Structural Characterization and Physical Properties of Double Perovskite La2FeReO6+δ Powders
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Synthesis of LFRO Powder
2.3. Sample Characterization
3. Results
3.1. Structural Characterization
3.2. XPS Spectra Analysis
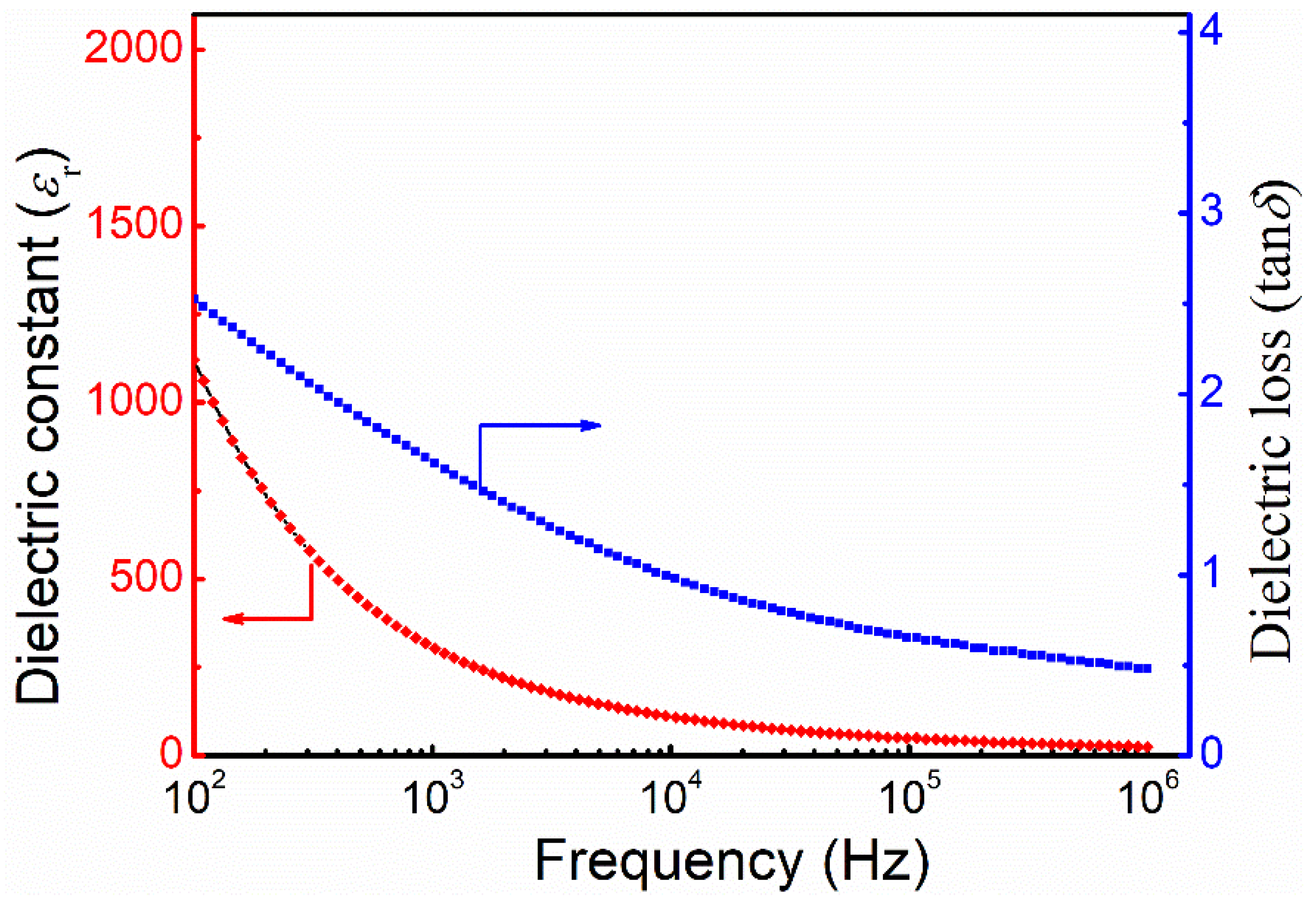
3.3. Dielectric Properties
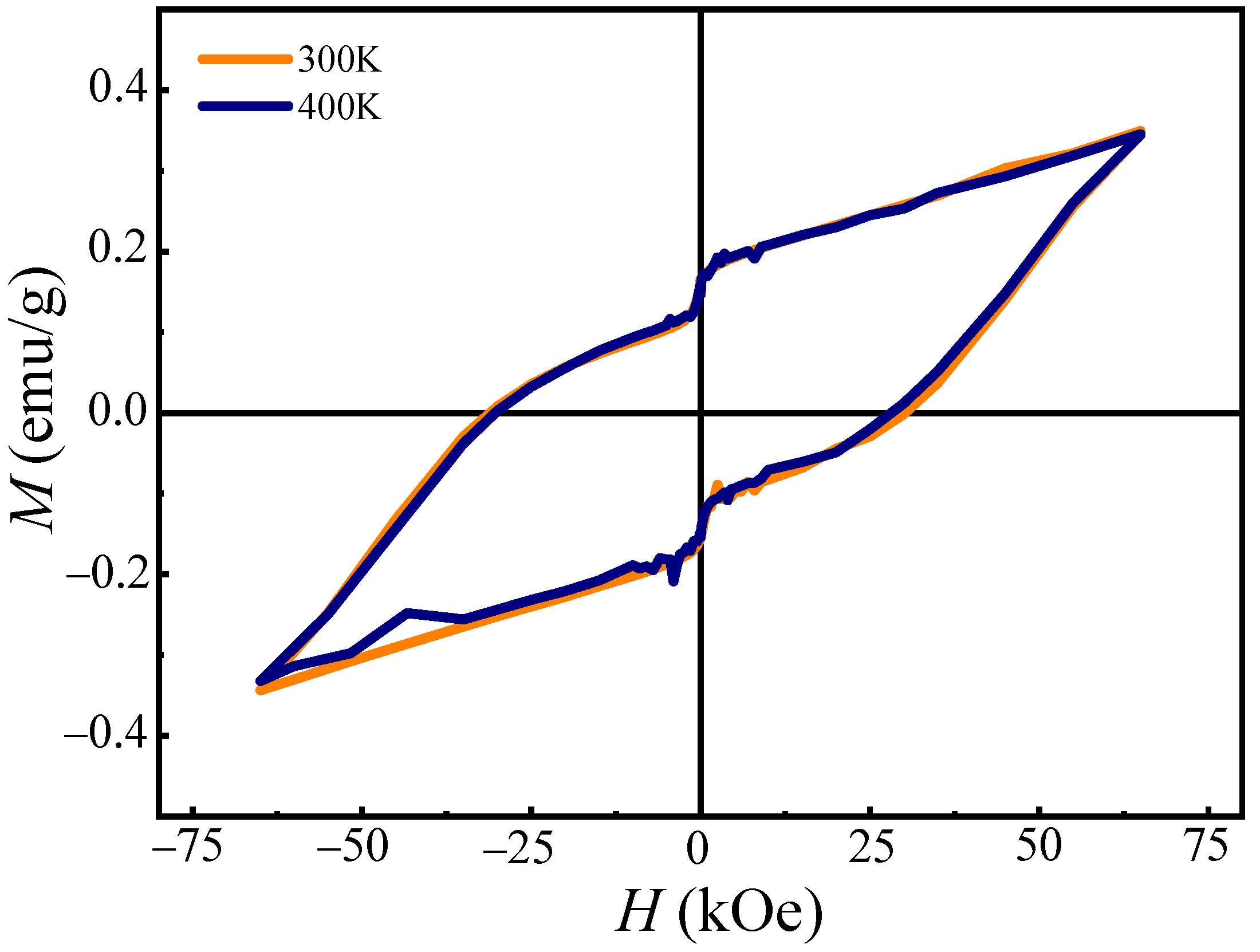
3.4. Magnetic Properties
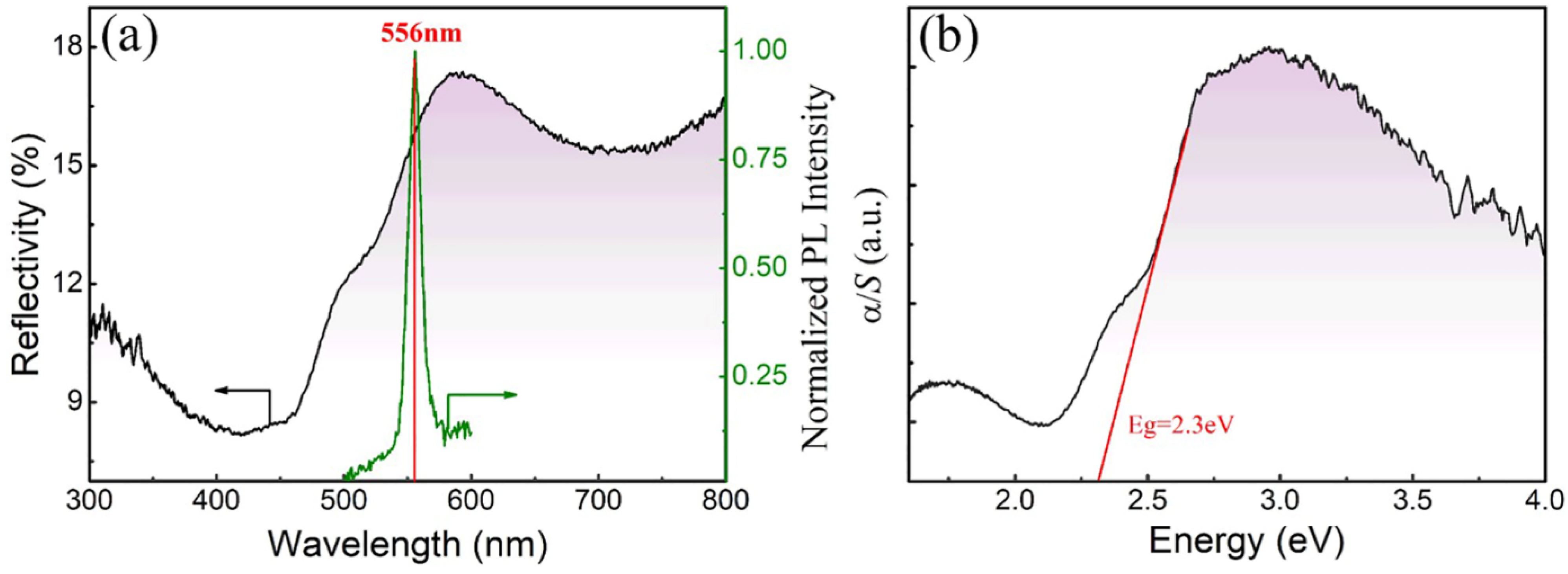
3.5. Optical Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wolf, S.A.; Awschalom, D.D.; Buhrman, R.A.; Daughton, J.M.; von Molnar, S.; Roukes, M.L.; Chtchelkanova, A.Y.; Treger, D.M. Spintronics: A Spin-based Electronics Vision for the Future. Science 2001, 294, 1488–1495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Makarov, A.; Windbacher, T.; Sverdlov, V.; Selberherr, S. CMOS-compatible Spintronic Devices: A Review. Semicond Sci. Technol. 2016, 31, 113006. [Google Scholar] [CrossRef] [Green Version]
- Bibes, M.; Barthelemy, A. Oxide Spintronics. IEEE Trans Electron. Devices 2007, 54, 1003–1023. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, K.I.; Kimura, T.; Sawada, H.; Terakura, K.; Tokura, Y. Room-temperature Magnetoresistance in an Oxide Material with an Ordered Double-perovskite Structure. Nature 1998, 395, 677–680. [Google Scholar] [CrossRef]
- Pickett, W.E.; Moodera, J.S. Half Metallic Magnets. Phys. Today 2001, 54, 39–44. [Google Scholar] [CrossRef]
- Groot, R.A.; Mueller, F.M.; Engen, P.G. New Class of Materials: Half-metallic Ferromagnets. Phys. Rev. Lett. 1983, 50, 2024–2027. [Google Scholar] [CrossRef] [Green Version]
- Attema, J.J.; Chioncel, L.; Fang, C.M.; Wijs, G.A.; Groot, R.A. Half-metals: Challenges in Spintronics and Routes toward Solutions. Lect. Notes Phys. 2005, 678, 201–216. [Google Scholar] [CrossRef]
- Felser, C.; Fecher, G.H.; Balke, B. Spintronics: A challenge for Materials Science and Solid-state Chemistry. Angew Chem Int. Ed. 2007, 46, 668–699. [Google Scholar] [CrossRef]
- Katsnelson, M.I.; Irkhin, V.Y.; Chioncel, L.; Groot, R.A. Half-metallic Ferromagnets: From Band Structure to Many-body Effects. Rev. Mod. Phys. 2008, 80, 315–378. [Google Scholar] [CrossRef] [Green Version]
- Galanakis, I. Surface Properties of the Half- and Full-Heusler Alloys. J. Phys Condens. Matter. 2002, 14, 6329–6340. [Google Scholar] [CrossRef]
- Schwarz, K. CrO2 Predicted as a Half-metallic Ferromagnet. J. Phys. 1986, 16, L211–L215. [Google Scholar] [CrossRef]
- Groot, R.A.; Bischow, K.H.J. Recent Developments in Half-metallic Magnetism. J. Magn. Magn. Mater. 1986, 54–57, 1377–1380. [Google Scholar] [CrossRef] [Green Version]
- Park, J.-H.; Vescovo, E.; Kim, H.-J.; Kwon, C.; Ramesh, R.; Venkatesan, T. Direct Evidence for a Half-metallic Ferromagnet. Nature 1998, 392, 794–796. [Google Scholar] [CrossRef]
- Balcells, L.; Navarro, J.; Bibes, M.; Roig, A.; Fontcuberta, J. Cationic Ordering Control of Magnetization in Sr2FeMoO6 Double Perovskite. Appl Phys Lett. 2001, 78, 781–783. [Google Scholar] [CrossRef] [Green Version]
- Ritter, C.; Ibarra, M.R.; Morellon, L.; Blasco, J.; Garcia, J.; Teresa, J.M.D. Structural and Magnetic Properties of Double Perovskites AA′FeMoO6 (AA′ = Ba2, BaSr, Sr2 and Ca2). J. Phys. Condens Matter. 2000, 12, 8295–8308. [Google Scholar] [CrossRef]
- Akinaga, H.; Manago, T.; Shirai, M. Material Design of Half-metallic Zinc-blende CrAs and the Synthesis by Molecular-beam Epitaxy. Jpn. J. Appl. Phys. 2000, 39, L1118–L1120. [Google Scholar] [CrossRef]
- Ohno, H.; Shen, A.; Matsukura, F.; Oiwa, A.; Endo, A.; Katsumoto, S.; Iye, Y. (Ga,Mn)As: A New Diluted Magnetic Semiconductor Based on GaAs. Appl. Phys. Lett. 1996, 69, 363–365. [Google Scholar] [CrossRef]
- Luo, S.J.; Yao, K.L. Electronic Structure of the Organic Half-metallic Magnet 2-(4-nitrophenyl)-4,4,5,5-tetramethyl-4,5-dihydro-1H-imidazol-1-oxyl 3-N-oxide. Phys. Rev. B 2003, 67, 214429. [Google Scholar] [CrossRef]
- Tomioka, Y.; Okuda, T.; Okimoto, Y.; Kumai, R.; Kobayashi, K.-I.; Tokura, Y. Magnetic and Electronic Properties of a Single Crystal of Ordered Double Perovskite Sr2FeMoO6. Phys. Rev. B 2000, 61, 422–427. [Google Scholar] [CrossRef]
- Kobayashi, K.I.; Kimura, T.; Tomioka, Y.; Sawada, H.; Terakura, K.; Tokura, Y. Intergrain Tunneling Magnetoresistance in Polycrystals of the Ordered Double Perovskite Sr2FeReO6. Phys. Rev. B 1999, 591, 11159. [Google Scholar] [CrossRef]
- Pei, Z.P.; Lu, Y.; Wu, H.; Xia, W.R.; Zhu, X.H. Advances on A2B′B″O6 Type Double Perovskite Oxides. J. Chin. Ceram. Soc. 2019, 47, 526–537. [Google Scholar] [CrossRef]
- Wang, H.L.; Frontera, C.; Herrero-Martin, J.; Pomar, A.; Roura, P.; Martinez, B.; Mestres, N. Aqueous Chemical Solution Deposition of Functional Double Perovskite Epitaxial Thin Films. Chem. A Eur. J. 2020, 26, 9338–9347. [Google Scholar] [CrossRef]
- Philipp, J.B.; Majewski, P.; Reisinger, D.; Geprags, S.; Opel, M.; Erb, A.; Alff, L.; Gross, R. Magnetorsistance and Magnetic Properties of the Double Perovskites. Acta Phys. Polonica A 2004, 105, 7–26. [Google Scholar] [CrossRef]
- Alff, L. Ferrimagnetic double perovskites as spintropnic materials. In Electron Correlation in New Materials and Nanosystems, 1st ed.; Scharnberg, K., Kruchinin, S., Eds.; Springer: Dordrecht, The Netherlands, 2007; Volume 241, pp. 393–400. [Google Scholar]
- Retuerto, M.; Li, M.-R.; Stephens, P.W.; Sánchez-Benítez, J.; Deng, X.; Kotliar, G.; Croft, M.C.; Ignatov, A.; Walker, D.; Greenblatt, M. Half-metallicity in Pb2CoReO6 Double Perovskite and High Magnetic Ordering Temperature in Pb2CrReO6 Perovskite. Chem. Mater. 2015, 27, 4450–4458. [Google Scholar] [CrossRef]
- Leng, K.; Tang, Q.K.; Wei, Y.; Yang, L.; Xie, Y.T.; Wu, Z.W.; Zhu, X.H. Recent Advances in Re-based Double Perovskites: Synthesis, Structural Characterization, Physical Properties, Advanced Applications, and Theoretical Studies. AIP Adv. 2020, 10, 120701. [Google Scholar] [CrossRef]
- Michalik, J.M.; Teresa, J.M.D.; Blasco, J.; Algarabel, P.A.; Ibarra, M.R.; Kapusta, C.; Zeitler, U. Temperature Dependence of Magnetization under High Fields in Re-based Double Perovskites. J Phys: Condens. Matter. 2007, 19, 506206. [Google Scholar] [CrossRef] [Green Version]
- Prellier, W.; Smolyaninova, V.; Biswat, A.; Gelley, C.; Greene, R.L.; Remesha, K.; Gopalakrishan, J. Properties of the Ferrimagnetic Double Perovskites A2FeReO6 (A= Ba and Ca). J. Phys Condens. Matter. 2000, 12, 965–973. [Google Scholar] [CrossRef] [Green Version]
- Serrate, D.; Teresa, J.M.D.; Ibarra, R. Double Perovskites with Ferromagnetism above Room Temperature. J. Phys: Condens. Matter. 2007, 19, 023201. [Google Scholar] [CrossRef]
- Liu, Y.P.; Fuh, H.R.; Wang, Y.K. Expansion Research on Half-metallic Materials in Double Perovskites of Sr2BB′O6 (B = Co, Cu, and Ni; B′ = Mo, W, Tc, and Re; and BB′ = FeTc). Comput. Mater. Sci. 2014, 92, 63–68. [Google Scholar] [CrossRef]
- Chen, S.H.; Xiao, Z.R.; Liu, Y.P.; Wang, Y.K. Investigation of Possible Half-metallic Antiferromagnets on Double Perovskites LaABB′O6 (A = Ca, Sr, Ba; B, B′ = transition elements). J. Appl. Phys. 2010, 108, 093908. [Google Scholar] [CrossRef]
- Chen, S.H.; Xiao, Z.R.; Liu, Y.P.; Lee, P.H.; Wang, Y.K. First-principle Calculation on nearly Half-metallic Antiferromagnetic Behavior of Double Perovskites La2VReO6. J. Mag. Mag. Mater. 2011, 323, 176–179. [Google Scholar] [CrossRef]
- Gueorguiev, G.K.; Goyenola, C.; Schmidt, S.; Hultman, L. CFx: A First-Principles Study of Structural Patterns Arising During Synthetic Growth. Chem Phys. Lett. 2011, 516, 62–67. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, S.; Greczynski, G.; Goyenola, C.; Gueorguiev, G.K.; Czigany, Z.; Jensen, J.; Ivanov, I.G.; Hultman, L. CFx Thin Solid Films Deposited by High Power Impulse Magnetron Sputtering: Synthesis and Characterization. Surf Coat Technol. 2011, 206, 646–653. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, S.; Goyenola, C.; Gueorguiev, G.K.; Jensen, J.; Greczynski, G.; Ivanov, I.G.; Czigany, Z.; Hultman, L. Reactive High Power Impulse Magnetron Sputtering of CFx Thin Films in Mixed Ar/C4F4 and Ar/C4F8 Discharges. Thin Solid Films 2013, 542, 21–30. [Google Scholar] [CrossRef]
- Zhuravlev, M.Y.; Sabirianov, R.F.; Jaswal, S.S.; Tsymbal, E.Y. Giant Electroresistance in Ferroelectric Tunnel Junctions. Phys. Rev. Lett. 2005, 94, 246802. [Google Scholar] [CrossRef]
- Fuh, H.R.; Weng, K.C.; Liu, Y.P.; Wang, Y.K. New Ferromagnetic Semiconductor Double Perovskites: La2FeMO6 (M = Co, Rh, and Ir). J. Alloy Compd. 2015, 622, 657–661. [Google Scholar] [CrossRef]
- Kaipamagalath, A.; Palakkal, J.P.; Varma, M.R. Griffiths-like Phase in High TC Perovskite La2FeReO6 Prepared in a Controlled Reducing Atmosphere. AIP Conf. Proc. 2018, 1953, 120041. [Google Scholar] [CrossRef]
- Larson, A.C.; Von Dreele, R.B. General Structure Analysis System (GSAS), Los Alamos National Laboratory Report No. LAUR. 2000; pp. 86–748. Available online: http://doc.wendoc.comnl.gov/public/gsas/ (accessed on 25 January 2007).
- Tandon, S.; Gupta, J. Measurement of Forbidden Energy Gap of Semiconductors by Diffuse Reflectance Technique. Phys Status Solidi B 1970, 38, 363–367. [Google Scholar] [CrossRef]
- Kakimoto, K.; Masuda, I.; Ohsato, H. Ferroelectric and Piezoelectric Properties of KNbO3 Ceramics Containing Small Amounts of LaFeO3. Jpn J. Appl. Phys. 2003, 42, 6102–6105. [Google Scholar] [CrossRef]
- Rae-Smith, A.R.; Cheetham, A.K.; Fuess, H. Preparation and Crystal Structure of La3ReO8. Z. Anorg. Allg. Chem. 1984, 510, 46–50. [Google Scholar] [CrossRef]
- Grosvenor, A.P.; Cavell, R.G.; Mar, A. X-ray Photoelectron Spectroscopy Study of Rare-Earth Filled Skutterudites LaFe4P12 and CeFe4P12. Chem Mater. 2006, 18, 1650–1657. [Google Scholar] [CrossRef]
- Yamashita, T.; Hayes, P. Analysis of XPS Spectra of Fe2+ and Fe3+ Ions in Oxide Materials. Appl. Surface Sci. 2008, 254, 2441–2449. [Google Scholar] [CrossRef]
- Muhler, M.; Schlögl, R.; Ertl, G. The Nature of the Iron Oxide-Based Catalyst for Dehydrogenation of Ethylbenzene to Styrene. J Catal. 1992, 138, 413–444. [Google Scholar] [CrossRef]
- Retuerto, M.; Jimenez-Villacorta, F.; Martinez-Lope, M.J.; Huttel, Y.; Roman, E.; Fernandez-Diazc, M.T.; Alonso, J.A. Study of the Valence State and Electronic Sstructure in Sr2FeMO6 (M = W, Mo, Re and Sb) Double Perovskites. Phys. Chem. Chem. Phys. 2010, 12, 13616–13625. [Google Scholar] [CrossRef] [PubMed]
- Murphy, N.R.; Gallagher, R.C.; Sun, L.; Jones, J.G.; Grant, J.T. Optical and Chemical Properties of Mixed-Valent Rhenium Oxide Films Synthesized by Reactive DC Magnetron Sputtering. Opt. Mater. 2015, 45, 191–196. [Google Scholar] [CrossRef]
- Kirlin, P.S.; Strohmeier, B.R.; Gates, B.C. The Effect of Carbon on the Reducibility of Pt-Re/Al2O3 Catalysts. J. Catal. 1986, 98, 308–316. [Google Scholar] [CrossRef]
- Tan, R.Q.; Zhu, Y.F. Poisoning Mechanism of Perovskite LaCoO3 Catalyst by Organophosphorous Gas. Appl. Catal. B-Environ. 2005, 58, 61–68. [Google Scholar] [CrossRef]
- Wu, H.; Zhu, X.H. Microstructures, Magnetic, and Dielectric Properties of Ba-doped BiFeO3 Nanoparticles Synthesized via Molten Salt Route. J. Am. Ceram. Soc. 2019, 102, 4698–4709. [Google Scholar] [CrossRef]
- Dupin, J.C.; Gonbeau, D.; Philippe Vinatier, P.; Levasseur, A. Systematic XPS Studies of Metal Oxides, Hydroxides and Peroxides. Phys. Chem. Chem. Phys. 2000, 2, 1319–1324. [Google Scholar] [CrossRef]
- Chu, F.; Reaney, I.M.; Setter, N. Spontaneous (zero-field) Relaxor–to–ferroelectric-phase Transition in Disordered Pb(Sc1/2Nb1/2)O3. J. Appl. Phys. 1995, 77, 1671–1676. [Google Scholar] [CrossRef]
- Yang, W.Z.; Liu, X.Q.; Zhao, H.J.; Lin, Y.Q.; Chen, X.M. Structure, Magnetic, and Dielectric Characteristics of Ln2NiMnO6 (Ln=Nd and Sm) Ceramics. J. Appl. Phys. 2012, 112, 064104. [Google Scholar] [CrossRef]
- Ihrig, H.; Hennings, D. Electrical Transport Properties of n-Type BaTiO3. Phys. Rev. B 1978, 17, 4593–4599. [Google Scholar] [CrossRef]
- Huang, F.; Wang, Z.; Lu, X.; Zhang, J.; Min, K.; Lin, W.; Ti, R.; Xu, T.T.; Ju, H.; Chen, Y.; et al. Nanoparticles with Size Approaching the Period of the Spiral Spin Structure. Sci. Rep. 2013, 3, 2907. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, M.; Fullem, S.I.; Suzuki, I.S.; Wang, L.; Zhong, C.J. Observation of Super Spin-glass Behavior in Fe3O4 Nanoparticles. Phys. Rev. B 2009, 79, 024418. [Google Scholar] [CrossRef]
- Rogado, N.S.; Li, J.; Sleight, A.; Subramanian, M. Magnetocapacitance and Magnetoresistance near Room Temperature in a Ferromagnetic Semiconductor: La2NiMnO6. Adv. Mater. 2005, 17, 2225–2227. [Google Scholar] [CrossRef]
- Taguchi, H. Relationship between Crystal Structure and Electrical Properties of Nd(Cr1-xFex)O3. J. Solid State Chem. 1997, 131, 108–114. [Google Scholar] [CrossRef]
- Buschow, K.H.J.; Boer, F.R. Physics of Magnetism and Magnetic Materials; Kluwer Academic/Plenum Publishers: Dordrecht, The Netherlands, 2003. [Google Scholar]
- Bray, A.J. Nature of the Griffiths phase. Phys. Rev. Lett. 1987, 59, 586–589. [Google Scholar] [CrossRef]
- Jiang, W.J.; Zhou, X.; Williams, G. Correlation between Phase Competition and the Nucleation of a Griffiths-like Phase in (La1-yPry)0.7Ca0.3Mn16/18O3. Europhys Lett. 2008, 84, 47009. [Google Scholar] [CrossRef] [Green Version]
- Zheng, X.; Gao, T.; Jing, W.; Wang, X.Y.; Liu, Y.S.; Chen, B.; Dong, H.L.; Chen, Z.Q.; Cao, S.X.; Cai, C.B.; et al. Evolution of Griffiths Phase and Spin Reorientation in Perovskite Manganites. J. Mag. Mag. Mater. 2019, 491, 165611. [Google Scholar] [CrossRef]
- Arima, T.; Tokura, Y.; Torrance, J.B. Variation of Optical Gaps in Perovskite-type 3d Transition-metal Oxides. Phys. Rev. B 1993, 48, 17006–17009. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, Q.; Zhu, X. Structural Characterization and Physical Properties of Double Perovskite La2FeReO6+δ Powders. Nanomaterials 2022, 12, 244. https://doi.org/10.3390/nano12020244
Tang Q, Zhu X. Structural Characterization and Physical Properties of Double Perovskite La2FeReO6+δ Powders. Nanomaterials. 2022; 12(2):244. https://doi.org/10.3390/nano12020244
Chicago/Turabian StyleTang, Qingkai, and Xinhua Zhu. 2022. "Structural Characterization and Physical Properties of Double Perovskite La2FeReO6+δ Powders" Nanomaterials 12, no. 2: 244. https://doi.org/10.3390/nano12020244





