of Rate Capability in Graphite Anode by Surface Modification with Zirconia. Electrochem. Solid State Lett. 2002, 5, A275. [Google Scholar] [CrossRef]
- Eftekhari, A. Surface Modification of Thin–Film Based LiCoPO4 5 V Cathode with Metal Oxide. J. Electrochem. Soc. 2004, 151, A1456. [Google Scholar] [CrossRef]
- Van Landschoot, N.; Kelder, E.M.; Kooyman, P.J.; Kwakernaak, C.; Schoonman, J. Electrochemical performance of Al2O3–coated Fe doped LiCoVO4. J. Power Source 2004, 138, 262. [Google Scholar] [CrossRef]
- Sun, Y.-K.; Yoon, C.S.; Oh, I.-H. Surface structural change of ZnO–coated LiNi0.5Mn1.5O4 spinel as 5 V cathode materials at elevated temperatures. Electrochim. Acta 2002, 48, 503. [Google Scholar]
- Miyashiro, H.; Yamanaka, A.; Tabuchi, M.; Seki, S.; Nakayama, M.; Ohno, Y.; Kobayashi, Y.; Mita, Y.; Usami, A.; Wakihara, M. Improvement of degradation at elevated temperature and at high state–of–charge storage by ZrO2 coating on LiCoO2. J. Electrochem. Soc. 2006, 153, A348. [Google Scholar] [CrossRef]
- Lin, Y.-M.; Wu, H.-C.; Yen, Y.-C.; Guo, Z.-Z.; Yang, M.-H.; Chen, H.-M.; Sheu, H.-S.; Wu, N.-L. Enhanced High–Rate Cycling Stability of LiMn2O4 Cathode by ZrO2 Coating for Li–Ion Battery. J. Electrochem. Soc. 2005, 152, A1526. [Google Scholar] [CrossRef]
- Zhao, T.; Li, L.; Chen, R.; Wu, H.; Zhang, X.; Chen, S.; Xie, M.; Wu, F.; Lu, J. Amine Design of surface protective layer of LiF/FeF3 nanoparticles in Li–rich cathode for high–capacity Li–ion batteries. Nano Energy 2015, 15, 164–176. [Google Scholar] [CrossRef]
- Zheng, J.; Gu, M.; Xiao, J.; Polzin, B.J.; Yan, P.; Chen, X.; Wang, C.; Zhang, G.-J. Functioning Mechanism of AlF3 Coating on the Li– and Mn–Rich Cathode Materials. Chem. Mater. 2014, 26, 6320–6327. [Google Scholar] [CrossRef]
- Xia, Q.; Zhao, X.; Xu, M.; Ding, Z.; Liu, J.; Chen, L.; Ivey, D.G.; Wei, W. A Li–rich Layered–Spinel–Carbon heterostructured cathode material for high capacity and high rate lithium–ion batteries fabricated via an in situ synchronous carbonization–reduction method. J. Mater. Chem. A 2015, 3, 3995–4003. [Google Scholar] [CrossRef]
- Sun, Y.-K.; Lee, M.-J.; Yoon, C.S.; Hassoun, J.; Amine, K.; Scrosati, B. The Role of AlF3 Coatings in Improving Electrochemical Cycling of Li–Enriched Nickel–Manganese Oxide Electrodes for Li–Ion Batteries. Adv. Mater. 2012, 24, 1192. [Google Scholar] [CrossRef] [PubMed]
- Croy, J.R.; Balasubramanian, M.; Kim, D.; Kang, J.-S.; Thackeray, M.M. Designing High–Capacity, Lithium–Ion Cathodes Using X–ray Absorption Spectroscopy. Chem. Mater. 2011, 23, 5415. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, S.; Wu, Q.; Wan, N.; Pan, D.; Bai, Y. Improved electrochemical and thermal performances of layered Li[Li0.2Ni0.17Co0.07Mn0.56]O2 via Li2ZrO3 surface modification. J. Power Source 2015, 282, 378–384. [Google Scholar] [CrossRef]
- Liu, W.; Oh, P.; Liu, X.; Myeong, S.; Cho, W.; Cho, J. Countering Voltage Decay and Capacity Fading of Lithium–Rich Cathode Material at 60 °C by Hybrid Surface Protection Layers. Adv. Energy Mater. 2015, 5, 1500274. [Google Scholar] [CrossRef]
- Lee, S.; Cho, Y.; Song, H.-K.; Lee, K.T.; Cho, J. Carbon–Coated Single–Crystal LiMn2O4 Nanoparticle Clusters as Cathode Material for High–Energy and High–Power Lithium–Ion Batteries. Angew. Chem. Int. Ed. 2012, 51, 8748–8752. [Google Scholar] [CrossRef]
- Zhou, L.; Zhao, D.; Lou, X.W. LiNi0.5Mn1.5O4 Hollow Structures as High–Performance Cathodes for Lithium–Ion Batteries. Angew. Chem. Int. Ed. 2012, 51, 239. [Google Scholar] [CrossRef]
- Thackeray, M.M. Lithium–ion batteries: An unexpected conductor. Nat. Mater. 2002, 1, 81. [Google Scholar] [CrossRef]
- Liao, C.; Li, F.; Liu, J. Challenges and Modification Strategies of Ni–Rich Cathode Materials Operating at High–Voltage. Nanomaterials 2022, 12, 1888. [Google Scholar] [CrossRef]
- Su, Y.; Chen, G.; Chen, L.; Li, Q.; Lu, Y.; Bao, L.; Li, N.; Chen, S.; Wu, F. Advances and Prospects of Surface Modification on Nickel–Rich Materials for Lithium–Ion Batteries. Chin. J. Chem. 2019, 37, 1817–1831. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, X.; Yu, R.; Jamil, S.; Cao, S.; Fang, S.; Wang, Y.; Tang, K.; Chen, G.; Luo, Z.; et al. Preparation and Performance of the Heterostructured Material with a Ni–Rich Layered Oxide Core and a LiNi0.5Mn1.5O4–like Spinel Shell. ACS Appl. Mater. Inter. 2019, 11, 16556–16566. [Google Scholar] [CrossRef] [PubMed]
- Min, K.; Jung, C.; Ko, D.-S.; Kim, K.; Jang, J.; Park, K.; Cho, E. High–Performance and Industrially Feasible Ni–Rich Layered Cathode Materials by Integrating Coherent Interphase. ACS Appl. Mater. Inter. 2018, 10, 20599–20610. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Chen, L.; Su, Y.; Tan, J.; Bao, L.; Lu, Y.; Wang, J.; Chen, R.; Chen, S.; Wu, F. An interfacial framework for breaking through the Li−ion transport barrier of Li−rich layered cathode materials. J. Mater. Chem. A 2017, 5, 24292. [Google Scholar] [CrossRef]
- Chen, S.; Zheng, Y.; Lu, Y.; Su, Y.; Bao, L.; Li, N.; Li, Y.; Wang, J.; Chen, R.; Wu, F. Enhanced Electrochemical Performance of Layered Lithium−Rich Cathode Materials by Constructing Spinel−Structure Skin and Ferric Oxide Islands. Appl. Mater. Interfaces 2017, 9, 8669–8678. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Shi, J.; Liang, J.; Yin, Y.; Zhang, J.; Yu, X.; Guo, Y. Suppressing Surface Lattice Oxygen Release of Li−Rich Cathode Materials via Heterostructured Spinel Li4Mn5O12 Coating. Adv. Mater. 2018, 30, 1801751. [Google Scholar] [CrossRef] [PubMed]
- Park, K.; Yeon, D.; Kim, J.H.; Park, J.H.; Doo, S.; Choi, B. Spinel–embedded lithium–rich oxide composites for Li–ion batteries. J. Power Source 2017, 360, 453–459. [Google Scholar] [CrossRef]
- Freitas, R.R.Q.; de Brito Mota, F.; Rivelino, R.; de Castilho, C.M.C.; Kakanakova–Georgieva, A.; Gueorguiev, G.K. Spin–orbit–induced gap modification in buckled honeycomb XBi and XBi3 (X = B, Al, Ga, and In) sheets. J. Phys. Condens. Matter 2015, 27, 485306. [Google Scholar] [CrossRef]
- Bakoglidis, K.D.; Palisaitis, J.; dos Santos, R.B.; Rivelino, R.; Persson, P.O.Å.; Gueorgui, K.; Gueorguiev Hultman, L. Self–Healing in Carbon Nitride Evidenced As Material Inflation and Superlubric Behavior. ACS Appl. Mater. Interfaces 2018, 10, 16238–16243. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Li, N.; Su, Y.; Zhang, L.; Bao, L.; Wang, J.; Chen, L.; Zheng, Y.; Dai, L.; Peng, J. Ultrathin Spinel Membrane−Encapsulated Layered Lithium−Rich Cathode Material for Advanced Li−Ion Batteries. Nano Lett. 2014, 14, 3550–3555. [Google Scholar]
- Yu, F.-D.; Que, L.-F.; Wang, Z.-B.; Zhang, Y.; Xue, Y.; Liu, B.-S.; Gu, D.-M. Layered−spinel capped nanotube assembled 3D Li−rich hierarchitectures for high performance Li−ion battery cathodes. Mater. Chem. A 2016, 4, 18416. [Google Scholar] [CrossRef]
- Cho, Y.; Lee, S.; Lee, Y.; Hong, T.; Cho, J. Spinel–Layered Core–Shell Cathode Materials for Li–Ion Batteries. Adv. Energy Mater. 2011, 1, 821. [Google Scholar] [CrossRef]
- Park, S.-H.; Kang, S.-H.; Johnson, C.-S.; Amine, K.; Thackeray, M.M. Lithium–manganese–nickel–oxide electrodes with integrated layered–spinel structures for lithium batteries. Electrochem. Commun. 2007, 9, 262. [Google Scholar] [CrossRef]
- Lee, E.-S.; Huq, A.; Chang, H.-Y.; Manthiram, A. High–Voltage, High–Energy Layered–Spinel Composite Cathodes with Superior Cycle Life for Lithium–Ion Batteries. Chem. Mater. 2012, 24, 600. [Google Scholar] [CrossRef]
- Yin, H.; Ji, S.; Gu, M.; Zhang, L.; Liu, J. Scalable Synthesis of Li1.2Mn0.54Ni0.13Co0.13O2/LiNi0.5Mn1.5O4 Sphere Composites as Stable and High Capacity Cathode for Li–Ion. RSC Adv. 2015, 5, 84673–84679. [Google Scholar] [CrossRef]
- Liu, W.; Liu, J.; Chen, K.; Ji, S.; Wan, Y.; Zhou, Y.; Xue, D.; Hodgson, P.; Li, Y. Enhancing the Electrochemical Performance of the LiMn2O4 Hollow Microsphere Cathode with a LiNi0.5Mn1.5O4 Coated Layer. Chem. Eur. J. 2014, 20, 824–830. [Google Scholar] [CrossRef] [PubMed]
- Kawai, H.; Nagata, M.; Kageyama, H.; Tukamoto, A.R.; West, A. New Lithium Cathode LiCoMnO4: Toward Practical 5 V Lithium Batteries. Electrochem. Solid State Lett. 1998, 1, 212. [Google Scholar] [CrossRef]
- Hu, M.; Tian, Y.; Su, L.; Wei, J.; Zhou, Z. Preparation and Ni−doping effect of nanosized truncated octahedral LiCoMnO4 as cathode materials for 5 V li−ion batteries. ACS Appl. Mater. Interfaces 2013, 5, 12185–12189. [Google Scholar] [CrossRef]
- Lin, H.-F.; Tsai, Y.R.; Cheng, C.-H.; Cheng, S.T.; Chen, D.Z.; Wu, N.-Y. Structural and electrochemical properties of LiCoMnO4 doped with Mg, La, and F as a high-voltage cathode material for lithium ion batteries. Electrochim. Acta 2022, 427, 140904. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, W.; Wang, Q.; Song, X.; Qian, Y. Synthesis of porous and hollow microspheres of nanocrystalline Mn2O3. Chem. Phys. Lett. 2006, 418, 46–49. [Google Scholar] [CrossRef]
- Li, Q.; Li, G.; Fu, C.; Luo, D.; Fan, J.; Xie, D.; Li, L. Balancing stability and specific energy in Li–rich cathodes for lithium ion batteries: A case study of a novel Li–Mn–Ni–Co oxide. J. Mater. Chem. A 2015, 3, 10592–10602. [Google Scholar] [CrossRef]
- Feng, X.; Yang, Z.; Tang, D.; Kong, Q.; Gu, L.; Wang, Z.; Chen, L. Performance improvement of Li–rich layer–structured Li1.2 Mn0.54Ni0.13Co0.13O2 by integration with spinel LiNi0.5Mn1.5O4. Phys. Chem. Chem. Phys. 2015, 17, 1257–1264. [Google Scholar]
- Hong, J.; Seo, D.H.; Kim, S.W.; Gwon, H.; Oh, S.T.; Kang, K. Structural evolution of layered Li1.2Ni0.2Mn0.6O2 upon electrochemical cycling in a Li rechargeable battery. J. Mater. Chem. 2010, 20, 10179–10186. [Google Scholar] [CrossRef]
- Yu, H.; Zhou, H. High–Energy Cathode Materials (Li2MnO3–LiMO2) for Lithium–Ion Batteries. J. Phys. Chem. Lett. 2013, 4, 1268–1280. [Google Scholar] [CrossRef] [PubMed]
- Baddour-Hadjean, R.; Pereira, J.P. Raman Microspectrometry Applied to the Study of Electrode Materials for Lithium Batteries. Chem. Rev. 2010, 110, 1278–1319. [Google Scholar] [CrossRef]
- Yi, L.; Liu, Z.; Yu, R.; Zhao, C.; Peng, H.; Liu, M.; Wu, B.; Chen, M.; Li-Rich, X.W. Layered/Spinel Heterostructured Special Morphology Cathode Material with High Rate Capability for Li-Ion Batteries. ACS Sustain. Chem. Eng. 2017, 5, 11–11005. [Google Scholar] [CrossRef]
- Moses, A.W.; Flores, H.G.G.; Kim, J.G.; Langell, A.M. Surface properties of LiCoO2, LiNiO2 and LiNi1-xCoxO2. Appl. Surf. Sci. 2007, 253, 4782–4791. [Google Scholar] [CrossRef]
- Treuil, N.; Labrugère, C.; Menetrier, M.; Portier, J.; Campet, G.; Deshayes, A.; Frison, J.-C.; Hwang, S.-J.; Song, S.-W.; Choy, J.-H. Relationship between Chemical Bonding Nature and Electrochemical Property of LiMn2O4 Spinel Oxides with Various Particle Sizes: “Electrochemical Grafting” Concept. J. Phys. Chem. B 1999, 103, 2100–2106. [Google Scholar] [CrossRef]
- Peng, H.; Zhao, S.-X.; Huangv, C.; Yu, L.Q.; Fang, Z.-Q.; Guo-Dan, W. In Situ Construction of Spinel Coating on the Surface of a Lithium- Rich Manganese-Based Single Crystal for Inhibiting Voltage Fade. ACS Appl. Mater. Interfaces 2020, 12, 11579–11588. [Google Scholar] [CrossRef]
- Thackeray, M.M.; David, W.I.F.; Bruce, P.G.; Goodenough, B.J. Lithium insertion into manganese spinels. Mater. Res. Bull. 1983, 18, 461–472. [Google Scholar] [CrossRef]
- Armstrong, A.R.; Holzapfel, M.; Novak, P.; Johnson, C.S.; Kang, S.-H.; Thackeray, M.M.; Bruce, P.G. Demonstrating Oxygen Loss and Associated Structural Reorganization in the Lithium Battery Cathode Li[Ni0.2Li0.2Mn0.6]O2. J. Am. Chem. Soc. 2006, 128, 8694–8698. [Google Scholar] [CrossRef]
- Lim, J.-H.; Bang, H.; Lee, K.-S.; Amine, K.; Sun, Y.-K. Electrochemical characterization of Li2MnO3–Li[Ni1/3Co1/3Mn1/3]O2–LiNiO2 cathode synthesized via co–precipitation for lithium secondary batteries. J. Power Source 2009, 189, 571–575. [Google Scholar]
- Zheng, J.; Gu, M.; Xiao, J.; Zuo, P.; Wangv, C.; Zhang, J.-G. Corrosion/fragmentation of layered composite cathode and related capacity/voltage fading during cycling process. Nano Lett. 2013, 13, 3824. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Xiao, J.; Gu, M.; Zuo, P.; Wang, C.; Zhang, J.G. Interface modifications by anion receptors for high energy lithium ion batteries. J. Power Source 2014, 250, 313–318. [Google Scholar] [CrossRef]
- Gu, M.; Belharouak, I.; Zheng, J.; Wu, H.; Xiao, J.; Genc, A.; Amine, K.; Thevuthasan, S.; Baer, D.R.; Zhang, J.-G.; et al. Formation of the Spinel Phase in the Layered Composite Cathode Used in Li-Ion Batteries. ACS Nano 2013, 7, 760–767. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, D.; Kalnaus, S.; Meisner, R.A.; Rhodes, K.J.; Li, J.L.; Payzant, E.A.; Wood, D.L.; Daniel, C. Structural transformation of a lithium–rich Li1.2Co0.1Mn0.55Ni0.15O2 cathode during high voltage cycling resolved by in situ X–ray diffraction. J. Power Sources 2013, 229, 239. [Google Scholar] [CrossRef]
- Fell, C.R.; Chi, M.; Meng, Y.S.; Jones, J.L. In situ X–ray diffraction study of the lithium excess layered oxide compound Li[Li0.2Ni0.2Mn0.6]O2 during electrochemical cycling. Solid State Ion. 2012, 207, 44. [Google Scholar] [CrossRef]
- Qiu, B.; Zhang, M.H.; Xia, Y.G.; Liu, Z.P.; Meng, Y.S. Understanding and Controlling Anionic Electrochemical Activity in High-Capacity Oxides for Next Generation Li-Ion Batteries. Chem. Mater. 2017, 29, 908–915. [Google Scholar] [CrossRef]
- Yan, P.F.; Nie, A.; Zheng, J.M.; Zhou, Y.G.; Lu, D.P.; Zhang, X.F.; Xu, R.; Belharouak, I.; Zu, X.A.; Xiao, J.; et al. Evolution of Lattice Structure and Chemical Composition of the Surface Reconstruction Layer in Li1.2Ni0.2Mn0.6O2 Cathode Material for Lithium Ion Batteries. Nano Lett. 2015, 15, 514–522. [Google Scholar] [CrossRef]
- Ye, D.L.; Zeng, G.; Nogita, K.; Ozawa, K.; Hankel, M.; Searles, D.J.; Wang, L.Z. Understanding the Origin of Li2MnO3 Activation in Li-Rich Cathode Materials for Lithium-Ion Batteries. Adv. Funct. Mater. 2015, 25, 7488–7496. [Google Scholar] [CrossRef]
- Wu, F.; Liu, J.R.; Li, L.; Zhang, X.X.; Luo, R.; Ye, Y.S.; Chen, R.J. Surface Modification of Li-Rich Cathode Materials for Lithium-Ion Batteries with a PEDOT:PSS Conducting Polymer. ACS Appl. Mater. Interfaces 2016, 8, 23095–23104. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, H.-F.; Cheng, S.-T.; Chen, D.-Z.; Wu, N.-Y.; Jiang, Z.-X.; Chang, C.-T. Stabilizing Li-Rich Layered Cathode Materials Using a LiCoMnO4 Spinel Nanolayer for Li-Ion Batteries. Nanomaterials 2022, 12, 3425. https://doi.org/10.3390/nano12193425
Lin H-F, Cheng S-T, Chen D-Z, Wu N-Y, Jiang Z-X, Chang C-T. Stabilizing Li-Rich Layered Cathode Materials Using a LiCoMnO4 Spinel Nanolayer for Li-Ion Batteries. Nanomaterials. 2022; 12(19):3425. https://doi.org/10.3390/nano12193425
Chicago/Turabian StyleLin, Hsiu-Fen, Si-Ting Cheng, De-Zhen Chen, Nian-Ying Wu, Zong-Xiao Jiang, and Chun-Ting Chang. 2022. "Stabilizing Li-Rich Layered Cathode Materials Using a LiCoMnO4 Spinel Nanolayer for Li-Ion Batteries" Nanomaterials 12, no. 19: 3425. https://doi.org/10.3390/nano12193425
APA StyleLin, H.-F., Cheng, S.-T., Chen, D.-Z., Wu, N.-Y., Jiang, Z.-X., & Chang, C.-T. (2022). Stabilizing Li-Rich Layered Cathode Materials Using a LiCoMnO4 Spinel Nanolayer for Li-Ion Batteries. Nanomaterials, 12(19), 3425. https://doi.org/10.3390/nano12193425




