Dynamic Fluid Flow Exacerbates the (Pro-)Inflammatory Effects of Aerosolised Engineered Nanomaterials In Vitro
Abstract
1. Introduction
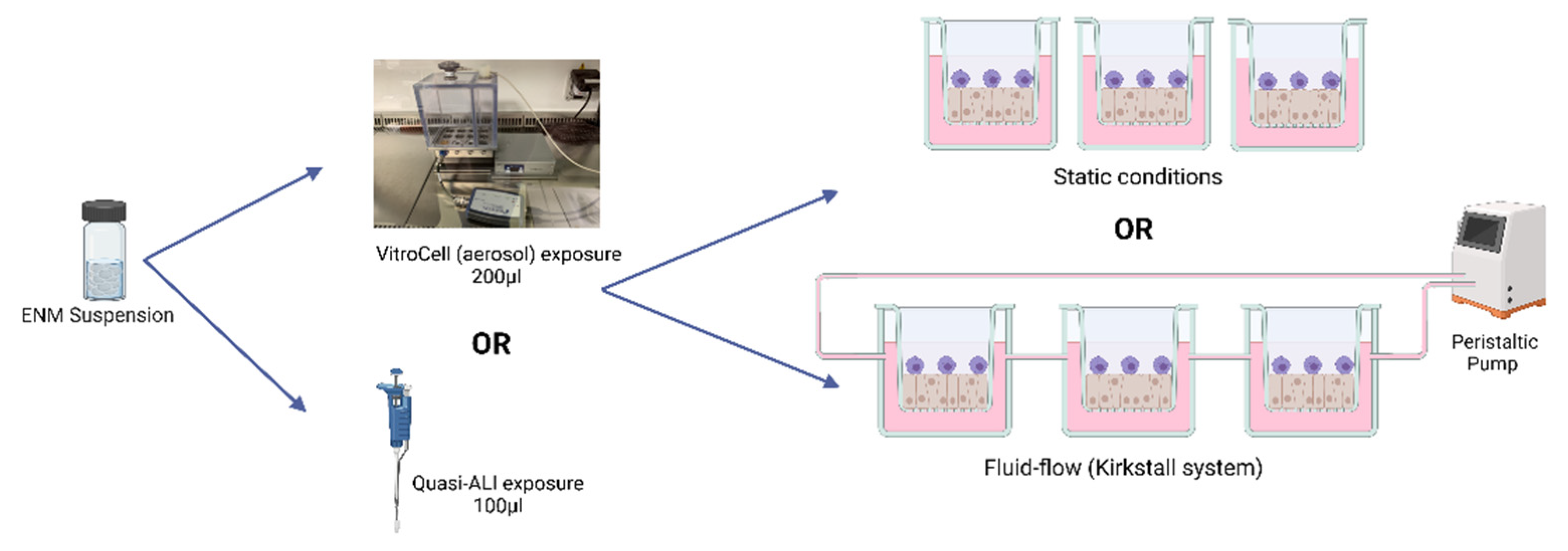
2. Materials and Methods
- Cell Cultures
- Fluid Flow System
- Characterisation
- ENP
| Z-Average (nm) | Polydispersity Index (PDI) | BET Surface m2/g | |
|---|---|---|---|
| TiO2 | 125.4 | 0.171 | 46.175 |
| DQ12 | 720 | 0.52 | 10.1 |
- Particle Exposures
- Biochemical Analysis
- Trypan Blue Exclusion Assay
- Blue Dextran—Membrane Integrity Analysis
- (Pro-)Inflammatory Response
- Statistical Analysis
3. Results
3.1. Model Characterisation
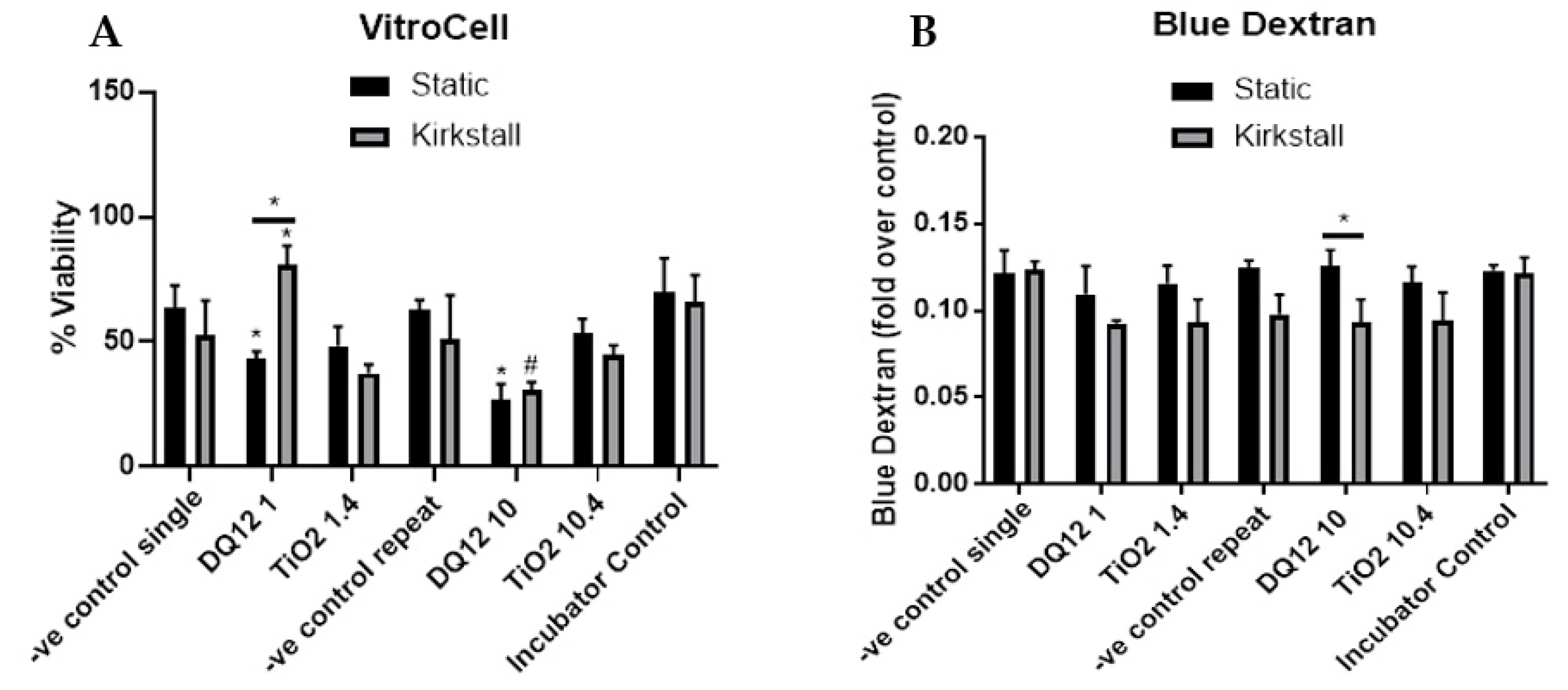
3.2. Quasi-ALI Exposure
- Viability and Membrane Integrity
- (Pro-)Inflammatory Response
3.3. Aerosol Exposure
- Viability and Membrane Integrity
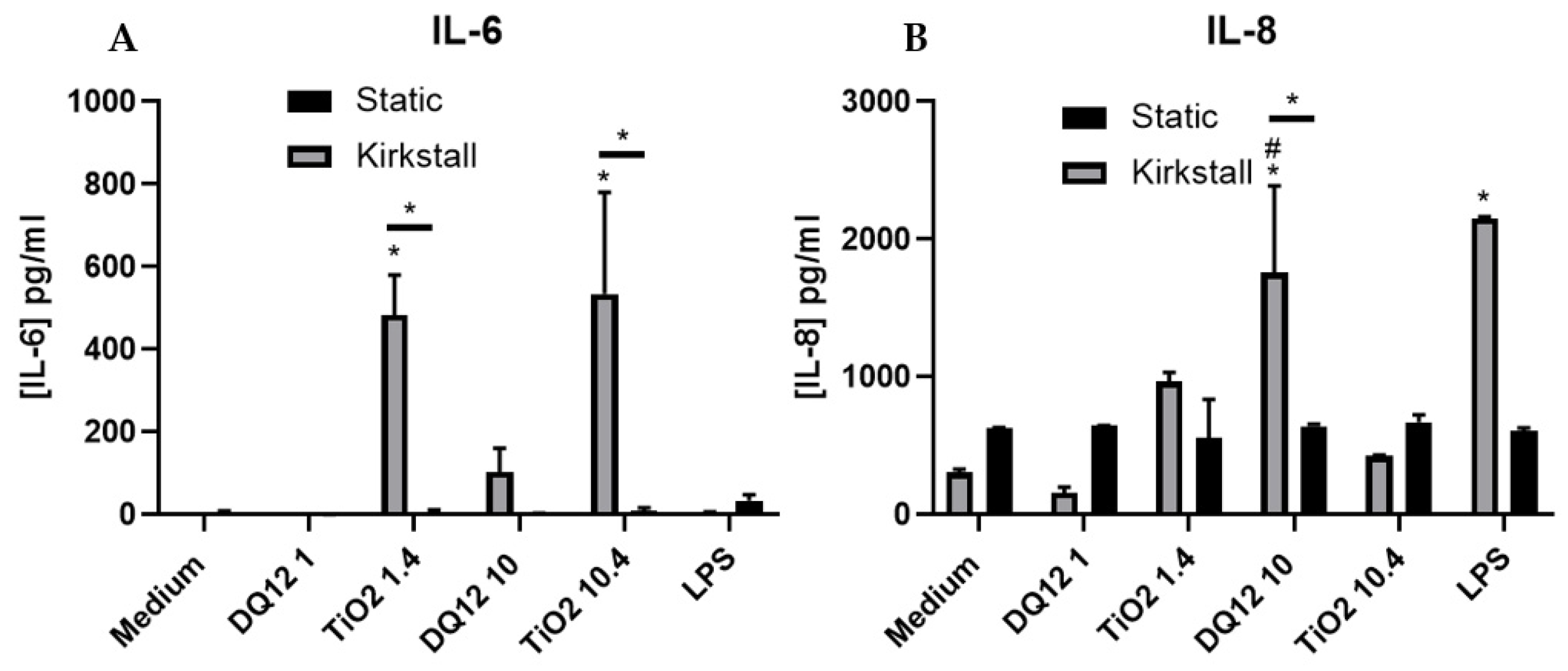
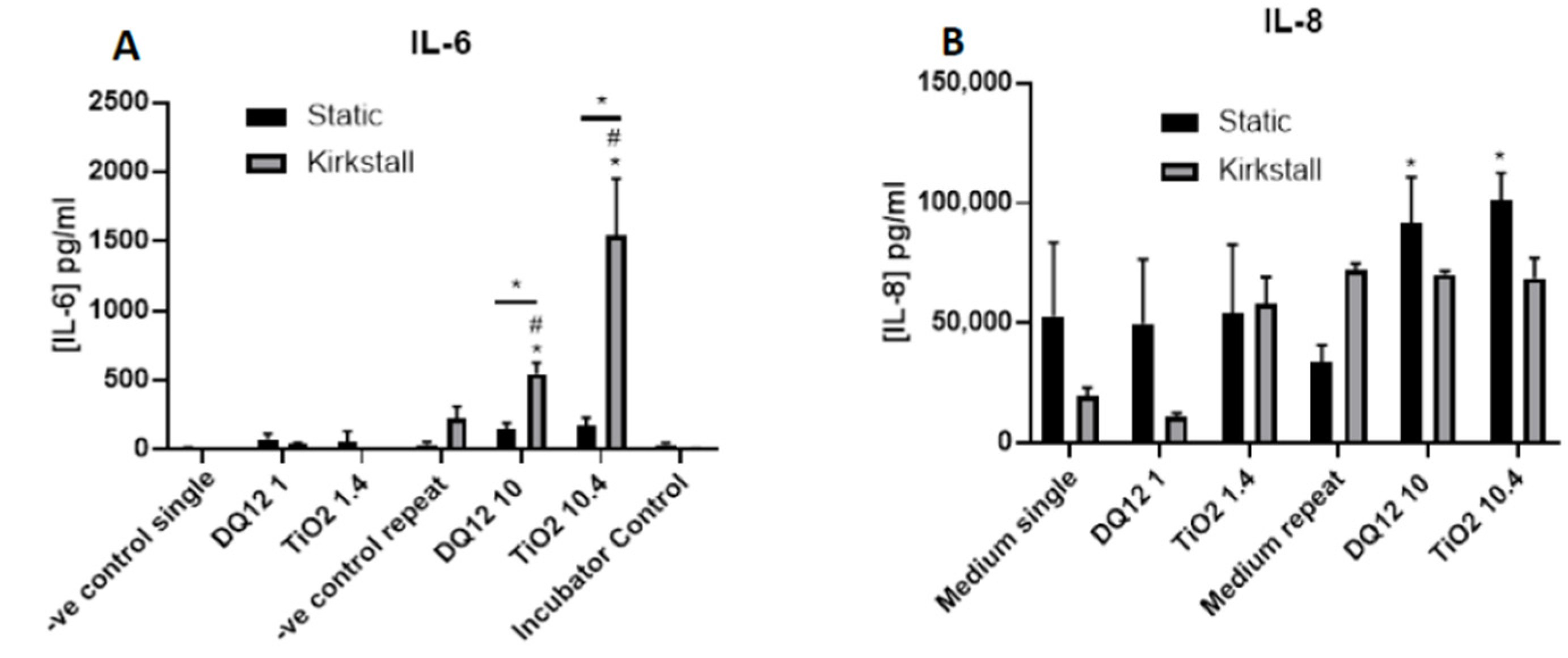
- (Pro-)inflammatory Response
4. Discussion
4.1. Addition of Dynamic Fluid Flow
4.2. Quasi-ALI and Aerosol Exposures
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sambandam, B.; Palanisami, E.; Abbugounder, R.; Prakhya, B.; Thiyagarajan, D. Characterizations of coal fly ash nanoparticles and induced in vitro toxicity in cell lines. J. Nanoparticle Res. 2014, 16, 2217. [Google Scholar] [CrossRef]
- Horie, M.; Nishio, K.; Kato, H.; Fujita, K.; Endoh, S.; Nakamura, A.; Miyauchi, A.; Kinugasa, S.; Yamamoto, K.; Niki, E.; et al. Cellular responses induced by cerium oxide nanoparticles: Induction of intracellular calcium level and oxidative stress on culture cells. J. Biochem. 2011, 150, 461–471. [Google Scholar] [CrossRef] [PubMed]
- Vert, M.; Doi, Y.; Hellwich, K.-H.; Hess, M.; Hodge, P.; Kubisa, P.; Rinaudo, M.; Schué, F. Terminology for biorelated polymers and applications (IUPAC Recommendations 2012). Pure Appl. Chem. 2012, 84, 377–410. [Google Scholar] [CrossRef]
- Hackenberg, S.; Scherzed, A.; Technau, A.; Kessler, M.; Froelich, K.; Ginzkey, C.; Koehler, C.; Burghartz, M.; Hagen, R.; Kleinsasser, N. Cytotoxic, genotoxic and pro-inflammatory effects of zinc oxide nanoparticles in human nasal mucosa cells in vitro. Toxicol. Vitr. 2011, 25, 657–663. [Google Scholar] [CrossRef]
- Duffin, R.; Tran, L.; Brown, D.; Stone, V.; Donaldson, K. Proinflammogenic effects of low-toxicity and metal nanoparticles in vivo and in vitro: Highlighting the role of particle surface area and surface reactivity. Inhal. Toxicol. 2007, 19, 849–856. [Google Scholar] [CrossRef]
- Schmid, O.; Stoeger, T. Surface area is the biologically most effective dose metric for acute nanoparticle toxicity in the lung. J. Aerosol Sci. 2016, 99, 133–143. [Google Scholar] [CrossRef]
- Skuland, T.; Ovrevik, J.; Låg, M.; Refsnes, M. Role of size and surface area for pro-inflammatory responses to silica nanoparticles in epithelial lung cells: Importance of exposure conditions. Toxicol. Vitr. Int. J. Publ. Assoc. BIBRA 2014, 28, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Noël, A.; Truchon, G.; Cloutier, Y.; Charbonneau, M.; Maghni, K.; Tardif, R. Mass or total surface area with aerosol size distribution as exposure metrics for inflammatory, cytotoxic and oxidative lung responses in rats exposed to titanium dioxide nanoparticles. Toxicol. Ind. Health 2016, 33, 351–364. [Google Scholar] [CrossRef]
- Kuhn, D.A.; Vanhecke, D.; Michen, B.; Blank, F.; Gehr, P.; Petri-Fink, A.; Rothen-Rutishauser, B. Different endocytotic uptake mechanisms for nanoparticles in epithelial cells and macrophages. Beilstein J. Nanotechnol. 2014, 5, 1625–1636. [Google Scholar] [CrossRef]
- Yokel, R.A.; MacPhail, R.C. Engineered nanomaterials: Exposures, hazards, and risk prevention. J. Occup. Med. Toxicol. 2011, 6, 7. [Google Scholar] [CrossRef]
- Chen, P.; Zhang, Z.; Gu, N.; Ji, M. Effect of the surface charge density of nanoparticles on their translocation across pulmonary surfactant monolayer: A molecular dynamics simulation. Mol. Simul. 2018, 44, 85–93. [Google Scholar] [CrossRef]
- Fulcher, M.L.; Randell, S.H. Human nasal and tracheo-bronchial respiratory epithelial cell culture. Methods Mol. Biol. 2013, 945, 109–121. [Google Scholar] [CrossRef]
- Ou, C.; Hang, J.; Deng, Q. Particle Deposition in Human Lung Airways: Effects of Airflow, Particle Size, and Mechanisms. Aerosol Air Qual. Res. 2020, 20, 2846–2858. [Google Scholar] [CrossRef]
- Tsuda, A.; Henry, F.S.; Butler, J.P. Particle transport and deposition: Basic physics of particle kinetics. Compr. Physiol. 2013, 3, 1437–1471. [Google Scholar]
- Ehrhardt, C.; Laue, M.; Kim, K.-J. In Vitro Models of the Alveolar Epithelial Barrier. In Drug Absorption Studies: In Situ, In vitro and In Silico Models; Ehrhardt, C., Kim, K.-J., Eds.; Springer: Boston, MA, USA, 2008; pp. 258–282. [Google Scholar] [CrossRef]
- Cooper, J.R.; Abdullatif, M.B.; Burnett, E.C.; Kempsell, K.E.; Conforti, F.; Tolley, H.; Collins, J.E.; Davies, D.E. Long Term Culture of the A549 Cancer Cell Line Promotes Multilamellar Body Formation and Differentiation towards an Alveolar Type II Pneumocyte Phenotype. PLoS ONE 2016, 11, e0164438. [Google Scholar] [CrossRef]
- Crapo, J.D.; Barry, B.E.; Gehr, P.; Bachofen, M.; Weibel, E.R. Cell number and cell characteristics of the normal human lung. Am. Rev. Respir. Dis. 1982, 126, 332–337. [Google Scholar]
- Stone, K.C.; Mercer, R.R.; Gehr, P.; Stockstill, B.; Crapo, J.D. Allometric relationships of cell numbers and size in the mammalian lung. Am. J. Respir. Cell Mol. Biol. 1992, 6, 235–243. [Google Scholar] [CrossRef]
- Chang, L.Y.; Crapo, J.D.; Gehr, P.; Rothen-Rutishauser, B.; Mühfeld, C.; Blank, F. 8.04—Alveolar Epithelium in Lung Toxicology. In Comprehensive Toxicology, 2nd ed.; McQueen, C.A., Ed.; Elsevier: Oxford, UK, 2010; pp. 59–91. [Google Scholar] [CrossRef]
- Franken, L.; Schiwon, M.; Kurts, C. Macrophages: Sentinels and regulators of the immune system. Cell. Microbiol. 2016, 18, 475–487. [Google Scholar] [CrossRef]
- Joshi, N.; Walter, J.M.; Misharin, A.V. Alveolar Macrophages. Cell. Immunol. 2018, 330, 86–90. [Google Scholar] [CrossRef]
- Holownia, A.; Wielgat, P.; Kwolek, A.; Jackowski, K.; Braszko, J.J. Crosstalk Between Co-cultured A549 Cells and THP1 Cells Exposed to Cigarette Smoke. Adv. Exp. Med. Biol. 2015, 858, 47–55. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, X.; Liu, X.; Zheng, J. Co-culture of human alveolar epithelial (A549) and macrophage (THP-1) cells to study the potential toxicity of ambient PM(2.5): A comparison of growth under ALI and submerged conditions. Toxicol. Res. (Camb) 2020, 9, 636–651. [Google Scholar] [CrossRef]
- Meindl, C.; Öhlinger, K.; Zrim, V.; Steinkogler, T.; Fröhlich, E. Screening for Effects of Inhaled Nanoparticles in Cell Culture Models for Prolonged Exposure. Nanomaterials 2021, 11, 606. [Google Scholar] [CrossRef]
- Meldrum, K.; Evans, S.J.; Vogel, U.; Tran, L.; Doak, S.H.; Clift, M.J.D. The influence of exposure approaches to in vitro lung epithelial barrier models to assess engineered nanomaterial hazard. Nanotoxicology 2022, 16, 114–134. [Google Scholar] [CrossRef]
- Kasurinen, S.; Happo, M.S.; Rönkkö, T.; Orasche, J.; Jokiniemi, J.; Kortelainen, M.; Tissari, J.; Zimmermann, R.; Hirvonen, M.-R.; Jalava, P.I. Differences between co-cultures and monocultures in testing the toxicity of particulate matter derived from log wood and pellet combustion. PLoS ONE 2018, 13, e0192453. [Google Scholar] [CrossRef]
- Wang, Y.; Adamcakova-Dodd, A.; Steines, B.R.; Jing, X.; Salem, A.K.; Thorne, P.S. Comparison of in vitro toxicity of aerosolized engineered nanomaterials using air-liquid interface mono-culture and co-culture models. NanoImpact 2020, 18, 100215. [Google Scholar] [CrossRef]
- Clippinger, A.J.; Ahluwalia, A.; Allen, D.; Bonner, J.C.; Casey, W.; Castranova, V.; David, R.M.; Halappanavar, S.; Hotchkiss, J.A.; Jarabek, A.M.; et al. Expert consensus on an in vitro approach to assess pulmonary fibrogenic potential of aerosolized nanomaterials. Arch. Toxicol. 2016, 90, 1769–1783. [Google Scholar] [CrossRef]
- Vuong, N.Q.; Breznan, D.; Goegan, P.; O’Brien, J.S.; Williams, A.; Karthikeyan, S.; Kumarathasan, P.; Vincent, R. In vitro toxicoproteomic analysis of A549 human lung epithelial cells exposed to urban air particulate matter and its water-soluble and insoluble fractions. Part. Fibre Toxicol. 2017, 14, 39. [Google Scholar] [CrossRef]
- Zhang, Y.; Darland, D.; He, Y.; Yang, L.; Dong, X.; Chang, Y. Reduction of Pm2.5 Toxicity on Human Alveolar Epithelial Cells A549 by Tea Polyphenols. J. Food Biochem. 2018, 42, e12496. [Google Scholar] [CrossRef]
- Griese, M. Pulmonary surfactant in health and human lung diseases: State of the art. Eur. Respir. J. 1999, 13, 1455–1476. [Google Scholar] [CrossRef]
- Blank, F.; Rothen-Rutishauser, B.M.; Schurch, S.; Gehr, P. An optimized in vitro model of the respiratory tract wall to study particle cell interactions. J. Aerosol Med. 2006, 19, 392–405. [Google Scholar] [CrossRef]
- Bruce, S.R.; Atkins, C.L.; Colasurdo, G.N.; Alcorn, J.L. Respiratory syncytial virus infection alters surfactant protein A expression in human pulmonary epithelial cells by reducing translation efficiency. Am. J. Physiol. Lung Cell. Mol. Physiol. 2009, 297, L559–L567. [Google Scholar] [CrossRef]
- Hiemstra, P.S.; Grootaers, G.; van der Does, A.M.; Krul, C.A.; Kooter, I.M. Human lung epithelial cell cultures for analysis of inhaled toxicants: Lessons learned and future directions. Toxicol. In Vitro 2018, 47, 137–146. [Google Scholar] [CrossRef]
- Lacroix, G.; Koch, W.; Ritter, D.; Gutleb, A.C.; Larsen, S.T.; Loret, T.; Zanetti, F.; Constant, S.; Chortarea, S.; Rothen-Rutishauser, B.; et al. Air–Liquid Interface In Vitro Models for Respiratory Toxicology Research: Consensus Workshop and Recommendations. Appl. In Vitro Toxicol. 2018, 4, 91–106. [Google Scholar] [CrossRef]
- Upadhyay, S.; Palmberg, L. Air-Liquid Interface: Relevant In Vitro Models for Investigating Air Pollutant-Induced Pulmonary Toxicity. Toxicol. Sci. 2018, 164, 21–30. [Google Scholar] [CrossRef]
- Romagnoli, S.; Roncalli, M.; Graziani, D.; Cassani, B.; Roz, E.; Bonavina, L.; Peracchia, A.; Bosari, S.; Coggi, G. Molecular alterations of Barrett’s esophagus on microdissected endoscopic biopsies. Lab. Investig. 2001, 81, 241–247. [Google Scholar] [CrossRef][Green Version]
- Öhlinger, K.; Kolesnik, T.; Meindl, C.; Gallé, B.; Absenger-Novak, M.; Kolb-Lenz, D.; Fröhlich, E. Air-liquid interface culture changes surface properties of A549 cells. Toxicol. In Vitro 2019, 60, 369–382. [Google Scholar] [CrossRef]
- Endes, C.; Schmid, O.; Kinnear, C.; Mueller, S.; Camarero-Espinosa, S.; Vanhecke, D.; Foster, E.J.; Petri-Fink, A.; Rothen-Rutishauser, B.; Weder, C.; et al. An in vitro testing strategy towards mimicking the inhalation of high aspect ratio nanoparticles. Part. Fibre Toxicol. 2014, 11, 40. [Google Scholar] [CrossRef]
- Ding, Y.; Weindl, P.; Lenz, A.G.; Mayer, P.; Krebs, T.; Schmid, O. Quartz crystal microbalances (QCM) are suitable for real-time dosimetry in nanotoxicological studies using VITROCELL®Cloud cell exposure systems. Part. Fibre Toxicol. 2020, 17, 44. [Google Scholar] [CrossRef]
- Hein, S.; Bur, M.; Kolb, T.; Muellinger, B.; Schaefer, U.F.; Lehr, C.-M. The Pharmaceutical Aerosol Deposition Device on Cell Cultures (PADDOCC) In Vitro System: Design and Experimental Protocol. Altern. Lab. Anim. 2010, 38, 285–295. [Google Scholar] [CrossRef]
- Pasman, T.; Baptista, D.; van Riet, S.; Truckenmüller, R.; Hiemstra, P.; Rottier, R.; Hamelmann, N.; Paulusse, J.; Stamatialis, D.; Poot, A. Development of an In Vitro Airway Epithelial-Endothelial Cell Culture Model on a Flexible Porous Poly(Trimethylene Carbonate) Membrane Based on Calu-3 Airway Epithelial Cells and Lung Microvascular Endothelial Cells. Membranes 2021, 11, 197. [Google Scholar] [CrossRef]
- Nossa, R.; Costa, J.; Cacopardo, L.; Ahluwalia, A. Breathing in vitro: Designs and applications of engineered lung models. J. Tissue Eng. 2021, 12, 20417314211008696. [Google Scholar] [CrossRef]
- Cei, D.; Doryab, A.; Lenz, A.; Schröppel, A.; Mayer, P.; Burgstaller, G.; Nossa, R.; Ahluwalia, A.; Schmid, O. Development of a dynamic in vitro stretch model of the alveolar interface with aerosol delivery. Biotechnol. Bioeng. 2021, 118, 690–702. [Google Scholar] [CrossRef]
- Mattei, G.; Giusti, S.; Ahluwalia, A. Design Criteria for Generating Physiologically Relevant In Vitro Models in Bioreactors. Processes 2014, 2, 548–569. [Google Scholar] [CrossRef]
- Doryab, A.; Taskin, M.B.; Stahlhut, P.; Schröppel, A.; Orak, S.; Voss, C.; Ahluwalia, A.; Rehberg, M.; Hilgendorff, A.; Stöger, T.; et al. A Bioinspired in vitro Lung Model to Study Particokinetics of Nano-/Microparticles Under Cyclic Stretch and Air-Liquid Interface Conditions. Front. Bioeng. Biotechnol. 2021, 9, 616830. [Google Scholar] [CrossRef]
- Ferroni, M.; Giusti, S.; Nascimento, D.; Silva, A.; Boschetti, F.; Ahluwalia, A. Modeling the fluid-dynamics and oxygen consumption in a porous scaffold stimulated by cyclic squeeze pressure. Med. Eng. Phys. 2016, 38, 725–732. [Google Scholar] [CrossRef]
- Huh, D.D. A human breathing lung-on-a-chip. Ann. Am. Thorac. Soc. 2015, 12 (Suppl. 1), S42–S44. [Google Scholar] [CrossRef]
- Barosova, H.; Meldrum, K.; Karakocak, B.B.; Balog, S.; Doak, S.H.; Petri-Fink, A.; Clift, M.J.D.; Rothen-Rutishauser, B. Inter-laboratory variability of A549 epithelial cells grown under submerged and air-liquid interface conditions. Toxicol. In Vitro 2021, 75, 105178. [Google Scholar] [CrossRef]
- Herzog, F.; Loza, K.; Balog, S.; Clift, M.J.; Epple, M.; Gehr, P.; Petri-Fink, A.; Rothen-Rutishauser, B. Mimicking exposures to acute and lifetime concentrations of inhaled silver nanoparticles by two different in vitro approaches. Beilstein J. Nanotechnol. 2014, 5, 1357–1370. [Google Scholar] [CrossRef]
- Herzog, F.; Clift, M.J.D.; Piccapietra, F.; Behra, R.; Schmid, O.; Petri-Fink, A.; Rothen-Rutishauser, B. Exposure of silver-nanoparticles and silver-ions to lung cells in vitro at the air-liquid interface. Part. Fibre Toxicol. 2013, 10, 11. [Google Scholar] [CrossRef]
- Klein, S.G.; Serchi, T.; Hoffmann, L.; Blmeke, B.; Gutleb, A.C. An improved 3D tetraculture system mimicking the cellular organisation at the alveolar barrier to study the potential toxic effects of particles on the lung. Part. Fibre Toxicol. 2013, 10, 31. [Google Scholar] [CrossRef]
- Centre, J.R.; Health, I.f.; Protection, C.; Rasmussen, K.; Mast, J.; De Temmerman, P.; Verleysen, E.; Waegeneers, N.; Van Steen, F.; Pizzolon, J.; et al. Titanium Dioxide, NM-100, NM-101, NM-102, NM-103, NM-104, NM-105: Characterisation and Physico-Chemical Properties; Publications Office of the European Union: Luxembourg, 2014. [Google Scholar] [CrossRef]
- Clouter, A.; Brown, D.; Höhr, D.; Borm, P.; Donaldson, K. Inflammatory effects of respirable quartz collected in workplaces versus standard DQ12 quartz: Particle surface correlates. Toxicol. Sci. Off. J. Soc. Toxicol. 2001, 63, 90–98. [Google Scholar] [CrossRef]
- Robock, K. Standard quartz dq12 greater than 5 micro m for experimental pneumoconiosis research projects in the Federal Republic of Germany. Ann. Occup. Hyg. 1973, 16, 63–66. [Google Scholar] [CrossRef]
- Maciaszek, K.; Brown, D.M.; Stone, V. An in vitro assessment of the toxicity of two-dimensional synthetic and natural layered silicates. Toxicol. In vitro 2022, 78, 105273. [Google Scholar] [CrossRef]
- Geiser, M.; Kreyling, W.G. Deposition and biokinetics of inhaled nanoparticles. Part. Fibre Toxicol. 2010, 7, 2. [Google Scholar] [CrossRef]
- Zavala, J.; Greenan, R.; Krantz, Q.T.; DeMarini, D.M.; Higuchi, M.; Gilmour, M.I.; White, P.A. Regulating temperature and relative humidity in air-liquid interface in vitro systems eliminates cytotoxicity resulting from control air exposures. Toxicol. Res. (Camb) 2017, 6, 448–459. [Google Scholar] [CrossRef]
- Miranda-Azpiazu, P.; Panagiotou, S.; Jose, G.; Saha, S. A novel dynamic multicellular co-culture system for studying individual blood-brain barrier cell types in brain diseases and cytotoxicity testing. Sci. Rep. 2018, 8, 8784. [Google Scholar] [CrossRef]
- Giusti, S.; Sbrana, T.; La Marca, M.; Di Patria, V.; Martinucci, V.; Tirella, A.; Domenici, C.; Ahluwalia, A. A novel dual-flow bioreactor simulates increased fluorescein permeability in epithelial tissue barriers. Biotechnol. J. 2014, 9, 1175–1184. [Google Scholar] [CrossRef]
- Chandorkar, P.; Posch, W.; Zaderer, V.; Blatzer, M.; Steger, M.; Ammann, C.G.; Binder, U.; Hermann, M.; Hörtnagl, P.; Lass-Flörl, C.; et al. Fast-track development of an in vitro 3D lung/immune cell model to study Aspergillus infections. Sci. Rep. 2017, 7, 11644. [Google Scholar] [CrossRef]
- Bickel, M. The role of interleukin-8 in inflammation and mechanisms of regulation. J. Periodontol. 1993, 64, 456–460. [Google Scholar]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef]
- Rincon, M.; Irvin, C.G. Role of IL-6 in asthma and other inflammatory pulmonary diseases. Int. J. Biol. Sci. 2012, 8, 1281–1290. [Google Scholar] [CrossRef]
- Smith, K.R.; Veranth, J.M.; Hu, A.A.; Lighty, J.S.; Aust, A.E. Interleukin-8 levels in human lung epithelial cells are increased in response to coal fly ash and vary with the bioavailability of iron, as a function of particle size and source of coal. Chem. Res. Toxicol. 2000, 13, 118–125. [Google Scholar] [CrossRef]
- Hirano, T. IL-6 in inflammation, autoimmunity and cancer. Int. Immunol. 2021, 33, 127–148. [Google Scholar] [CrossRef]
- Petrache, I.; Serban, K. Lost in Trans-IL-6 Signaling: Alveolar Type II Cell Death in Emphysema. Am. J. Respir. Crit. Care Med. 2016, 194, 1441–1443. [Google Scholar] [CrossRef]
- Kida, H.; Yoshida, M.; Hoshino, S.; Inoue, K.; Yano, Y.; Yanagita, M.; Kumagai, T.; Osaki, T.; Tachibana, I.; Saeki, Y.; et al. Protective effect of IL-6 on alveolar epithelial cell death induced by hydrogen peroxide. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2005, 288, L342–L349. [Google Scholar] [CrossRef]
- Diabate, S.; Armand, L.; Murugadoss, S.; Dilger, M.; Fritsch-Decker, S.; Schlager, C.; Beal, D.; Arnal, M.E.; Biola-Clier, M.; Ambrose, S.; et al. Air-Liquid Interface Exposure of Lung Epithelial Cells to Low Doses of Nanoparticles to Assess Pulmonary Adverse Effects. Nanomater 2020, 11, 65. [Google Scholar] [CrossRef]
- Medina-Reyes, E.I.; Delgado-Buenrostro, N.L.; Leseman, D.L.; Deciga-Alcaraz, A.; He, R.; Gremmer, E.R.; Fokkens, P.H.B.; Flores-Flores, J.O.; Cassee, F.R.; Chirino, Y.I. Differences in cytotoxicity of lung epithelial cells exposed to titanium dioxide nanofibers and nanoparticles: Comparison of air-liquid interface and submerged cell cultures. Toxicol. In Vitro 2020, 65, 104798. [Google Scholar] [CrossRef]
- Panas, A.; Comouth, A.; Saathoff, H.; Leisner, T.; Al-Rawi, M.; Simon, M.; Seemann, G.; Dössel, O.; Mülhopt, S.; Paur, H.-R.; et al. Silica nanoparticles are less toxic to human lung cells when deposited at the air-liquid interface compared to conventional submerged exposure. Beilstein J. Nanotechnol. 2014, 5, 1590–1602. [Google Scholar] [CrossRef]
- Loven, K.; Dobric, J.; Bolukbas, D.A.; Karedal, M.; Tas, S.; Rissler, J.; Wagner, D.E.; Isaxon, C. Toxicological effects of zinc oxide nanoparticle exposure: An in vitro comparison between dry aerosol air-liquid interface and submerged exposure systems. Nanotoxicology 2021, 15, 494–510. [Google Scholar] [CrossRef]
- Loret, T.; Peyret, E.; Dubreuil, M.; Aguerre-Chariol, O.; Bressot, C.; le Bihan, O.; Amodeo, T.; Trouiller, B.; Braun, A.; Egles, C.; et al. Air-liquid interface exposure to aerosols of poorly soluble nanomaterials induces different biological activation levels compared to exposure to suspensions. Part. Fibre Toxicol. 2016, 13, 58. [Google Scholar] [CrossRef]
- Raemy, D.O.; Grass, R.N.; Stark, W.J.; Schumacher, C.M.; Clift, M.J.; Gehr, P.; Rothen-Rutishauser, B. Effects of flame made zinc oxide particles in human lung cells—A comparison of aerosol and suspension exposures. Part. Fibre Toxicol. 2012, 9, 33. [Google Scholar] [CrossRef]
- Ozbey, G.; Gorczynski, R.; Erin, N. Stability of cytokines in supernatants of stimulated mouse immune cells. Eur. Cytokine Netw. 2014, 25, 30–34. [Google Scholar] [CrossRef]

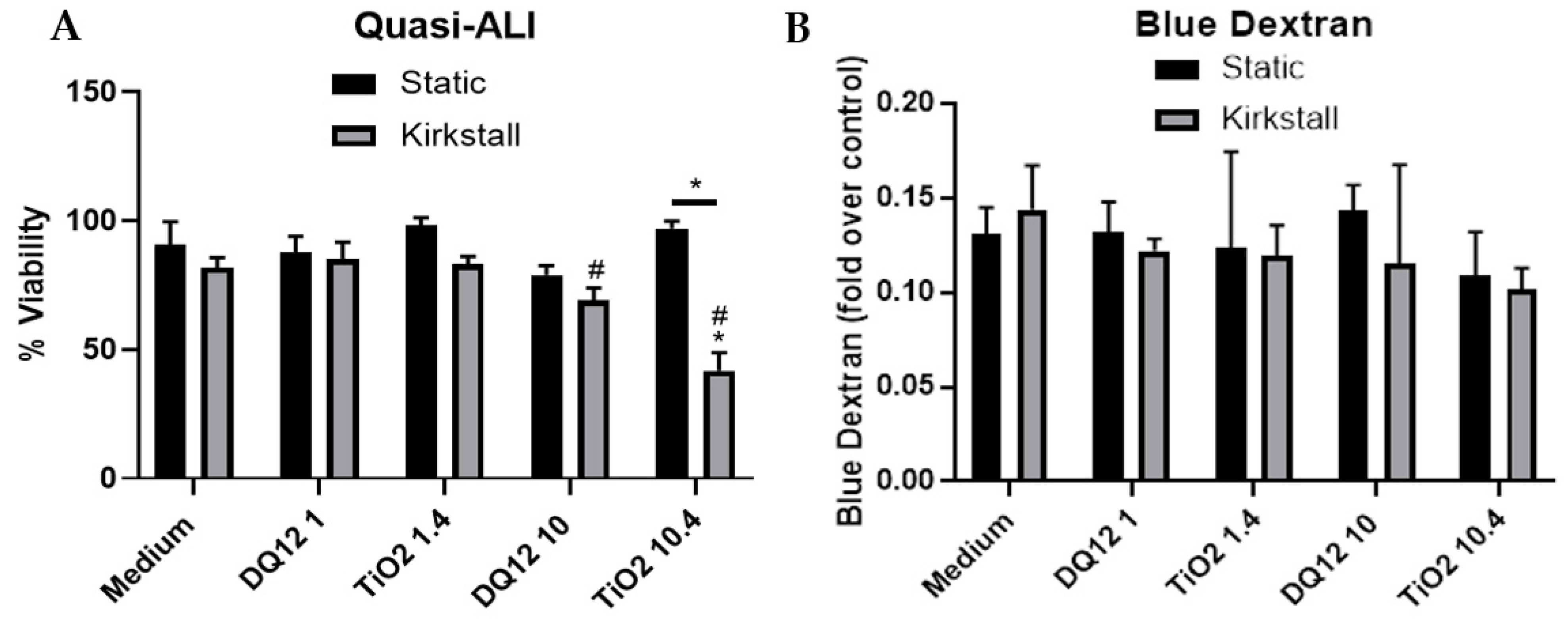
| Quasi-ALI | Aerosol | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Static | Kirkstall | Static | Kirkstall | ||||||||||
| Viability | IL-8 | IL-6 | Viability | IL-8 | IL-6 | Viability | IL-8 | IL-6 | Viability | IL-8 | IL-6 | ||
| DQ12 | 1 | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | ↓*$ | ↔ | ↔ | ↑ | ↔ | ↔ |
| 10 | ↔ | ↓* | ↔ | ↔ | ↑#$ | ↑ | ↓$ | ↑$ | ↓* | ↓# | ↔ | ↑#$ | |
| TiO2 | 1.4 | ↔ | ↔ | ↓* | ↓# | ↔ | ↑$ | ↓ | ↔ | ↔ | ↓ | ↑ | ↔ |
| 10.4 | ↑* | ↔ | ↓* | ↓#$ | ↔ | ↑$ | ↓ | ↑$ | ↓* | ↓ | ↔ | ↑#$ | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meldrum, K.; Moura, J.A.; Doak, S.H.; Clift, M.J.D. Dynamic Fluid Flow Exacerbates the (Pro-)Inflammatory Effects of Aerosolised Engineered Nanomaterials In Vitro. Nanomaterials 2022, 12, 3431. https://doi.org/10.3390/nano12193431
Meldrum K, Moura JA, Doak SH, Clift MJD. Dynamic Fluid Flow Exacerbates the (Pro-)Inflammatory Effects of Aerosolised Engineered Nanomaterials In Vitro. Nanomaterials. 2022; 12(19):3431. https://doi.org/10.3390/nano12193431
Chicago/Turabian StyleMeldrum, Kirsty, Joana A. Moura, Shareen H. Doak, and Martin J. D. Clift. 2022. "Dynamic Fluid Flow Exacerbates the (Pro-)Inflammatory Effects of Aerosolised Engineered Nanomaterials In Vitro" Nanomaterials 12, no. 19: 3431. https://doi.org/10.3390/nano12193431
APA StyleMeldrum, K., Moura, J. A., Doak, S. H., & Clift, M. J. D. (2022). Dynamic Fluid Flow Exacerbates the (Pro-)Inflammatory Effects of Aerosolised Engineered Nanomaterials In Vitro. Nanomaterials, 12(19), 3431. https://doi.org/10.3390/nano12193431







