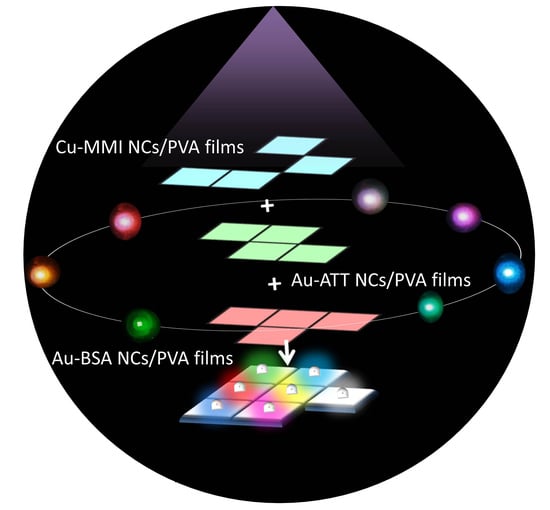
Metal Nanoclusters/Polyvinyl Alcohol Composite Films as the Alternatives for Fabricating Remote-Type White Light-Emitting Diodes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Cu-MMI NCs
2.3. Preparation of Au-ATT NCs
2.4. Preparation of Au-BSA NCs
2.5. Preparation of MNCs/PVA Composite Film
2.6. Characterization
3. Results and Discussion
3.1. Preparation and Optical Properties of MNCs/PVA Solutions
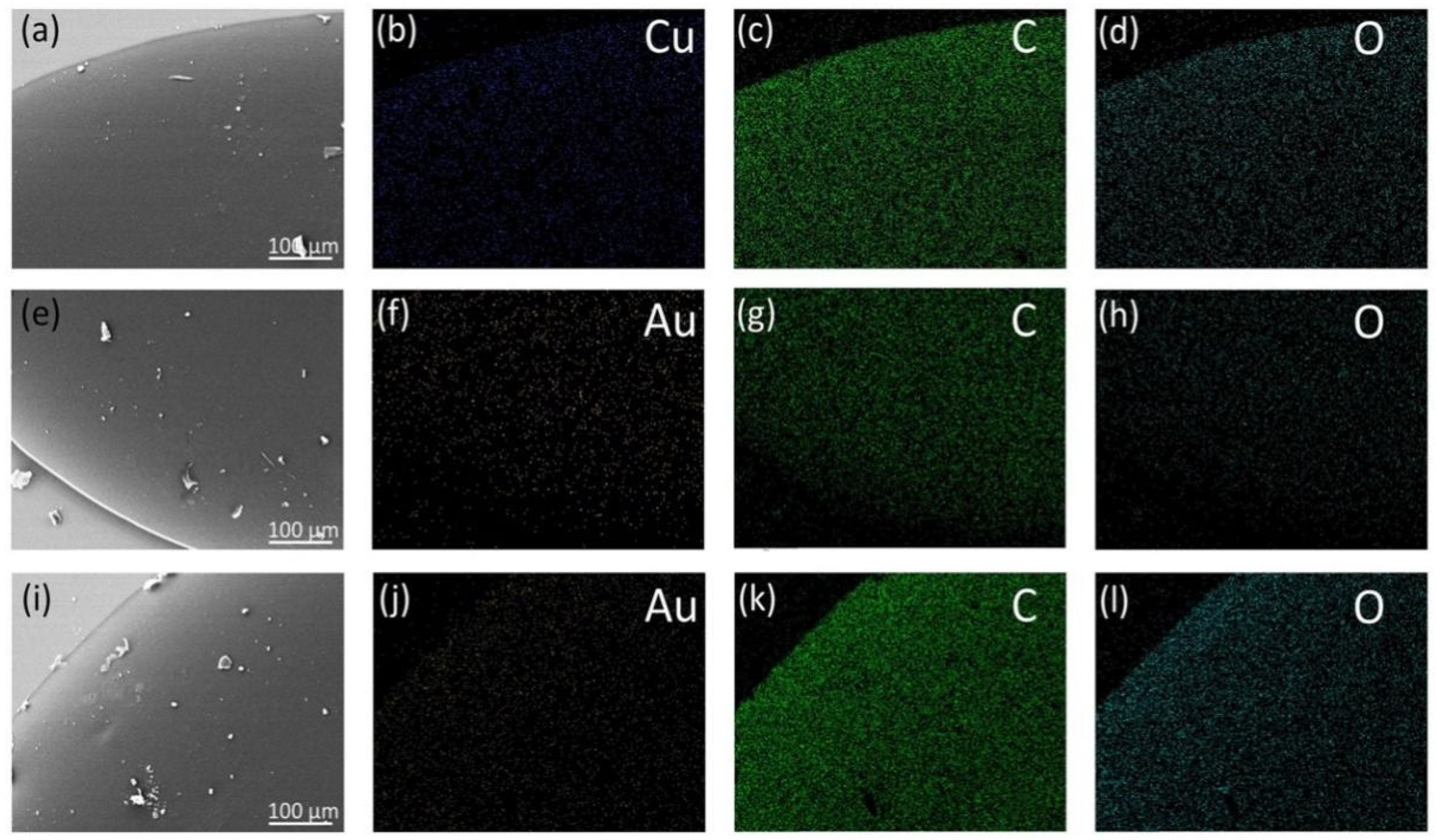
3.2. Structural and Optical Characterization of the MNCs/PVA Composite Films
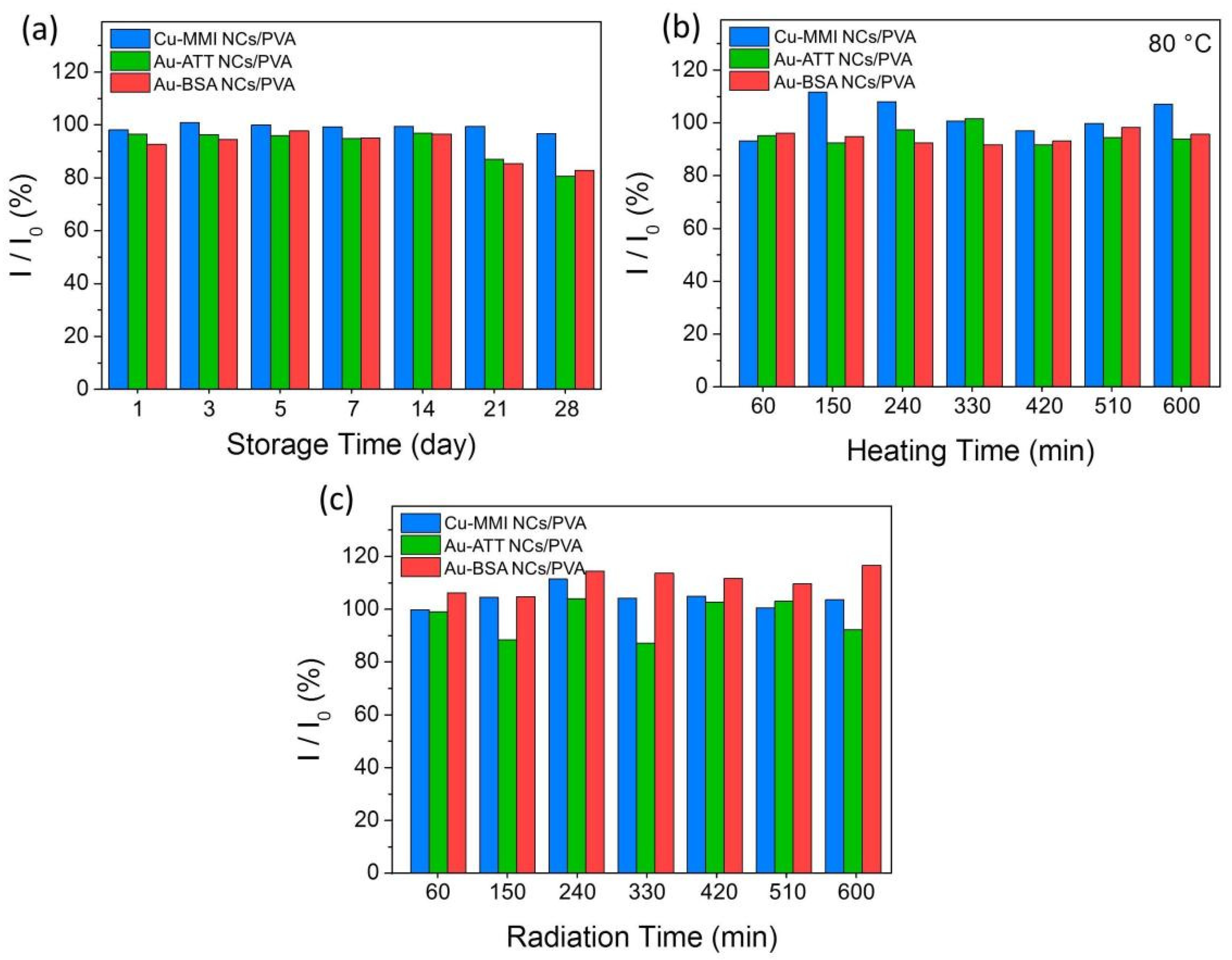
3.3. PL Stability of MNCs/PVA Composite Films
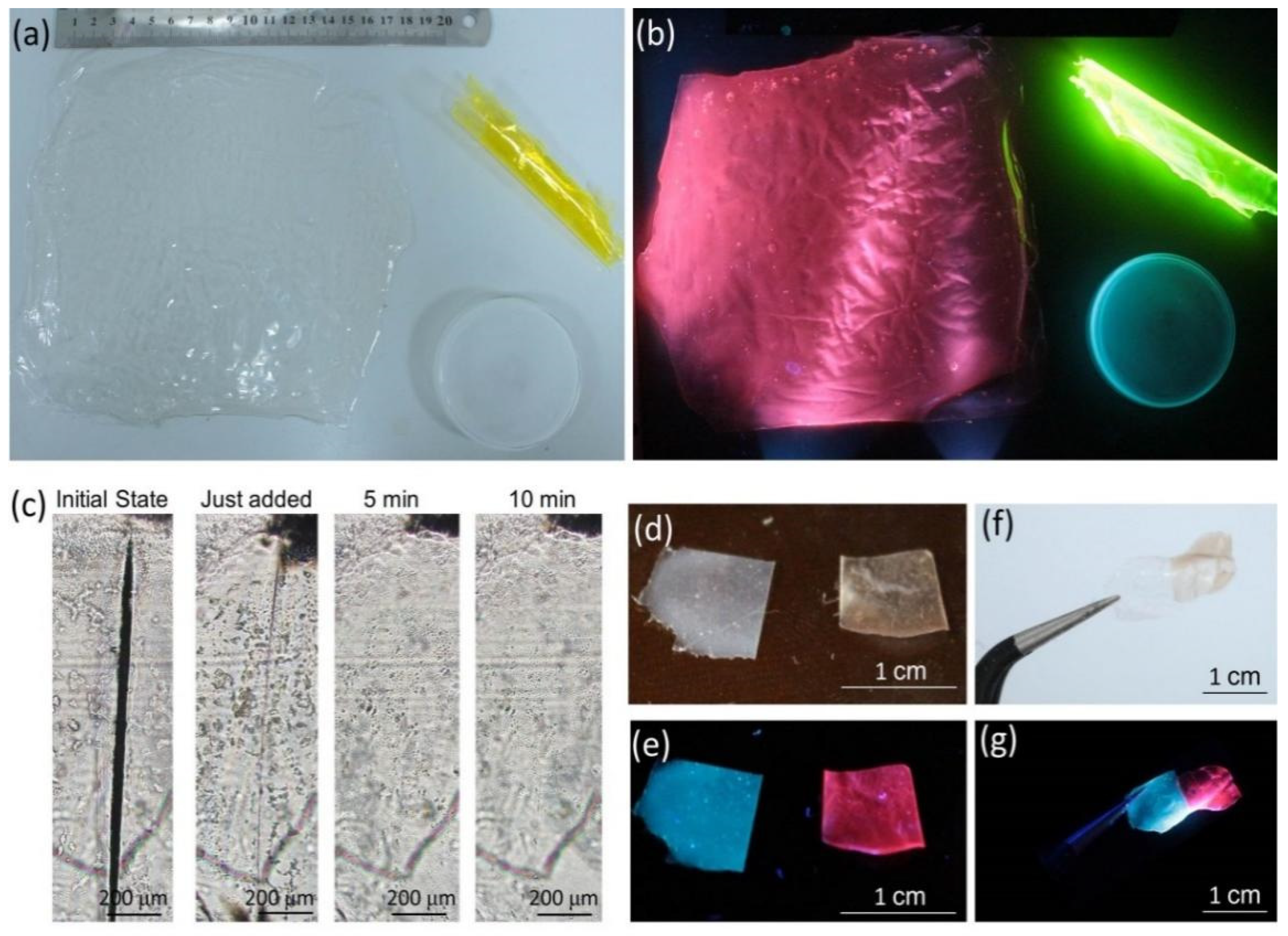
3.4. Fabricability and Self-Healing Ability of the MNCs/PVA Composite Films
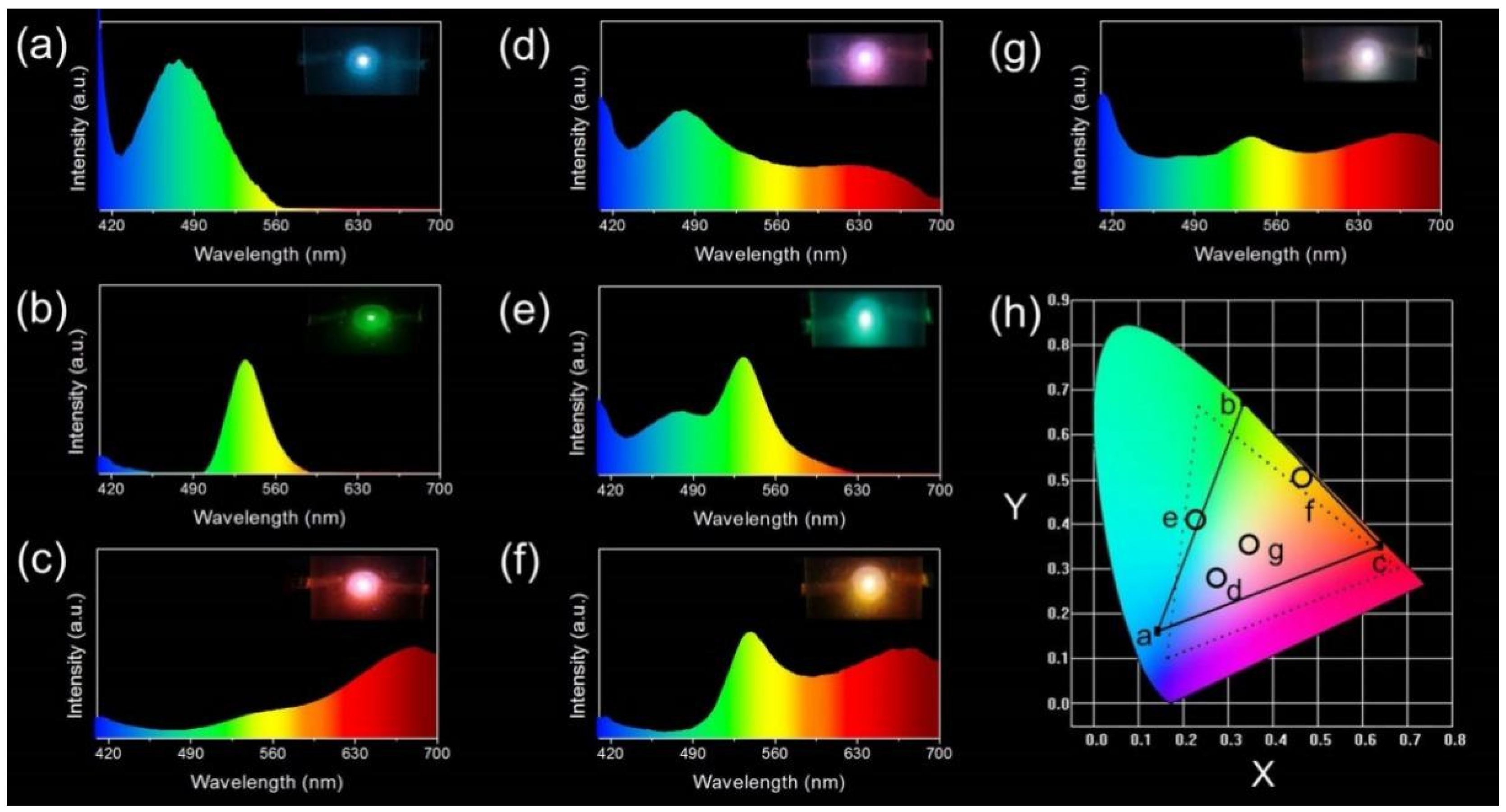
3.5. Application of MNCs/PVA Composite Films in Remote-Type LEDs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Dasog, M.; Kehrle, J.; Rieger, B.; Veinot, J.G. Silicon Nanocrystals and Silicon-Polymer Hybrids: Synthesis, Surface Engineering, and Applications. Angew. Chem. Int. Ed. Engl. 2016, 55, 2322–2339. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Liu, Z.; Xu, W.; Yang, L.; Liu, Y.; Yao, D.; Zhang, D.; Zhang, H.; Yang, B. Engineering the Photoluminescence of CsPbX3 (X = Cl, Br, and I) Perovskite Nanocrystals Across the Full Visible Spectra with the Interval of 1 nm. ACS Appl. Mater. Interfaces 2019, 11, 14256–14265. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zha, T.; Gong, X.; Hu, Q.; Yu, M.; Wu, J.; Li, R.; Wang, J.; Chen, Y. Cu–Cd–Zn–S/ZnS Core/Shell Quantum Dot/Polyvinyl Alcohol Flexible Films for White Light-emitting Diodes. RSC Adv. 2020, 10, 24425–24433. [Google Scholar] [CrossRef]
- Song, S.; Wang, X.; Wang, C.; Wen, Z.; Yang, Y.; Liu, H.; Yang, M.; Lin, Q. Large-Scale Synthesis of Flexible, Stable, and Transparent MoS2 Quantum Dots-Polyvinyl Alcohol Sensing Film. Part. Part. Syst. Charact. 2018, 35, 1800189. [Google Scholar] [CrossRef]
- Xu, L.; Li, Y.; Gao, S.; Niu, Y.; Liu, H.; Mei, C.; Cai, J.; Xu, C. Preparation and Properties of Cyanobacteria-Based Carbon Quantum Dots/Polyvinyl Alcohol/ Nanocellulose Composite. Polymers 2020, 12, 1143. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.C.; Kang, H.; Lee, S.; Oh, J.H.; Yang, H.; Do, Y.R. Study of Perovskite QD Down-Converted LEDs and Six-Color White LEDs for Future Displays with Excellent Color Performance. ACS Appl. Mater. Interfaces 2016, 8, 18189–18200. [Google Scholar] [CrossRef]
- Wang, C.; Lin, H.; Xu, Z.; Huang, Y.; Humphrey, M.G.; Zhang, C. Tunable Carbon-Dot-Based Dual-Emission Fluorescent Nanohybrids for Ratiometric Optical Thermometry in Living Cells. ACS Appl. Mater. Interfaces 2016, 8, 6621–6628. [Google Scholar] [CrossRef]
- Lei, J.; Huang, Z.; Gao, P.; Sun, J.; Zhou, L. Polyvinyl Alcohol Enhanced Fluorescent Sulfur Quantum Dots for Highly Sensitive Detection of Fe3+ and Temperature in Cells. Part. Part. Syst. Charact. 2021, 38, 2000332. [Google Scholar] [CrossRef]
- Kalytchuk, S.; Polakova, K.; Wang, Y.; Froning, J.P.; Cepe, K.; Rogach, A.L.; Zboril, R. Carbon Dot Nanothermometry: Intracellular Photoluminescence Lifetime Thermal Sensing. ACS Nano 2017, 11, 1432–1442. [Google Scholar] [CrossRef]
- Lai, W.F.; Wong, W.T.; Rogach, A.L. Development of Copper Nanoclusters for In Vitro and In Vivo Theranostic Applications. Adv. Mater. 2020, 32, 1906872. [Google Scholar] [CrossRef]
- Jin, R.; Zeng, C.; Zhou, M.; Chen, Y. Atomically Precise Colloidal Metal Nanoclusters and Nanoparticles: Fundamentals and Opportunities. Chem. Rev. 2016, 116, 10346–10413. [Google Scholar] [CrossRef]
- An, Y.; Ren, Y.; Bick, M.; Dudek, A.; Waworuntu, E.H.; Tang, J.; Chen, J.; Chang, B. Highly Fluorescent Copper Nanoclusters for Sensing and Bioimaging. Biosens. Bioelectron. 2020, 154, 112078. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Chen, B.; Rogach, A.L. Synthesis, Optical Properties and Applications of Light-emitting Copper Nanoclusters. Nanoscale Horiz. 2017, 2, 135–146. [Google Scholar] [CrossRef]
- Liu, X.; Astruc, D. Atomically Precise Copper Nanoclusters and Their Applications. Coord. Chem. Rev. 2018, 359, 112–126. [Google Scholar] [CrossRef]
- Diez, I.; Ras, R.H. Fluorescent Silver Nanoclusters. Nanoscale 2011, 3, 1963–1970. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.Y.; Wang, C.W.; Yuan, Z.; Chang, H.T. Fluorescent Gold Nanoclusters: Recent Advances in Sensing and Imaging. Anal. Chem. 2015, 87, 216–229. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Luo, Z.; Zhang, Q.; Zhang, X.; Zheng, Y.; Lee, J.Y.; Xie, J. Synthesis of Highly Fluorescent Metal (Ag, Au, Pt, and Cu) Nanoclusters by Electrostatically Induced Reversible Phase Transfer. ACS Nano 2011, 5, 8800–8808. [Google Scholar] [CrossRef] [PubMed]
- Shang, L.; Dong, S.; Nienhaus, G.U. Ultra-small Fluorescent Metal Nanoclusters: Synthesis and Biological Applications. Nano Today 2011, 6, 401–418. [Google Scholar] [CrossRef]
- Rao, T.U.B.; Pradeep, T. Luminescent Ag7 and Ag8 Clusters by Interfacial Synthesis. Angew. Chem. Int. Ed. Engl. 2010, 49, 3925–3929. [Google Scholar]
- Zhang, L.; Wang, E. Metal Nanoclusters: New Fluorescent Probes for Sensors and Bioimaging. Nano Today 2014, 9, 132–157. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, Y. Developing Fluorescent Copper Nanoclusters: Synthesis, Properties, and Applications. Colloids Surf. B 2020, 195, 111244. [Google Scholar] [CrossRef]
- Wang, Z.; Xiong, Y.; Kershaw, S.V.; Chen, B.; Yang, X.; Goswami, N.; Lai, W.F.; Xie, J.; Rogach, A.L. In Situ Fabrication of Flexible, Thermally Stable, Large-Area, Strongly Luminescent Copper Nanocluster/Polymer Composite Films. Chem. Mater. 2017, 29, 10206–10211. [Google Scholar] [CrossRef]
- Lin, H.; Wang, B.; Xu, J.; Zhang, R.; Chen, H.; Yu, Y.; Wang, Y. Phosphor-in-glass for High-powered Remote-type White AC-LED. ACS Appl. Mater. Interfaces 2014, 6, 21264–21269. [Google Scholar] [CrossRef] [PubMed]
- Peppas, N.A.; Hilt, J.Z.; Khademhosseini, A.; Langer, R. Hydrogels in Biology and Medicine: From Molecular Principles to Bionanotechnology. Adv. Mater. 2006, 18, 1345–1360. [Google Scholar] [CrossRef]
- Song, Y.H.; Ji, E.K.; Bak, S.H.; Kim, Y.N.; Lee, D.B.; Jung, M.K.; Jeong, B.W.; Yoon, D.H. New Design of Hybrid Remote Phosphor with Single-layer Graphene for Application in High-power LEDs. Chem. Eng. J. 2016, 287, 511–515. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, B.; Zhu, M.; Kershaw, S.V.; Zhi, C.; Zhong, H.; Rogach, A.L. Stretchable and Thermally Stable Dual Emission Composite Films of On-Purpose Aggregated Copper Nanoclusters in Carboxylated Polyurethane for Remote White Light-Emitting Devices. ACS Appl. Mater. Interfaces 2016, 8, 33993–33998. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, J.; Huang, L.; Pan, F.; Chen, Y.; Lei, B.; Peng, M.; Wu, M. Tunable Luminescent Properties and Concentration-Dependent, Site-Preferable Distribution of Eu2+ Ions in Silicate Glass for White LEDs Applications. ACS Appl. Mater. Interfaces 2015, 7, 10044–10054. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Wen, Z.; Wang, C.; Thomas, T.; Wang, C.; Song, Q.; Yang, M. Temperature-controlled Spectral Tuning of Full-color Carbon Dots and Their Strongly Fluorescent Solid-state Polymer Composites for Light-emitting Diodes. Nanoscale Adv. 2019, 1, 1413–1420. [Google Scholar] [CrossRef] [Green Version]
- Zeng, Q.; Zhang, X.; Feng, X.; Lu, S.; Chen, Z.; Yong, X.; Redfern, S.A.T.; Wei, H.; Wang, H.; Shen, H.; et al. Polymer-Passivated Inorganic Cesium Lead Mixed-Halide Perovskites for Stable and Efficient Solar Cells with High Open-Circuit Voltage over 1.3 V. Adv. Mater. 2018, 30, 1705393. [Google Scholar] [CrossRef] [PubMed]
- Qu, F.; Zhang, S.; Huang, C.; Guo, X.; Zhu, Y.; Thomas, T.; Guo, H.; Attfield, J.P.; Yang, M. Surface Functionalized Sensors for Humidity-Independent Gas Detection. Angew. Chem. Int. Ed. 2021, 60, 6561–6566. [Google Scholar] [CrossRef] [PubMed]
- Chong, S.F.; Smith, A.A.; Zelikin, A.N. Microstructured, functional PVA Hydrogels through Bioconjugation with Oligopeptides under Physiological Conditions. Small 2013, 9, 942–950. [Google Scholar] [CrossRef]
- Hu, Z.; Ma, Z.; Peng, M.; He, X.; Zhang, H.; Li, Y.; Qiu, J. Composite Film Polarizer Based on the Oriented Assembly of Electrospun Nanofibers. Nanotechnology 2016, 27, 135301. [Google Scholar] [CrossRef]
- Shin, E.J.; Lyoo, W.S.; Lee, Y.H. Polarizer Effect and Structure of Iodinated Before and After Casting Poly(vinyl alcohol) Film. J. Appl. Polym. Sci. 2011, 120, 397–405. [Google Scholar] [CrossRef]
- Yao, R.; You, Q.; Liu, P.; Xu, Y. Synthesis and pH-induced Phase Transition Behavior of PAA/PVA Nanogels in Aqueous Media. J. Appl. Polym. Sci. 2009, 111, 358–362. [Google Scholar] [CrossRef]
- Kamoun, E.A.; Kenawy, E.S.; Chen, X. A Review on Polymeric Hydrogel Membranes for Wound Dressing Applications: PVA-based Hydrogel Dressings. J. Adv. Res. 2017, 8, 217–233. [Google Scholar] [CrossRef] [PubMed]
- Alipoori, S.; Mazinani, S.; Aboutalebi, S.H.; Sharif, F. Review of PVA-based Gel Polymer Electrolytes in Flexible Solid-state Supercapacitors: Opportunities and Challenges. J. Energy Storage 2020, 27, 101072. [Google Scholar] [CrossRef]
- Deng, H.; Zhuang, Q.; Huang, K.; Balasubramanian, P.; Lin, Z.; Peng, H.; Xia, X.; Chen, W. Solid-state Thiolate-stabilized Copper Nanoclusters with Ultrahigh Photoluminescence Quantum Yield for White Light-emitting Devices. Nanoscale 2020, 12, 15791–15799. [Google Scholar] [CrossRef]
- Deng, H.; Shi, X.; Wang, F.; Peng, H.; Liu, A.; Xia, X.; Chen, W. Fabrication of Water-Soluble, Green-Emitting Gold Nanoclusters with a 65% Photoluminescence Quantum Yield via Host-Guest Recognition. Chem. Mater. 2017, 29, 1362–1369. [Google Scholar] [CrossRef]
- Xie, J.; Zheng, Y.; Ying, J. Protein-Directed Synthesis of Highly Fluorescent Gold Nanoclusters. J. Am. Chem. Soc. 2009, 131, 888–889. [Google Scholar] [CrossRef]
- Peng, Z.; Kong, L.X. Morphology of Self-assembled Polyvinyl Alcohol/Silica Nanocomposites Studied with Atomic Force Microscopy. Polym. Bull. 2007, 59, 207–216. [Google Scholar] [CrossRef]
- Ingrosso, C.; Fakhfouri, V.; Striccoli, M.; Agostiano, A.; Voigt, A.; Gruetzner, G.; Curri, M.L.; Brugger, J. An Epoxy Photoresist Modified by Luminescent Nanocrystals for the Fabrication of 3D High-Aspect-Ratio Microstructures. Adv. Funct. Mater. 2007, 17, 2009–2017. [Google Scholar] [CrossRef]
- Bhushan, B.; Qi, J. Phase Contrast Imaging of Nanocomposites and Molecularly Thick Lubricant Films in Magnetic Media. Nanotechnology 2003, 14, 886–895. [Google Scholar] [CrossRef]
- Sreeja, S.; Sreedhanya, S.; Smijesh, N.; Philip, R.; Muneera, C.I. Organic Dye Impregnated Poly(vinyl alcohol) Nanocomposite as An Efficient Optical Limiter: Structure, Morphology and Photophysical Properties. J. Mater. Chem. C 2013, 1, 3851–3861. [Google Scholar] [CrossRef]
- Bhajantri, R.F.; Ravindrachary, V.; Harisha, A.; Crasta, V.; Nayak, S.P.; Poojary, B. Microstructural Studies on BaCl2 Doped Poly(vinyl alcohol). Polymer 2006, 47, 3591–3598. [Google Scholar] [CrossRef]
- Shi, Y.; Zhuang, X.; Cao, L.; Gou, S.; Xiong, Y.; Lai, W.-F.; Wang, Z.; Rogach, A.L. Copper-Nanocluster-Based Transparent Ultraviolet-Shielding Polymer Films. ChemNanoMat 2019, 5, 110–115. [Google Scholar] [CrossRef]
- Xiong, Y.; Zhu, M.; Wang, Z.; Schneider, J.; Huang, H.; Kershaw, S.V.; Zhi, C.; Rogach, A.L. A Building Brick Principle to Create Transparent Composite Films with Multicolor Emission and Self-Healing Function. Small 2018, 14, 1800315. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Yao, D.; Liu, H.; Zhang, H. Metal Nanoclusters/Polyvinyl Alcohol Composite Films as the Alternatives for Fabricating Remote-Type White Light-Emitting Diodes. Nanomaterials 2022, 12, 204. https://doi.org/10.3390/nano12020204
Liu Z, Yao D, Liu H, Zhang H. Metal Nanoclusters/Polyvinyl Alcohol Composite Films as the Alternatives for Fabricating Remote-Type White Light-Emitting Diodes. Nanomaterials. 2022; 12(2):204. https://doi.org/10.3390/nano12020204
Chicago/Turabian StyleLiu, Zhaoyu, Dong Yao, Huiwen Liu, and Hao Zhang. 2022. "Metal Nanoclusters/Polyvinyl Alcohol Composite Films as the Alternatives for Fabricating Remote-Type White Light-Emitting Diodes" Nanomaterials 12, no. 2: 204. https://doi.org/10.3390/nano12020204