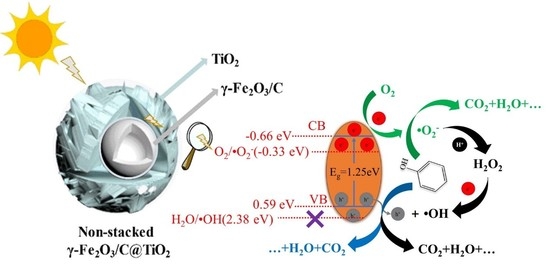
Non-Stacked γ-Fe2O3/C@TiO2 Double-Layer Hollow Nanoparticles for Enhanced Photocatalytic Applications under Visible Light
Abstract
:1. Introduction
2. Materials and Methods
2.1. Catalyst Preparation
2.1.1. Materials
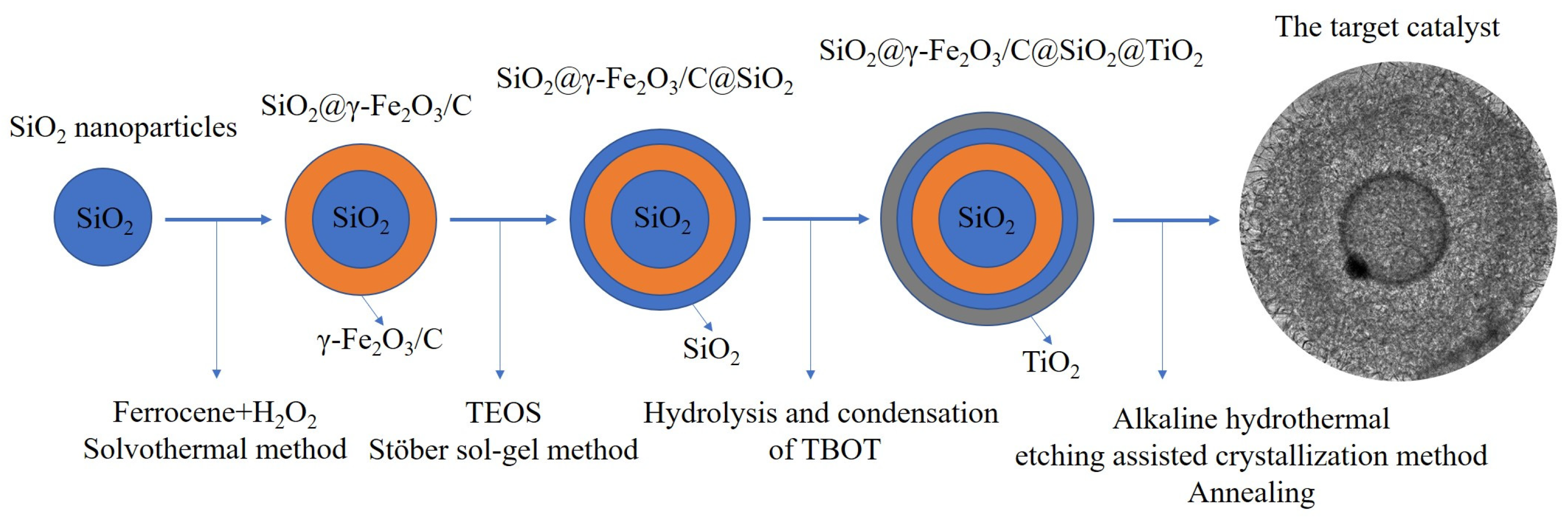
2.1.2. Synthesis of Non-Stacked γ-Fe2O3/C@TiO2 Double-Layer Hollow Nanoparticles
- (1)
- Synthesis of SiO2@γ-Fe2O3/C nanoparticles
- (2)
- Synthesis of SiO2@γ-Fe2O3/C@SiO2 nanoparticles
- (3)
- Synthesis of SiO2@γ-Fe2O3/C@SiO2@TiO2 nanoparticles
- (4)
- Synthesis of non-stacked γ-Fe2O3/C@TiO2 double-layer hollow nanoparticles
2.2. Catalyst Characterization
2.3. Catalytic Activity Measurement
- A0—the initial absorbance of phenol.
- At—Absorption of phenol at t min.
3. Results and Discussion
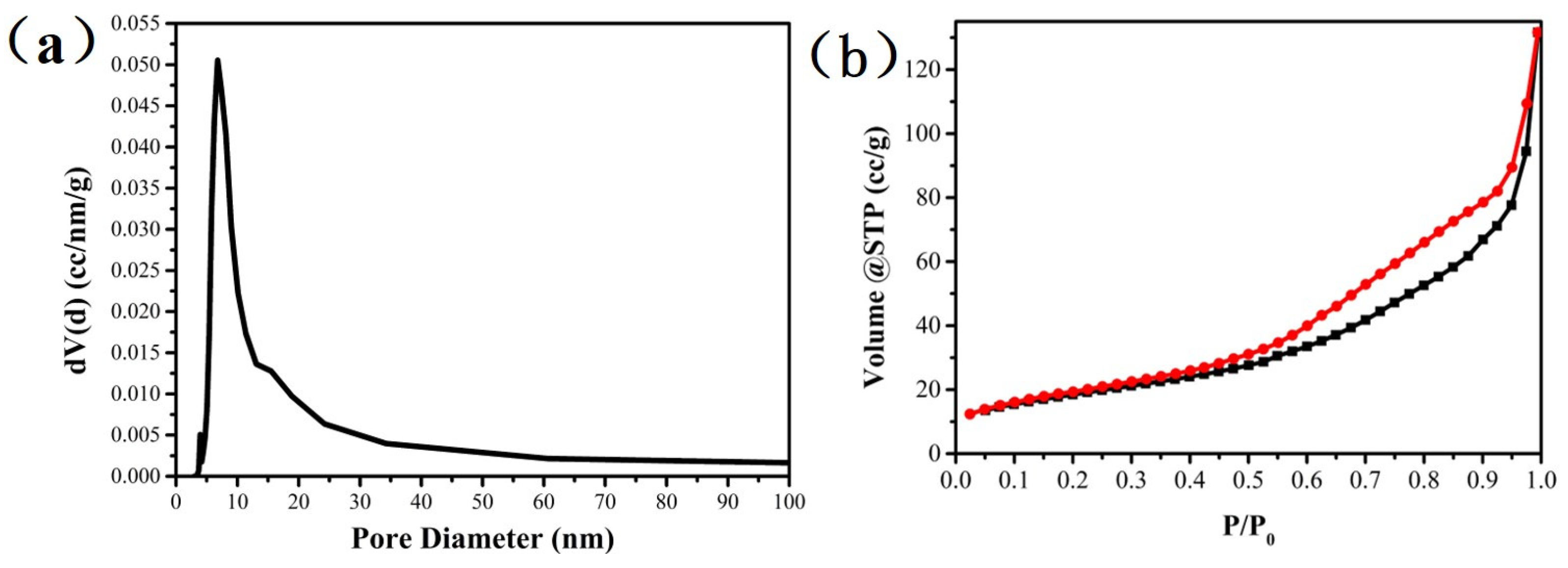
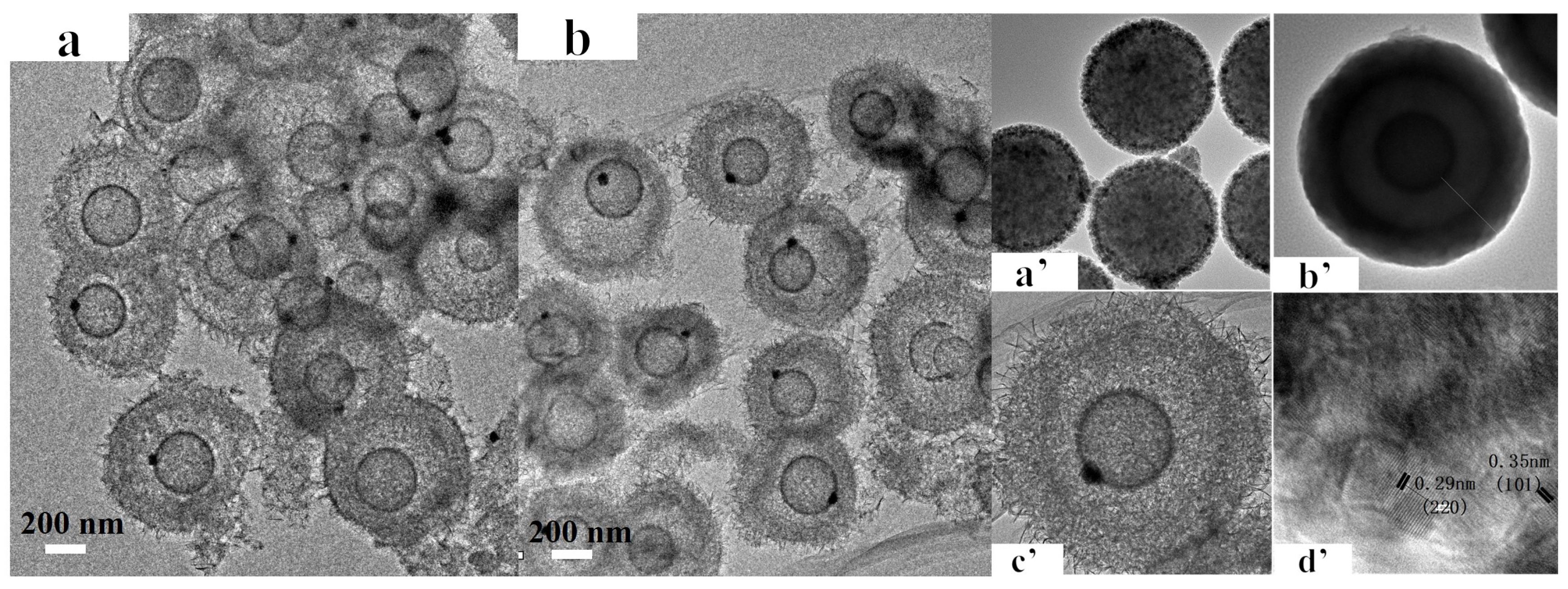
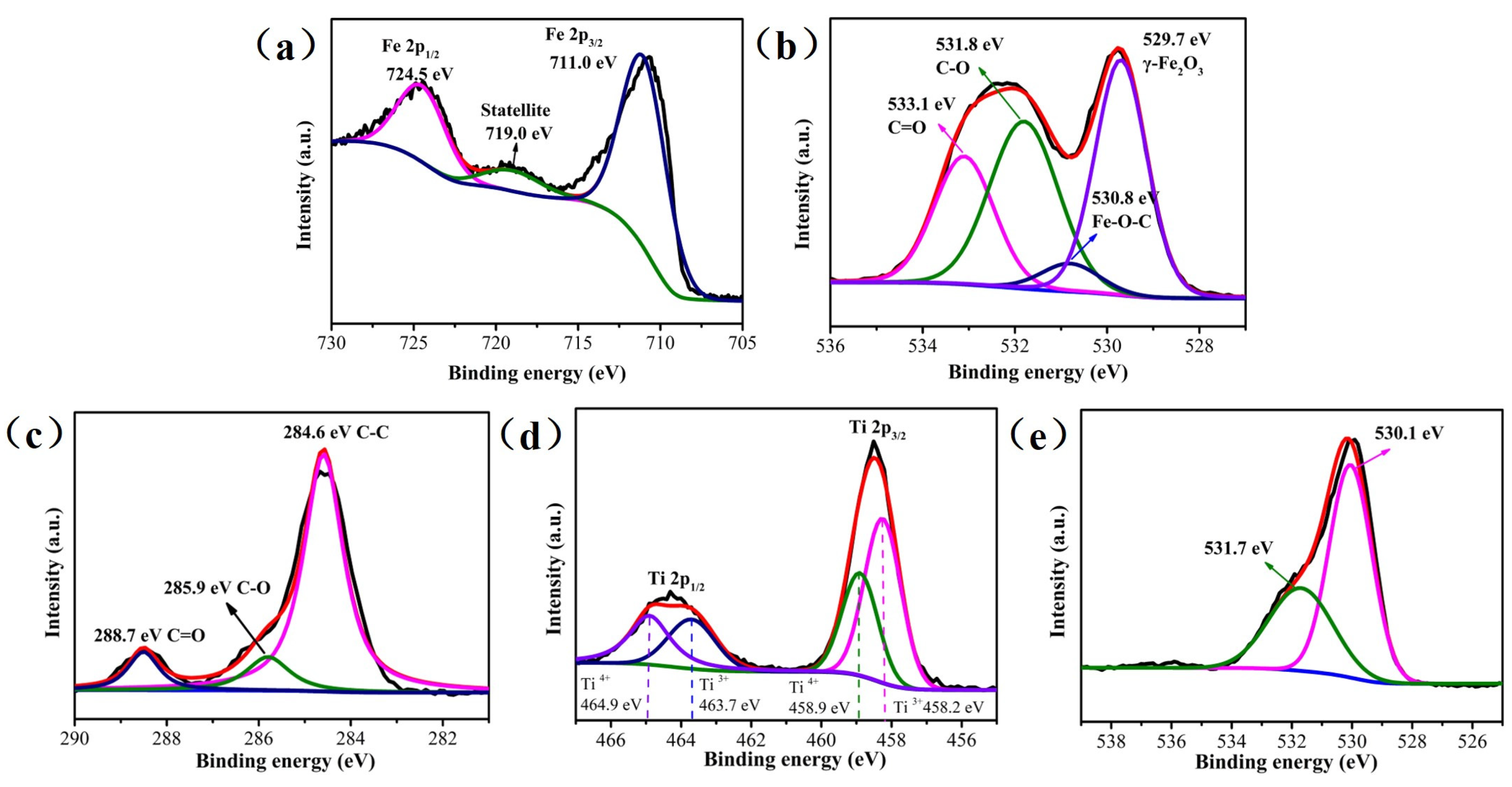
3.1. Textural Properties of Catalysts
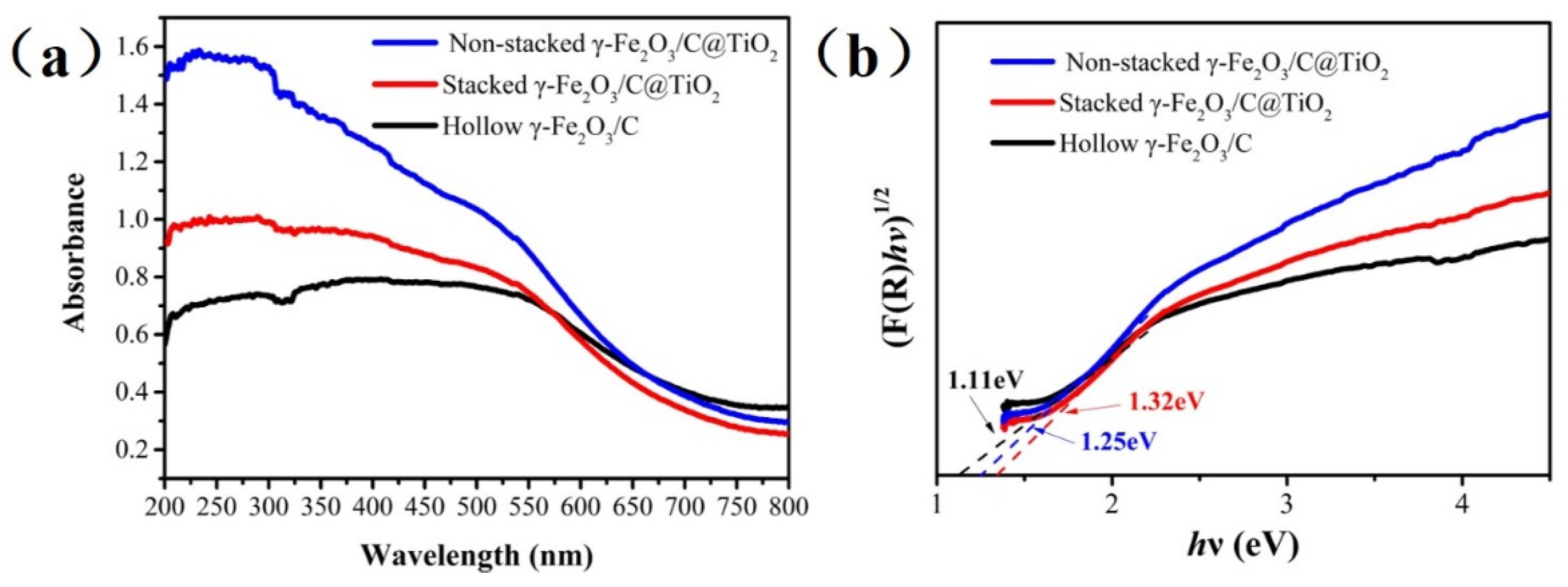
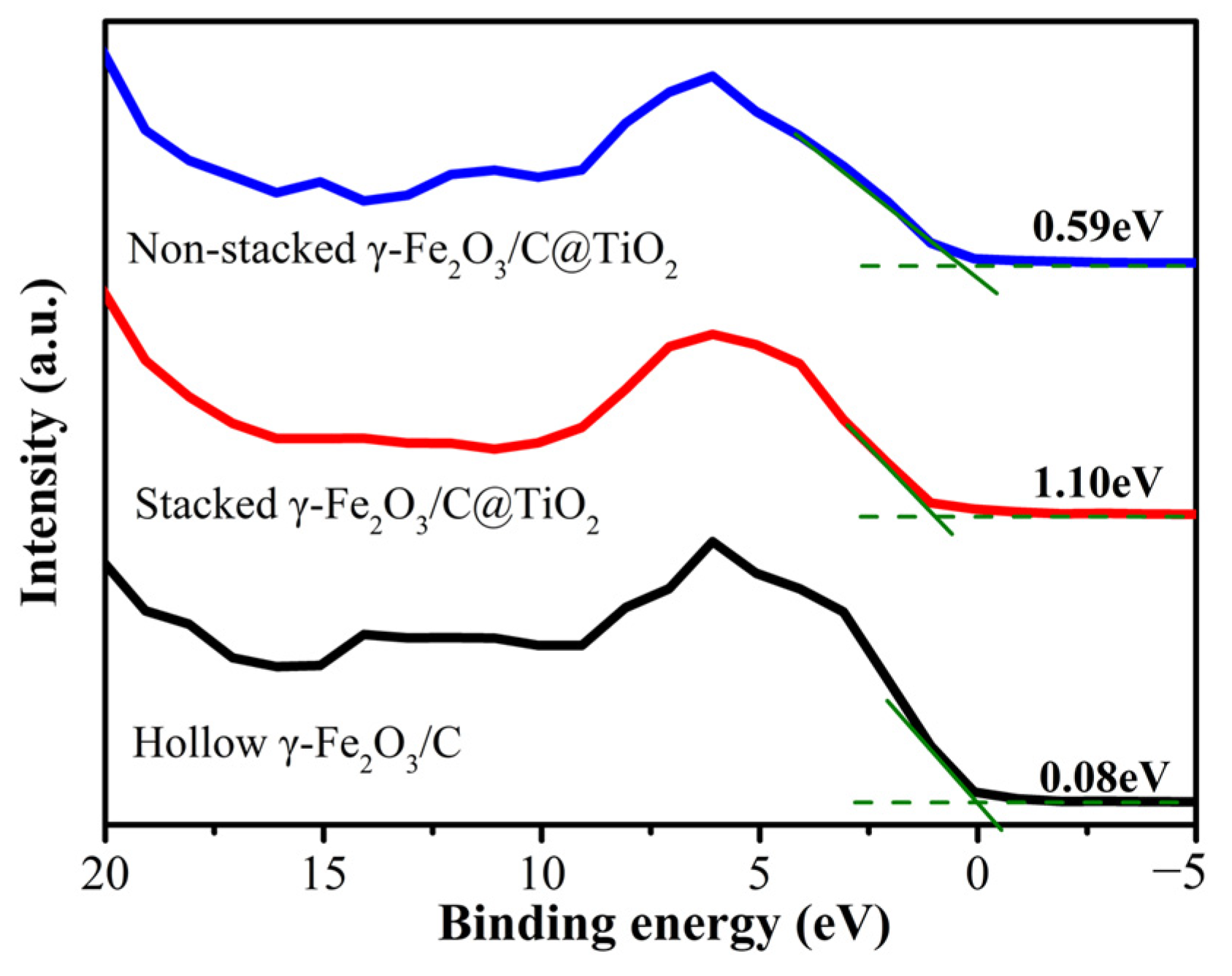
3.2. UV-Vis Absorbance Spectra of Non-Stacked γ-Fe2O3/C@TiO2
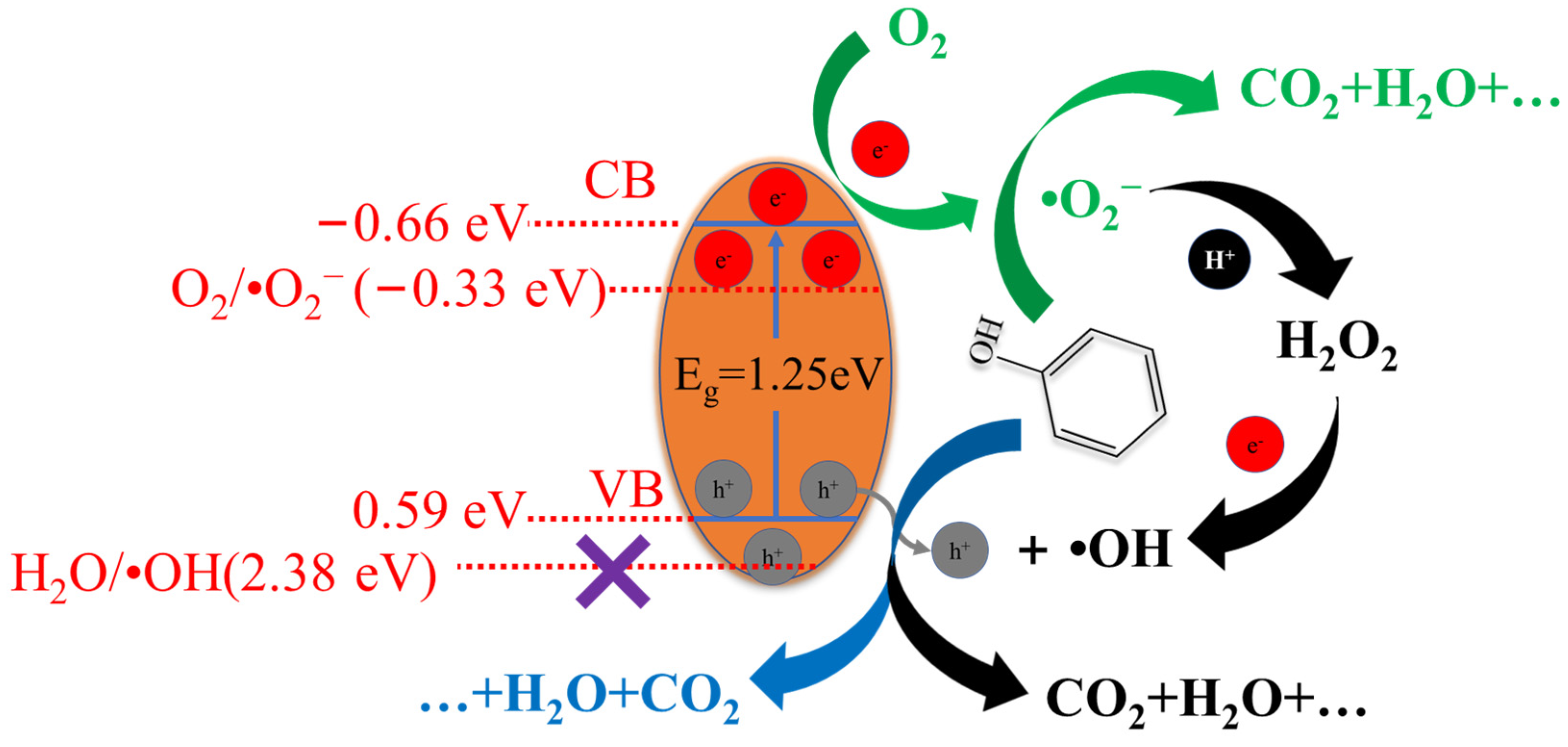
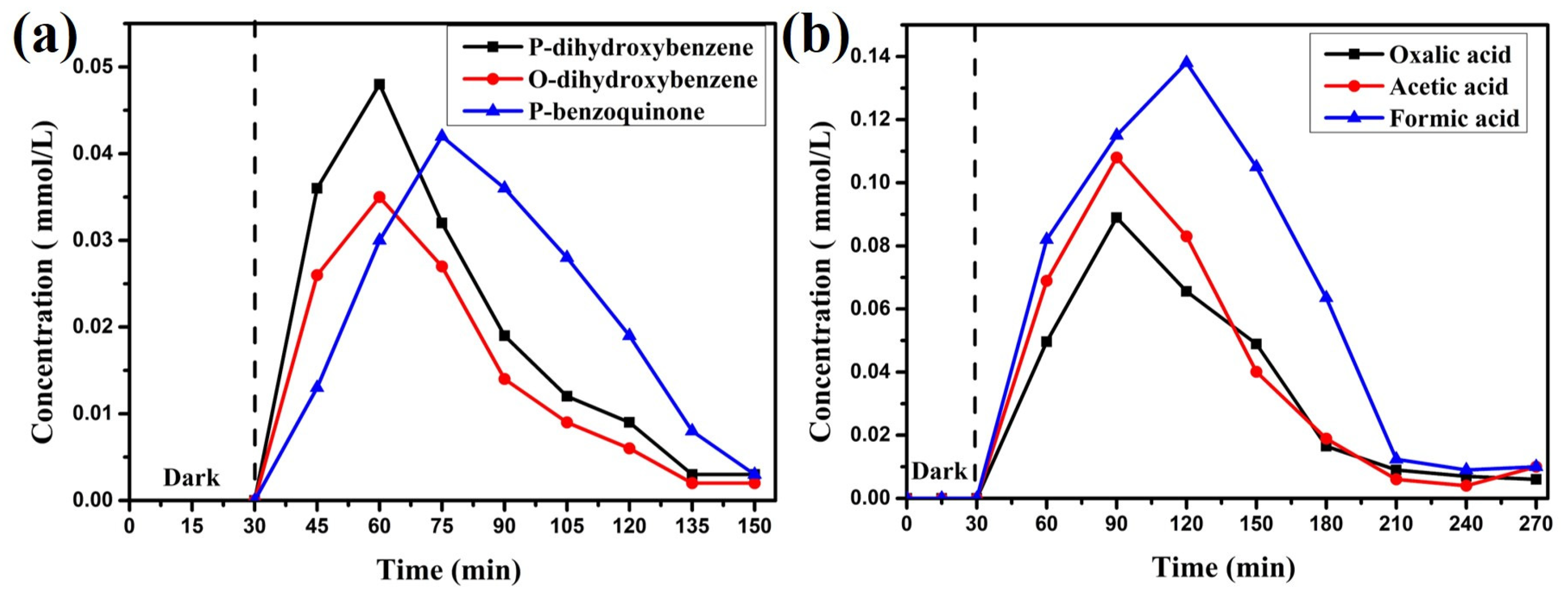
3.3. Photocatalytic Degradation of Phenol
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cao, T.T.; Xu, T.F.; Zhao, N.M.; Jiang, X.; Cui, C.W. Application of vacuum-ultraviolet (VUV) for phenolic homologues removal in humic acid solution: Efficiency, pathway and DFT calculation. J. Hazard. Mater. 2020, 384, 121464. [Google Scholar] [CrossRef]
- Busca, G.; Berardinelli, S.; Resini, C.; Arrighi, L. Technologies for the removal of phenol from fluid streams: A short review of recent developments. J. Hazard. Mater. 2008, 160, 265–288. [Google Scholar] [CrossRef]
- Ahmed, S.; Rasul, M.G.; Martens, W.N.; Brown, R.; Ashib, M.A.H. Heterogeneous photocatalytic degradation of phenols in wastewater: A review on current status and developments. Desalination 2010, 261, 3–18. [Google Scholar] [CrossRef] [Green Version]
- Qian, H.L.; Hou, Q.D.; Duan, E.H.; Niu, J.R.; Nie, Y.F.; Bai, C.Y.L.; Bai, X.Y.; Ju, M.T. Honeycombed Au@C-TiO2-Xcatalysts for enhanced photocatalytic mineralization of Acid red 3R under visible light. J. Hazard. Mater. 2020, 391, 122246. [Google Scholar] [CrossRef] [PubMed]
- Pal, U.; Sandoval, A.; Madrid, S.I.U.; Corro, G.; Sharma, V. Mohanty, Mixed titanium, silicon, and aluminum oxide nanostructures as novel adsorbent for removal of rhodamine 6G and methylene blue as cationic dyes from aqueous solution. Chemosphere 2016, 163, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Streit, A.F.M.; Côrtes, L.N.; Druzian, S.P.; Godinho, M.; Collazzo, G.C.; Perondi, D.; Dotto, G.L. Development of high quality activated carbon from biological sludge and its application for dyes removal from aqueous solutions. Sci. Total Environ. 2019, 660, 277–287. [Google Scholar] [CrossRef]
- Oladipo, A.A.; Ifebajo, A.O.; Gazi, M. Magnetic LDH-based CoO–NiFe2O4 catalyst with enhanced performance and recyclability for efficient decolorization of azo dye via Fenton-like reactions. Appl. Catal. B Environ. 2019, 243, 243–252. [Google Scholar] [CrossRef]
- Amanulla, B.; Krishnan, D.; Vellaichamy, B.; Krishnan, N.P.; Chandran, M.; Ramaraj, S.K. Synthesis of highly efficient g-CN@CuO nanocomposite for photocatalytic degradation of phenol under visible light. J. Alloy. Compd. 2021, 886, 161167. [Google Scholar]
- Soheila, A.K.; Aziz, H.Y.; Kunio, Y. Novel g-C3N4 nanosheets/CDs/BiOCl photocatalysts with exceptional activity under visible light. J. Am. Ceram. Soc. 2019, 102, 1435–1453. [Google Scholar]
- Pan, X.; Yang, M.Q.; Fu, X.; Zhang, N.; Xu, Y.J. Defective TiO2 with oxygen vacancies: Synthesis, properties and photocatalytic applications. Nanoscale 2013, 5, 3601–3614. [Google Scholar] [CrossRef]
- Wu, Y.Y.; Ji, H.D.; Liu, Q.M.; Sun, Z.Y.; Li, P.S.; Ding, P.R.; Guo, M.; Yi, X.H.; Xu, W.L.; Wang, C.C.; et al. Visible light photocatalytic degradation of sulfanilamide enhanced by Mo doping of BiOBr nanoflowers. J. Hazard. Mater. 2022, 424, 127563. [Google Scholar] [CrossRef]
- Serga, V.; Burve, R.; Krumina, A.; Romanova, M.; Kotomin, E.A.; Popov, A.I. Extraction–pyrolytic method for TiO2 polymorphs production. Crystals 2021, 11, 431. [Google Scholar] [CrossRef]
- Zhang, W.Q.; Liu, H.; Liu, Z.C.; An, Y.H.; Zhong, Y.; Hu, Z.C.; Li, S.J.; Chen, Z.J.; Wang, S.G.; Sheng, X.L.; et al. Eu-dopedzeolitic imidazolate framework-8 modified mixed-crystal TiO2 for efficient removal of basic fuchsin from effluent. Materials 2021, 14, 7265. [Google Scholar] [CrossRef]
- Tsebriienko, T.; Popov, A.I. Effect of poly(Titanium Oxide) on the viscoelastic and thermophysical properties of interpenetrating polymer networks. Crystals 2021, 11, 794. [Google Scholar] [CrossRef]
- Shao, M.; Han, J.; Wei, M.; Evans, D.G.; Duan, X. The synthesis of hierarchical Zn–Ti layered double hydroxide for efficient visible-light photocatalysis. Chem. Eng. J. 2011, 168, 519–524. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, B.; Zhao, X.Y.; Chen YSh Wei, D.D.; Zhang, L.J.; Su, X.T. Facile synthesis of ultrathin γ-Fe2O3 magnetic nanosheets rich in oxygen vacancies and their photocatalytic activity for water oxidation. Appl. Surf. Sci. 2022, 578, 151999. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, J.; Zhao, S.; Chen, X.; Yu, Y. Synergistic effect of adsorption and visible-light photocatalysis for organic pollutant removal over BiVO4/carbon sphere nanocomposites. Appl. Surf. Sci. 2018, 453, 394–404. [Google Scholar] [CrossRef]
- Waghmode, T.R.; Kurade, M.B.; Sapkal, R.T.; Bhosale, C.H.; Jeon, B.-H.; Govindwar, S.P. Sequential photocatalysis and biological treatment for the enhanced degradation of the persistent azo dye methyl red. J. Hazard. Mater. 2019, 371, 115–122. [Google Scholar] [CrossRef]
- Nguyen, C.H.; Fu, C.-C.; Juang, R.-S. Degradation of methylene blue and methyl orange by palladium-doped TiO2 photocatalysis for water reuse: Efficiency and degradation pathways. J. Clean. Prod. 2018, 202, 413–427. [Google Scholar] [CrossRef]
- Li, W.; Deng, Y.H.; Wu, Z.X.; Qian, X.F.; Yang, J.P.; Wang, Y.; Gu, D.; Zhang, F.; Tu, B.; Zhao, D.Y. Hydrothermal etching assisted crystallization: A facile route to functional yolk-shell titanate microspheres with ultrathin nanosheets-assembled double shells. J. Am. Chem. Soc. 2011, 133, 15830–15833. [Google Scholar] [CrossRef]
- Xu, Y.; Ahmed, R.; Klein, D.; Cap, S.; Freedy, K.; McDonnell, S.; Zangari, G. Improving photo-oxidation activity of water by introducing Ti3+ in self-ordered TiO2 nanotube arrays treated with Ar/NH3. J. Power Sources 2019, 414, 242–249. [Google Scholar] [CrossRef]
- Zarrin, S.; Heshmatpour, F. Photocatalytic activity of TiO2/Nb2O5/PANI and TiO2/Nb2O5/RGO as new nanocomposites for degradation of organic pollutants. J. Hazard. Mater. 2018, 351, 147–159. [Google Scholar] [CrossRef]
- Sangjan, S.; Wisasa, K.; Deddeaw, N. Enhanced photodegradation of reactive blue dye using Ga and Gd as catalyst in reduced graphene oxide-based TiO2 composites. Mater. Today Proc. 2019, 6, 19–23. [Google Scholar] [CrossRef]
- Yin, L.; Gao, Y.J.; Jeon, I.; Yang, H.; Kim, J.-P.; Jeong, S.Y.; Cho, C.R. Rice-panicle-like γ-Fe2O3@C nanofibers as high-rate anodes for superior lithium-ion batteries. Chem. Eng. J. 2019, 356, 60–68. [Google Scholar] [CrossRef]
- Han, W.; Wang, L.; Li, Z.; Tang, H.; Li, Y.; Huo, C.; Lan, G.; Yang, X.; Liu, H. γ-Fe2O3 as the precursor of iron based catalyst prepared by solid-state reaction at room temperature for Fischer-Tropsch to olefins. Appl. Catal. A Gen. 2019, 572, 158–167. [Google Scholar] [CrossRef]
- Zeb, F.; Khan, M.S.; Nadeem, K.; Kamran, M.; Abbas, H.; Krenn, H.; Szabo, D.V. Reduced surface spin disorder in ZrO2 coated γ-Fe2O3 nanoparticles. Solid State Commun. 2018, 284–286, 69–74. [Google Scholar] [CrossRef]
- Pan, X.; Duan, X.; Lin, X.; Zong, F.; Tong, X.; Li, Q.; Wang, T. Rapid synthesis of Cr-doped γ-Fe2O3/reduced graphene oxide nanocomposites as high performance anode materials for lithium ion batteries. J. Alloys Compd. 2018, 732, 270–279. [Google Scholar] [CrossRef]
- Ibrahim, E.M.M.; Abdel-Rahman, L.H.; Abu-Dief, A.M.; Elshafaie, A.; Hamdan, S.K.; Ahmed, A.M. Electric, thermoelectric and magnetic characterization of γ-Fe2O3 and Co3O4 nanoparticles synthesized by facile thermal decomposition of metal-Schiff base complexes. Mater. Res. Bull. 2018, 99, 103–108. [Google Scholar] [CrossRef]
- Lushchik, A.; Feldbach, E.; Kotomin, E.A.; Kudryavtseva, I.; Kuzovkov, V.N.; Popov , A.I.; Seeman, V.; Shablonin, E. Distinctive features of diffusion controlled radiation defect recombination in stoichiometric magnesium aluminate spinel single crystals and ransparent polycrystalline ceramics. Sci. Rep. 2020, 10, 7810. [Google Scholar] [CrossRef]
- Mykhailovych, V.; Kanak, A.; Cojocaru, S.; Chitoiu-Arsene, E.; Palamaru, M.N.; Iordan, A.R.; Korovyanko, O.; Diaconu, A.; Ciobanu, V.G.; Caruntu, G.; et al. Structural, Optical, and Catalytic Properties of MgCr2O4 Spinel-Type Nanostructures Synthesized by Sol–Gel Auto-Combustion Method. Catalysts 2021, 11, 1476. [Google Scholar] [CrossRef]
- Rajkumar, S.; Prabaharan, M. Multi-functional core-shell Fe3O4@Au nanoparticles for cancer diagnosis and therapy. Colloids Surf. B Biointerfaces 2019, 174, 252–259. [Google Scholar]
- Li, P.; He, Z.; Luo, C.; Xiao, Y.; Wang, Y.; Hu, J.; Li, G.; Jiang, H.; Zhang, W. α-Fe2O3@dopamine core-shell nanocomposites and their highly enhanced photoacoustic performance. Appl. Surf. Sci. 2019, 466, 185–192. [Google Scholar] [CrossRef]
- Li, M.; Hu, Y.; Fang, Y.; Tan, T. Coating mesoporous ZSM-5 by thin microporous Silicalite-1 shell: Formation of core/shell structure, improved hydrothermal stability and outstanding catalytic performance. Catal. Today 2020, 339, 312–320. [Google Scholar] [CrossRef]
- Al-Fahdi, T.; Al Marzouqi, F.; Kuvarega, A.T.; Mamba, B.B.; Al Kindy, S.M.Z.; Kim, Y.; Selvaraj, R. Visible light active CdS@TiO2 core-shell nanostructures for the photodegradation of chlorophenols. J. Photochem. Photobiol. A Chem. 2019, 374, 75–83. [Google Scholar] [CrossRef]
- Zhang, J.; Du, Y.; Zhang, L.; Zheng, X.; Ma, Y.; Dong, S.; Zhou, D. Preparation of sponge carrier supported photocatalyst by self-assembly technique for phenol photodegradation in visible light. Mol. Catal. 2017, 432, 1–7. [Google Scholar] [CrossRef]
- Mohamed, M.A.; Salleh, W.N.W.; Jaafar, J.; Ismail, A.F.; Nor, N.A.M. Photodegradation of phenol by N-doped TiO2 anatase/rutile nanorods assembled microsphere under UV and visible light irradiation. Mater. Chem. Phys. 2015, 162, 113–123. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Zhao, C.C.; Duan, Y.S.; Wang, C.; Zhao, Z.C.; Wang, H.J.; Gao, Y.J. Phosphorus-doped TiO2 for visible light-driven oxidative coupling of benzyl amines and photodegradation of phenol. Appl. Surf. Sci. 2020, 527, 146693. [Google Scholar] [CrossRef]
- Yao, J.; Chen, H.; Jiang, F.; Jiao, Z.; Jin, M. Titanium dioxide and cadmium sulfide cosensitized graphitic carbon nitride nanosheets composite photocatalysts with superior performance in phenol degradation under visible-light irradiation. J. Colloid Interface Sci. 2017, 490, 154–162. [Google Scholar] [CrossRef]
- Wang, J.L.; W, B.; Xu, L.L.; Gao, H.; Sun, W.J.; Che, J.X. Multilayered MoS2 coated TiO2 hollow spheres for efficient photodegradation of phenol under visible light irradiation. Mater. Lett. 2016, 179, 42–46. [Google Scholar] [CrossRef] [Green Version]



| Photo Catalyst | Phenol | Characteristics | % Degradation |
|---|---|---|---|
| Er3+: YAlO3/TiO2: 0.39 g | 50 mgL−1 | Visible light | 58% (8 h) [35] |
| N-doped TiO2: 0.3 g | 50 mgL−1 | λ = 312 nm UV | 99.6% (540 min) [36] |
| 2P-TiO2-500: 50 mg | 10 mgL−1 | Xenon lamp (300 W) | 100% (180 min) [37] |
| TiO2-CdS-gCNNSs: 50 mg | 10 mgL−1 | Visible light | 80% (300 min) [38] |
| TiO2/MoS2 heterostructures | - | Visible light | 78% (150 min) [39] |
| This work: 0.1 g | 20 mgL−1 | Visible light | Almost 100% (135 min) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, X.; Yan, X.; Su, H.; Sun, L.; Zhao, L.; Shi, J.; Wang, Z.; Niu, J.; Qian, H.; Duan, E. Non-Stacked γ-Fe2O3/C@TiO2 Double-Layer Hollow Nanoparticles for Enhanced Photocatalytic Applications under Visible Light. Nanomaterials 2022, 12, 201. https://doi.org/10.3390/nano12020201
Sun X, Yan X, Su H, Sun L, Zhao L, Shi J, Wang Z, Niu J, Qian H, Duan E. Non-Stacked γ-Fe2O3/C@TiO2 Double-Layer Hollow Nanoparticles for Enhanced Photocatalytic Applications under Visible Light. Nanomaterials. 2022; 12(2):201. https://doi.org/10.3390/nano12020201
Chicago/Turabian StyleSun, Xun, Xiao Yan, Huijuan Su, Libo Sun, Lijun Zhao, Junjie Shi, Zifan Wang, Jianrui Niu, Hengli Qian, and Erhong Duan. 2022. "Non-Stacked γ-Fe2O3/C@TiO2 Double-Layer Hollow Nanoparticles for Enhanced Photocatalytic Applications under Visible Light" Nanomaterials 12, no. 2: 201. https://doi.org/10.3390/nano12020201