Synthesis of CNT@CoS/NiCo Layered Double Hydroxides with Hollow Nanocages to Enhance Supercapacitors Performance
Abstract
:1. Introduction
2. Materials and Methods
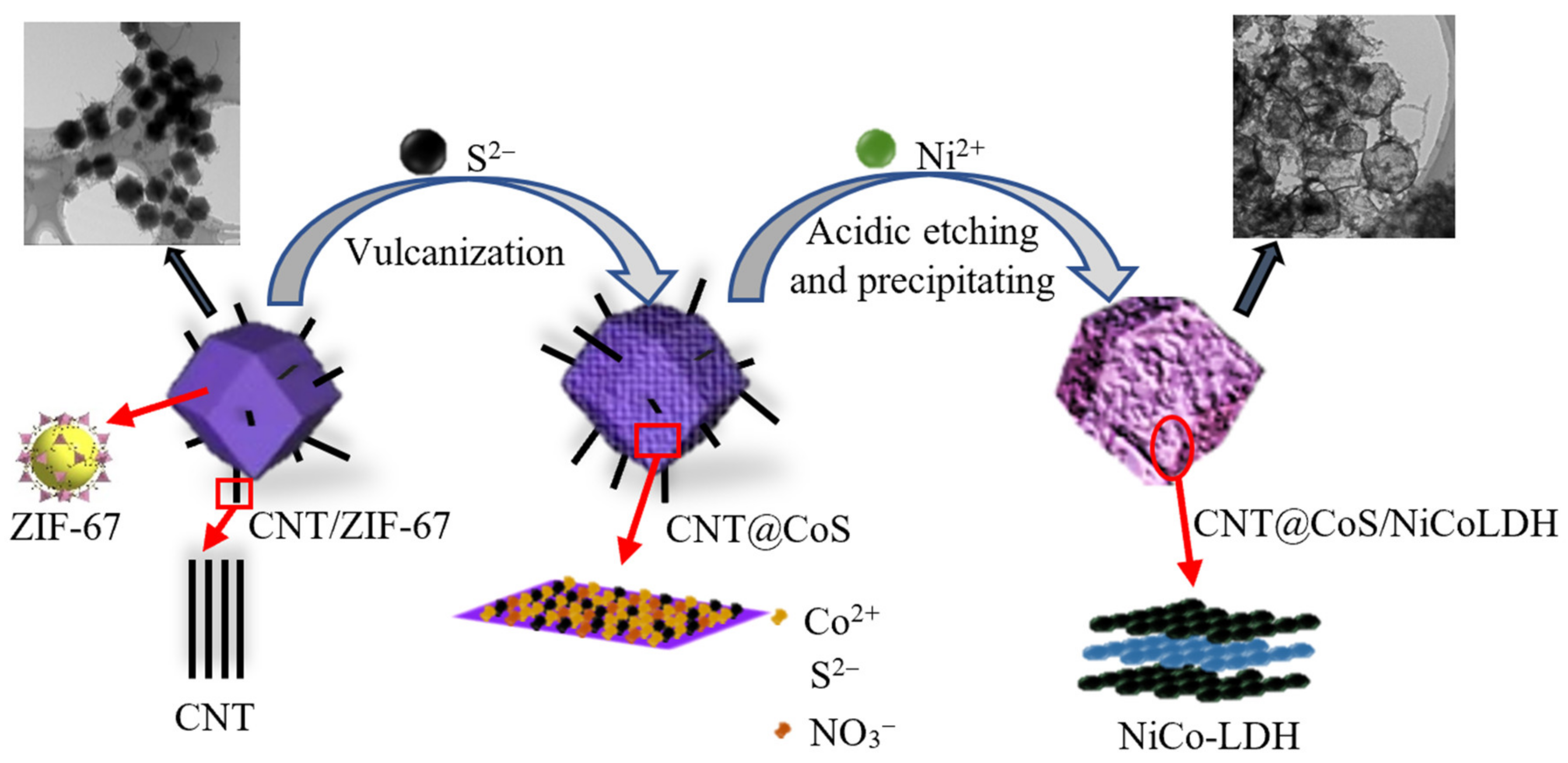
2.1. Preparation of CNT@CoS Precursor
2.2. Preparation of CNT@CoS/NiCo-LDH
2.3. Characterization
2.4. Electrochemical Measurements
3. Results and Discussion
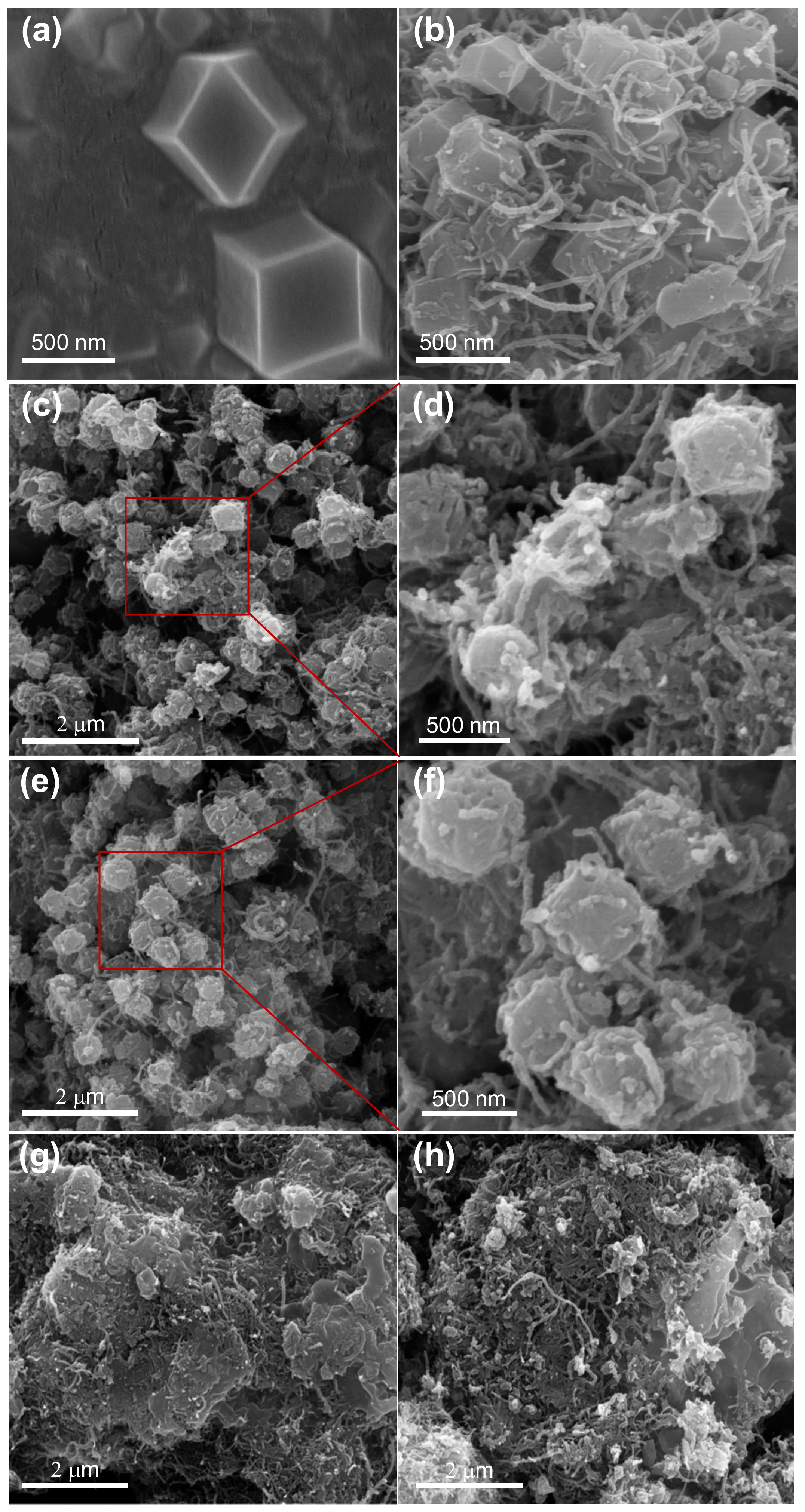
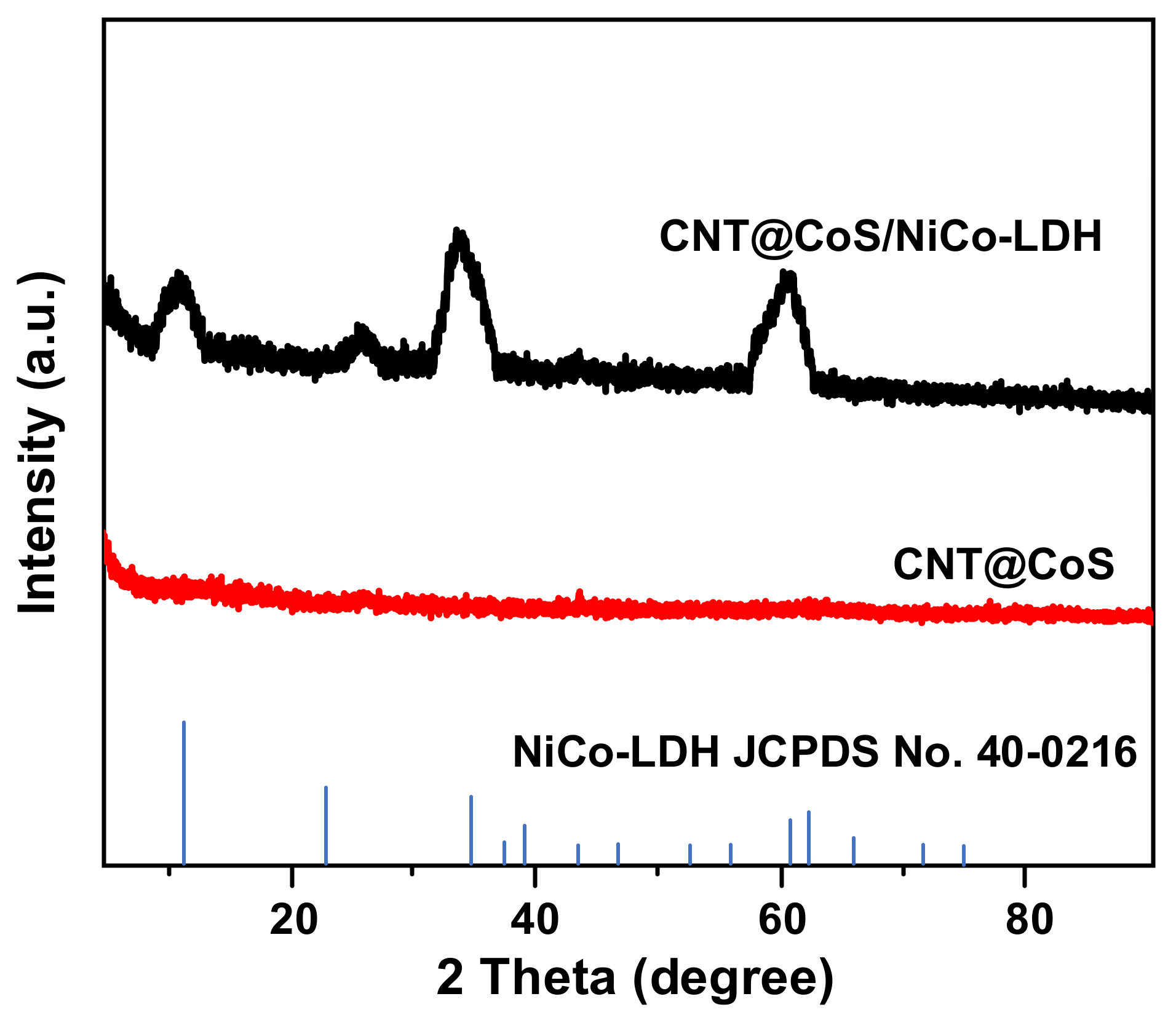
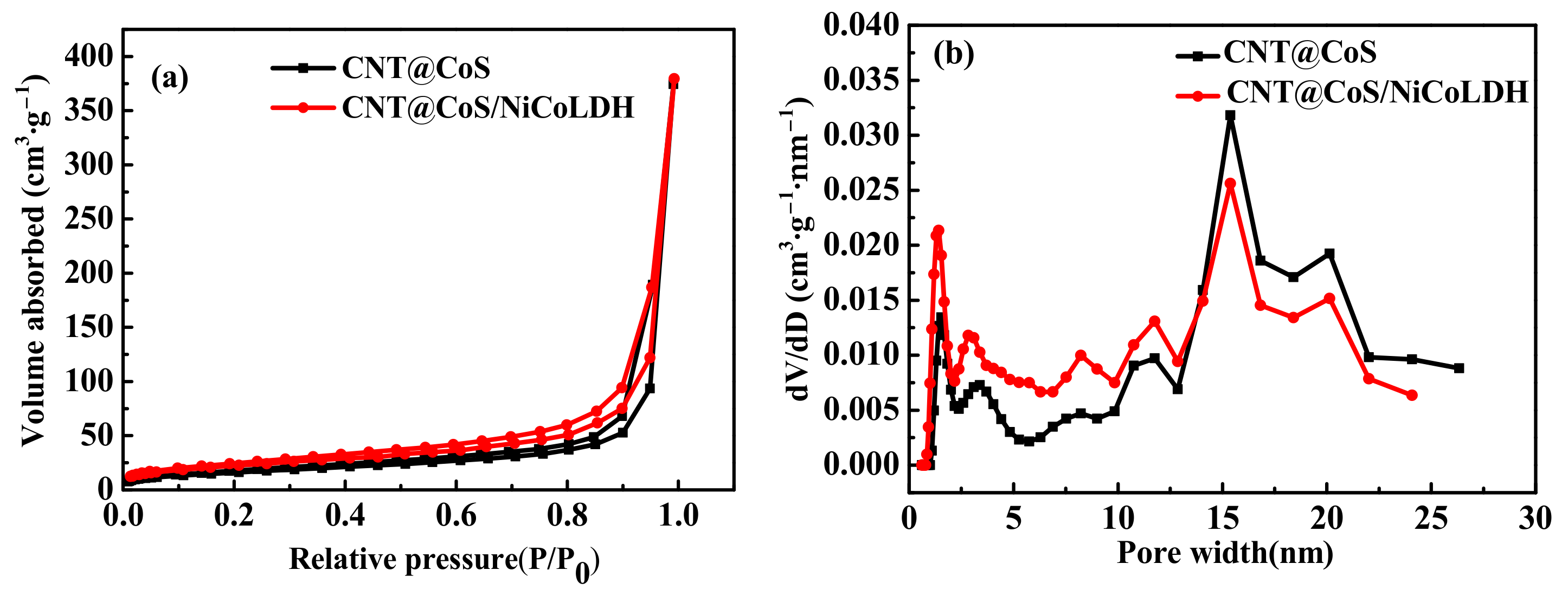
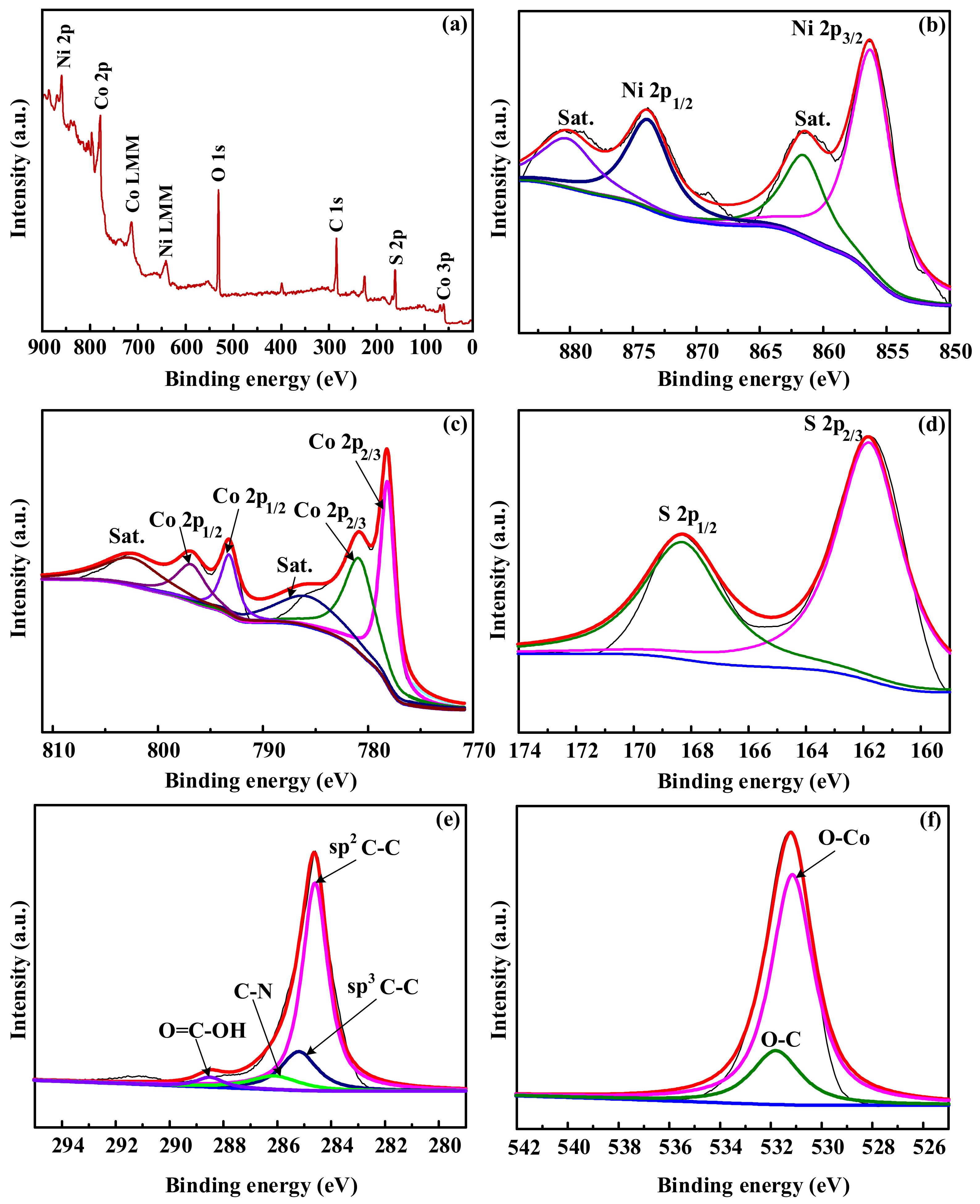
3.1. Characterization of the Samples
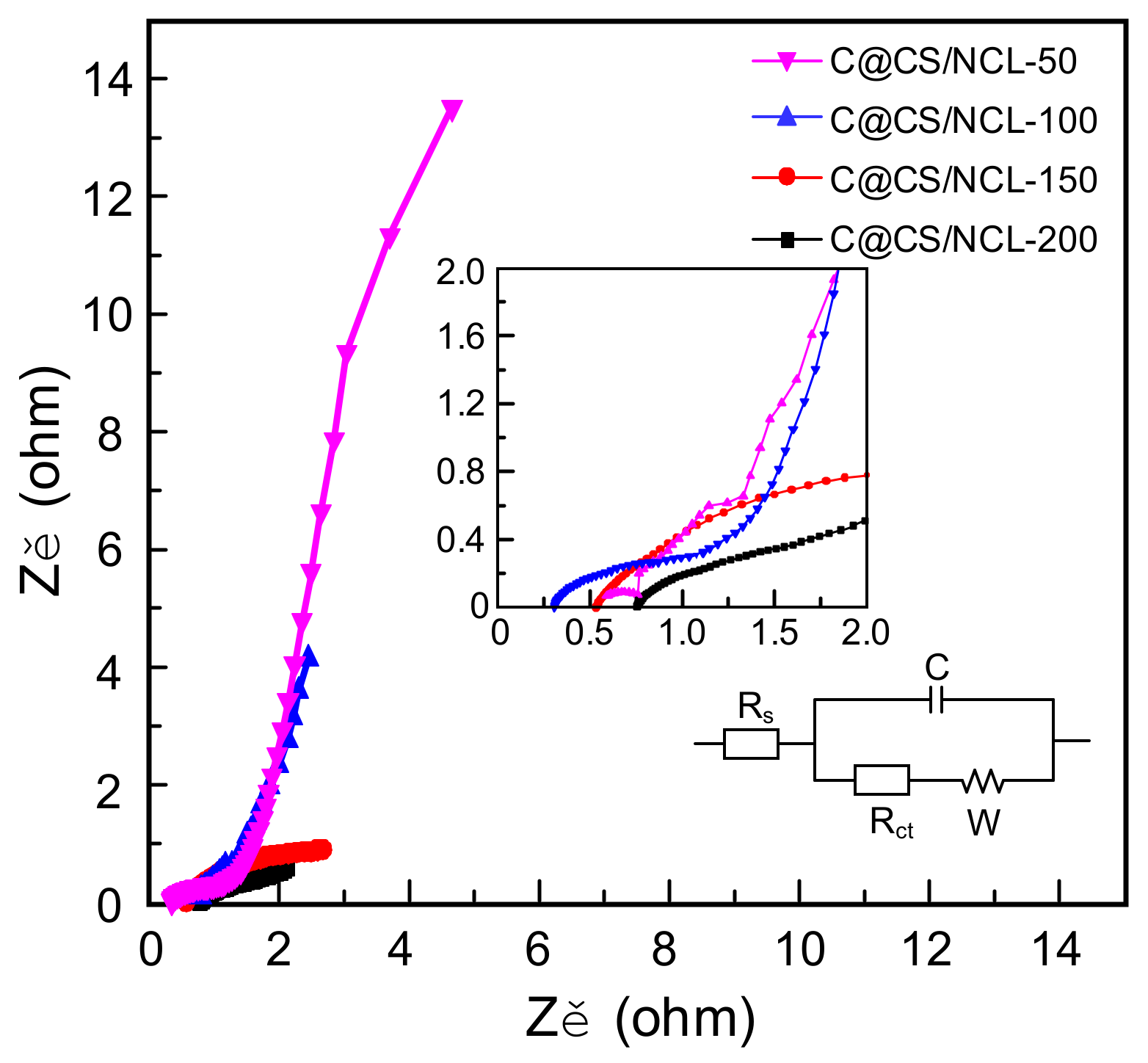
3.2. Electrochemical Measurements
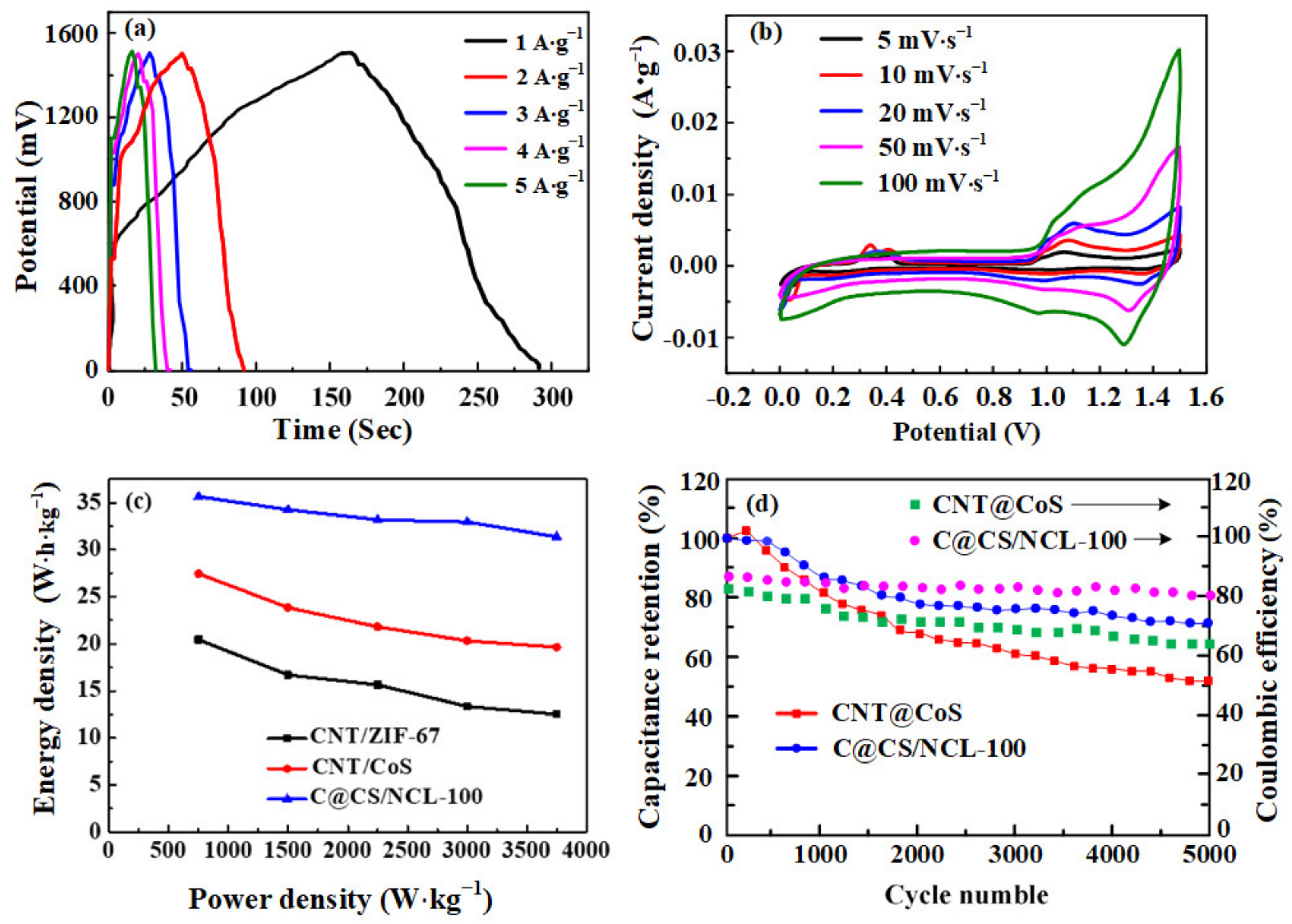
3.3. Assembly of All-Solid-State Asymmetric Supercapacitor
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Arico, A.S.; Bruce, P.; Scrosati, B.; Tarascon, J.M.; Van Schalkwijk, W. Nanostructured materials for advanced energy conversion and storage devices. Nat. Mater. 2005, 4, 366–377. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, L.; Zhang, J. A review of electrode materials for electrochemical supercapacitors. Chem. Soc. Rev. 2021, 41, 797–828. [Google Scholar] [CrossRef] [Green Version]
- Yi, T.; Chang, H.; Wei, T.; Qi, S.; Li, Y.; Zhu, Y. Approaching high-performance electrode materials of ZnCo2S4 nanoparticle wrapped carbon nanotubes for supercapacitors. J. Mater. 2021, 7, 563–576. [Google Scholar] [CrossRef]
- Li, M.; Yang, W.; Huang, Y.; Yu, Y. Hierarchical mesoporous Co3O4@ZnCo2O4 hybrid nanowire arrays supported on Ni foam for high-performance asymmetric supercapacitors. Sci. China-Mater. 2018, 61, 1167–1176. [Google Scholar] [CrossRef] [Green Version]
- Yan, S.; Luo, S.; Feng, J.; Li, P.; Guo, R.; Wang, Q.; Zhang, Y.; Liu, Y.; Bao, S. Rational design of flower-like FeCo2S4/reduced graphene oxide films: Novel binder-free electrodes with ultra-high conductivity flexible substrate for high-performance all-solid-state pseudocapacitor. Chem. Eng. J. 2020, 381, 122695. [Google Scholar] [CrossRef]
- Elsaid, M.A.M.; Hassan, A.A.; Mohamed, S.G.; Sayed, A.Z.; Ashmawy, A.M.; Waheed, A.F. Synthesis and electrochemical performance of porous FeCo2S4 nanorods as an electrode material for supercapacitor. J. Energy Storage 2021, 44, 103330. [Google Scholar] [CrossRef]
- Tian, D.; Ao, Y.; Li, W.; Xu, J.; Wang, C. General fabrication of metal-organic frameworks on electrospun modified carbon nanofibers for high-performance asymmetric supercapacitors. J. Colloid Interface Sci. 2021, 603, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Pershaanaa, M.; Bashir, S.; Ramesh, S.; Ramesh, K. Every bite of Supercap: A brief review on construction and enhancement of supercapacitor. J. Energy Storage 2022, 50, 104599. [Google Scholar] [CrossRef]
- Béguin, F.; Presser, V.; Balducci, A.; Frackowiak, E. Carbons and Electrolytes for Advanced Supercapacitors. Adv. Mater. 2014, 26, 2219–2251. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Zhang, H.; Hu, B.; Wang, M.; Zhang, S.; Jia, Q.; He, L.; Zhang, Z. The intergrated nanostructure of bimetallic CoNi-based zeolitic imidazolate framework and carbon nanotubes as high-performance electrochemical supercapacitors. J. Colloid Interface Sci. 2022, 608, 1257–1267. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Zheng, R.; Liu, Y.; Ying, Y.; Shi, W. Carbon nanotubes interpenetrating MOFs-derived Co-Ni-S composite spheres with interconnected architecture for high performance hybrid supercapacitor. J. Colloid Interface Sci. 2021, 602, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Wei, J.; Zhao, Y.; Shen, Y.; Pan, Y.; Wei, Y.; Mao, L. Metal-organic framework derived petal-like Co3O4@CoNi2S4 hybrid on carbon cloth with enhanced performance for supercapacitors. Inorg. Chem. Front. 2020, 7, 1428–1436. [Google Scholar] [CrossRef]
- Ahmad, R.; Khan, U.A.; Iqbal, N.; Noor, T. Zeolitic imidazolate framework (ZIF)-derived porous carbon materials for supercapacitors: An overview. RSC Adv. 2020, 10, 43733–43750. [Google Scholar] [CrossRef]
- Sun, S.; Wang, Y.; Chen, L.; Chu, M.; Dong, Y.; Liu, D.; Liu, P.; Qu, D.; Duan, J.; Li, X. MOF(Ni)/CNT composites with layer structure for high capacitive performance. Colloids Surf. A Physicochem. Eng. Asp. 2022, 643, 128802. [Google Scholar] [CrossRef]
- Chu, X.; Meng, F.; Yang, H.; Zhang, W.; Qin, T.; Wang, Z.; Molin, S.; Jasinski, P.; Zheng, W. Cu-Doped Layered Double Hydroxide Constructs the Performance-Enhanced Supercapacitor Via Band Gap Reduction and Defect Triggering. ACS Appl. Energy Mater. 2022, 5, 2192–2201. [Google Scholar] [CrossRef]
- Tong, H.; Meng, Q.; Liu, J.; Li, T.; Gong, D.; Xiao, J.; Shen, L.; Zhang, T.; Bing, D.; Zhang, X. Cross-linked NiCo2O4 nanosheets with low crystallinity and rich oxygen vacancies for asymmetric supercapacitors. J. Alloys Compd. 2020, 822, 153689. [Google Scholar] [CrossRef]
- Kim, K.S.; Shinde, N.M.; Yun, J.M.; Kim, K.H. Sulfur and phosphorus co-doped nickel-cobalt layered double hydroxides for enhancing electrochemical reactivity and supercapacitor performance. RSC Adv. 2021, 11, 12449–12459. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Duan, Y.; Teng, Y.; Fan, N.; Huo, Y. MOF-derived tremelliform Co3O4/NiO/Mn2O3 with excellent capacitive performance. Appl. Surf. Sci. 2019, 478, 247–254. [Google Scholar] [CrossRef]
- Li, Q.; Yue, L.; Li, L.; Liu, H.; Yao, W.; Wu, N.; Zhang, L.; Guo, H.; Yang, W. Metal-organic frameworks derived N, S co-doped bimetal nanocomposites as high-performance electrodes materials for supercapacitor. J. Alloys Compd. 2019, 810, 151961. [Google Scholar] [CrossRef]
- Liu, L.; Guan, T.; Fang, L.; Wu, F.; Lu, Y.; Luo, H.; Song, X.; Zhou, M.; Hu, B.; Wei, D.; et al. Self-supported 3D NiCo-LDH/Gr composite nanosheets array electrode for high-performance supercapacitor. J. Alloys Compd. 2018, 763, 926–934. [Google Scholar] [CrossRef]
- Shi, Z.; Zhu, J.; Li, Z.; Xiao, Q.; Zhu, J. Sulfur-Doped Nickel-Cobalt Double Hydroxide Electrodes for High-Performance Asymmetric Supercapacitors. ACS Appl. Energy Mater. 2020, 3, 11082–11090. [Google Scholar] [CrossRef]
- Li, P.; Liu, X.; Arif, M.; Yan, H.; Hu, C.; Chen, S.; Liu, X. In situ growth of glucose-intercalated LDHs on NiCo2S4 hollow nanospheres to enhance energy storage capacity for hybrid supercapacitors. Colloids Surf. A Physicochem. Eng. Asp. 2022, 644, 128823. [Google Scholar] [CrossRef]
- Saraf, M.; Rajak, R.; Mobin, S.M. MOF Derived High Surface Area Enabled Porous Co3O4 Nanoparticles for Supercapacitors. ChemistrySelect 2019, 4, 8142–8149. [Google Scholar] [CrossRef]
- Liu, Z.; Qiu, Y.; Zhang, A.; Yang, W.; Barrow, C.J.; Razal, J.M.; Liu, J. In situ embedding of cobalt sulfide quantum dots among transition metal layered double hydroxides for high performance all-solid-state asymmetric supercapacitors. J. Mater. Chem. A 2021, 9, 22573–22584. [Google Scholar] [CrossRef]
- Li, Y.; Wang, H.; Shu, T.; Yuan, J.; Lu, G.; Lin, B.; Gao, Z.; Wei, F.; Ma, C.; Qi, J.; et al. Two-dimensional hierarchical MoS2 lamella inserted in CoS2 flake as an advanced supercapacitor electrode. J. Energy Storage 2022, 51, 104299. [Google Scholar] [CrossRef]
- Yao, L.; Zhao, J.; Pan, Q.; Li, X.; Xing, B.; Jiang, S.; Song, J.; Pang, M. Tailoring NiO@NiFe2O4/CNTs triphase hybrids towards high-performance anode for lithium-ion batteries. J. Alloys Compd. 2022, 912, 165209. [Google Scholar] [CrossRef]
- Rajesh, D.; Francis, M.K.; Bhargav, P.B.; Nafis, A.; Balaji, C. 2D layered nickel-cobalt double hydroxide nano sheets @ 1D silver nanowire-graphitic carbon nitrides for high performance super capacitors. J. Alloys Compd. 2022, 898, 162803. [Google Scholar] [CrossRef]
- Wang, M.; Feng, Y.; Zhang, Y.; Li, S.; Wu, M.; Xue, L.; Zhao, J.; Zhang, W.; Ge, M.; Lai, Y.; et al. Ion regulation of hollow nickel cobalt layered double hydroxide nanocages derived from ZIF-67 for High-Performance supercapacitors. Appl. Surf. Sci. 2022, 596, 153582. [Google Scholar] [CrossRef]
- Jiang, Z.; Lu, W.; Li, Z.; Ho, K.H.; Li, X.; Jiao, X.; Chen, D. Synthesis of amorphous cobalt sulfide polyhedral nanocages for high performance supercapacitors. J. Mater. Chem. A 2014, 2, 8603–8606. [Google Scholar] [CrossRef]
- Zheng, K.; Liao, L.; Zhang, Y.; Tan, H.; Liu, J.; Li, C.; Jia, D. Hierarchical NiCo-LDH core/shell homostructural electrodes with MOF-derived shell for electrochemical energy storage. J. Colloid Interface Sci. 2022, 619, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Hung, P.; Wang, G.; Chung, W.; Chiang, T.; Wu, P. Green Synthesis of Ni@PEDOT and Ni@PEDOT/Au (Core@Shell) Inverse Opals for Simultaneous Detection of Ascorbic Acid, Dopamine, and Uric Acid. Nanomaterials 2020, 10, 1722. [Google Scholar] [CrossRef] [PubMed]
- Liao, M.; Zhang, Q.; Tang, F.; Xu, Z.; Zhou, X.; Li, Y.; Zhang, Y.; Yang, C.; Ru, Q.; Zhao, L. Nanosized CoO Loaded on Copper Foam for High-Performance, Binder-Free Lithium-Ion Batteries. Nanomaterials 2018, 8, 183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bai, X.; Liu, J.; Liu, Q.; Chen, R.; Jing, X.; Li, B.; Wang, J. In-Situ Fabrication of MOF-Derived Co-Co Layered Double Hydroxide Hollow Nanocages/Graphene Composite: A Novel Electrode Material with Superior Electrochemical Performance. Chem.-Eur. J. 2017, 23, 14839–14847. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Han, C.; Song, X. Facile synthesis of novel CoNi2S4/carbon nanofibers composite for high-performance supercapacitor. Mater. Chem. Phys. 2022, 283, 126038. [Google Scholar] [CrossRef]
- Zhou, X.; Zhu, L.; Yang, Y.; Xu, L.; Qian, X.; Zhou, J.; Dong, W.; Jiang, M. High-yield and nitrogen self-doped hierarchical porous carbon from polyurethane foam for high-performance supercapacitors. Chemosphere 2022, 300, 134552. [Google Scholar] [CrossRef]
- Li, J.; Zou, Y.; Jin, L.; Xu, F.; Sun, L.; Xiang, C. Polydopamine-assisted NiMoO4 nanorods anchored on graphene as an electrode material for supercapacitor applications. J. Energy Storage 2022, 50, 104639. [Google Scholar] [CrossRef]
- Peçenek, H.; Dokan, F.K.; Onses, M.S.; Yılmaz, E.; Sahmetlioglu, E. Outstanding supercapacitor performance with intertwined flower-like NiO/MnO2/CNT electrodes. Mater. Res. Bull. 2022, 149, 111745. [Google Scholar] [CrossRef]
- Dong, K.; Yang, Z.; Shi, D.; Chen, M.; Dong, W. N-doped carbon coating for stabilizing metal sulfides on carbon materials for high cycle life asymmetric supercapacitors. J. Mater. Sci. Mater. Electron. 2022, 33, 10928–10938. [Google Scholar] [CrossRef]
- Sun, X.; Xu, W.; Zhang, X.; Lei, T.; Lee, S.; Wu, Q. ZIF-67@Cellulose nanofiber hybrid membrane with controlled porosity for use as Li-ion battery separator. J. Energy Chem. 2021, 52, 170–180. [Google Scholar] [CrossRef]
- Li, K.; Zhao, B.; Bai, J.; Ma, H.; Fang, Z.; Zhu, X.; Sun, Y. A High-Energy-Density Hybrid Supercapacitor with P-Ni(OH)2@Co(OH)2 Core-Shell Heterostructure and Fe2O3 Nanoneedle Arrays as Advanced Integrated Electrodes. Small 2020, 16, 2001974. [Google Scholar]
- Chen, H.; Wang, G.; Chen, L.; Dai, B.; Yu, F. Three-Dimensional Honeycomb-Like Porous Carbon with Both Interconnected Hierarchical Porosity and Nitrogen Self-Doping from Cotton Seed Husk for Supercapacitor Electrode. Nanomaterials 2018, 8, 412. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Liu, Y.; Yu, S.; Guo, W.; Mu, S.; Wang, H.; Zhao, Y.; Hou, L.; Fan, Y.; Gao, F. Template-free hydrothermal synthesis of nickel cobalt hydroxide nanoflowers with high performance for asymmetric supercapacitor. Electrochim. Acta 2015, 161, 279–289. [Google Scholar] [CrossRef]
- Zang, Y.; Luo, H.; Zhang, H.; Xue, H. Polypyrrole Nanotube-Interconnected NiCo-LDH Nanocages Derived by ZIF-67 for Supercapacitors. ACS Appl. Energ. Mater. 2021, 4, 1189–1198. [Google Scholar] [CrossRef]
- Guan, X.; Huang, M.; Yang, L.; Wang, G.; Guan, X. Facial design and synthesis of CoSx/Ni-Co LDH nanocages with rhombic dodecahedral structure for high-performance asymmetric supercapacitors. Chem. Eng. J. 2019, 372, 151–162. [Google Scholar] [CrossRef]
- Niu, H.; Zhang, Y.; Liu, Y.; Xin, N.; Shi, W. NiCo-layered double-hydroxide and carbon nanosheets microarray derived from MOFs for high performance hybrid supercapacitors. J. Colloid Interface Sci. 2019, 539, 545–552. [Google Scholar] [CrossRef] [PubMed]

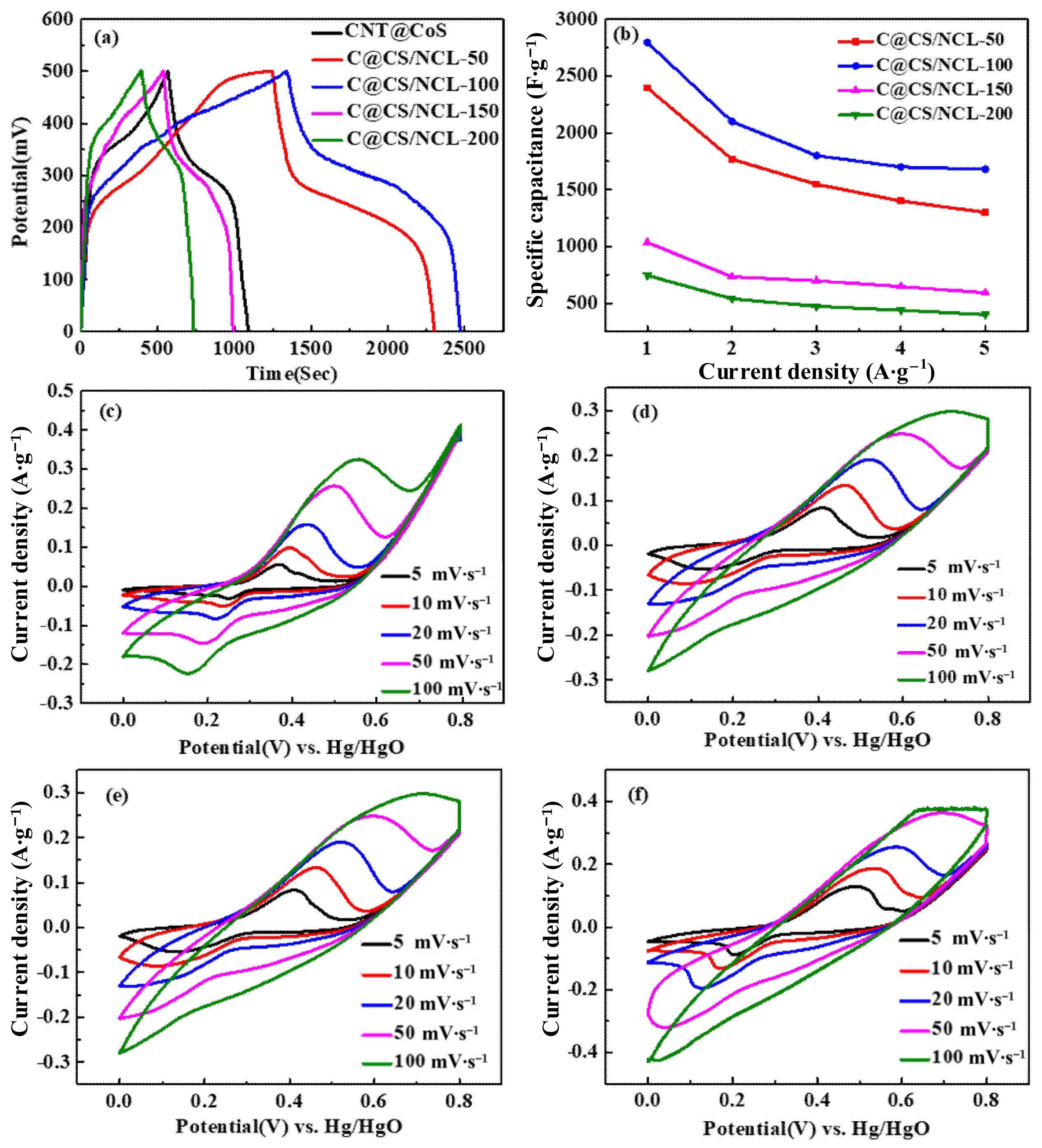
| Current Density (A·g−1) | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| C@CS/NCL-50 | 2396.7 | 1768.1 | 1547.4 | 1399.6 | 1299.7 |
| C@CS/NCL-100 | 2794.6 | 2100.5 | 1800.4 | 1700.7 | 1680.0 |
| C@CS/NCL-150 | 1035.5 | 733.9 | 699.0 | 648.7 | 592.7 |
| C@CS/NCL-200 | 746.0 | 733.9 | 473.9 | 439.9 | 400.0 |
| CNT@CoS | 1920.9 | 1679.8 | 1550.1 | 1414.8 | 1284.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yue, X.; Chen, Z.; Xiao, C.; Song, G.; Zhang, S.; He, H. Synthesis of CNT@CoS/NiCo Layered Double Hydroxides with Hollow Nanocages to Enhance Supercapacitors Performance. Nanomaterials 2022, 12, 3509. https://doi.org/10.3390/nano12193509
Yue X, Chen Z, Xiao C, Song G, Zhang S, He H. Synthesis of CNT@CoS/NiCo Layered Double Hydroxides with Hollow Nanocages to Enhance Supercapacitors Performance. Nanomaterials. 2022; 12(19):3509. https://doi.org/10.3390/nano12193509
Chicago/Turabian StyleYue, Xiaoming, Zihua Chen, Cuicui Xiao, Guohao Song, Shuangquan Zhang, and Hu He. 2022. "Synthesis of CNT@CoS/NiCo Layered Double Hydroxides with Hollow Nanocages to Enhance Supercapacitors Performance" Nanomaterials 12, no. 19: 3509. https://doi.org/10.3390/nano12193509





