Effect of Post-Deposition Annealing on the Structural Evolution and Optoelectronic Properties of In2O3:H Thin Films
Abstract
:1. Introduction
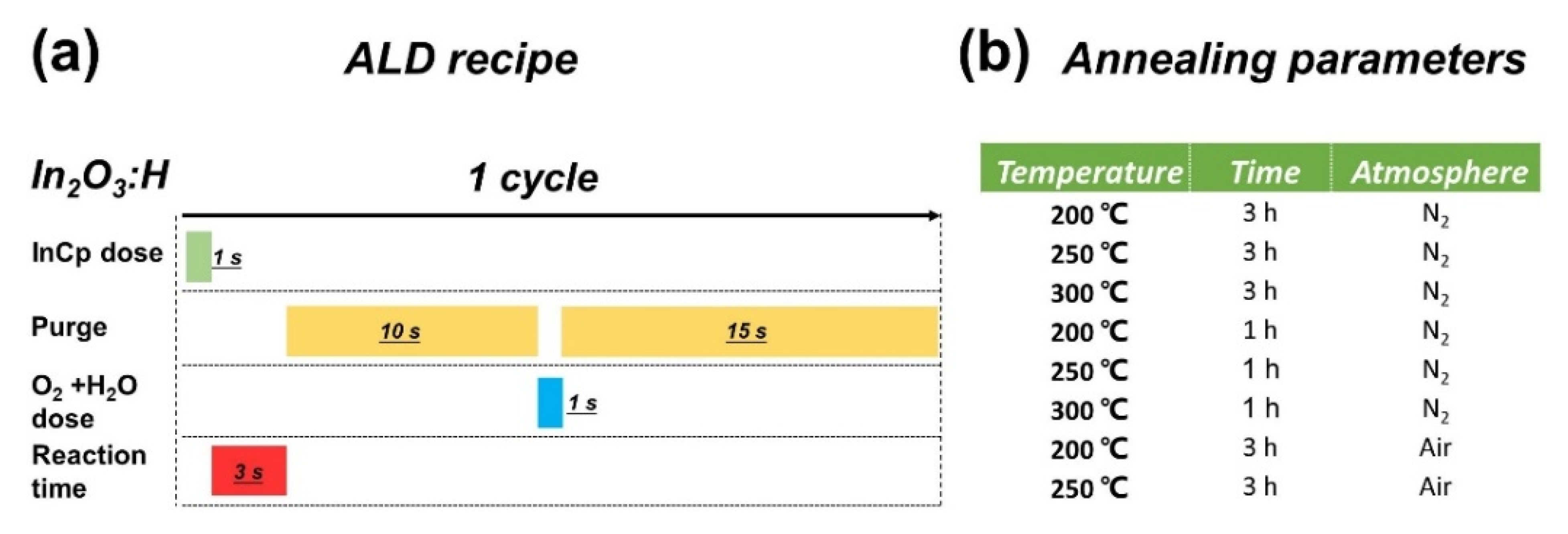
2. Materials and Methods
3. Results and Discussion
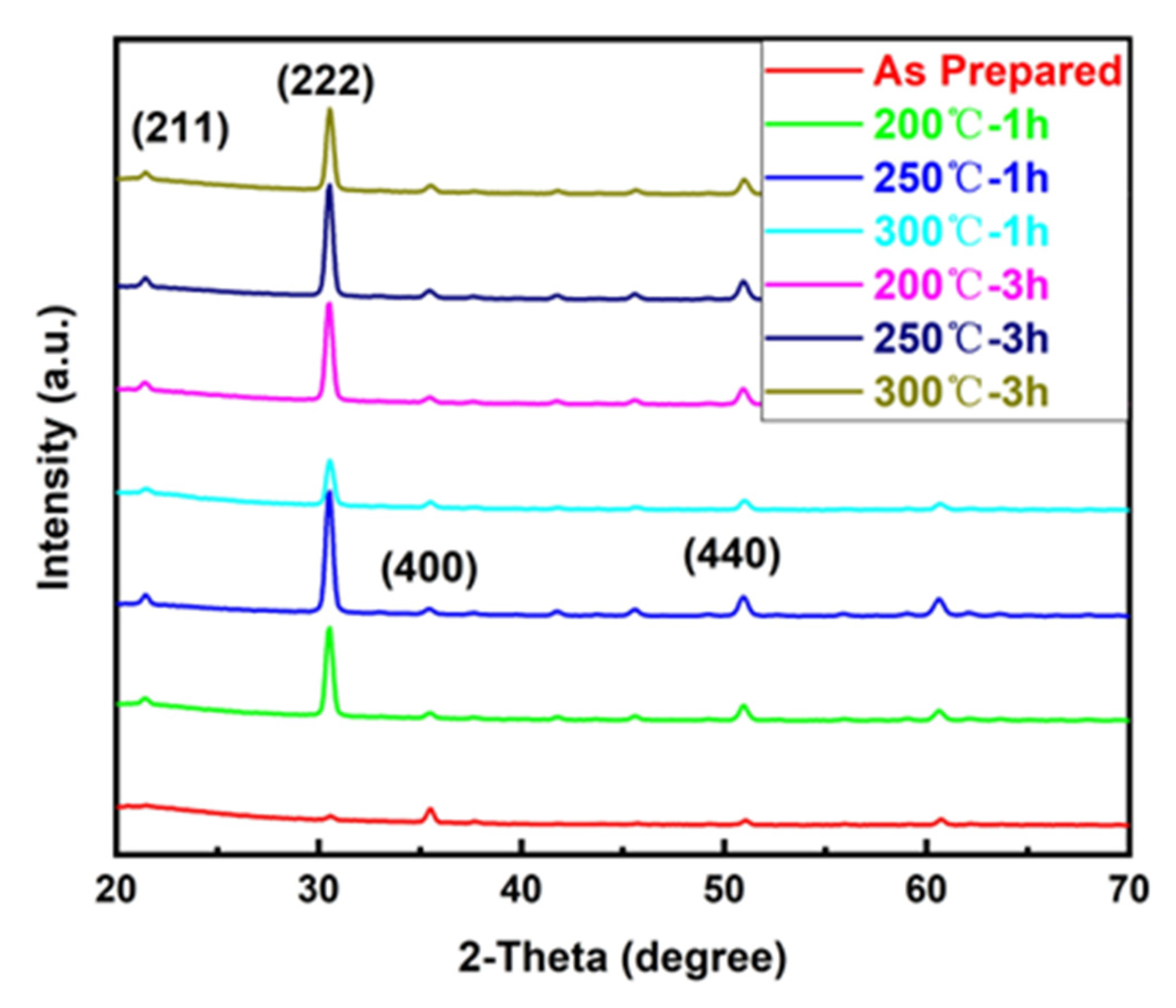
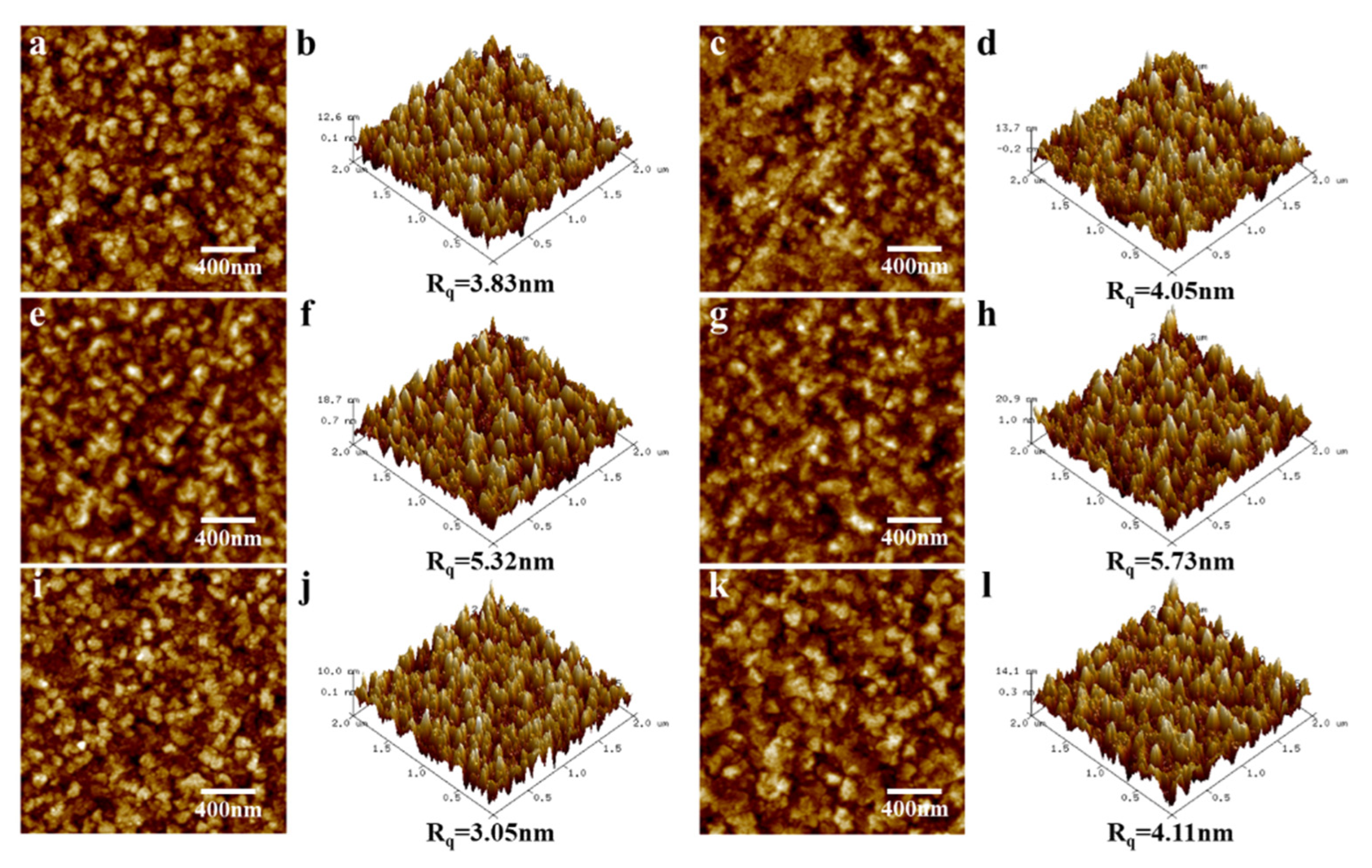
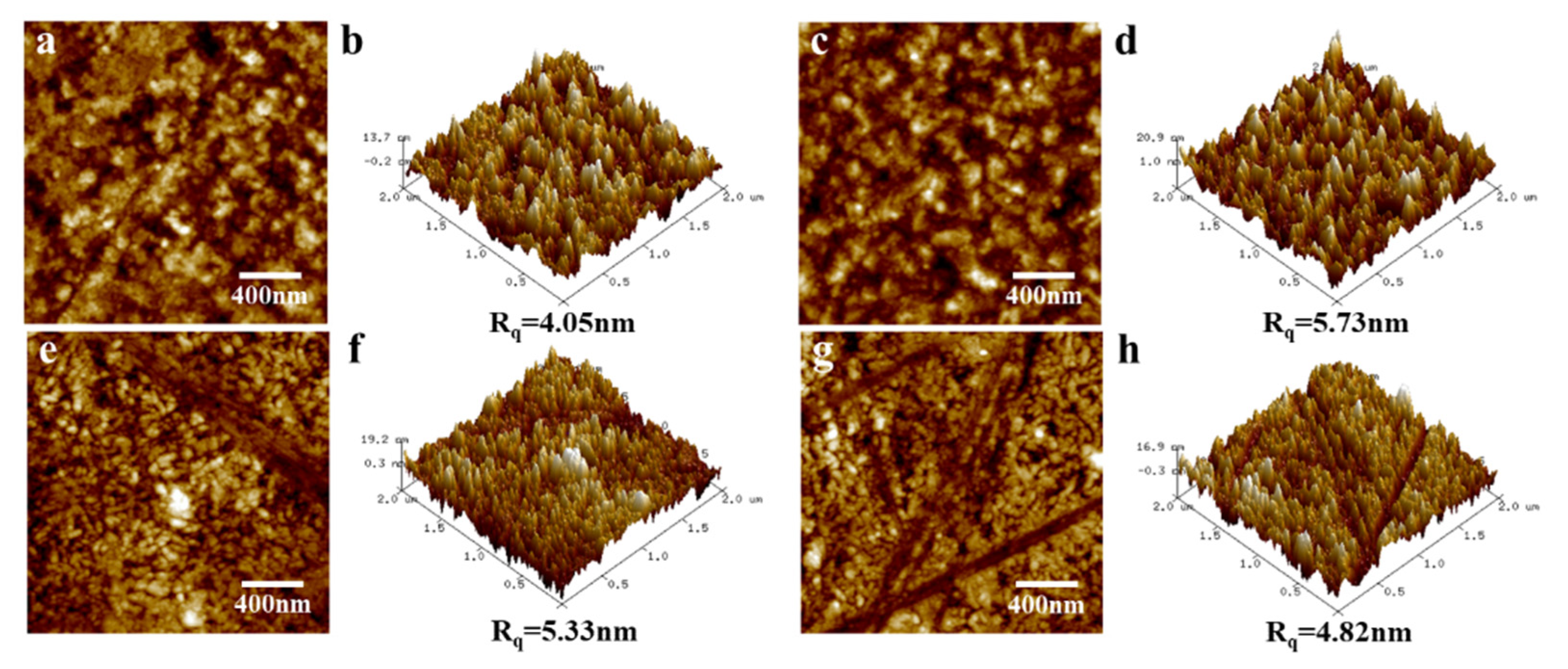
3.1. Effect of Annealing Temperature and Time on Microstructure
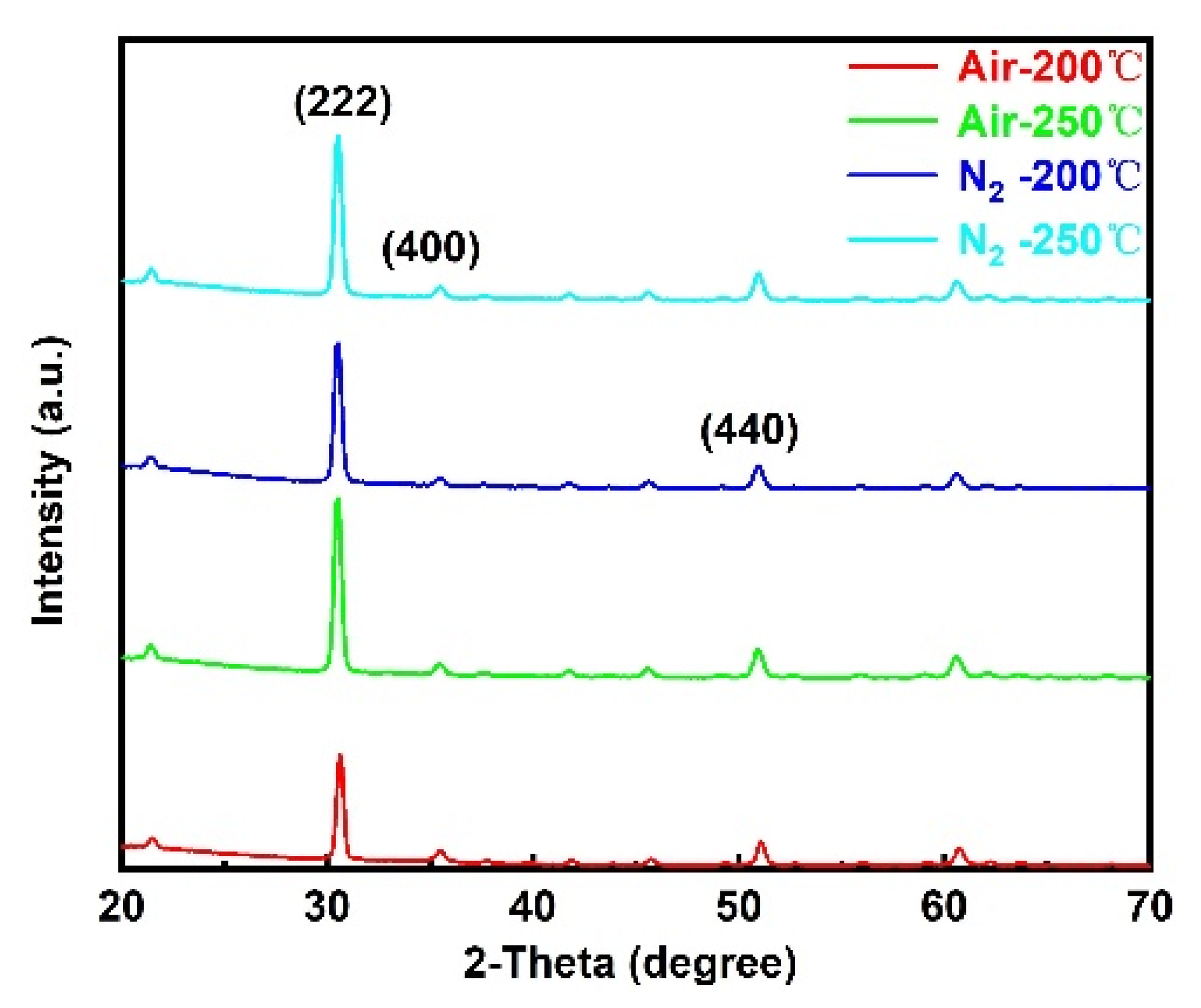
3.2. Effect of Annealing Atmosphere on Microstructure
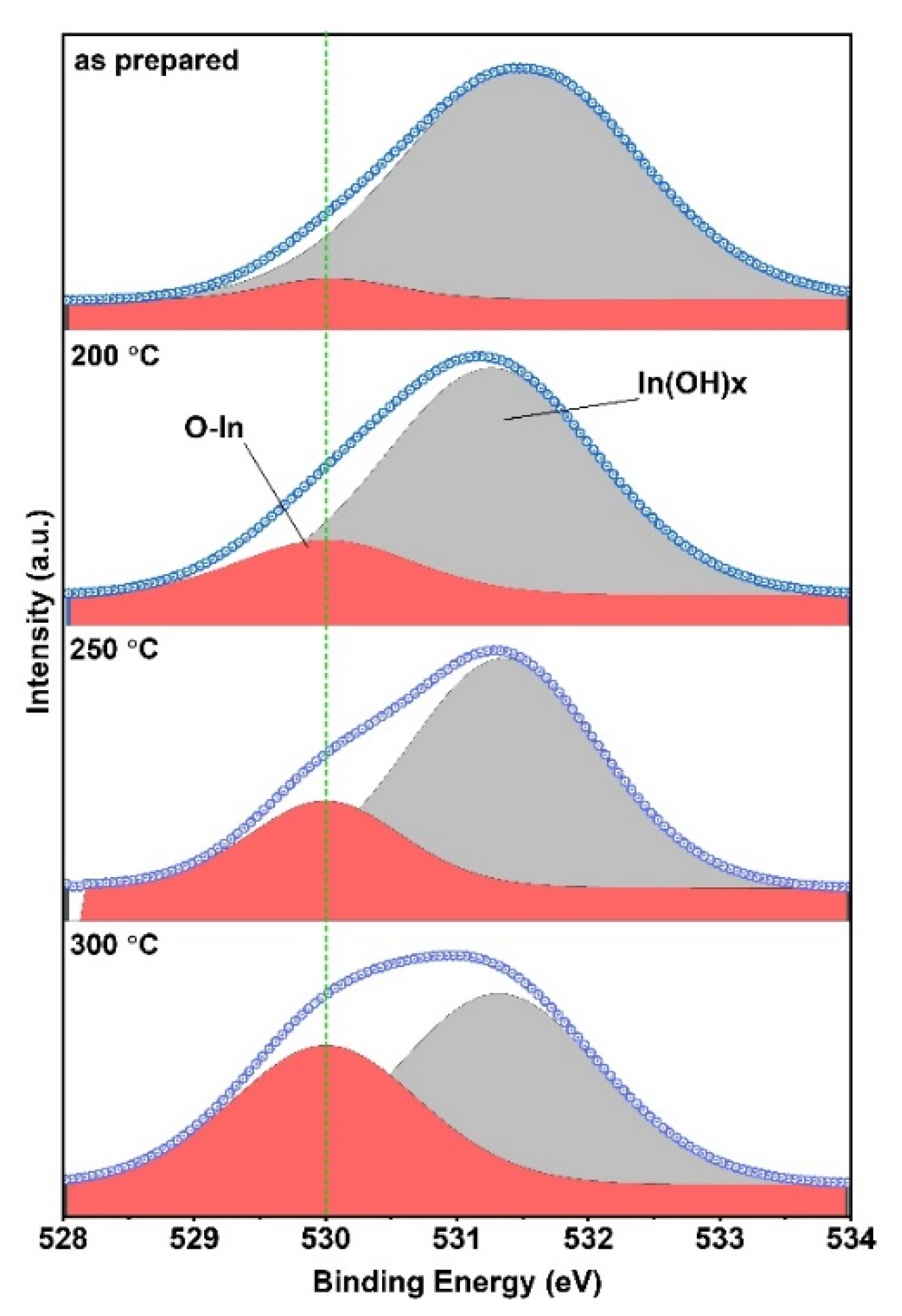
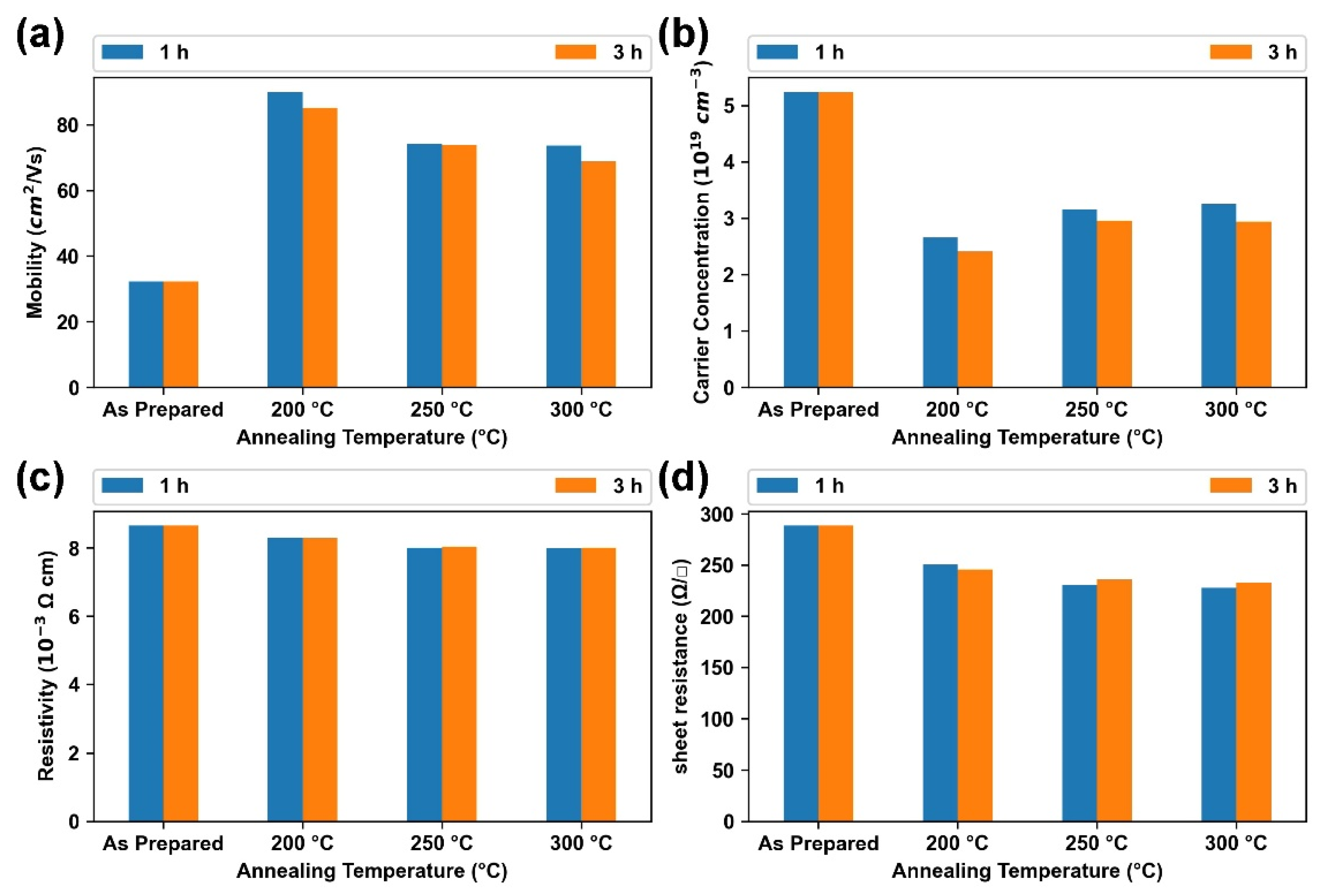
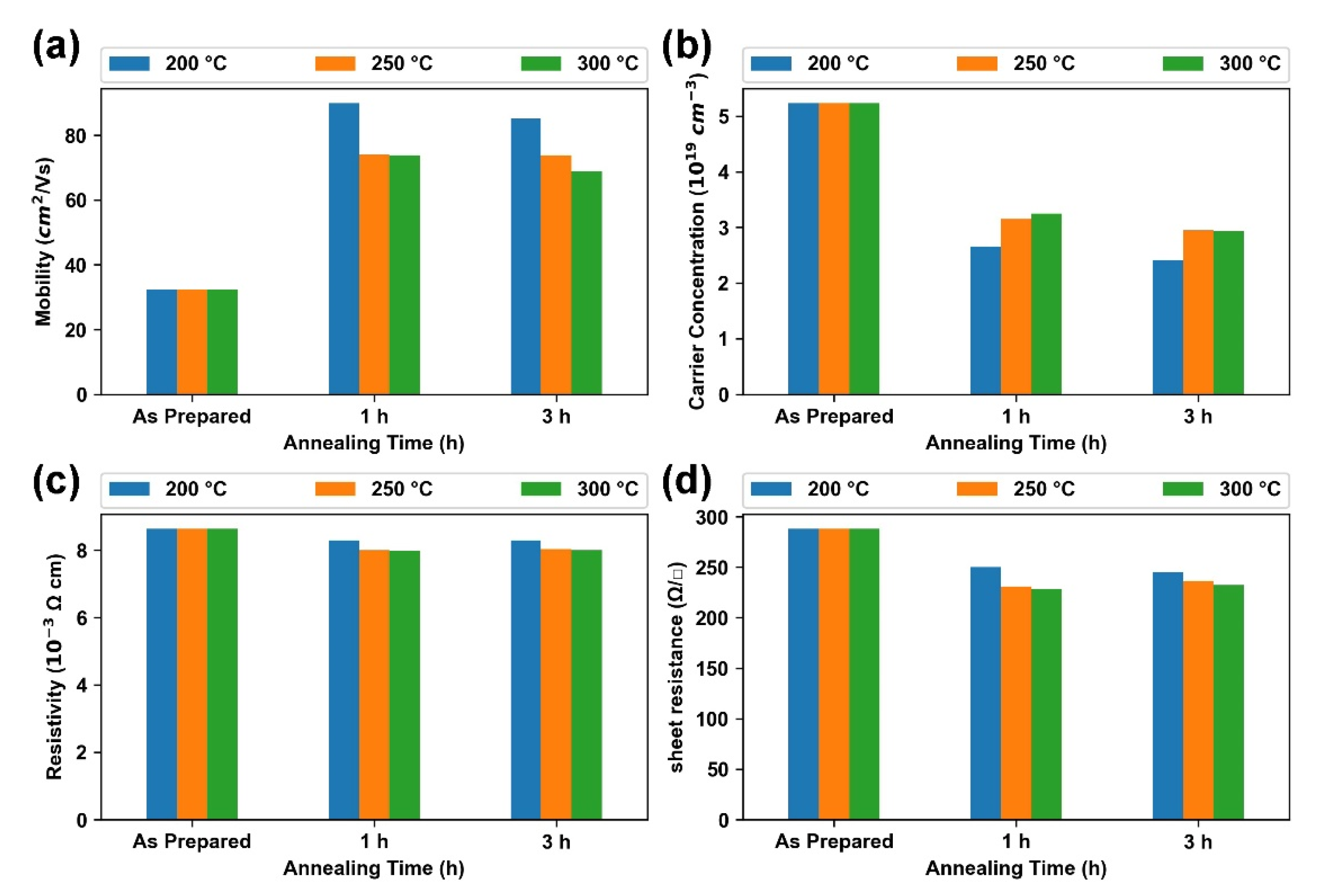
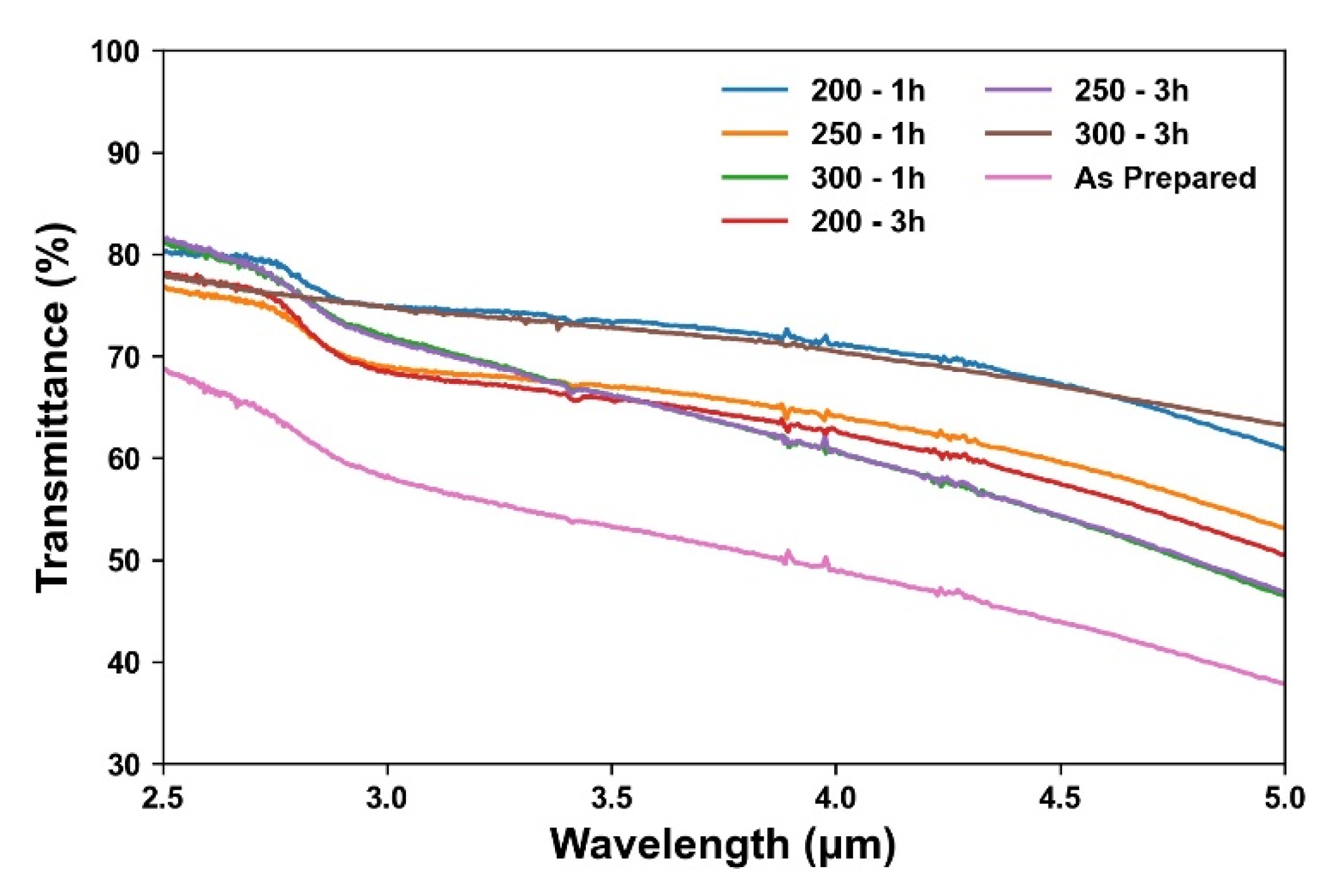
3.3. Effect of Annealing Temperature and Time on Optoelectronic Properties
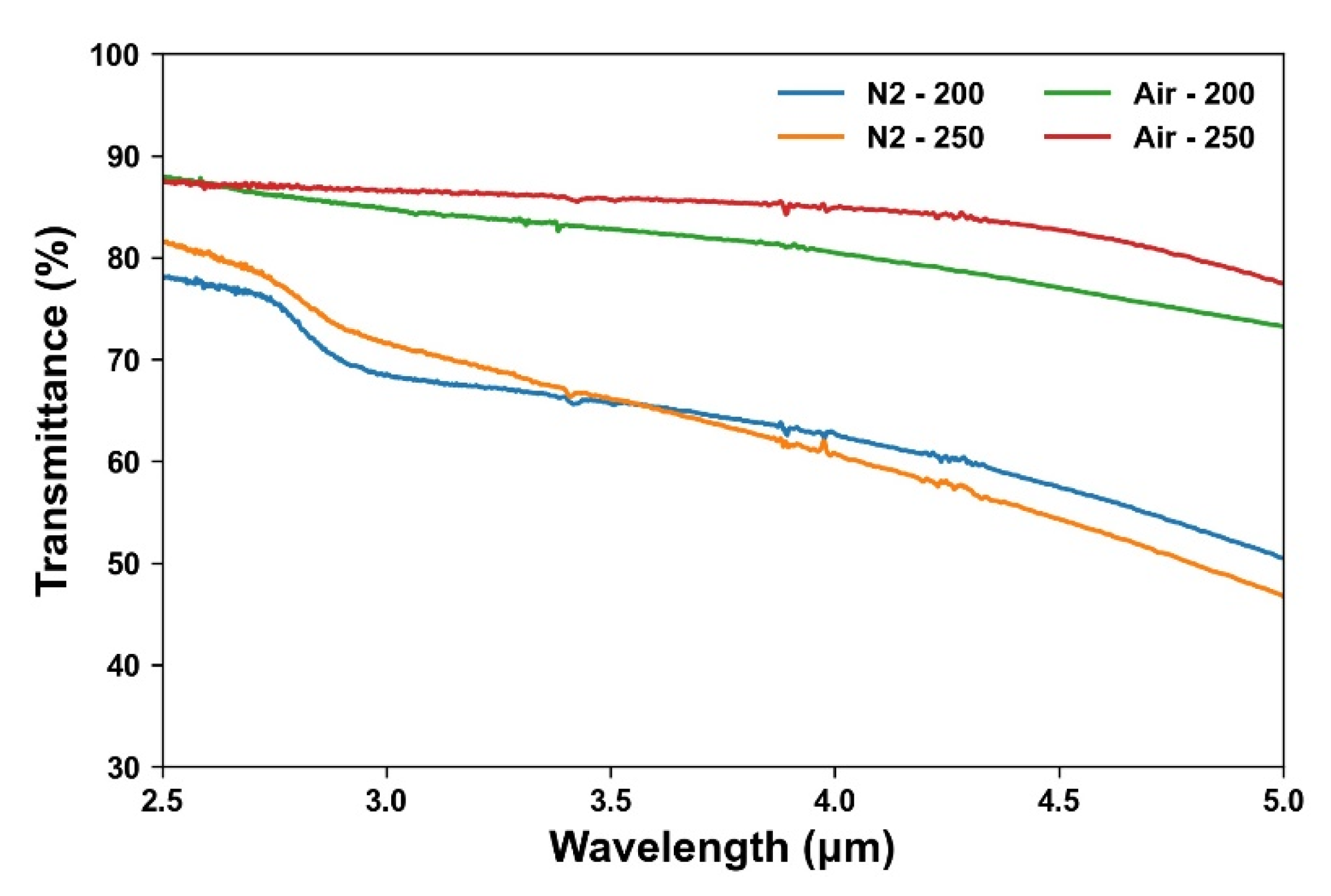
3.4. Effect of Annealing Atmosphere on the Photoelectric Properties
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Xu, L.; Yang, Z.; Zhang, Z.; Yang, L.; Xia, F.; Wang, P.; Gao, G.; Sun, C.; Yang, J.; Geng, F.; et al. Reaction mode-controlled crystal structure and optical and electrical properties of SnOx infrared transparent conducting films. Surf. Coat. Technol. 2021, 422, 127506. [Google Scholar] [CrossRef]
- Guo, S.; Yang, L.; Dai, B.; Geng, F.J.; Ralchenko, V.; Han, J.C.; Zhu, J.Q. Past Achievements and Future Challenges in the Development of Infrared Antireflective and Protective Coatings. Phys. Status Solidi A 2020, 217, 2000149. [Google Scholar] [CrossRef]
- Hsu, C.H.; Zhang, Z.X.; Shi, C.Y.; Huang, P.H.; Wu, W.Y.; Wuu, D.S.; Gao, P.; Huang, C.J.; Lien, S.Y.; Zhu, W.Z. Single-crystalline-like indium tin oxide thin films prepared by plasma enhanced atomic layer deposition. J. Mater. Chem. C 2022, 10, 12350–12358. [Google Scholar] [CrossRef]
- Zhang, Z.X.; Hsu, C.H.; Huang, P.H.; Zhao, M.J.; Peng, D.C.; Wu, W.Y.; Huang, C.J.; Zhu, W.Z.; Lien, S.Y. Carbon content in PEALD-In2O3 thin films impact to its electrical and structural properties. Opt. Mater. 2021, 121, 111621. [Google Scholar] [CrossRef]
- Zhao, M.J.; Zhang, Z.X.; Hsu, C.H.; Wu, W.Y.; Zhang, X.Y.; Lien, S.Y.; Zhu, W.Z. In2O3 film prepared by a PEALD process with balanced oxygen radical supply and ion bombardment damage. Vacuum 2021, 192, 110411. [Google Scholar] [CrossRef]
- Zhao, M.J.; Zhang, Z.X.; Hsu, C.H.; Zhang, X.Y.; Wu, W.Y.; Lien, S.Y.; Zhu, W.Z. Properties and Mechanism of PEALD-In2O3 Thin Films Prepared by Different Precursor Reaction Energy. Nanomaterials 2021, 11, 978. [Google Scholar] [CrossRef] [PubMed]
- Macco, B.; Wu, Y.; Vanhemel, D.; Kessels, W.M.M. High mobility In2O3:H transparent conductive oxides prepared by atomic layer deposition and solid phase crystallization. Phys. Status Solidi RRL-Rapid Res. Lett. 2014, 8, 987–990. [Google Scholar] [CrossRef]
- Macco, B.; Verheijen, M.A.; Black, L.E.; Barcones, B.; Melskens, J.; Kessels, W.M.M. On the solid phase crystallization of In2O3:H transparent conductive oxide films prepared by atomic layer deposition. J. Appl. Phys. 2016, 120, 085314. [Google Scholar] [CrossRef] [Green Version]
- Macco, B.; Knoops, H.C.M.; Kessels, W.M.M. Electron Scattering and Doping Mechanisms in Solid-Phase-Crystallized In2O3:H Prepared by Atomic Layer Deposition. ACS Appl. Mater. Interfaces 2015, 7, 16723–16729. [Google Scholar] [CrossRef] [PubMed]
- Kuang, Y.; Macco, B.; Karasulu, B.; Ande, C.K.; Bronsveld, P.C.P.; Verheijen, M.A.; Wu, Y.; Kessels, W.M.M.; Schropp, R.E.I. Towards the implementation of atomic layer deposited In2O3:H in silicon heterojunction solar cells. Sol. Energy Mater. Sol. Cells 2017, 163, 43–50. [Google Scholar] [CrossRef]
- Wu, Y.; Macco, B.; Vanhemel, D.; Kolling, S.; Verheijen, M.A.; Koenraad, P.M.; Kessels, W.M.; Roozeboom, F. Atomic Layer Deposition of In2O3:H from InCp and H2O/O2: Microstructure and Isotope Labeling Studies. ACS Appl. Mater. Interfaces 2017, 9, 592–601. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.L.; Zhang, X.Y.; Xin, Z.Q.; Deng, M.M.; Wen, Y.Q.; Song, Y.L. Controlled Inkjetting of a Conductive Pattern of Silver Nanoparticles Based on the Coffee-Ring Effect. Adv. Mater. 2013, 25, 6714–6718. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.W.; Mao, L.; Gao, Y.L.; Zhang, P.; Li, C.; Ma, C.Q.; Tu, Y.F.; Cui, Z.; Chen, L.W. ITO-free photovoltaic cell utilizing a high-resolution silver grid current collecting layer. Sol. Energy Mat. Sol. Cells 2013, 113, 85–89. [Google Scholar] [CrossRef]
- Yang, H.J.; Seul, H.J.; Kim, M.J.; Kim, Y.; Cho, H.C.; Cho, M.H.; Song, Y.H.; Yang, H.; Jeong, J.K. High-Performance Thin-Film Transistors with an Atomic-Layer-Deposited Indium Gallium Oxide Channel: A Cation Combinatorial Approach. ACS Appl. Mater. Interfaces 2020, 12, 52937–52951. [Google Scholar] [CrossRef] [PubMed]
- Mamoru Furuta, K.S.; Kataoka, T.; Tanaka, D.; Matsumura, T.; Magari, Y.; Velichko, R. High mobility hydrogenated polycrystalline In–Ga–O (IGO:H) thin-film transistors formed by Solid phase crystallization. SID Symp. Dig. Tech. Pap. 2021, 52, 69. [Google Scholar] [CrossRef]
- Magari, Y.; Yeh, W.; Ina, T.; Furuta, M. Influence of Grain Boundary Scattering on the Field-Effect Mobility of Solid-Phase Crystallized Hydrogenated Polycrystalline In2O3 (In2O3:H). Nanomaterials 2022, 12, 2958. [Google Scholar] [CrossRef] [PubMed]
- Magari, Y.; Kataoka, T.; Yeh, W.; Furuta, M. High-mobility hydrogenated polycrystalline In2O3 (In2O3:H) thin-film transistors. Nat. Commun. 2022, 13, 1078. [Google Scholar] [CrossRef]
- Shao, Y.; Ding, S.-J. Effects of hydrogen impurities on performances and electrical reliabilities of indium-gallium-zinc oxide thin film transistors. Acta Phys. Sin.-Chin. Ed. 2018, 67, 098502. [Google Scholar] [CrossRef]
- Nomura, K.; Kamiya, T.; Hosono, H. Effects of Diffusion of Hydrogen and Oxygen on Electrical Properties of Amorphous Oxide Semiconductor, In-Ga-Zn-O. ECS J. Solid State Sci. Technol. 2013, 2, P5–P8. [Google Scholar] [CrossRef]
- Faur, M.; Faur, M.; Jayne, D.T.; Goradia, M.; Goradia, C. XPS investigation of anodic oxides grown on p-type InP. Surf. Interface Anal. 1990, 15, 641–650. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, L.; Yang, J.; Li, K.; Yang, L.; Zhu, J. Effect of Post-Deposition Annealing on the Structural Evolution and Optoelectronic Properties of In2O3:H Thin Films. Nanomaterials 2022, 12, 3533. https://doi.org/10.3390/nano12193533
Xu L, Yang J, Li K, Yang L, Zhu J. Effect of Post-Deposition Annealing on the Structural Evolution and Optoelectronic Properties of In2O3:H Thin Films. Nanomaterials. 2022; 12(19):3533. https://doi.org/10.3390/nano12193533
Chicago/Turabian StyleXu, Liangge, Jinye Yang, Kun Li, Lei Yang, and Jiaqi Zhu. 2022. "Effect of Post-Deposition Annealing on the Structural Evolution and Optoelectronic Properties of In2O3:H Thin Films" Nanomaterials 12, no. 19: 3533. https://doi.org/10.3390/nano12193533
APA StyleXu, L., Yang, J., Li, K., Yang, L., & Zhu, J. (2022). Effect of Post-Deposition Annealing on the Structural Evolution and Optoelectronic Properties of In2O3:H Thin Films. Nanomaterials, 12(19), 3533. https://doi.org/10.3390/nano12193533







