Polystyrene Nanoplastics Induce Lung Injury via Activating Oxidative Stress: Molecular Insights from Bioinformatics Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and PS-NPs Treatment
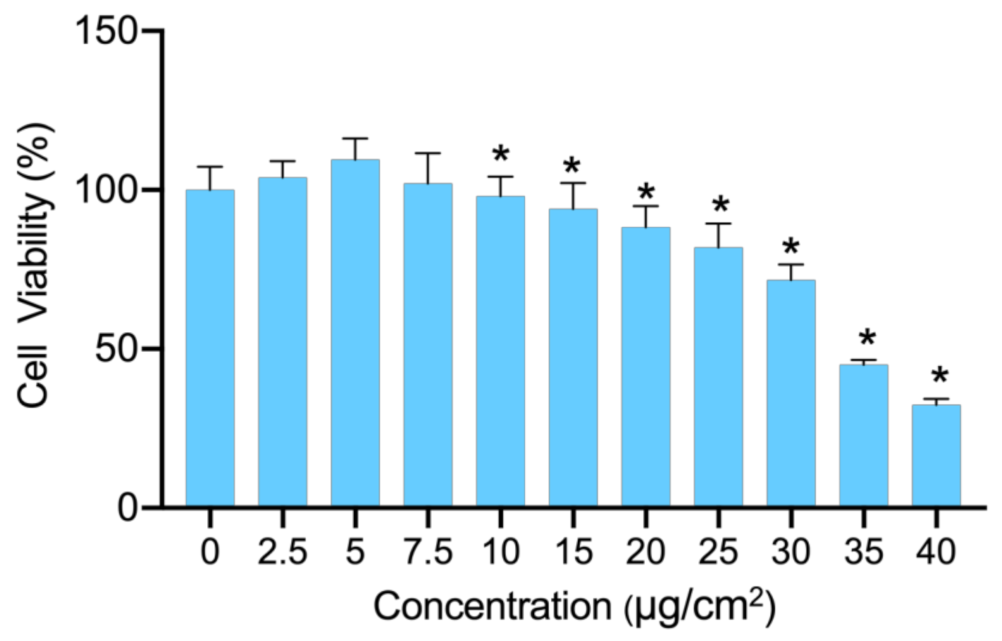
2.2. Cell Viability Assay
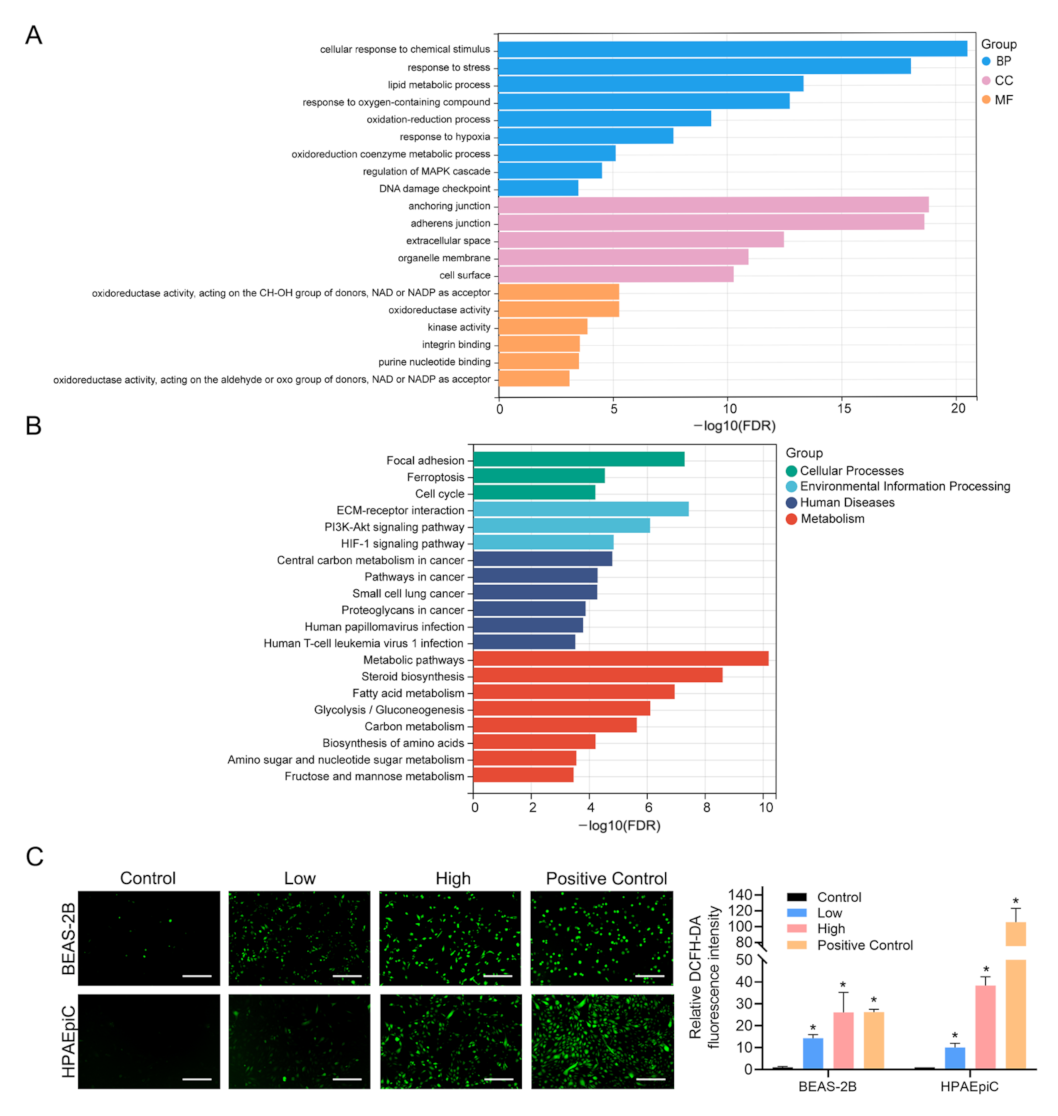
2.3. Measurement of Intracellular Reactive Oxygen Species (ROS) Levels
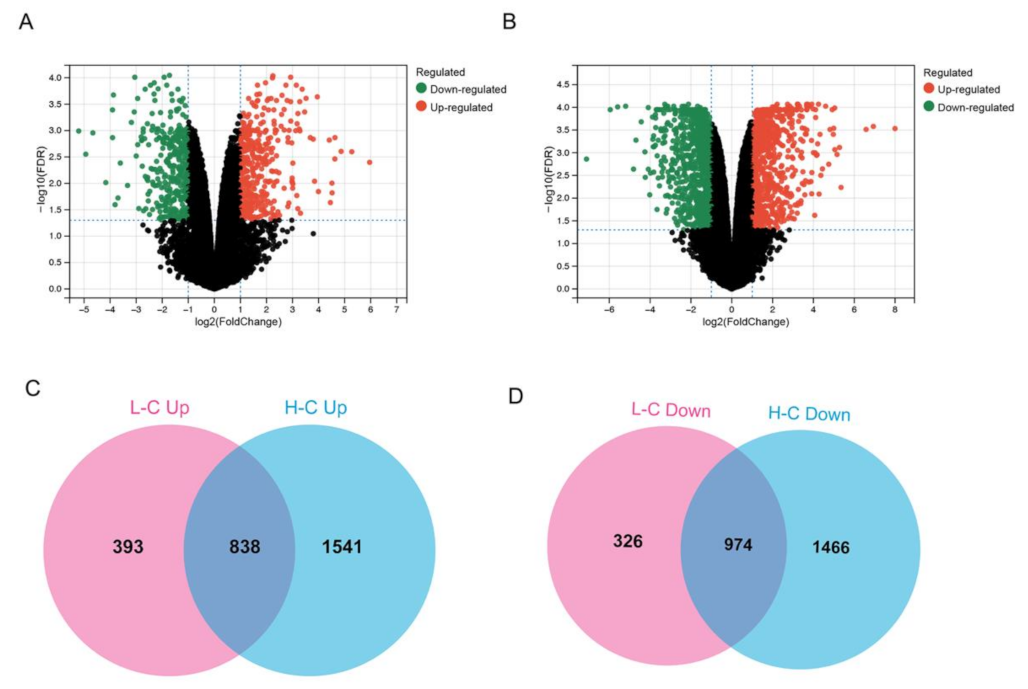
2.4. RNA-Sequencing
2.5. Bioinformatics Analysis
2.6. Prediction of Transcription Factors (TFs) for DEGs and Construction of Oxidative Stress-Associated TF-mRNA Regulatory Network
2.7. Gene Expression of Rat Lung Tissues with Acute Pulmonary Embolism Induced by Polystyrene
2.8. RNA Extraction and Real-Time PCR
2.9. Statistical Analysis
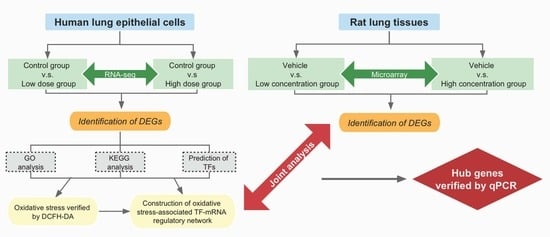
3. Results
3.1. Cytotoxicity of PS-NPs
3.2. Gene Expression Alternation in PS-NP-treated BEAS-2B Cells
3.3. Functional Annotation of DEGs Induced by PS-NPs and Validation of ROS
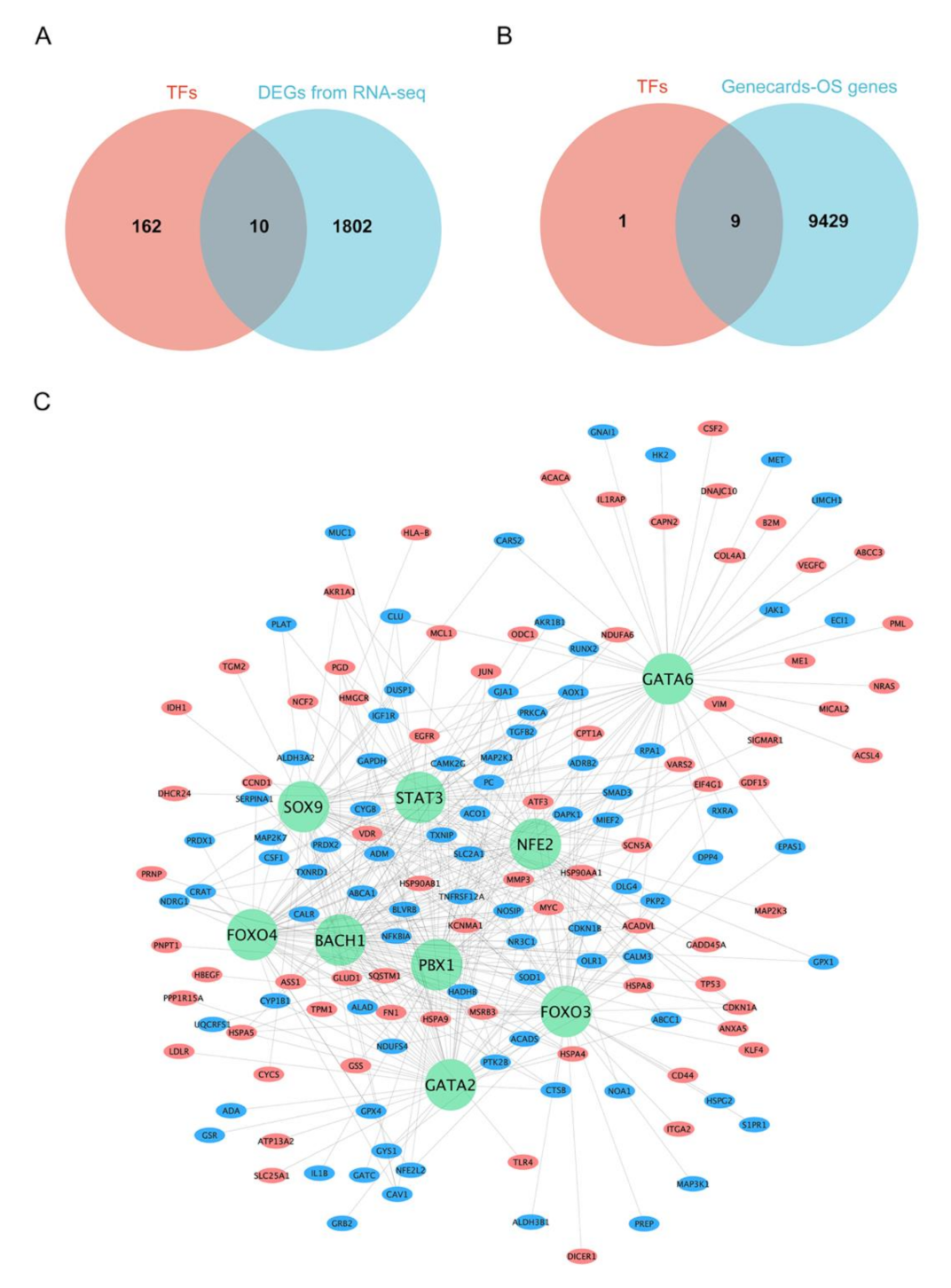
3.4. Prediction of TFs for DEGs and Construction of Oxidative Stress-Associated TF-mRNA Regulatory Network
3.5. Combined Analysis with Rat Lung Injury Models
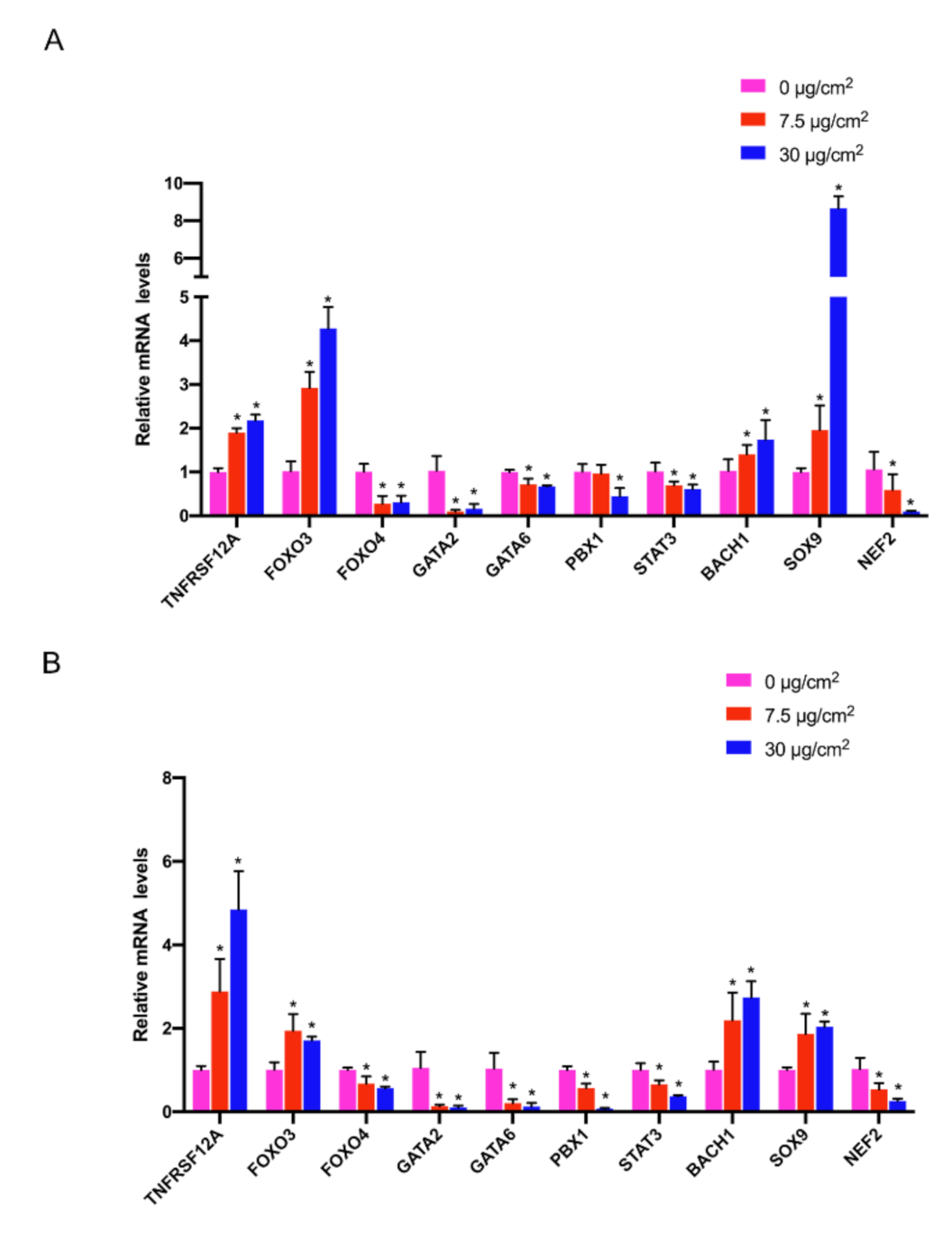
3.6. Gene Expression Validation by qRT-PCR
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, S.; Cheng, Y.; Liu, T.; Huang, S.; Yin, L.; Pu, Y.; Liang, G. Impact of waste of COVID-19 protective equipment on the environment, animals and human health: A review. Environ. Chem. Lett. 2022, 20, 2951–2970. [Google Scholar] [CrossRef] [PubMed]
- Mu, Y.; Sun, J.; Li, Z.; Zhang, W.; Liu, Z.; Li, C.; Peng, C.; Cui, G.; Shao, H.; Du, Z. Activation of pyroptosis and ferroptosis is involved in the hepatotoxicity induced by polystyrene microplastics in mice. Chemosphere 2022, 291 Pt 2, 132944. [Google Scholar] [CrossRef] [PubMed]
- Plastics Europe, Plastics—the Facts 2021. 2021. Available online: https://plasticseurope.org/knowledge-hub/plastics-the-facts-2021/ (accessed on 8 July 2022).
- Lehner, R.; Weder, C.; Petri-Fink, A.; Rothen-Rutishauser, B. Emergence of Nanoplastic in the Environment and Possible Impact on Human Health. Environ. Sci. Technol. 2019, 53, 1748–1765. [Google Scholar] [CrossRef] [PubMed]
- Vethaak, A.D.; Legler, J. Microplastics and human health. Science 2021, 371, 672–674. [Google Scholar] [CrossRef] [PubMed]
- ISO/DIS. Principles for the Analysis of Plastics and Microplastics Present in the Environment. Available online: https://www.iso.org/obp/ui#iso:std:iso:24187:dis:ed-1:v1:en (accessed on 30 September 2022).
- Vivekanand, A.C.; Mohapatra, S.; Tyagi, V.K. Microplastics in aquatic environment: Challenges and perspectives. Chemosphere 2021, 282, 131151. [Google Scholar] [CrossRef] [PubMed]
- Kvale, K.; Prowe, A.E.F.; Chien, C.-T.; Landolfi, A.; Oschlies, A. Zooplankton grazing of microplastic can accelerate global loss of ocean oxygen. Nat. Commun. 2021, 12, 2358. [Google Scholar] [CrossRef] [PubMed]
- Oberbeckmann, S.; Labrenz, M. Marine Microbial Assemblages on Microplastics: Diversity, Adaptation, and Role in Degradation. Ann. Rev. Mar. Sci. 2020, 12, 209–232. [Google Scholar] [CrossRef] [PubMed]
- Kole, P.J.; Löhr, A.J.; Van Belleghem, F.G.A.J.; Ragas, A.M.J. Wear and Tear of Tyres: A Stealthy Source of Microplastics in the Environment. Int. J. Environ. Res. Public Health 2017, 14, 1265. [Google Scholar] [CrossRef] [PubMed]
- Byrley, P.; Wallace, M.A.G.; Boyes, W.K.; Rogers, K. Particle and volatile organic compound emissions from a 3D printer filament extruder. Sci. Total Environ. 2020, 736, 139604. [Google Scholar] [CrossRef]
- O’Brien, S.; Okoffo, E.D.; Rauert, C.; O’Brien, J.W.; Ribeiro, F.; Burrows, S.D.; Toapanta, T.; Wang, X.; Thomas, K.V. Quantification of selected microplastics in Australian urban road dust. J. Hazard. Mater. 2021, 416, 125811. [Google Scholar] [CrossRef]
- Amato-Lourenço, L.F.; dos Santos Galvão, L.; de Weger, L.A.; Hiemstra, P.S.; Vijver, M.G.; Mauad, T. An emerging class of air pollutants: Potential effects of microplastics to respiratory human health? Sci. Total Environ. 2020, 749, 141676. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Wang, J.; Peng, J.; Tan, Z.; Zhan, Z.; Tan, X.; Chen, Q. Characteristic of microplastics in the atmospheric fallout from Dongguan city, China: Preliminary research and first evidence. Environ. Sci. Pollut. Res. Int. 2017, 24, 24928–24935. [Google Scholar] [CrossRef]
- Liu, K.; Wang, X.; Fang, T.; Xu, P.; Zhu, L.; Li, D. Source and potential risk assessment of suspended atmospheric microplastics in Shanghai. Sci. Total Environ. 2019, 675, 462–471. [Google Scholar] [CrossRef] [PubMed]
- Wright, S.; Ulke, J.; Font, A.; Chan, K.; Kelly, F. Atmospheric microplastic deposition in an urban environment and an evaluation of transport. Environ. Int. 2020, 136, 105411. [Google Scholar] [CrossRef] [PubMed]
- Allen, S.; Allen, D.; Phoenix, V.; Le Roux, G.; Jiménez, P.D.; Simonneau, A.; Binet, S.; Galop, D. Atmospheric transport and deposition of microplastics in a remote mountain catchment. Nat. Geosci. 2019, 12, 339–344. [Google Scholar] [CrossRef]
- Jenner, L.C.; Rotchell, J.M.; Bennett, R.T.; Cowen, M.; Tentzeris, V.; Sadofsky, L.R. Detection of microplastics in human lung tissue using μFTIR spectroscopy. Sci. Total Environ. 2022, 831, 154907. [Google Scholar] [CrossRef] [PubMed]
- Amato-Lourenço, L.F.; Carvalho-Oliveira, R.; Júnior, G.R.; Galvão, L.D.S.; Ando, R.A.; Mauad, T. Presence of airborne microplastics in human lung tissue. J. Hazard. Mater. 2021, 416, 126124. [Google Scholar] [CrossRef]
- Baeza-Martínez, C.; Olmos, S.; González-Pleiter, M.; López-Castellanos, J.; García-Pachón, E.; Masiá-Canuto, M.; Hernández-Blasco, L.; Bayo, J. First evidence of microplastics isolated in European citizens’ lower airway. J. Hazard. Mater. 2022, 438, 129439. [Google Scholar] [CrossRef]
- Lambert, S.; Wagner, M. Characterisation of nanoplastics during the degradation of polystyrene. Chemosphere 2016, 145, 265–268. [Google Scholar] [CrossRef]
- Xu, G.; Cheng, H.; Jones, R.; Feng, Y.; Gong, K.; Li, K.; Fang, X.; Tahir, M.A.; Valev, V.K.; Zhang, L. Surface-Enhanced Raman Spectroscopy Facilitates the Detection of Microplastics <1 μm in the Environment. Environ. Sci. Technol. 2020, 54, 15594–15603. [Google Scholar] [CrossRef]
- Xu, M.; Halimu, G.; Zhang, Q.; Song, Y.; Fu, X.; Li, Y.; Li, Y.; Zhang, H. Internalization and toxicity: A preliminary study of effects of nanoplastic particles on human lung epithelial cell. Sci. Total Environ. 2019, 694, 133794. [Google Scholar] [CrossRef]
- Halimu, G.; Zhang, Q.; Liu, L.; Zhang, Z.; Wang, X.; Gu, W.; Zhang, B.; Dai, Y.; Zhang, H.; Zhang, C.; et al. Toxic effects of nanoplastics with different sizes and surface charges on epithelial-to-mesenchymal transition in A549 cells and the potential toxicological mechanism. J. Hazard. Mater. 2022, 430, 128485. [Google Scholar] [CrossRef]
- Lim, S.L.; Ng, C.T.; Zou, L.; Lu, Y.; Chen, J.; Bay, B.H.; Shen, H.-M.; Ong, C.N. Targeted metabolomics reveals differential biological effects of nanoplastics and nanoZnO in human lung cells. Nanotoxicology 2019, 13, 1117–1132. [Google Scholar] [CrossRef]
- Xu, D.; Ma, Y.; Han, X.; Chen, Y. Systematic toxicity evaluation of polystyrene nanoplastics on mice and molecular mechanism investigation about their internalization into Caco-2 cells. J. Hazard. Mater. 2021, 417, 126092. [Google Scholar] [CrossRef]
- Yang, S.; Cheng, Y.; Chen, Z.; Liu, T.; Yin, L.; Pu, Y.; Liang, G. In vitro evaluation of nanoplastics using human lung epithelial cells, microarray analysis and co-culture model. Ecotoxicol. Environ. Saf. 2021, 226, 112837. [Google Scholar] [CrossRef]
- Madrigal-Matute, J.; Fernandez-Laso, V.; Sastre, C.; Llamas-Granda, P.; Egido, J.; Martin-Ventura, J.L.; Zalba, G.; Blanco-Colio, L.M. TWEAK/Fn14 interaction promotes oxidative stress through NADPH oxidase activation in macrophages. Cardiovasc. Res. 2015, 108, 139–147. [Google Scholar] [CrossRef]
- Liu, H.; Peng, H.; Xiang, H.; Guo, L.; Chen, R.; Zhao, S.; Chen, W.; Chen, P.; Lu, H.; Chen, S. TWEAK/Fn14 promotes oxidative stress through AMPK/PGC-1α/MnSOD signaling pathway in endothelial cells. Mol. Med. Rep. 2018, 17, 1998–2004. [Google Scholar] [CrossRef] [PubMed]
- Lai, H.; Liu, X.; Qu, M. Nanoplastics and Human Health: Hazard Identification and Biointerface. Nanomaterials 2022, 12, 1298. [Google Scholar] [CrossRef]
- Hadian, K.; Stockwell, B.R. SnapShot: Ferroptosis. Cell 2020, 181, 1188–1188.e1. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Stockwell, B.R.; Conrad, M. Ferroptosis: Mechanisms, biology and role in disease. Nat. Rev. Mol. Cell Biol. 2021, 22, 266–282. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Chen, X.; Kang, R.; Kroemer, G. Ferroptosis: Molecular mechanisms and health implications. Cell Res. 2021, 31, 107–125. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhao, X.; Cai, H.; Sun, H.; Hu, Y.; Huang, X.; Kong, W.; Kong, W. The role of sodium hydrosulfide in attenuating the aging process via PI3K/AKT and CaMKKβ/AMPK pathways. Redox. Biol. 2017, 12, 987–1003. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Gehrke, S.; Haque, E.; Imai, Y.; Kosek, J.; Yang, L.; Beal, M.F.; Nishimura, I.; Wakamatsu, K.; Ito, S.; et al. Inactivation of Drosophila DJ-1 leads to impairments of oxidative stress response and phosphatidylinositol 3-kinase/Akt signaling. Proc. Natl. Acad. Sci. USA 2005, 102, 13670–13675. [Google Scholar] [CrossRef]
- Chen, P.-J.; Weng, J.-Y.; Hsu, P.-H.; Shew, J.-Y.; Huang, Y.-S.; Lee, W.-H. NPGPx modulates CPEB2-controlled HIF-1α RNA translation in response to oxidative stress. Nucleic Acids Res. 2015, 43, 9393–9404. [Google Scholar] [CrossRef]
- Prabhakar, N.R.; Semenza, G.L. Adaptive and maladaptive cardiorespiratory responses to continuous and intermittent hypoxia mediated by hypoxia-inducible factors 1 and 2. Physiol. Rev. 2012, 92, 967–1003. [Google Scholar] [CrossRef]
- Hu, M.; Palić, D. Micro- and nano-plastics activation of oxidative and inflammatory adverse outcome pathways. Redox Biol. 2020, 37, 101620. [Google Scholar] [CrossRef] [PubMed]
- Francois, M.; Donovan, P.; Fontaine, F. Modulating transcription factor activity: Interfering with protein-protein interaction networks. Semin. Cell Dev. Biol. 2020, 99, 12–19. [Google Scholar] [CrossRef]
- Morris, B.J.; Willcox, D.C.; Donlon, T.A.; Willcox, B.J. FOXO3: A Major Gene for Human Longevity--A Mini-Review. Gerontology 2015, 61, 515–525. [Google Scholar] [CrossRef] [PubMed]
- Aucello, M.; Dobrowolny, G.; Musarò, A. Localized accumulation of oxidative stress causes muscle atrophy through activation of an autophagic pathway. Autophagy 2009, 5, 527–529. [Google Scholar] [CrossRef] [PubMed]
- Marinkovic, D.; Zhang, X.; Yalcin, S.; Luciano, J.P.; Brugnara, C.; Huber, T.; Ghaffari, S. Foxo3 is required for the regulation of oxidative stress in erythropoiesis. J. Clin. Investig. 2007, 117, 2133–2144. [Google Scholar] [CrossRef]
- Gupta, R.K.; Gracias, D.T.; Figueroa, D.S.; Miki, H.; Miller, J.; Fung, K.; Ay, F.; Burkly, L.; Croft, M. TWEAK functions with TNF and IL-17 on keratinocytes and is a potential target for psoriasis therapy. Sci. Immunol. 2021, 6, eabi8823. [Google Scholar] [CrossRef] [PubMed]
- Johnston, A.J.; Murphy, K.; Jenkinson, L.; Laine, D.; Emmrich, K.; Faou, P.; Weston, R.; Jayatilleke, K.M.; Schloegel, J.; Talbo, G.; et al. Targeting of Fn14 Prevents Cancer-Induced Cachexia and Prolongs Survival. Cell 2015, 162, 1365–1378. [Google Scholar] [CrossRef]
- Weinberg, J.M. TWEAK-Fn14 as a mediator of acute kidney injury. Kidney Int. 2011, 79, 151–153. [Google Scholar] [CrossRef][Green Version]
- Sidler, D.; Wu, P.; Herro, R.; Claus, M.; Wolf, D.; Kawakami, Y.; Kawakami, T.; Burkly, L.; Croft, M. TWEAK mediates inflammation in experimental atopic dermatitis and psoriasis. Nat. Commun. 2017, 8, 15395. [Google Scholar] [CrossRef]
- Wang, M.; Xie, Z.; Xu, J.; Feng, Z. TWEAK/Fn14 axis in respiratory diseases. Clin. Chim. Acta 2020, 509, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Bao, S.; Wang, F.; Guo, L.; Zhu, J.; Wang, J.; Deng, X.; Li, J. FN14 Blockade on Pulmonary Microvascular Endothelial Cells Improves the Outcome of Sepsis-Induced Acute Lung Injury. Shock 2018, 49, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Szabo, Y.Z.; Slavish, D.C.; Graham-Engeland, J.E. The effect of acute stress on salivary markers of inflammation: A systematic review and meta-analysis. Brain Behav. Immun. 2020, 88, 887–900. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, T.; Yang, S.; Ge, Y.; Wan, X.; Zhu, Y.; Li, J.; Yin, L.; Pu, Y.; Liang, G. Polystyrene Nanoplastics Induce Lung Injury via Activating Oxidative Stress: Molecular Insights from Bioinformatics Analysis. Nanomaterials 2022, 12, 3507. https://doi.org/10.3390/nano12193507
Zhang T, Yang S, Ge Y, Wan X, Zhu Y, Li J, Yin L, Pu Y, Liang G. Polystyrene Nanoplastics Induce Lung Injury via Activating Oxidative Stress: Molecular Insights from Bioinformatics Analysis. Nanomaterials. 2022; 12(19):3507. https://doi.org/10.3390/nano12193507
Chicago/Turabian StyleZhang, Tianyi, Sheng Yang, Yiling Ge, Xin Wan, Yuxin Zhu, Jie Li, Lihong Yin, Yuepu Pu, and Geyu Liang. 2022. "Polystyrene Nanoplastics Induce Lung Injury via Activating Oxidative Stress: Molecular Insights from Bioinformatics Analysis" Nanomaterials 12, no. 19: 3507. https://doi.org/10.3390/nano12193507
APA StyleZhang, T., Yang, S., Ge, Y., Wan, X., Zhu, Y., Li, J., Yin, L., Pu, Y., & Liang, G. (2022). Polystyrene Nanoplastics Induce Lung Injury via Activating Oxidative Stress: Molecular Insights from Bioinformatics Analysis. Nanomaterials, 12(19), 3507. https://doi.org/10.3390/nano12193507