Recent Developments in the Application of Inorganic Nanomaterials and Nanosystems for the Protection of Cultural Heritage Organic Artifacts
Abstract
:1. Introduction
2. Cultural Heritage Objects of an Organic Nature
- -
- Cellulose-based artifacts are subjected to acidic degradation of cellulose chains (due to the action of environmental or internal acids), alkaline degradation, photodegradation, and oxidative degradation;
- -
- -
- Ivory and archaeological bones undergo similar degradation phenomena: while the collagen part of the artifacts can degrade by chemical hydrolysis [21,22], the degradation also affects the inorganic part, which can undergo mineral recrystallization and degradation of mechanical and morphological properties (such as increased porosity).
3. Inorganic Nanomaterials for the Protection of Paper Artifacts
- -
- Formulation of the deacidification solution, as the presence of some surfactants/stabilizers could lead to a reduction in nanoparticle reactivity, creating a too alkaline environment that could result in the alkaline depolymerization process [31];
- -
- Compatibility of the proposed recipes with other elements present on the paper artifacts (such as inks, dyes, or pigments) [23].
4. Inorganic Nanomaterials for the Protection of Historical Wood
5. Inorganic Nanomaterials for the Protection of Other Types of Cultural Heritage Artifacts of an Organic Nature
6. Conclusions and Future Perspectives
- -
- Enhancement/protection of mechanical and esthetic characteristics;
- -
- Protection from acid or UV-induced degradation (pH regulation, UV adsorption);
- -
- Antimicrobial protection.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mcintyre-Tamwoy, S. The Impact of Global Climate Change and Cultural Heritage: Grasping the Issues and Defining the Problem. Hist. Environ. 2008, 21, 1–9. [Google Scholar]
- Bayda, S.; Adeel, M.; Tuccinardi, T.; Cordani, M.; Rizzolio, F. The History of Nanoscience and Nanotechnology: From Chemical–Physical Applications to Nanomedicine. Molecules 2020, 25, 112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, D.; Xie, G.; Luo, J. Mechanical Properties of Nanoparticles: Basics and Applications. J. Phys. D Appl. Phys. 2013, 47, 013001. [Google Scholar] [CrossRef] [Green Version]
- Buzea, C.; Pacheco, I. Nanomaterials and Their Classification. Adv. Struct. Mat. 2017, 62, 3–45. [Google Scholar] [CrossRef]
- Serafini, I.; Ciccola, A. Nanotechnologies and Nanomaterials: An Overview for Cultural Heritage. In Nanotechnologies and Nanomaterials for Diagnostic, Conservation and Restoration of Cultural Heritage; Lazzara, G., Fakhrullin, R., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 325–380. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, P.; Ding, J.; Dong, Y.; Cao, Y.; Dong, W.; Zhao, X.; Li, X.; Camaiti, M. Nano Ca(OH)2: A review on synthesis, properties and applications. J. Cult. Herit. 2021, 50, 25–42. [Google Scholar] [CrossRef]
- Walsh-Korbs, Z.; Avérous, L. Recent developments in the conservation of materials properties of historical wood. Progr. Mat. Sci. 2019, 102, 167–221. [Google Scholar] [CrossRef]
- Girginova, P.I.; Galacho, C.; Veiga, R.; Santos Silva, A.; Candeias, A. Inorganic Nanomaterials for Restoration of Cultural Heritage: Synthesis Approaches towards Nanoconsolidants for Stone and Wall Paintings. ChemSusChem 2018, 11, 4168–4182. [Google Scholar] [CrossRef]
- Ricca, M.; La Russa, M.F. Challenges for the protection of underwater cultural heritage (UCH), from waterlogged and weathered stone materials to conservation strategies: An overview. Heritage 2020, 3, 402–411. [Google Scholar] [CrossRef]
- Franco-Castillo, I.; Hierro, L.; de la Fuente, J.M.; Seral-Ascaso, A.; Mitchell, S.G. Perspectives for antimicrobial nanomaterials in cultural heritage conservation. Chem 2021, 7, 629–669. [Google Scholar] [CrossRef]
- Dahlin, E. Damage assessment-causes, mechanisms and measurements. In Proceedings of the 5th EC Conference Cultural Heritage Research: A Pan-European Challenge, Cracow, Poland, 16–18 May 2002; Institute of Catalysis and Surface Chemistry: Cracow, Poland, 2003; pp. 57–60. [Google Scholar]
- Fabbri, K.; Bonora, A. Two New Indices for Preventive Conservation of the Cultural Heritage: Predicted Risk of Damage and Heritage Microclimate Risk. J. Cult. Herit. 2021, 47, 208–217. [Google Scholar] [CrossRef]
- Baxter, M. Artifact Classification: A Conceptual and Methodological Approach, by Dwight W. Read, 2007. Walnut Creek (CA): Left Coast Press; ISBN 978-1-59874-102-5 Hardback £30 & US$89; 363 Pp., 61 Figs., 31 Tables. Cambridge Archaeol. J. 2009, 19, 260–261. [Google Scholar] [CrossRef]
- Pyzik, A.; Ciuchcinski, K.; Dziurzynski, M.; Dziewit, L. The Bad and the Good—Microorganisms in Cultural Heritage Environments—An Update on Biodeterioration and Biotreatment Approaches. Materials 2021, 14, 177. [Google Scholar] [CrossRef] [PubMed]
- Sterflinger, K.; Piñar, G. Microbial deterioration of cultural heritage and works of art—tilting at windmills? Appl. Microbiol. Biotechnol. 2013, 97, 9637–9646. [Google Scholar] [CrossRef] [Green Version]
- Blanchette, R.A.; Nilsson, T.; Daniel, G.; Abad, A. Biological Degradation of Wood Author. In Archaeological Wood Properties, Chemistry, and Preservation; Rowell, R.M., Barbour, R.J., Eds.; American Chemical Society: Washington, DC, USA, 1990; pp. 141–174. [Google Scholar]
- Gjelstrup Björdal, G. Microbial degradation of waterlogged archaeological wood. J. Cult. Herit. 2012, 13, S118–S122. [Google Scholar] [CrossRef]
- Gutarowska, B.; Pietrzak, K.; Machnowski, W.; Milczarek, J.M. Historical textiles – a review of microbial deterioration analysis and disinfection methods. Textile Res. J. 2016, 87, 2388–2406. [Google Scholar] [CrossRef]
- Boyatzis, S.C.; Velivasaki, G.; Malea, E. A study of the deterioration of aged parchment marked with laboratory iron gall inks using FTIR-ATR spectroscopy and micro hot table. Herit. Sci. 2016, 4, 13. [Google Scholar] [CrossRef] [Green Version]
- Dolgin, B.; Bulatov, V.; Schechter, I. Non-destructive assessment of parchment deterioration by optical methods. Anal. Bioanal. Chem. 2007, 388, 1885–1896. [Google Scholar] [CrossRef]
- Doménech-Carbó, M.T.; Buendía-Ortuño, M.; Pasies-Oviedo, T.; Osete-Cortina, L. Analytical study of waterlogged ivory from the Bajo de la campana site (Murcia, Spain). Microchem. J. 2016, 126, 381–405. [Google Scholar] [CrossRef] [Green Version]
- Matthiesen, H.; Høier Eriksen, A.M.; Hollesen, J.; Collins, M. Bone degradation at five Arctic archaeological sites: Quantifying the importance of burial environment and bone characteristics. J. Archaeol. Sci. 2021, 125, 105296. [Google Scholar] [CrossRef]
- Baglioni, P.; Chelazzi, D.; Giorgi, R. Deacidification of Paper, Canvas and Wood. In Nanotechnologies in the Conservation of Cultural Heritage; Springer: Berlin, Germany, 2015; pp. 117–144. [Google Scholar] [CrossRef]
- Wójciak, A. Deacidification of Paper with Mg(OH)2 Nanoparticles: The Impact of Dosage on Process Effectiveness. Wood Res. 2016, 61, 937–950. [Google Scholar]
- Poggi, G.; Sistach, M.C.; Marin, E.; Garcia, J.F.; Giorgi, R.; Baglioni, P. Calcium Hydroxide Nanoparticles in Hydroalcoholic Gelatin Solutions (GeolNan) for the Deacidification and Strengthening of Papers Containing Iron Gall Ink. J. Cul. Herit. 2016, 18, 250–257. [Google Scholar] [CrossRef]
- Afsharpour, M.; Imani, S. Preventive Protection of Paper Works by Using Nanocomposite Coating of Zinc Oxide. J. Cult. Herit. 2017, 25, 142–148. [Google Scholar] [CrossRef]
- Poggi, G.; Giorgi, R.; Mirabile, A.; Xing, H.; Baglioni, P. A Stabilizer-Free Non-Polar Dispersion for the Deacidification of Contemporary Art on Paper. J. Cult. Herit. 2017, 26, 44–52. [Google Scholar] [CrossRef]
- Bastone, S.; Chillura Martino, D.F.; Renda, V.; Saladino, M.L.; Poggi, G.; Caponetti, E. Alcoholic Nanolime Dispersion Obtained by the Insolubilisation-Precipitation Method and Its Application for the Deacidification of Ancient Paper. Colloids Surf. A 2017, 513, 241–249. [Google Scholar] [CrossRef]
- Rushdy, A.M.; Wahba, W.N.; Abd-Aziz, M.S.; El Samahy, M.; Kamel, S. A Comparative Study of Consolidation Materials for Paper Conservation. Int. J. Conserv. Sci. 2017, 8, 441–452. [Google Scholar]
- Ariafar, A.A.; Afsharpour, M.; Samanian, K. Use of TiO2/Chitosan Nanoparticles for Enhancing the Preservative Effects of Carboxymethyl Cellulose in Paper-Art-Works against Biodeterioration. Int. Biodeter. Biodegrad. 2018, 131, 67–77. [Google Scholar] [CrossRef]
- Huang, J.; Liang, G.; Lu, G.; Zhang, J. Conservation of Acidic Papers Using a Dispersion of Oleic Acid-Modified MgO Nanoparticles in a Non-Polar Solvent. J. Cult. Herit. 2018, 34, 61–68. [Google Scholar] [CrossRef]
- Amornkitbamrung, L.; Marnul, M.C.; Palani, T.; Hribernik, S.; Kovalcik, A.; Kargl, R.; Stana-Kleinschek, K.; Mohan, T. Strengthening of Paper by Treatment with a Suspension of Alkaline Nanoparticles Stabilized by Trimethylsilyl Cellulose. Nano-Struct. Nano-Objects 2018, 16, 363–370. [Google Scholar] [CrossRef]
- Saoud, K.M.; Saeed, S.; al Soubaihi, R.; Samara, A.; Ibala, I.; el Ladki, D.; Ezzeldeen, O. Application of Mg(OH)2 Nanosheets for Conservation and Restoration of Precious Documents and Cultural Archives. BioResources 2018, 13, 3259–3274. [Google Scholar] [CrossRef] [Green Version]
- Hassan, R.R.A.; Mohamed, W.S. The Impact of Methyl Methacrylate Hydroxyethyl Methacrylate Loaded with Silver Nanoparticles on Mechanical Properties of Paper. Appl. Phys. A 2018, 124, 551. [Google Scholar] [CrossRef]
- Jia, M.; Zhang, X.; Weng, J.; Zhang, J.; Zhang, M. Protective Coating of Paper Works: ZnO/Cellulose Nanocrystal Composites and Analytical Characterization. J. Cult. Herit. 2019, 38, 64–74. [Google Scholar] [CrossRef]
- Fouda, A.; Abdel-Maksoud, G.; Abdel-Rahman, M.A.; Eid, A.M.; Barghoth, M.G.; El-Sadany, M.A.H. Monitoring the Effect of Biosynthesized Nanoparticles against Biodeterioration of Cellulose-Based Materials by Aspergillus Niger. Cellulose 2019, 26, 6583–6597. [Google Scholar] [CrossRef]
- Franco Castillo, I.; Garciá Guillén, E.; de la Fuente, J.M.; Silva, F.; Mitchell, S.G. Preventing Fungal Growth on Heritage Paper with Antifungal and Cellulase Inhibiting Magnesium Oxide Nanoparticles. J. Mat. Chem. B 2019, 7, 6412–6419. [Google Scholar] [CrossRef]
- Fouda, A.; Abdel-Maksoud, G.; Abdel-Rahman, M.A.; Salem, S.S.; Hassan, S.E.D.; El-Sadany, M.A.H. Eco-Friendly Approach Utilizing Green Synthesized Nanoparticles for Paper Conservation against Microbes Involved in Biodeterioration of Archaeological Manuscript. Int. Biodeter. Biodegrad. 2019, 142, 160–169. [Google Scholar] [CrossRef]
- He, B.; Lin, Q.; Chang, M.; Liu, C.; Fan, H.; Ren, J. A New and Highly Efficient Conservation Treatment for Deacidification and Strengthening of Aging Paper by In-Situ Quaternization. Carbohydr. Polym. 2019, 209, 250–257. [Google Scholar] [CrossRef]
- Nemoykina, A.L.; Shabalina, A.V.; Svetlichnyi, V.A. Restoration and Conservation of Old Low-Quality Book Paper Using Aqueous Colloids of Magnesium Oxyhydroxide Obtained by Pulsed Laser Ablation. J. Cult. Herit. 2019, 39, 42–48. [Google Scholar] [CrossRef]
- Weng, J.; Zhang, X.; Jia, M.; Zhang, J. Deacidification of Aged Papers Using Dispersion of Ca(OH)2 Nanoparticles in Subcritical 1,1,1,2-Tetrafluoroethane (R134a). J. Cult. Herit. 2019, 37, 137–147. [Google Scholar] [CrossRef]
- Malešič, J.; Kadivec, M.; Kunaver, M.; Skalar, T.; Cigić, I.K. Nano Calcium Carbonate versus Nano Calcium Hydroxide in Alcohols as a Deacidification Medium for Lignocellulosic Paper. Herit. Sci. 2019, 7, 50. [Google Scholar] [CrossRef]
- Taghiyari, H.R.; Kalantari, A.; Kalantari, A.; Avramidis, S. Effect of wollastonite nanofibers and exposure to Aspergillus niger fungus on air flow rate in paper. Measurement 2019, 136, 307–313. [Google Scholar] [CrossRef]
- Xu, Q.; Poggi, G.; Resta, C.; Baglioni, M.; Baglioni, P. Grafted Nanocellulose and Alkaline Nanoparticles for the Strengthening and Deacidification of Cellulosic Artworks. J. Colloid Interface Sci. 2020, 576, 147–157. [Google Scholar] [CrossRef]
- Bergamonti, L.; Potenza, M.; Haghighi Poshtiri, A.; Lorenzi, A.; Sanangelantoni, A.M.; Lazzarini, L.; Lottici, P.P.; Graiff, C. Ag-Functionalized Nanocrystalline Cellulose for Paper Preservation and Strengthening. Carbohydr. Polym. 2020, 231, 115773. [Google Scholar] [CrossRef]
- Fan, H.; Guo, M.; Mou, H.; Shi, W.; Li, J.; Liu, J. Deacidification and Reinforcement of Old Books Using Sodium Carbonate and Latex Composites. Bioresources 2020, 15, 302–316. [Google Scholar] [CrossRef]
- Lisuzzo, L.; Cavallaro, G.; Milioto, S.; Lazzara, G. Halloysite Nanotubes Filled with MgO for Paper Reinforcement and Deacidification. Appl. Clay Sci. 2021, 213, 106231. [Google Scholar] [CrossRef]
- Zając, I.; Szulc, J.; Gutarowska, B. The Effect of Ethylene Oxide and Silver Nanoparticles on Photographic Models in the Context of Disinfection of Photo Albums. J. Cult. Herit. 2021, 51, 59–70. [Google Scholar] [CrossRef]
- Longo, E.; Senapeschi, A.N.; Varela, J.A.; Whittemore, O.J. Mechanisms of Water Interaction with an MgO Surface. Langmuir 1985, 1, 456–461. [Google Scholar] [CrossRef]
- Baty, J.W.; Maitland, C.L.; Minter, W.; Hubbe, M.A.; Jordan-Mowery, S.K. Deacidification for the conservation and preservation of paper-based works: A review. Bioresources 2010, 5, 1955–2023. [Google Scholar] [CrossRef]
- Hassan, R.R.A.; Mahmoud, S.M.A.; Nessem, M.A.; Aty, R.T.A.; Ramzy, M.G.; Dessoky, E.S.; Abdelkhalek, A.; Salem, M.Z.M. Hydroxypropyl Cellulose Loaded with ZnO Nanoparticles for Enhancing the Mechanical Properties of Papyrus (Cyperus Papyrus L.) Strips. BioResources 2021, 16, 2607–2625. [Google Scholar] [CrossRef]
- Zhou, K.; Li, A.; Xie, L.; Wang, C.C.; Wang, P.; Wang, X. Mechanism and Effect of Alkoxysilanes on the Restoration of Decayed Wood Used in Historic Buildings. J. Cult. Herit. 2020, 43, 64–72. [Google Scholar] [CrossRef]
- Fierascu, R.C.; Doni, M.; Fierascu, I. Selected Aspects Regarding the Restoration/Conservation of Traditional Wood and Masonry Building Materials: A Short Overview of the Last Decade Findings. Appl. Sci. 2020, 10, 1164. [Google Scholar] [CrossRef] [Green Version]
- Poggi, G.; Toccafondi, N.; Chelazzi, D.; Canton, P.; Giorgi, R.; Baglioni, P. Calcium Hydroxide Nanoparticles from Solvothermal Reaction for the Deacidification of Degraded Waterlogged Wood. J. Colloid Interface Sci. 2016, 473, 1–8. [Google Scholar] [CrossRef]
- Harandi, D.; Ahmadi, H.; Mohammadi Achachluei, M. Comparison of TiO2 and ZnO Nanoparticles for the Improvement of Consolidated Wood with Polyvinyl Butyral against White Rot. Int. Biodeter. Biodegrad. 2016, 108, 142–148. [Google Scholar] [CrossRef]
- Schofield, E.J.; Sarangi, R.; Mehta, A.; Jones, A.M.; Smith, A.; Mosselmans, J.F.W.; Chadwick, A.V. Strontium Carbonate Nanoparticles for the Surface Treatment of Problematic Sulfur and Iron in Waterlogged Archaeological Wood. J. Cult. Herit. 2016, 18, 306–312. [Google Scholar] [CrossRef] [Green Version]
- Terzi, E.; Kartal, S.N.; Yılgor, N.; Rautkari, L.; Yoshimura, T. Role of various nano-particles in prevention of fungal decay, mold growth and termite attack in wood, and their effect on weathering properties and water repellency. Int. Biodeter. Biodegrad. 2016, 107, 77–87. [Google Scholar] [CrossRef]
- Andriulo, F.; Braovac, S.; Kutzke, H.; Giorgi, R.; Baglioni, P. Nanotechnologies for the restoration of alum-treated archaeological wood. Appl. Phys. A 2016, 122, 322. [Google Scholar] [CrossRef]
- Muhcu, D.; Terzi, E.; Kartal, S.N.; Yoshimura, T. Biological performance, water absorption, and swelling of wood treated with nano-particles combined with the application of Paraloid B72®. J. For. Res. 2017, 28, 381–394. [Google Scholar] [CrossRef]
- Guo, H.; Bachtiar, E.V.; Ribera, J.; Heeb, M.; Schwarze, F.W.M.R.; Burgert, I. Non-biocidal preservation of wood against brown-rot fungi with a TiO2/Ce xerogel. Green Chem. 2018, 20, 1375–1382. [Google Scholar] [CrossRef]
- Cavallaro, G.; Milioto, S.; Parisi, F.; Lazzara, G. Halloysite Nanotubes Loaded with Calcium Hydroxide: Alkaline Fillers for the Deacidification of Waterlogged Archeological Woods. ACS Appl. Mat. Interfac. 2018, 10, 27355–27364. [Google Scholar] [CrossRef]
- Ion, R.M.; Nyokong, T.; Nwahara, N.; Suica-Bunghez, I.R.; Iancu, L.; Teodorescu, S.; Dulama, I.D.; Stirbescu, R.M.; Gheboianu, A.; Grigorescu, R.M. Wood Preservation with Gold Hydroxyapatite System. Herit. Sci. 2018, 6, 37. [Google Scholar] [CrossRef] [Green Version]
- De Peres, M.L.; Delucis, R.D.A.; Amico, S.C.; Gatto, D.A. Zinc oxide nanoparticles from microwave-assisted solvothermal process: Photocatalytic performance and use for wood protection against xylophagous fungus. Nanomat. Nanotechnol. 2019, 9, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Abbasi, J.; Samanian, K.; Afsharpour, M. Consolidation of Historical Woods Using Polyvinyl Butyral/Zinc Oxide Nano-Composite: Investigation of Water Absorption, Wettability, and Resistance to Weathering. Int. J. Conserv. Sci. 2020, 11, 15–24. [Google Scholar]
- Janesch, J.; Czabany, I.; Hansmann, C.; Mautner, A.; Rosenau, T.; Gindl-Altmutter, W. Transparent Layer-by-Layer Coatings Based on Biopolymers and CeO2 to Protect Wood from UV Light. Progr. Org. Coat. 2020, 138, 105409. [Google Scholar] [CrossRef]
- Nabil, E.; Mahmoud, N.; Youssef, A.M.; Kamel, S. Influence of Polymers Loaded with ZnO and TiO2 Nanoparticles on Thermal Resistance of Archaeological Wood. Egypt. J. Chem. 2020, 63, 4645–4657. [Google Scholar] [CrossRef]
- Taglieri, G.; Daniele, V.; Macera, L.; Schweins, R.; Zorzi, S.; Capron, M.; Chaumat, G.; Mondelli, C. Sustainable Nanotechnologies for Curative and Preventive Wood Deacidification Treatments: An Eco-Friendly and Innovative Approach. Nanomaterials 2020, 10, 1744. [Google Scholar] [CrossRef] [PubMed]
- Yves, K.G.; Chen, T.; Aladejana, J.T.; Wu, Z.; Xie, Y. Preparation, Test, and Analysis of a Novel Aluminosilicate-Based Antimildew Agent Applied on the Microporous Structure of Wood. ACS Omega 2020, 5, 8784–8793. [Google Scholar] [CrossRef] [Green Version]
- Parisi, F.; Bernardini, F.; Cavallaro, G.; Mancini, L.; Milioto, S.; Prokop, D.; Lazzara, G. Halloysite Nanotubes/Pluronic Nanocomposites for Waterlogged Archeological Wood: Thermal Stability and X-Ray Microtomography. J. Thermal Anal. Calorim. 2020, 141, 981–989. [Google Scholar] [CrossRef]
- Lisuzzo, L.; Hueckel, T.; Cavallaro, G.; Sacanna, S.; Lazzara, G. Pickering Emulsions Based on Wax and Halloysite Nanotubes: An Ecofriendly Protocol for the Treatment of Archeological Woods. ACS Appl. Mat. Interfac. 2021, 13, 1651–1661. [Google Scholar] [CrossRef]
- Weththimuni, M.L.; Milanese, C.; Licchelli, M.; Malagodi, M. Improving the Protective Properties of Shellac-Based Varnishes by Functionalized Nanoparticles. Coatings 2021, 11, 419. [Google Scholar] [CrossRef]
- Pietrzak, K.; Otlewska, A.; Puchalski, M.; Gutarowska, B.; Guiamet, P. Antimicrobial Properties of Silver Nanoparticles against Biofilm Formation by Pseudomonas Aeruginosa on Archaeological Textiles. Appl. Environ. Biotechnol. 2017, 2, 1–9. [Google Scholar] [CrossRef]
- Baglioni, M.; Bartoletti, A.; Bozec, L.; Chelazzi, D.; Giorgi, R.; Odlyha, M.; Pianorsi, D.; Poggi, G.; Baglioni, P. Nanomaterials for the Cleaning and PH Adjustment of Vegetable-Tanned Leather. Appl. Phys. A 2016, 122, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Pietrzak, K.; Puchalski, M.; Otlewska, A.; Wrzosek, H.; Guiamet, P.; Piotrowska, M.; Gutarowska, B. Microbial Diversity of Pre-Columbian Archaeological Textiles and the Effect of Silver Nanoparticles Misting Disinfection. J. Cult. Herit. 2017, 23, 138–147. [Google Scholar] [CrossRef]
- Osman, E.M.; Ibrahim, S.F.; Essa, D.M. Evaluation the Using of Nano Materials as Self Cleaning Agents of Different Kinds of Stained Archeological Textiles. Egypt. J. Chem. 2017, 60, 945–956. [Google Scholar] [CrossRef]
- Taghiyari, H.R.; Majidinajafabadi, R.; Vahidzadeh, R. Wollastonite to hinder growth of Aspergillus niger fungus on cotton textile. An. Acad. Bras. Ciênc. 2018, 90, 2797–2804. [Google Scholar] [CrossRef]
- Gong, W.; Yang, S.; Zheng, L.; Xiao, H.; Zheng, J.; Wu, B.; Zhou, Z. Consolidating Effect of Hydroxyapatite on the Ancient Ivories from Jinsha Ruins Site: Surface Morphology and Mechanical Properties Study. J. Cult. Herit. 2019, 35, 116–122. [Google Scholar] [CrossRef]
- Salvatore, A.; Vai, S.; Caporali, S.; Caramelli, D.; Lari, M.; Carretti, E. Evaluation of Diammonium Hydrogen Phosphate and Ca(OH)2 Nanoparticles for Consolidation of Ancient Bones. J. Cult. Herit. 2020, 41, 1–12. [Google Scholar] [CrossRef]
- Palladino, N.; Hacke, M.; Poggi, G.; Nechyporchuk, O.; Kolman, K.; Xu, Q.; Persson, M.; Giorgi, R.; Holmberg, K.; Baglioni, P.; et al. Nanomaterials for Combined Stabilisation and Deacidification of Cellulosic Materials—the Case of Iron-Tannate Dyed Cotton. Nanomaterials 2020, 10, 900. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Ding, J.; Zhang, P.; Dong, W.; Zhao, X.; Camaiti, M.; Li, X. In-Situ Growth Synthesis of Nanolime/Kaolin Nanocomposite for Strongly Consolidating Highly Porous Dinosaur Fossil. Constr. Build. Mat. 2021, 300, 124312. [Google Scholar] [CrossRef]
- Saada, N.S.; Abdel-Maksoud, G.; Abd El-Aziz, M.S.; Youssef, A.M. Green Synthesis of Silver Nanoparticles, Characterization, and Use for Sustainable Preservation of Historical Parchment against Microbial Biodegradation. Biocatal. Agricult. Biotechnol. 2021, 32, 101948. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, Q.; Zhang, K.; Yang, F.; Yang, L.; Wang, L. In-situ growth of calcium sulfate dihydrate as a consolidating material for the archaeological bones. Mat. Lett. 2021, 282, 128713. [Google Scholar] [CrossRef]
- Fierascu, I.; Fierascu, I.C.; Brazdis, R.I.; Baroi, A.M.; Fistos, T.; Fierascu, R.C. Phytosynthesized Metallic Nanoparticles—between Nanomedicine and Toxicology. A Brief Review of 2019’s Findings. Materials 2020, 13, 574. [Google Scholar] [CrossRef] [Green Version]
- Fierascu, I.; Fierascu, R.C.; Somoghi, R.; Ion, R.M.; Moanta, A.; Avramescu, S.M.; Damian, C.M.; Ditu, L.M. Tuned Apatitic Materials: Synthesis, Characterization and Potential Antimicrobial Applications. Appl. Surf. Sci. 2018, 438, 127–135. [Google Scholar] [CrossRef]


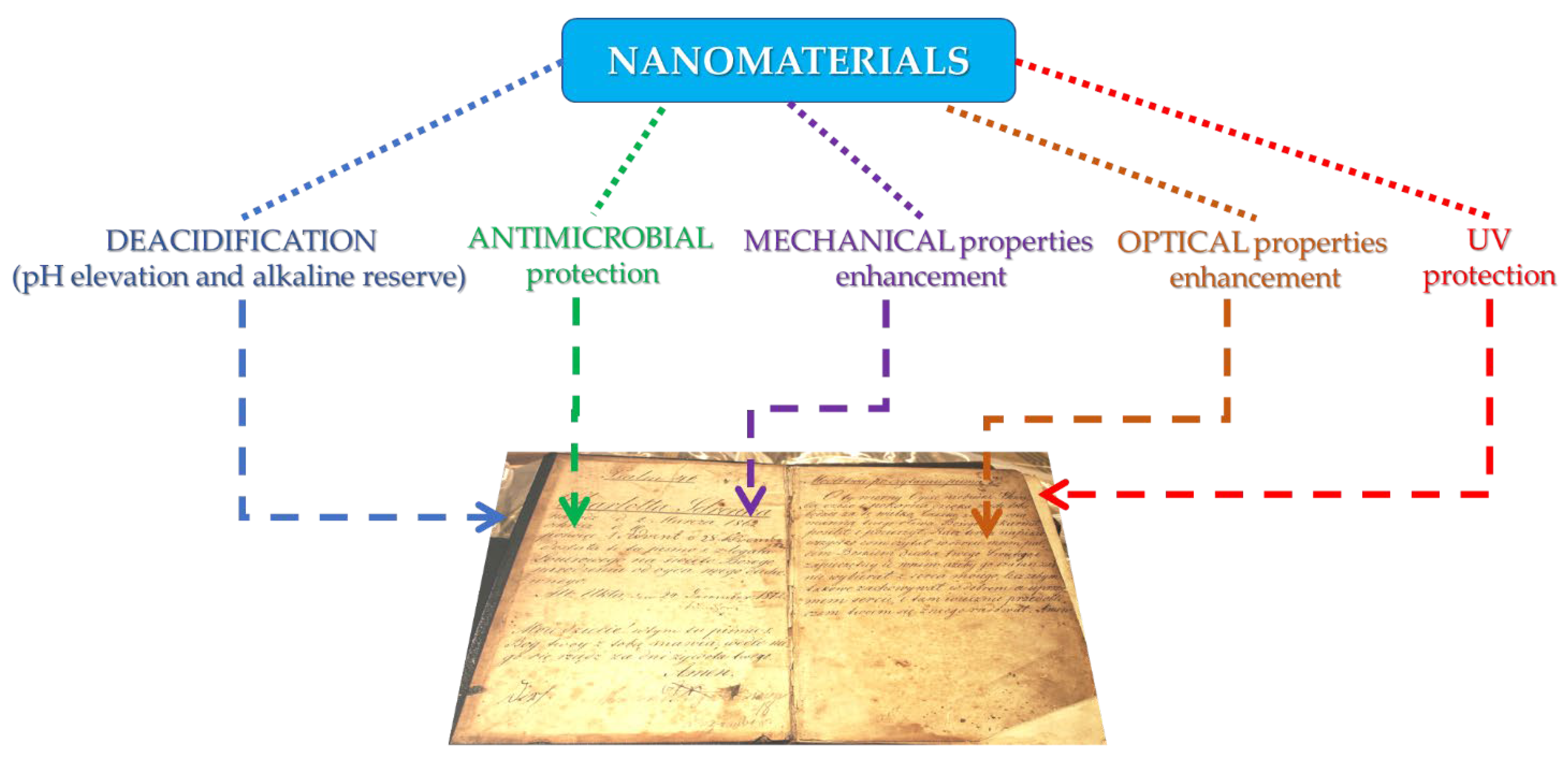
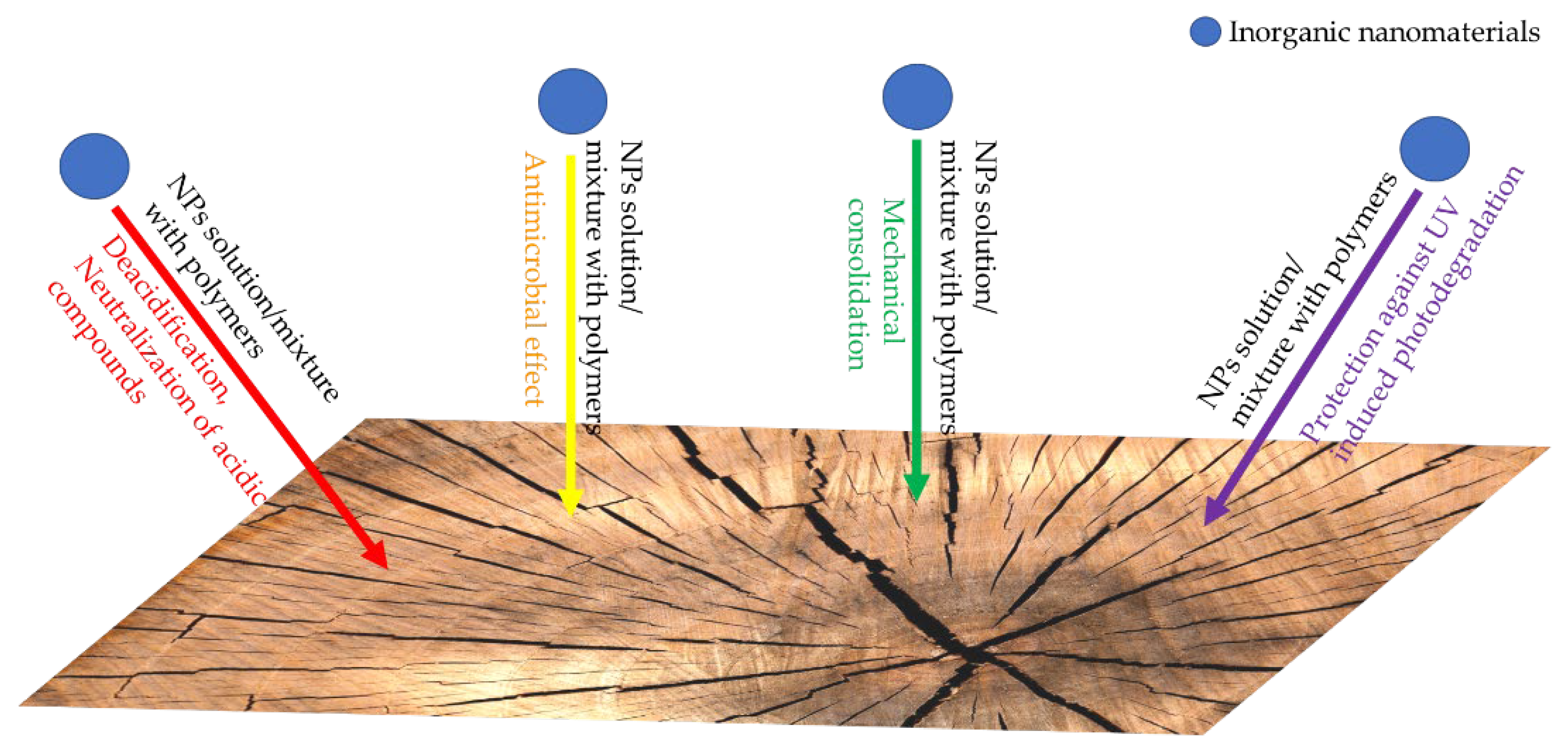
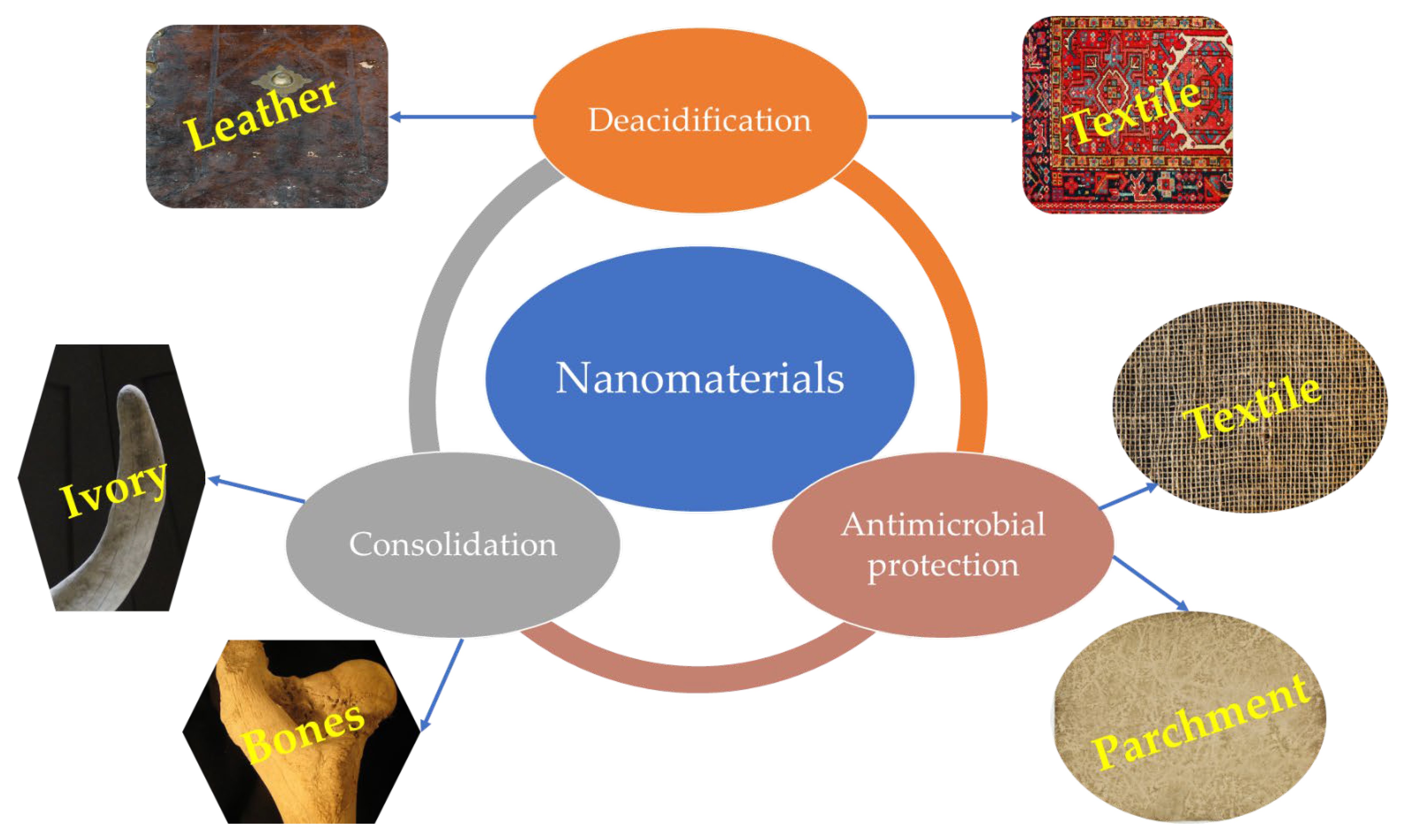
| Nanomaterial Characteristics | Application Method | Obtained Results | References |
|---|---|---|---|
| MgO, nanorods (<100 nm) | Immersion of acidified paper in nanoparticle solution in propanol | Neutralization of a part of the sulfuric acid molecules. Treatment protected against hydrolytic degradation and depolymerization, regardless of the presence of gall ink. Deacidification led to an imperceptible increase in the brightness and yellowing. | [24] |
| Ca(OH)2, solvothermal method, dispersed in n-propanol in gelatin solution | Brush application of solution on paper containing gall ink (both simulated and real artifacts) | Simulated artifacts: significant differences in the cellulose degree of polymerization between treated and untreated samples were observed upon artificial aging. pH preservation at 9 after accelerated aging. Historical paper: pH preservation at 6.5. | [25] |
| ZnO, 150 nm, dispersed in ethanol with hydroxypropyl cellulose | Spray coating of different types of paper | Protection of paper against UV radiation, fungi, and bacteria. | [26] |
| Ca(OH)2, solvothermal method, dispersed in cyclohexane | Airbrush application on acidic paper and on historical paper artwork | Acidic paper: resilience to aging, lower cellulose depolymerization, and less color changes. Paper artwork: safeguarding of the original shape and topography of the support. | [27] |
| Ca(OH)2, dispersed in 2-propanol | Soaking in the NP solution | Stabilization of the pH values (7–8.2) 1 year after treatment and restoration of the alkaline reserve. | [28] |
| Ag, in combination with carboxymethyl cellulose, chitosan, soya beans flour, BEVA 371 | Direct formation on the surface of the paper, followed by consolidant application on cotton linter and an 1887 book sample | Antimicrobial effect against Staphylococcus aureus, Aspergillus niger, Candida albicans, and Pseudomonas aeruginosa and improvement in the mechanical properties with a detrimental effect (color change) in combination with CMC and chitosan. | [29] |
| TiO2, 25 nm, dispersed in carboxymethyl cellulose with and without chitosan | Brush coating of Whatman filter paper | Developed adhesive led to protection against Aspergillus flavus and A. niger, increasing the tensile strength of the paper with a slight reduction in pH; protection against yellowing. | [30] |
| MgO–oleic acid in cyclohexane | Impregnation and immersion of different types of paper | Reduction in the surface pH of all types of acidic papers to ~8.0 without affecting the tensile strengths. After accelerated aging tests, surface pH and tensile strength values of treated samples were greater than the untreated ones; application in the inter-layer crevice and on the surface led to a change in the hydrophobicity of the papers (from hydrophilic to hydrophobic). | [31] |
| Mg(OH)2 (<100 nm) in trimethylsilyl cellulose | Dip coating of paper samples | The treatment reduced water wettability and increased mechanical strength of the paper. Mechanical properties (i.e., strength and elasticity) were improved with an increased number of coating steps | [32] |
| Mg(OH)2 (microwave-assisted synthesized nanosheets) in alcoholic aqueous solution | Air-spray coating of acidified and old paper samples | The treatment increased the pH value (from 2.5 to 10.5 for old paper), alkaline reserve (up to 372 mmol/kg for old paper), and mechanical properties (up to 6 GPa for Young’s modulus for old paper). | [33] |
| AgNPs (spherical, 7–13 nm) loaded in methyl methacrylate hydroxyethyl methacrylate | Brush coating on accelerated aged cotton and wood paper | Preservation of tensile strength and elongation rate upon accelerated aging. | [34] |
| ZnO, obtained by chemical route, with cellulose nanocrystals, 50 nm | In situ coating of newspaper | Increased color stability of the paper coated; antimicrobial effect of the nanocomposite against fungi (A. niger, A. versicolor, Rhizopus nigricans, Saccharomycetes, and Mucor) and bacteria (Staphylococcus aureus and Escherichia coli). | [35] |
| Biosynthesized spherical Ag (26–62 nm) and ZnO (8–23 nm) | Spray deposition of nanoparticles on paper inoculated with A. niger from old book paper | 2 mM NP solutions prevented fungal biodeterioration and enhanced tensile strength. | [36] |
| MgO (sol-gel method), 12 nm | Impregnation of XVIIIth century paper with NP solution | 10 mg/mL dispersion of MgO NPs provided complete inhibition of the Trichoderma reesei, A. niger, and Cladosporium cladosporioides fungal strains, avoiding color changes. Inhibited A. niger and T. reesei cellulase enzymes. | [37] |
| Biosynthesized Ag and ZnO nanoparticles | Deposition of nanoparticles on paper inoculated with Bacillus subtilis and Penicllium chrysogenum strains on a XVIIth century manuscript | 1 mM AgNP and 2mM ZnO-NPs led to 100% microbial inhibition. Treated paper exhibited a slight color change and a similar structural analysis as the original paper. | [38] |
| MgO (50 nm) dispersed in hexamethyldisiloxane | Spray coating on 1954 wheat straw pulp paper, followed by the addition of saturated Ca(OH)2 solution | pH value increased to 7.5–9.0 and alkali storage to 220 mmol/kg. Tensile strength and folding degree increased by 28.05% and 80%. Color difference was negligible. The treatment provided good antimicrobial (A. niger) and anti-aging performances. | [39] |
| Mg5O(OH)8 lamellas, 5–10 nm thickness, obtained by pulsed laser ablation | 80 year old paper washed in NP solution | Increased and stabilized the paper pH (no significant decrease during aging); enhanced the paper’s mechanical properties. | [40] |
| Ca(OH)2 (hexagonal, 60–90 nm) dispersed in in subcritical 1,1,1,2-tetrafluoroethane | Non-aqueous coating of naturally aged acidic paper including different pigments | The treatment neutralized the acidic functions of the papers, increased the alkaline reserve, and increased the mechanical strength, even compared to the classical spraying method. | [41] |
| CaCO3 (20 nm) alcoholic solutions, Ca(OH)2 commercial products | Immersion in nanoparticle solution followed by accelerated aging | All treatments led to alkaline pH values above 9 for commercial products and below 9 for carbonate; pH values should be under 9, as undesirable reactions could appear for lignocellulosic papers. Sufficient alkaline reserve (corresponding to the ISO/TS 18344/2016 standard). Less color change induced by carbonate compared with commercial products. | [42] |
| Nano-wollastonite (CaSiO3), 30–110 nm, in gel form, commercial | Impregnation of a 75 year old book | Significant hindering of A. niger growth at 20% and decreased permeability. | [43] |
| Ca(OH)2 (hexagonal platelets, 20–30 nm in thickness, 140 nm in diameter) and CaCO3 (70 nm) by solvothermal method | Brush coating of nanoparticles dispersed in oleic acid-grafted cellulose nanocrystals on acidified and aged paper | The treatment proved to be highly effective in the strengthening and deacidification of acidic and degraded paper, without significant alterations in the visual aspect of samples. | [44] |
| AgNPs (synthesized by chemical reduction, 8–10 nm)/nanocrystalline cellulose composites | Brush application on paper samples | The treatment enhanced the plastic properties of the paper, increasing inter-fiber interactions (leading to higher tensile strain resistance). Good biocidal activity against A. niger, while not affecting aesthetic appearance. | [45] |
| Na2CO3 solution and styrene acrylic latex composite | Ultrasonic atomization deposition on paper samples | Na2CO3 latex led to a pH higher than 7, only slight color change. Breaking length and the tear index were increased. Ink and handwriting were not diffused or smudged. | [46] |
| MgO in halloysite nanotubes in carboxymethyl cellulose | Impregnation of paper samples in nanocomposite solution | At 10% nanocomposite, treated paper retains a neutral pH value on exposure to acidic atmosphere. The tensile properties of the paper were improved after impregnation. Colorimetric properties and the writing quality were not modified after treatment. | [47] |
| AgNPs (10–80 nm) in aqueous solution | Disinfection in a misting chamber of photographic paper models | The AgNPs treatment proved to be less efficient disinfectant, compared with the ethylene oxide, against Bacillus subtilis, Streptomyces sp., A. versicolor and T. viride. AgNPs had less impact on the photographic models’ material properties (including color change and mechanical properties) | [48] |
| Nanomaterial Characteristics | Application Method | Obtained Results | References |
|---|---|---|---|
| Ca(OH)2 hexagonal particles, 180 nm | Vacuum suction of NP solution into waterlogged archaeological wood (Vasa ship samples) | Deacidification of the treated samples, to neutral values, up to 8. | [54] |
| TiO2 (10–15 nm) and ZnO2 (10–30 nm) embedded in polyvinyl butyral | Treatment under vacuum of poplar wood | Antimicrobial protection against Trametes versicolor (white rot fungi) at 1% under light, color stability, and lignin degradation prevention. | [55] |
| SrCO3, 50 nm | Immersion and surface brushing of nanoparticle solution of oak waterlogged wood (Mary Rose ship) | Neutralization of sulfur-containing acidic compounds, with the formation of insoluble strontium sulfate; pH increased from 3 to up to 5. | [56] |
| ZnO, <100 nm, B2O3, <30 nm, CuO, 23–37 nm, TiO2, < 25 nm, CeO2, <25 nm, SnO2, <100 nm, commercial | Vacuum-treated sapwood portions of Scots pine, according to the BS EN 113 standard test method | CuO and SnO2 inhibited fungal decay by T. versicolor in weathered and unweathered specimens; all materials prevented decay by Gloeophyllum trabeum except for the B2O3-treated and weathered sample. CuO and B2O3 inhibited termite feeding. ZnO and CeO2 caused moderate termite resistance. ZnO and B2O3 inhibited mold growth. | [57] |
| Ca(OH)2 dispersed in ethanol (5 g/L) | Direct dipping of nanocomposite solution for consolidation of waterlogged archaeological wood (softwood and hardwood), alum-treated archaeological wood (Oseberg find), and sound oak | pH increased (2–3 units); on degraded samples with very small amounts of cellulose, a pH of 5.5 was reached; stabilization after 1 month. | [58] |
| ZnO, <100 nm, B2O3, <30 nm, CuO, 23–37 nm, TiO2, <25 nm, CeO2, <25 nm, SnO2, <100 nm, commercial, combined with Paraloid B72 consolidant | Vacuum-treated sapwood portions of Scots pine, according to the BS EN 113 standard test method | Nanomaterials used in combination with the consolidant slowed fungal degradation and did not inhibit surface mold growth; improved water resistance. Consolidant treatment reduced nanoparticle leaching. | [59] |
| TiO2/Ce xerogel | Soaking of Norway spruce wood followed by evaluation of antifungal efficiency (G. trabeum, Rhodonia placenta, and Coniophora puteana, acc. EN 113 standard) and mechanical assays | The treatment led to increased resistance against brown rot decay, maintaining the mechanical properties. | [60] |
| Ca(OH)2 encapsulated in halloysite nanotubes in PEG 1500 solution | Immersion in nanocomposite solution for consolidation of waterlogged archaeological wood (Chretienne C ship) | Mechanical consolidation (increase in the elastic modulus and stress at the breaking point) and deacidification of the treated samples (pH = 7.6 12 months after treatment). | [61] |
| AuNPs (50 nm)/hydroxyapatite composites | Brushing of young and aged hazelnut wood | Stopping the wood weathering process (increased surface hardness, increased hydroscopic stability). | [62] |
| ZnO rod-shaped particles obtained by microwave-assisted solvothermal method | Impregnation of pine tree samples | Improved pinewood decay resistance against white rot fungi (Ganoderma applanatum) above 2.5% content without affecting the hardness results. | [63] |
| ZnO, 29 nm, in polyvinyl butyral matrix | Immersion on consolidant solution of historic wood and oriental plane samples | ZnO addition lowered the degradation rate at accelerated aging and decreased water penetration and wettability. Optimum concentration was 1 wt% ZnO. | [64] |
| CeO2 embedded in biopolymers (chitosan or cationic starch) | Immersion in NP/biopolymer solution of spruce wood | Reducing UV-related color changes (especially yellowing). | [65] |
| ZnO2 (spherical, 8–15 nm, solvothermal method) and TiO2 (under 25 nm, hydrothermal method) embedded in polymer | Immersion in NP/polymer solutions of cedar and sycamore woods | Increased mechanical properties (increased bending and compression resistances). | [66] |
| Ca(OH)2—hexagonal lamellas, 10 nm, Mg(OH)2—hexagonal lamellas, under 10 nm | Immersion on NP solution for preventive and curative treatment of waterlogged wood (Gallo–Roman wreck) | Deacidification of the treated samples to neutral pH values. | [67] |
| Al2(SO4)3, CuSO4·5H2O, H3BO3 introduced into H3PO4 | Treatment of sapwood | Efficient mildew resistance after 28 days of exposure to A. niger and T. viride. | [68] |
| Halloysite nanotubes/pluronic nanocomposites | Treatment of waterlogged wood | Nanotubes added reaching the internal part of the wood (consolidating not only the surface) through the lignin channels. | [69] |
| Halloysite nanotubes in molten paraffin wax | Immersion on composite solution of waterlogged wood | Overall, improvement in the mechanical properties; Young’s modulus/stress at breaking increased, the elongation at break decreased, in the absence of side effects. | [70] |
| ZnO (30–110 nm), ZrO2 (90-230 nm), functionalized with 3-glycidoxypropyltrimethoxysilane (GPTMS) in shellac-based varnishes | Brushing of maple wood samples | Increased resistance to alcoholic media; no negative effect on the chromatic properties of the coating; improved water-repellence behavior; ZrO2 varnish increased resistance to scratches; ZnO varnish increased resistance to UV aging and enhanced resistance to mold growth. | [71] |
| Artifact Type | Nanomaterial Characteristics | Application Method | Obtained Results | References |
|---|---|---|---|---|
| Pre-Columbian archaeological textiles | AgNPs, 10–15 nm and 50–80 nm | Applied using a patented method to a concentration of 4.5 ppm/g of textile/disinfection cycle | Application of AgNPs led to the protection of textiles against Pseudomonas aeruginosa (by 63–97%, depending on the strain and exposition time). | [72] |
| Historical leather (XVIIIth century) | Ca(OH)2, solvothermal method, hexagonal platelets, 20–30 nm (thickness) | Mixed with calcium lactate nanoparticles, applied by dipping on real and historical leather | After treatment application, the pH of the historical leather was adjusted to 4.5 with no detrimental effect on the collagen. | [73] |
| Pre-Columbian archaeological textiles | AgNPs (10–80 nm) | Misting disinfection using patented installation | The treatment led to a reduction in microbial contamination by 30.8–99.9% (depending on the microbial species and initial level of contamination) for several microbial lines (most resistant—Bacillus spp.; more sensitive—Oceanobacillus, Kocuria, Paracoccus, Cladosporium, and Penicillium spp.) No changes were recorded in the pH values and esthetic characteristics of the treated samples. | [74] |
| Linen fabric samples (simulating old stained samples) | TiO2, ZnO, 3–18 nm, commercial | Spraying the simulated old stained samples | TiO2 treatment led to higher fading of the stains, compared with ZnO; TiO2 had higher hydrophobicity than ZnO. Due to the fact of safety reasons, to protect the artifacts, the authors suggest the use of ZnO as self-cleaning agent. | [75] |
| Cotton exposed to fungi commonly found on ancient textiles | Wollastonite (CaSiO3) nanofibers, 30–110 nm, commercial | Immersion of cotton strips in nanomaterial gel (20%) | Impregnation led to significant limitation of A. niger activity on cotton as demonstrated by the tensile tests. | [76] |
| Ivory (from ancient elephant tusks) | Hydroxyapatite (HAP): spherical, 50 nm, hydrothermal method | Immersion in the colloidal solution, dried | After treatment, an HAP layer formed (protecting the ivory from further deterioration), repairing the loose and porous surface. Hardness, elastic modulus, and anti-scratch performance were significantly improved. No esthetic changes were recorded. | [77] |
| Archaeological human bone remains, Iron age | Ca(OH)2 nanoparticles dispersed in 2-propanol and diammonium hydrogen phosphate (DAP) | Bones soaked for in Ca(OH)2, dried, soaked in DAP | In situ synthesis of hydroxyapatite, led to an increase in hardness (up to 56%) and mineral density, and there was a significant reduction in pore volume and surface area. No substantial effect on the ability to recover endogenous DNA molecules was recorded. | [78] |
| Iron tannate dyed cotton | CaCO3 (90 nm), SiO2 (spherical, 35 nm), in diverse complex combination with polyethyleneimine, carboxymethylcellulose, cellulose nanofibers, or polyvinylpyrrolidone | Nebulization/brush application on naturally and accelerated aged samples | SiO2 nanoparticles, in combination with Nanocellulose, stabilized the naturally aged samples, while calcium carbonate nanoparticles were used as deacidification treatment (pH changed from 3.6 to 7.5). CaCO3 also protected strengthening agents, which led to an increase in the mechanical properties of the samples; after artificial aging, the deacidified samples revealed a slowing down of cotton degradation. | [79] |
| Pterosaur fossils | Ca(OH)2 (hexagonal, 20 nm)/kaolin (nanosheets, 4–12 nm thickness) nanocomposite, dispersed in ethanol | Brush application to saturation | The treatment had no significant effect on the breathability of the fossil, significantly enhanced the consolidation strength of the fossil, porosity was reduced to 51%; no eye-detected effects on the color of the fossil. | [80] |
| Parchment from goat skin | Tea leaf-mediated AgNPs, spherical, oval, and hexagonal shapes, 20–50 nm | Deposited on parchment samples, artificially aged | Antimicrobial treatment effective against bacteria and fungi (Streptomyces Albidoflavus, Cladosporium xanthochromaticum, A. fumigatus, Byssochlamys spectabilis) at a 0.025% concentration. The treatment did not significantly influence the chemical and mechanical characteristics of treated parchment even after accelerated thermal aging. | [81] |
| Simulated bone artifacts | Ca(OH)2, 217 nm, dispersed in 2-propanol | In situ growth of Ca(SO4)2 by the drip-permeance method | A Ca(SO4)2·2H2O continuous phase formed in situ which filled the holes, bridged the cracks, and conferred strength to the bones, maintaining their original appearance. Microhardness increased by 3 times, porosity reduced by 10%, and the color difference by 2.7. | [82] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fistos, T.; Fierascu, I.; Fierascu, R.C. Recent Developments in the Application of Inorganic Nanomaterials and Nanosystems for the Protection of Cultural Heritage Organic Artifacts. Nanomaterials 2022, 12, 207. https://doi.org/10.3390/nano12020207
Fistos T, Fierascu I, Fierascu RC. Recent Developments in the Application of Inorganic Nanomaterials and Nanosystems for the Protection of Cultural Heritage Organic Artifacts. Nanomaterials. 2022; 12(2):207. https://doi.org/10.3390/nano12020207
Chicago/Turabian StyleFistos, Toma, Irina Fierascu, and Radu Claudiu Fierascu. 2022. "Recent Developments in the Application of Inorganic Nanomaterials and Nanosystems for the Protection of Cultural Heritage Organic Artifacts" Nanomaterials 12, no. 2: 207. https://doi.org/10.3390/nano12020207
APA StyleFistos, T., Fierascu, I., & Fierascu, R. C. (2022). Recent Developments in the Application of Inorganic Nanomaterials and Nanosystems for the Protection of Cultural Heritage Organic Artifacts. Nanomaterials, 12(2), 207. https://doi.org/10.3390/nano12020207









