Negatively Charged Composite Nanofibrous Hydrogel Membranes for High-Performance Protein Adsorption
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
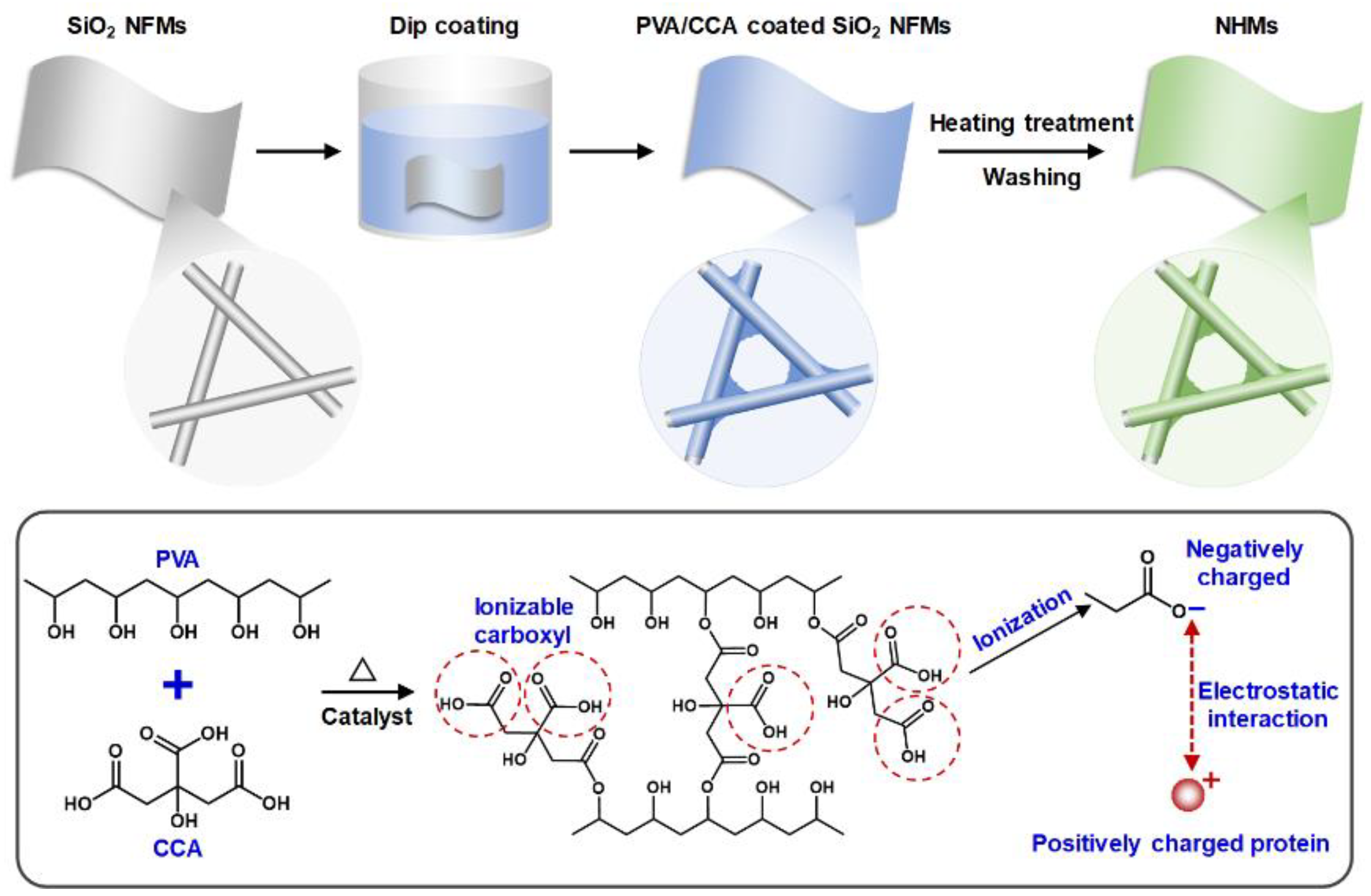
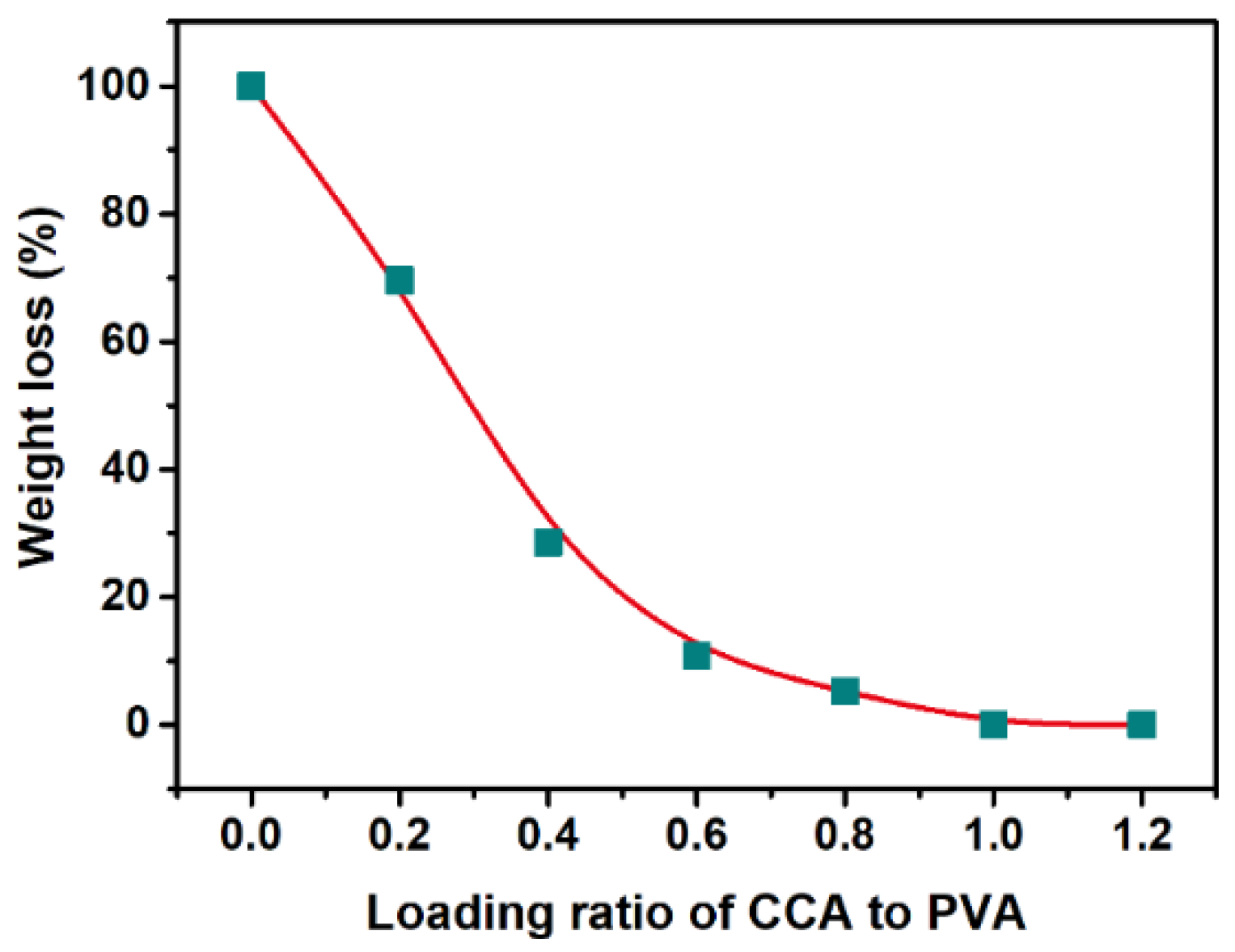
2.2. Fabrication of NHMs
2.3. Measurements of Protein Adsorption Capability
2.4. Kinetic and Isothermal Adsorption Process Analysis of the NHMs
2.5. Measurements of Influence Factors on the Performance of NHMs
2.6. Instruments and Characterizations
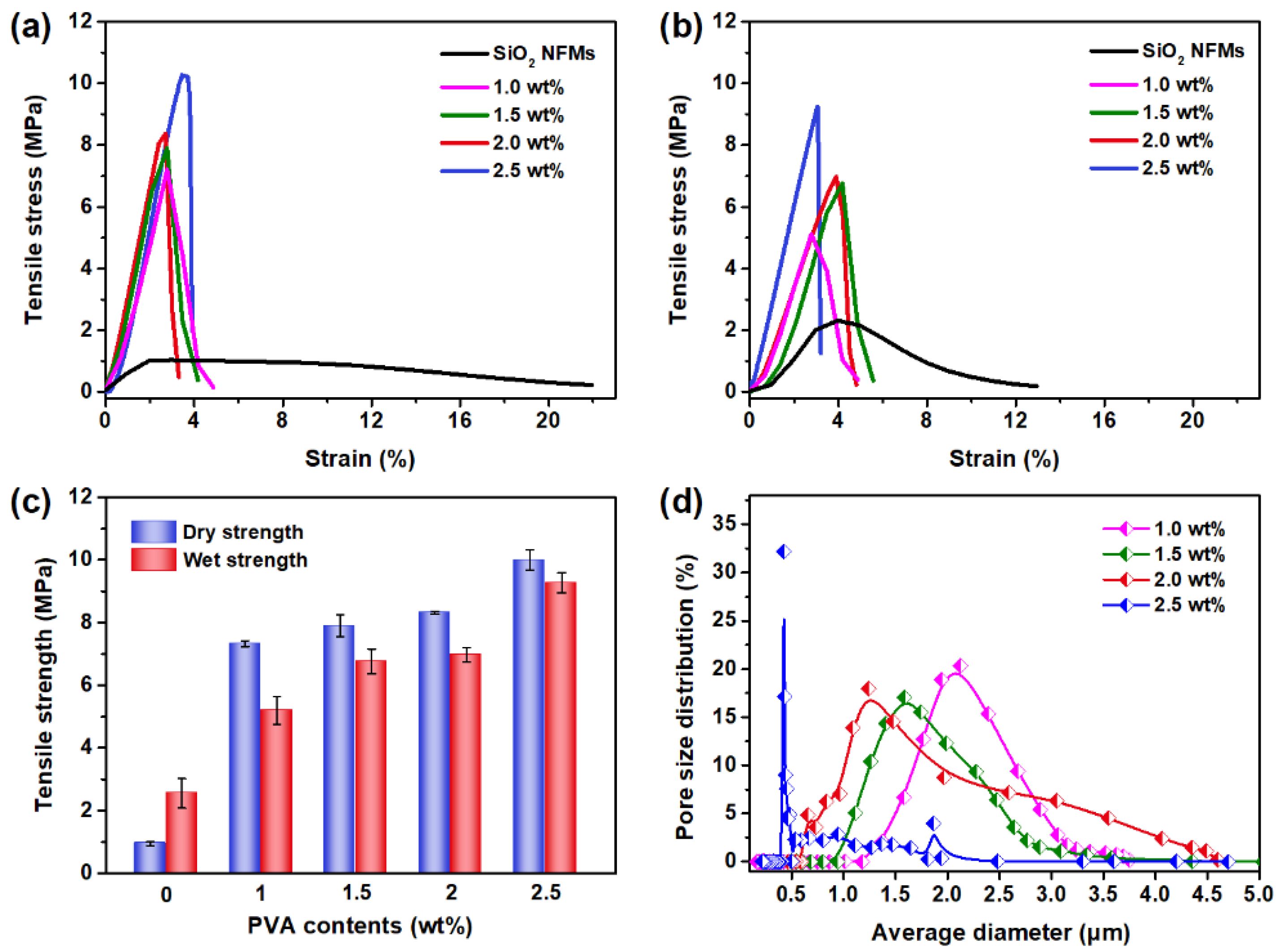
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xiu, L.C.; Valeja, S.G.; Alpert, A.J.; Jin, S.; Ge, Y. Effective protein separation by coupling hydrophobic interaction and reverse phase chromatography for top-down proteomics. Anal. Chem. 2014, 86, 7899–7906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amaly, N.; Ma, Y.; El-Moghazy, A.Y.; Sun, G. Copper complex formed with pyridine rings grafted on cellulose nanofibrous membranes for highly efficient lysozyme adsorption. Sep. Purif. Technol. 2020, 250, 117086. [Google Scholar] [CrossRef]
- Nfor, B.K.; Verhaert, P.D.; van der Wielen, L.A.; Hubbuch, J.; Ottens, M. Rational and systematic protein purification process development: The next generation. Trends Biotechnol. 2009, 27, 673–679. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Sun, Y. Recent advances in protein chromatography with polymer-grafted media. J. Chromatogr. A 2021, 1638, 461865. [Google Scholar] [CrossRef]
- Orr, V.; Zhong, L.; Moo-Young, M.; Chou, C.P. Recent advances in bioprocessing application of membrane chromatography. Biotechnol. Adv. 2013, 31, 450–465. [Google Scholar] [CrossRef]
- Hardick, O.; Dods, S.; Stevens, B.; Bracewell, D.G. Nanofiber adsorbents for high productivity continuous downstream processing. J. Biotechnol. 2015, 213, 74–82. [Google Scholar] [CrossRef] [Green Version]
- Qiao, X.; Chen, R.; Yan, H.; Shen, S. Polyhedral oligomeric silsesquioxane-based hybrid monolithic columns: Recent advances in their preparation and their applications in capillary liquid chromatography. TrAC-Trend. Anal. Chem. 2017, 97, 50–64. [Google Scholar] [CrossRef]
- Salimi, K.; Usta, D.D.; Kocer, I.; Celik, E.; Tuncel, A. Protein A and protein A/G coupled magnetic SiO2 microspheres for affinity purification of immunoglobulin G. Int. J. Biol. Macromol. 2018, 111, 178–185. [Google Scholar] [CrossRef]
- Saikia, D.; Deka, J.R.; Wu, C.E.; Yang, Y.C.; Kao, H.M. pH responsive selective protein adsorption by carboxylic acid functionalized large pore mesoporous silica nanoparticles SBA-1. Mater. Sci. Eng. C-Mater. 2019, 94, 344–356. [Google Scholar] [CrossRef]
- Hardick, O.; Dods, S.; Stevens, B.; Bracewell, D.G. Nanofiber adsorbents for high productivity downstream processing. Biotechnol. Bioeng. 2013, 110, 1119–1128. [Google Scholar] [CrossRef]
- Zhu, J.; Sun, G. Facile fabrication of hydrophilic nanofibrous membranes with an immobilized metal-chelate affinity complex for selective protein separation. ACS Appl. Mater. Interfaces 2014, 6, 925–932. [Google Scholar] [CrossRef]
- Wang, H.; Duan, Y.; Zhong, W. ZrO2 nanofiber as a versatile tool for protein analysis. ACS Appl. Mater. Interfaces 2015, 7, 26414–26420. [Google Scholar] [CrossRef]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and electrospun nanofibers: Methods, materials, and applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef]
- Lyu, C.; Zhao, P.; Xie, J.; Dong, S.; Liu, J.; Rao, C.; Fu, J. Electrospinning of nanofibrous membrane and its applications in air filtration: A review. Nanomaterials 2021, 11, 1501. [Google Scholar] [CrossRef]
- Zhang, L.; Menkhaus, T.; Fong, H. Fabrication and bioseparation studies of adsorptive membranes/felts made from electrospun cellulose acetate nanofibers. J. Membr. Sci. 2008, 319, 176–184. [Google Scholar] [CrossRef]
- Lan, T.; Shao, Z.Q.; Gu, M.J.; Zhou, Z.W.; Wang, Y.L.; Wang, W.J.; Wang, F.J.; Wang, J.Q. Electrospun nanofibrous cellulose diacetate nitrate membrane for protein separation. J. Membr. Sci. 2015, 489, 204–211. [Google Scholar] [CrossRef]
- Rajesh, S.; Schneiderman, S.; Crandall, C.; Fong, H.; Menkhaus, T.J. Synthesis of cellulose-graft-polypropionic acid nanofiber cation-exchange membrane adsorbers for high-efficiency separations. ACS Appl. Mater. Interfaces 2017, 9, 41055–41065. [Google Scholar] [CrossRef]
- Fu, Q.; Wang, X.; Si, Y.; Liu, L.; Yu, J.; Ding, B. Scalable fabrication of electrospun nanofibrous membranes functionalized with citric acid for high-performance protein adsorption. ACS Appl. Mater. Interfaces 2016, 8, 11819–11829. [Google Scholar] [CrossRef]
- Yi, S.; Dai, F.; Ma, Y.; Yan, T.; Si, Y.; Sun, G. Ultrafine silk-derived nanofibrous membranes exhibiting effective lysozyme adsorption. ACS Sustain. Chem. Eng. 2017, 5, 8777–8784. [Google Scholar] [CrossRef]
- Wang, X.; Fu, Q.; Wang, X.; Si, Y.; Yu, J.; Wang, X.; Ding, B. In situ cross-linked and highly carboxylated poly(vinyl alcohol) nanofibrous membranes for efficient adsorption of proteins. J. Mater. Chem. B 2015, 3, 7281–7290. [Google Scholar] [CrossRef]
- Dou, X.; Wang, Q.; Li, Z.; Ju, J.; Wang, S.; Hao, L.; Sui, K.; Xia, Y.; Tan, Y. Seaweed-derived electrospun nanofibrous membranes for ultrahigh protein adsorption. Adv. Funct. Mater. 2019, 29, 1905610. [Google Scholar] [CrossRef]
- Lan, T.; Shao, Z.Q.; Wang, J.Q.; Gu, M.J. Fabrication of hydroxyapatite nanoparticles decorated cellulose triacetate nanofibers for protein adsorption by coaxial electrospinning. Chem. Eng. J. 2015, 260, 818–825. [Google Scholar] [CrossRef]
- Esfahani, H.; Prabhakaran, M.P.; Salahi, E.; Tayebifard, A.; Rahimipour, M.R.; Keyanpour-Rad, M.; Ramakrishna, S. Electrospun nylon 6/zinc doped hydroxyapatite membrane for protein separation: Mechanism of fouling and blocking model. Mater. Sci. Eng. C-Mater. 2016, 59, 420–428. [Google Scholar] [CrossRef]
- Wang, L.; Fu, Q.; Yu, J.; Liu, L.; Ding, B. Nanoparticle-doped polystyrene/polyacrylonitrile nanofiber membrane with hierarchical structure as promising protein hydrophobic interaction chromatography media. Compos. Commun. 2019, 16, 33–40. [Google Scholar] [CrossRef]
- Fu, Q.; Duan, C.; Yan, Z.; Si, Y.; Liu, L.; Yu, J.; Ding, B. Electrospun nanofibrous composite materials: A versatile platform for high efficiency protein adsorption and separation. Compos. Commun. 2018, 8, 92–100. [Google Scholar] [CrossRef]
- Fu, Q.; Si, Y.; Duan, C.; Yan, Z.; Liu, L.; Yu, J.; Ding, B. Highly carboxylated, cellular structured, and underwater superelastic nanofibrous aerogels for efficient protein separation. Adv. Funct. Mater. 2019, 29, 1808234. [Google Scholar] [CrossRef]
- Shan, H.; Wang, X.; Shi, F.; Yan, J.; Yu, J.; Ding, B. Hierarchical porous structured SiO2/SnO2 nanofibrous membrane with superb flexibility for molecular filtration. ACS Appl. Mater. Interfaces 2017, 9, 18966–18976. [Google Scholar] [CrossRef]
- Fu, Q.; Si, Y.; Liu, L.; Yu, J.; Ding, B. Elaborate design of ethylene vinyl alcohol (EVAL) nanofiber-based chromatographic media for highly efficient adsorption and extraction of proteins. J. Colloid Interface Sci. 2019, 555, 11–21. [Google Scholar] [CrossRef]
- Singh, I.; Birajdar, B. Effective La-Na Co-doped TiO2 nano-particles for dye adsorption: Synthesis, characterization and study on adsorption kinetics. Nanomaterials 2019, 9, 400. [Google Scholar] [CrossRef] [Green Version]
- Plazinski, W.; Rudzinski, W.; Plazinska, A. Theoretical models of sorption kinetics including a surface reaction mechanism: A review. Adv. Colloid Interface Sci. 2009, 152, 2–13. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Y.; Jin, S.; Lu, Q.; Ji, J. Adsorption isotherm, kinetic and mechanism of expanded graphite for sulfadiazine antibiotics removal from aqueous solutions. Environ. Technol. 2017, 38, 2629–2638. [Google Scholar] [CrossRef] [PubMed]
- Teng, F.; Wu, J.; Su, B.; Wang, Y. Enhanced tribological properties of vulcanized natural rubber composites by applications of carbon nanotube: A molecular dynamics study. Nanomaterials 2021, 11, 2464. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.X.; Chu, B.; Gao, Y.; Wu, C.X.; Zhang, L.M.; Chen, P.; Wang, X.Y.; Tang, S.Q. Modification of agarose with carboxylation and grafting dopamine for promotion of its cell-adhesiveness. Carbohydr. Polym. 2013, 92, 2245–2251. [Google Scholar] [PubMed]
- Nweke, M.C.; McCartney, R.G.; Bracewell, D.G. Mechanical characterisation of agarose-based chromatography resins for biopharmaceutical manufacture. J. Chromatogr. A 2017, 1530, 129–137. [Google Scholar] [CrossRef]
- Louf, J.F.; Lu, N.B.; O’Connell, M.G.; Cho, H.J.; Datta, S.S. Under pressure: Hydrogel swelling in a granular medium. Sci. Adv. 2021, 7, eabd2711. [Google Scholar] [CrossRef]
- Tian, Y.; Zhang, X.; Feng, X.; Zhang, J.; Zhong, T. Shapeable and underwater super-elastic cellulose nanofiber/alginate cryogels by freezing-induced oxa-Michael reaction for efficient protein purification. Carbohydr. Polym. 2021, 272, 118498. [Google Scholar] [CrossRef]
- Qiu, J.; Feng, Y.; Zhang, X.; Jia, M.; Yao, J. Acid-promoted synthesis of UiO-66 for highly selective adsorption of anionic dyes: Adsorption performance and mechanisms. J. Colloid Interface Sci. 2017, 499, 151–158. [Google Scholar] [CrossRef]
- Lv, H.; Wang, X.; Fu, Q.; Si, Y.; Yin, X.; Li, X.; Sun, G.; Yu, J.; Ding, B. A versatile method for fabricating ion-exchange hydrogel nanofibrous membranes with superb biomolecule adsorption and separation properties. J. Colloid Interface Sci. 2017, 506, 442–451. [Google Scholar] [CrossRef]
- Latour, R.A. The Langmuir isotherm: A commonly applied but misleading approach for the analysis of protein adsorption behavior. J. Biomed. Mater. Res. A 2015, 103, 949–958. [Google Scholar] [CrossRef]
- Aramesh, M.; Shimoni, O.; Ostrikov, K.; Prawer, S.; Cervenka, J. Surface charge effects in protein adsorption on nanodiamonds. Nanoscale 2015, 7, 5726–5736. [Google Scholar] [CrossRef]
- Losdorfer Bozic, A.; Podgornik, R. pH Dependence of charge multipole moments in proteins. Biophys. J. 2017, 113, 1454–1465. [Google Scholar] [CrossRef] [Green Version]
- Szymanski, J.; Pobozy, E.; Trojanowicz, M.; Wilk, A.; Garstecki, P.; Holyst, R. Net Charge and Electrophoretic Mobility of Lysozyme Charge Ladders in Solutions of Nonionic Surfactant. J. Phys. Chem. B 2007, 111, 5503–5510. [Google Scholar] [CrossRef]
- Rahmatika, A.M.; Goi, Y.; Kitamura, T.; Widiyastuti, W.; Ogi, T. TEMPO-oxidized cellulose nanofiber (TOCN) decorated macroporous silica particles: Synthesis, characterization, and their application in protein adsorption. Mater. Sci. Eng. C-Mater. 2019, 105, 110033. [Google Scholar] [CrossRef]
- Lan, H.; Liu, H.; Ye, Y.; Yin, Z. The role of surface properties on protein aggregation behavior in aqueous solution of different pH values. AAPS PharmSciTech 2020, 21, 122. [Google Scholar] [CrossRef]
- Jungbauer, A. Chromatographic media for bioseparation. J. Chromatogr. A 2005, 1065, 3–12. [Google Scholar] [CrossRef]
- Zhang, F.; Xu, G.; Zhu, L.; Jiang, J. Effects of hydrolysis treatment on the structure and properties of semi-interpenetrating superabsorbent polymers. J. Appl. Polym. Sci. 2021, 138, e51307. [Google Scholar] [CrossRef]
- Stone, M.C.; Tao, Y.; Carta, G. Protein adsorption and transport in agarose and dextran-grafted agarose media for ion exchange chromatography: Effect of ionic strength and protein characteristics. J. Chromatogr. A 2009, 1216, 4465–4474. [Google Scholar] [CrossRef]
- Eweida, B.Y.; El-Moghazy, A.Y.; Pandey, P.K.; Amaly, N. Fabrication and simulation studies of high-performance anionic sponge alginate beads for lysozyme separation. Colloid Surf. A 2021, 619, 126556. [Google Scholar] [CrossRef]
- Brorson, K.; Brown, J.; Hamilton, E.; Stein, K.E. Identification of protein A media performance attributes that can be monitored as surrogates for retrovirus clearance during extended re-use. J. Chromatogr. A 2003, 989, 155–163. [Google Scholar] [CrossRef]



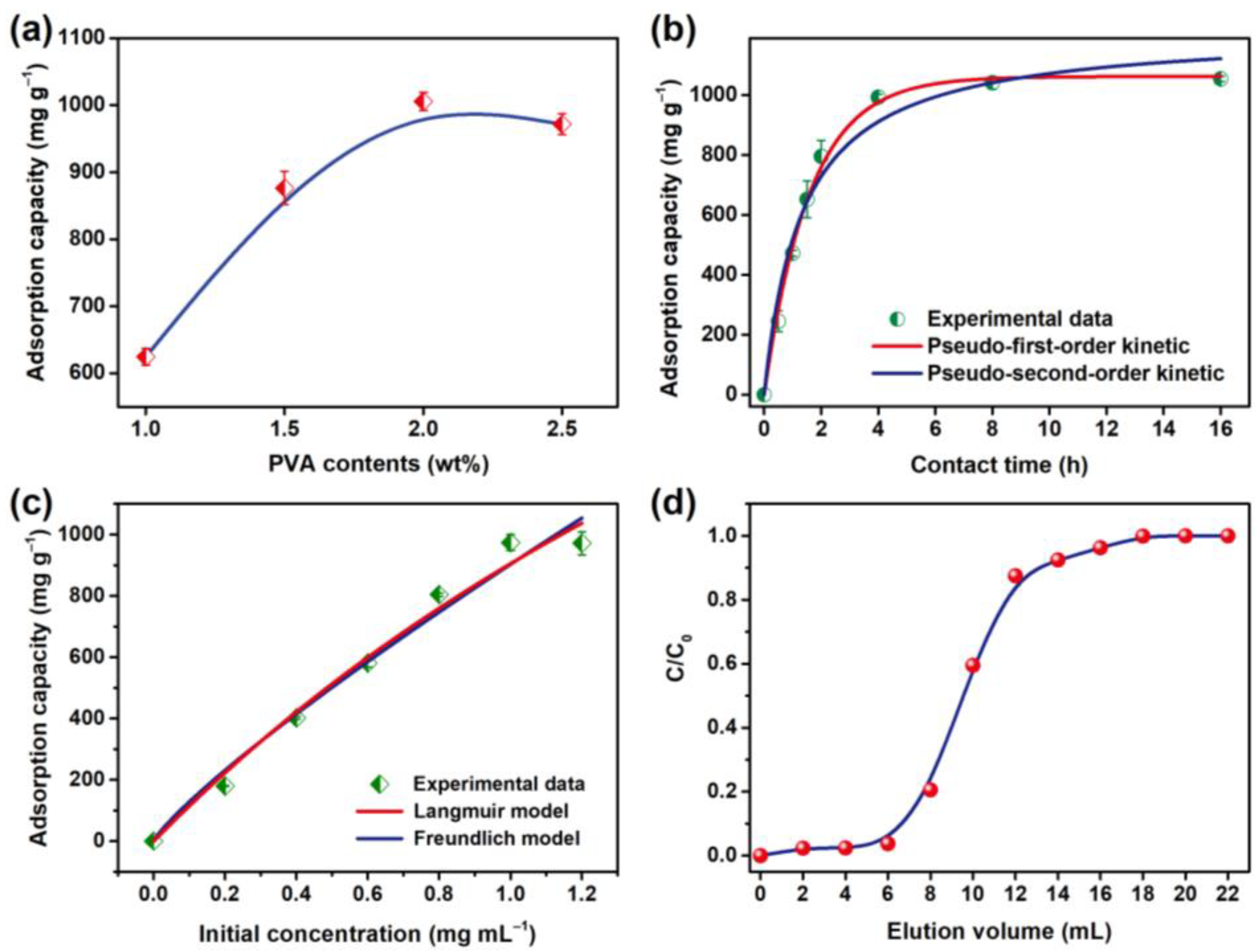
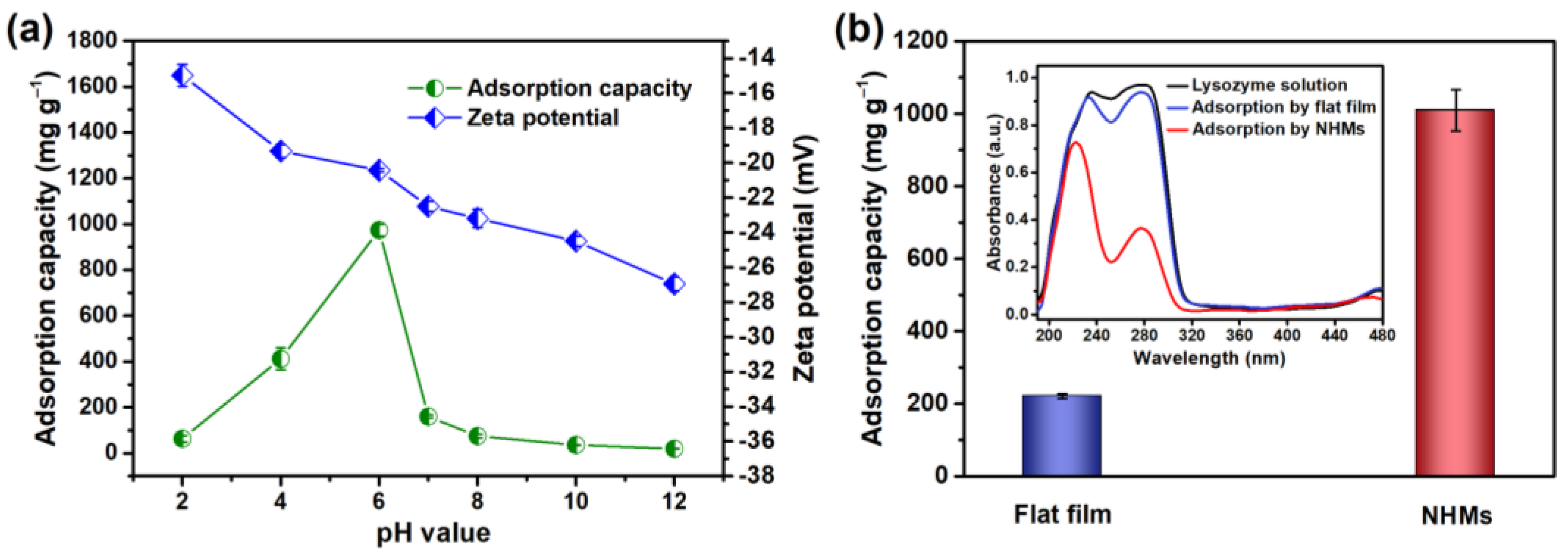

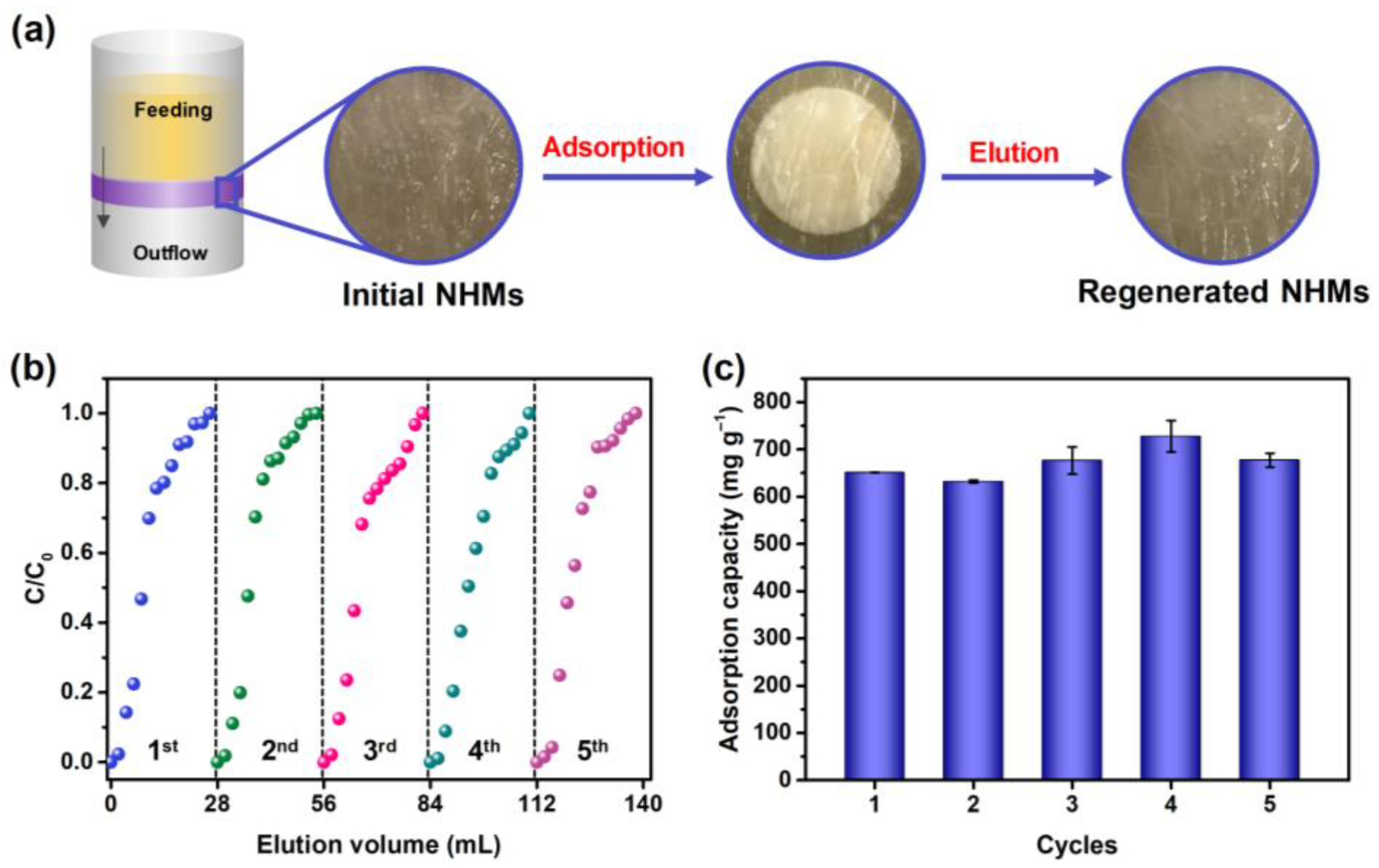
| Pseudo-First-Order Kinetic Model | Pseudo-Second-Order Kinetic Model | ||||
|---|---|---|---|---|---|
| Qe (mg g−1) | k1 (min−1) | R2 | Qe (mg g−1) | k2 (min−1) | R2 |
| 1062.0 | 1.447 | 0.996 | 1214.959 | 0.617 | 0.972 |
| Langmuir Model | Freundlich Model | ||||
|---|---|---|---|---|---|
| Qm (mg g−1) | KL (mL mg−1) | R2 | KF | 1/n | R2 |
| 3846.8 | 0.307 | 0.982 | 902.8 | 1.180 | 0.976 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, Q.; Xie, D.; Ge, J.; Zhang, W.; Shan, H. Negatively Charged Composite Nanofibrous Hydrogel Membranes for High-Performance Protein Adsorption. Nanomaterials 2022, 12, 3500. https://doi.org/10.3390/nano12193500
Fu Q, Xie D, Ge J, Zhang W, Shan H. Negatively Charged Composite Nanofibrous Hydrogel Membranes for High-Performance Protein Adsorption. Nanomaterials. 2022; 12(19):3500. https://doi.org/10.3390/nano12193500
Chicago/Turabian StyleFu, Qiuxia, Dandan Xie, Jianlong Ge, Wei Zhang, and Haoru Shan. 2022. "Negatively Charged Composite Nanofibrous Hydrogel Membranes for High-Performance Protein Adsorption" Nanomaterials 12, no. 19: 3500. https://doi.org/10.3390/nano12193500





