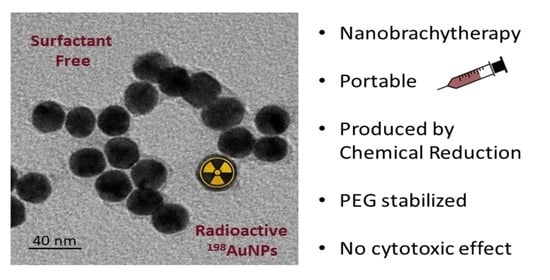
Synthesis, In Vitro Testing, and Biodistribution of Surfactant-Free Radioactive Nanoparticles for Cancer Treatment
Abstract
:1. Introduction
1.1. Radiation Therapy and Biology
1.2. Radioactive Material
1.3. Gold Nanoparticles (AuNPs)
1.4. Examples of Radioactive 198AuNPs
2. Materials and Methods
2.1. Reactants for Synthesis
2.2. Nuclear Activation
2.3. Synthesis of H198AuCl4
- Gold metal disks with ≈0.7 g are placed in 50 mL of the special setup described below. Then, they are placed in 70 mL of aqua regia;
- The solution is progressively heated, and the final temperature is 50 °C. Within 1 min, the Gold will be dissolved in the solution, and the temperature will increase to 75 °C;
- Heat is maintained until the volume reaches ≈30 mL;
- Next, 10 mL of HCl is slowly added, which will remove the nitric component;
- The same temperature is maintained until it is concentrated to ≈30 mL;
- Steps 4 and 5 are repeated three times;
- The concentration is conducted by heating to achieve the final volume of 15 mL;
- The chloroauric acid precursor preparation is complete;
- A trap is used to control the pressure in the closed system.
2.4. Synthesis of 198AuNPs
2.5. Characterization
2.6. Cell Culture
2.7. Cell Viability Assay
2.8. In Vivo Study
2.8.1. Animals
2.8.2. Tumor Xenograft Models
2.8.3. Treatment
3. Results and Discussion
3.1. Synthesis and Characterization
3.2. In Vitro Studies—Cell Viability
3.3. In Vivo Tests—Therapeutic Efficacy
3.4. In Vivo Tests—µPET/SPECT/CT and Biodistribution Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- International Agency for Research on Cancer. All Cancers. Available online: https://gco.iarc.fr/today/data/factsheets/cancers/39-All-cancers-fact-sheet.pdf (accessed on 26 October 2021).
- Weinberg, R.A. The Biology of Cancer, 2nd ed.; W. W. Norton & Company: New York, NY, USA, 2006. [Google Scholar]
- Daruich de Souza, C.; Kim, J.J.; Hong, J.T. Start Here When Performing Radiochemical Reactions. In Radiopharmaceuticals, 1st ed.; InTechOPEN: London, UK, 2021; Available online: https://www.intechopen.com/online-first/77528 (accessed on 10 November 2021).
- Khan, M.K.; Minc, L.D.; Nigavekar, S.S.; Kariapper, M.S.T.; Nair, B.M.; Schipper, M.; Cook, A.C.; Lesniak, W.G.; Balogh, L.P. Fabrication of 198Au0 radioactive composite nanodevices and their use for nanobrachytherapy. Nanomed. Nanotechnol. Biol. Med. 2008, 4, 57–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mettler, F.A., Jr.; Upton, A.C. Medical Effects of Ionizing Radiation, 1st ed.; Saunders Elsevier: Philadelphia, PA, USA, 1995. [Google Scholar]
- Daruich de Souza, C. Materials for the Course: TNA5805 Brachytherapy: Fundamentals, Production, Application, Dosimetry and Quality. University of São Paulo. Available online: https://carlepy.wixsite.com/website/blank-page-1 (accessed on 10 November 2021).
- Daruich de Souza, C.; Peleias, F.D.S., Jr.; Rostelato, M.E.C.M.; Zeituni, C.A.; Tiezzi, R.; Rodrigues, B.T.; Feher, A.; Moura, J.A.; Costa, O.L. Work with Iodine-125: 8 years experience in brachytherapy sources production lab. In Proceedings of the InMed, Kioto, Japan, 11–12 September 2015. [Google Scholar]
- Rostelato, E.C.M.; Daruich De Souza, C.; Gonzalez, A.D.C.C.; Nogueira, B.R.; Zeituni, C.A.; Fortin, M.A.; Chevallier, P. Nanobrachytherapy and its challenges. In Proceedings of the Pannnano—1st Pan American Congress of Nanotechnology, Guarujá, Brazil, 27–30 November 2017. [Google Scholar]
- Nishida, N.; Yano, H.; Nishida, T.; Kamura, T.; Kojiro, M. Angiogenesis in cancer. Vasc. Health Risk Manag. 2006, 2, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Holmgren, L.; O’reilly, M.S.; Folkman, J. Dormancy of micrometastases: Balanced proliferation and apoptosis in the presence of angiogenesis suppression. Nat. Med. 1995, 1, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Parangi, S.; O’reilly, M.; Christofori, G.; Holmgren, L.; Grosfeld, J.; Folkman, J.; Hanahan, D. Antiangiogenic therapy of transgenic mice impairs de novo tumor growth. Proc. Natl. Acad. Sci. USA 1996, 93, 2002–2007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rostelato, M.E.C.M.; De Souza, C.D.; Zeituini, C.; Rosero, W.A.A.; Nogueira, B.R. Síntese de Nanopartículas Radioativas e Não-Radioativas de Ouro Para Uso Terapêutico. INPI. BR 10 2020 007225-0. 2020. Available online: https://busca.inpi.gov.br/pePI/BR10202000722508 (accessed on 10 October 2021).
- Daruich de Souza, C. Materials for the Course: TNA 5744 Dicipline—Applications of Intense Radiation Sources. University of São Paulo. Available online: https://carlepy.wixsite.com/website/lecture-1 (accessed on 10 November 2021).
- Podgorsak, E.B. Radiation Oncology Physics: A Handbook for Teachers and Students, 1st ed.; IAEA: Vienna, Austria, 2005. [Google Scholar]
- Polte, J. Fundamental growth principles of colloidal metal nanoparticles—A new perspective. CrystEngComm 2015, 17, 6809–6830. [Google Scholar] [CrossRef] [Green Version]
- De Souza, C.D.; Ribeiro Nogueira, B.; Zeituni, C.A.; Rostelato, M.E.C.M. Nanobrachytherapy: The use of radioactive nanoparticles for cancer treatment. In Nanoparticle Therapeutics: Production Technologies, Types of Nanoparticles, and Regulatory Aspects, 1st ed.; Elsevier: Cambridge, MA, USA, 2021. [Google Scholar]
- Toma, H.E.; Zamarion, V.M.; Toma, S.H.; Araki, K. The coordination chemistry at gold nanoparticles. J. Braz. Chem. Soc. 2010, 21, 1158–1176. [Google Scholar] [CrossRef]
- Daruich de Souza, C.; Ribeiro Nogueira, B.; Rostelato, M.E.C.M. Review of the methodologies used in the synthesis gold nanoparticles by chemical reduction. J. Alloys Compd. 2019, 798, 714–740. [Google Scholar] [CrossRef]
- Bakshi, M.S. How Surfactants Control Crystal Growth of Nanomaterials. Cryst. Growth Des. 2016, 16, 1104–1133. [Google Scholar] [CrossRef]
- Kumar, S. Role of Surfactants in Synthesis and Stabilization of Nanoparticles Spectroscopic and Physicochemical Aspects. Ph.D. Thesis, Panjab University, Chandigarh, India, 2011. [Google Scholar]
- Kimling, J.; Maier, M.; Okenve, B.; Kotaidis, V.; Ballot, H.; Plech, A. Turkevich Method for Gold Nanoparticle Synthesis Revisited. J. Phys. Chem. B 2006, 110, 15700–15707. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.C.; Meisel, D. Adsorption and surface-enhanced Raman of dyes on silver and gold sols. J. Phys. Chem. 1982, 86, 3391–3395. [Google Scholar] [CrossRef]
- Chanda, N.; Kan, P.; Watkinson, L.D.; Shukla, R.; Zambre, A.; Carmack, T.L.; Engelbrecht, H.; Lever, J.R.; Katti, K.; Fent, G.M.; et al. Radioactive gold nanoparticles in cancer therapy: Therapeutic efficacy studies of GA-198AuNP nanoconstruct in prostate tumor–bearing mice. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Daniel, M.-C.; Astruc, D. Gold Nanoparticles: Assembly, Supramolecular Chemistry, Quantum-Size-Related Properties, and Applications toward Biology, Catalysis, and Nanotechnology. Chem. Rev. 2004, 104, 293–346. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Hirst, D.G.; O’sullivan, J.M. Gold nanoparticles as novel agents for cancer therapy. Br. J. Radiol. 2012, 85, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Hahn, P.F.; Lareau, D.G.; Feaster, B.L.; Carothers, E.L.; Gollan, F.; Meneely, G.R.; Sherman, D. Intravenous radioactive gold in the treatment of chronic leukemia. Acta Radiol. 1958, 50, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Hahn, P.F.; Carothers, E.L. Use of radioactive colloidal metallic gold in the treatment of malignancies. Nucleonics 1950, 6, 54–62. [Google Scholar] [PubMed]
- Hahn, P.F.; Goodell, J.P.; Sheppard, C.W.; Cannon, R.O.; Francis, H.C. Direct infiltration of radioactive isotopes as a means of delivering ionizing radiation to discrete tissues. J. Lab. Clin. Med. 1947, 32, 1442–1453. [Google Scholar] [PubMed]
- International Atomic Nuclear Agency. Live Chart of Nuclides: Nuclear Structure and Decay Data. Available online: http://www-nds.iaea.org/relnsd/vcharthtml/VChartHTML.html (accessed on 26 February 2020).
- Djoumessi, D.; Laprise-Pelletier, M.; Chevallier, P.; Lagueux, J.; Cote, M.F.; Fortin, M.A. Rapid, one-pot procedure to synthesise 103Pd:Pd@Au nanoparticles en route for radiosensitisation and radiotherapeutic applications. J. Mater. Chem. B 2015, 3, 2192–2205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choppin, G.; Liljenzin, J.-O.; Rydberg, J.; Ekberg, C. Chapter 17—Production of Radionuclides. In Radiochemistry and Nuclear Chemistry, 4th ed.; Academic Press: Oxford, UK, 2013. [Google Scholar]
- Saifullah, S.; Ali, I.; Kawish, M.; El-Shabasy, R.M.; Chen, L.; El-Seedi, H.R. Chapter 12—Surface functionalized magnetic nanoparticles for targeted cancer therapy and diagnosis. In Metal Nanoparticles for Drug Delivery and Diagnostic Applications, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Bahmanpour, A.H.; Navaei, T.; Ahadi, F. Chapter 28—Pulmonary system responses to biomaterials. In Handbook of Biomaterials Biocompatibility, 1st ed.; Woodhead Publishing: Duxford, UK, 2020. [Google Scholar]
- Souza, T.; Ciminelli, V.; Mohallem, N. A comparison of TEM and DLS methods to characterize size distribution of ceramic nanoparticles. J. Phys. Conf. Ser. 2016, 733, 012039. [Google Scholar] [CrossRef] [Green Version]







| Organ | % ID g−1 | Organ | % ID g−1 |
|---|---|---|---|
| Blood | 0.04 | Intestine | 0.00 |
| Heart | 0.02 | Pancreas | 0.01 |
| Lungs | 0.04 | Bone | 0.04 |
| Liver | 0.63 | Muscle | 0.00 |
| Kidneys | 0.03 | Brain | 0.00 |
| Spleen | 0.13 | Bladder | 0.00 |
| Stomach | 0.00 | Tumor | 10.07 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daruich de Souza, C.; Bueno Barbezan, A.; Arcos Rosero, W.A.; Nascimento dos Santos, S.; Vergaças de Sousa Carvalho, D.; Zeituni, C.A.; Soares Bernardes, E.; Perez Vieira, D.; Spencer, P.J.; Simões Ribeiro, M.; et al. Synthesis, In Vitro Testing, and Biodistribution of Surfactant-Free Radioactive Nanoparticles for Cancer Treatment. Nanomaterials 2022, 12, 187. https://doi.org/10.3390/nano12020187
Daruich de Souza C, Bueno Barbezan A, Arcos Rosero WA, Nascimento dos Santos S, Vergaças de Sousa Carvalho D, Zeituni CA, Soares Bernardes E, Perez Vieira D, Spencer PJ, Simões Ribeiro M, et al. Synthesis, In Vitro Testing, and Biodistribution of Surfactant-Free Radioactive Nanoparticles for Cancer Treatment. Nanomaterials. 2022; 12(2):187. https://doi.org/10.3390/nano12020187
Chicago/Turabian StyleDaruich de Souza, Carla, Angelica Bueno Barbezan, Wilmmer Alexander Arcos Rosero, Sofia Nascimento dos Santos, Diego Vergaças de Sousa Carvalho, Carlos Alberto Zeituni, Emerson Soares Bernardes, Daniel Perez Vieira, Patrick Jack Spencer, Martha Simões Ribeiro, and et al. 2022. "Synthesis, In Vitro Testing, and Biodistribution of Surfactant-Free Radioactive Nanoparticles for Cancer Treatment" Nanomaterials 12, no. 2: 187. https://doi.org/10.3390/nano12020187