Contrast Enhanced Ultrasound Molecular Imaging of Spontaneous Chronic Inflammatory Bowel Disease in an Interleukin-2 Receptor α−/− Transgenic Mouse Model Using Targeted Microbubbles
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of P- and E- Dual Selectin Targeted Microbubbles
2.2. Animals
2.3. Ultrasound Molecular Imaging
2.4. Analysis of Ultrasound Molecular Imaging Data
2.5. Ex Vivo Analysis of Colon Tissues
2.6. Statistical Analysis
3. Results
3.1. General Conditions
3.2. In Vivo Ultrasound Molecular Imaging
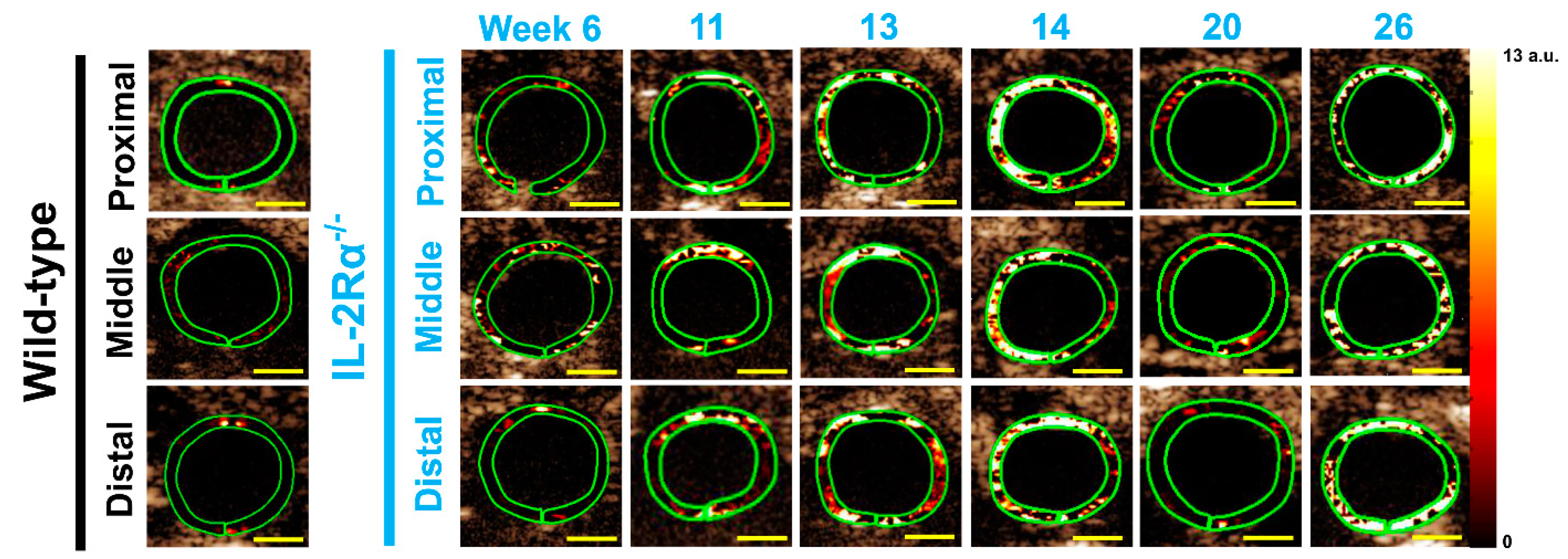
3.2.1. Spontaneous Colitis in IL-2Rα−/− Mice with 100% Penetrance
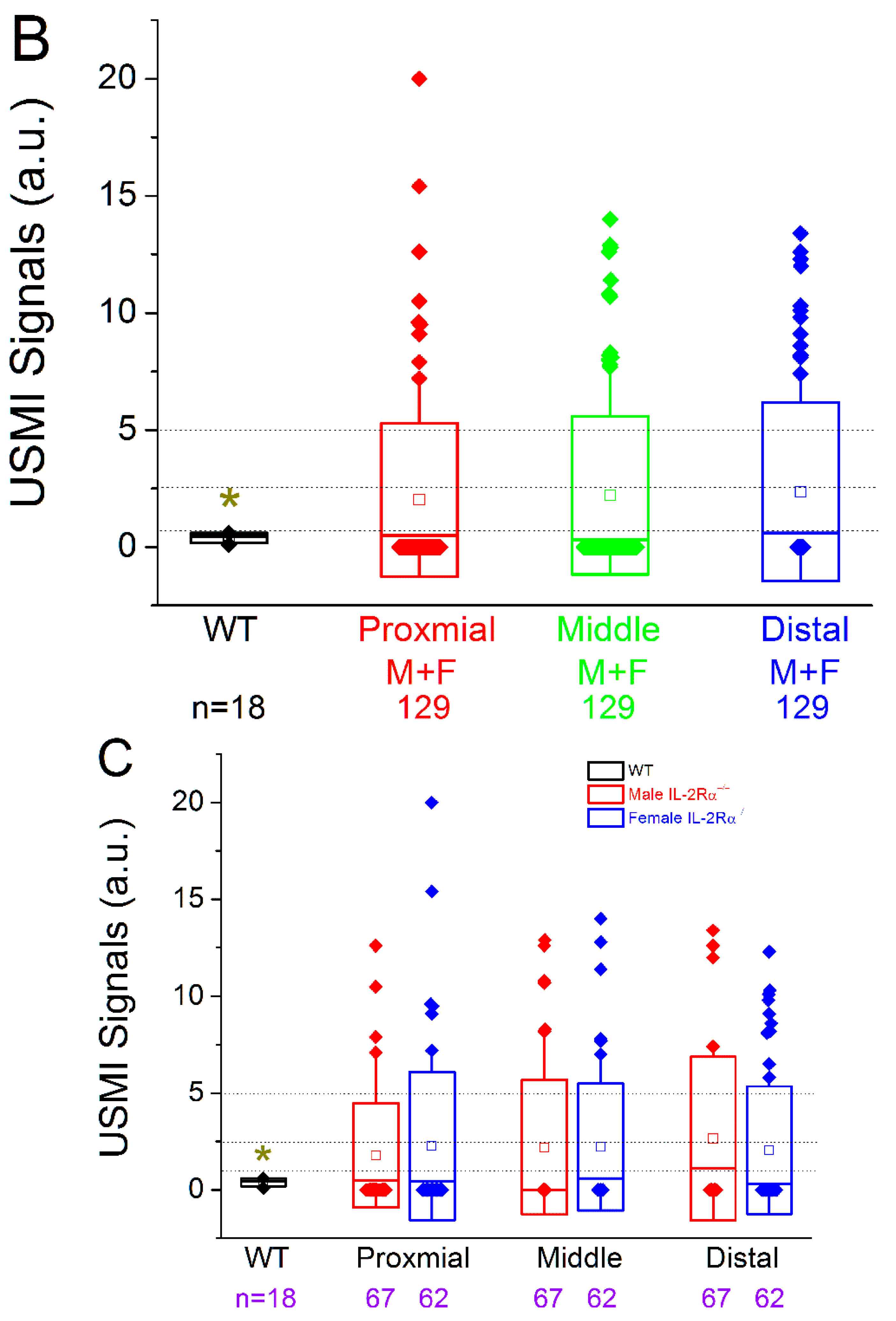
3.2.2. Spontaneous Colitis in IL-2Rα−/− Mice without Sex or Location Difference
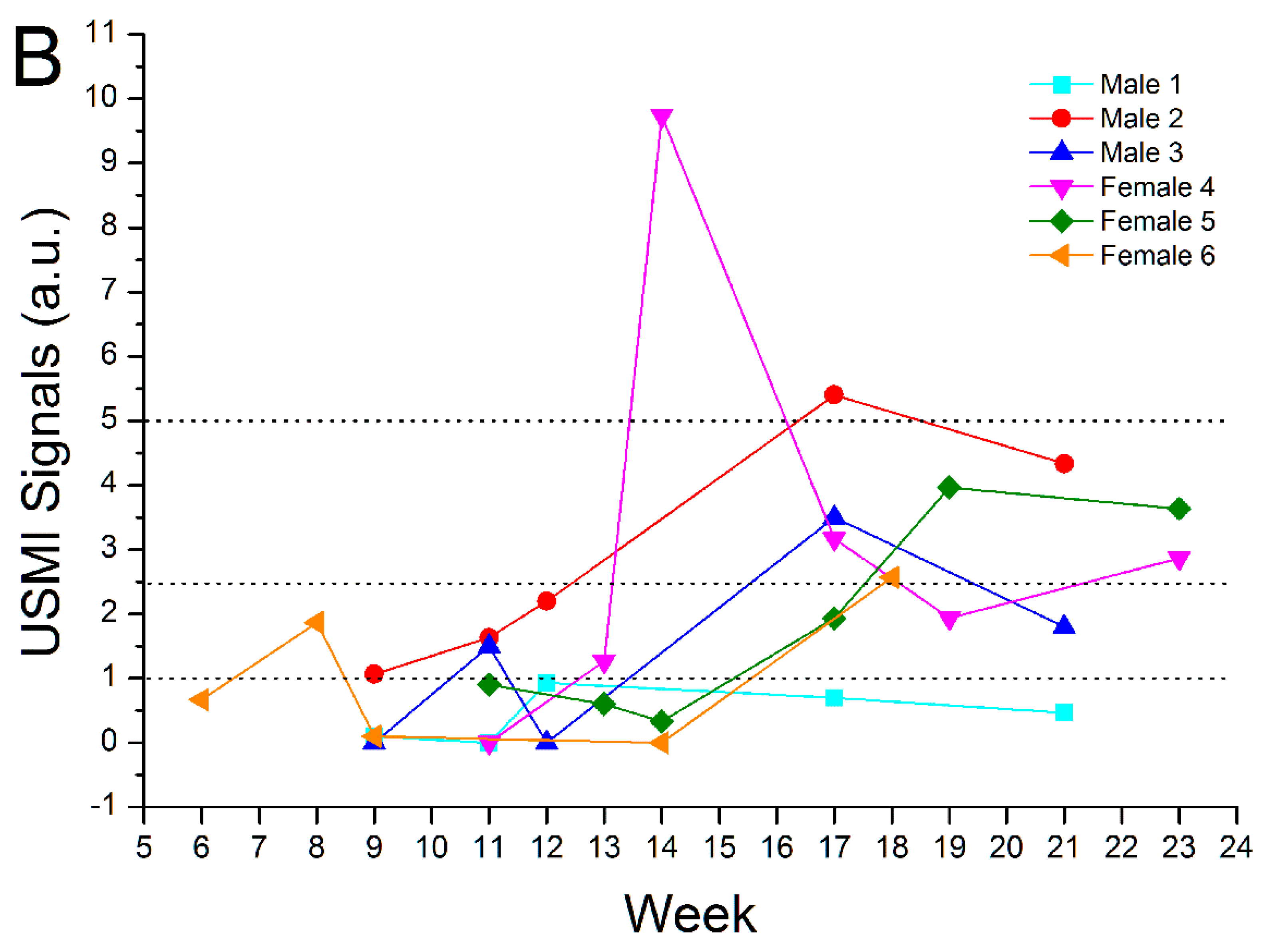
3.3. Remission Flare-Like Pattern of Spontaneous Chronic Colitis in IL-2Rα−/− Mice
3.4. Ex Vivo Analyses
3.4.1. Hematoxylin and Eosin (H&E) Staining
3.4.2. Immunofluorescence Staining
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| a.u. | arbitrary unit |
| CEUS | contrast-enhanced ultrasound |
| FOV | field of view |
| H&E | hematoxylin and eosin |
| IBD | inflammatory bowel disease |
| IL-2Rα−/− | interleukin 2 receptor α deficient |
| MBSelectin | dual P- and E-selectin targeted microbubbles |
| PBS | phosphate buffered saline |
| PFA | paraformaldehyde |
| OCT | optimal cutting temperature compound |
| ROI | region of interest |
| Treg | T regulatory |
| USMI | ultrasound molecular imaging |
References
- Loftus, E.V., Jr. Clinical epidemiology of inflammatory bowel disease: Incidence, prevalence, and environmental influences. Gastroenterology 2004, 126, 1504–1517. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Wu, J.C.Y.; Chan, F.K.L.; et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet 2018, 390, 2769–2778. [Google Scholar] [CrossRef]
- Liverani, E.; Scaioli, E.; Digby, R.J.; Bellanova, M.; Belluzzi, A. How to predict clinical relapse in inflammatory bowel disease patients. World J. Gastroenterol. 2016, 22, 1017–1033. [Google Scholar] [CrossRef] [PubMed]
- Kingsley, M.J.; Abreu, M.T. A Personalized Approach to Managing Inflammatory Bowel Disease. Gastroenterol. Hepatol. 2016, 12, 308–315. [Google Scholar]
- Martinez-Montiel, M.P.; Casis-Herce, B.; Gomez-Gomez, G.J.; Masedo-Gonzalez, A.; Yela-San Bernardino, C.; Piedracoba, C.; Castellano-Tortajada, G. Pharmacologic therapy for inflammatory bowel disease refractory to steroids. Clin. Exp. Gastroenterol. 2015, 8, 257–269. [Google Scholar] [CrossRef] [PubMed]
- Nylund, K.; Hausken, T.; Gilja, O.H. Ultrasound and inflammatory bowel disease. Ultrasound Q. 2010, 26, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Strobel, D.; Goertz, R.S.; Bernatik, T. Diagnostics in inflammatory bowel disease: Ultrasound. World J. Gastroenterol. 2011, 17, 3192–3197. [Google Scholar]
- Serra, C.; Menozzi, G.; Labate, A.M.; Giangregorio, F.; Gionchetti, P.; Beltrami, M.; Robotti, D.; Fornari, F.; Cammarota, T. Ultrasound assessment of vascularization of the thickened terminal ileum wall in Crohn’s disease patients using a low-mechanical index real-time scanning technique with a second generation ultrasound contrast agent. Eur. J. Radiol. 2007, 62, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Quaia, E.; Migaleddu, V.; Baratella, E.; Pizzolato, R.; Rossi, A.; Grotto, M.; Cova, M.A. The diagnostic value of small bowel wall vascularity after sulfur hexafluoride-filled microbubble injection in patients with Crohn’s disease. Correlation with the therapeutic effectiveness of specific anti-inflammatory treatment. Eur. J. Radiol. 2009, 69, 438–444. [Google Scholar] [CrossRef]
- Ripolles, T.; Martinez, M.J.; Paredes, J.M.; Blanc, E.; Flors, L.; Delgado, F. Crohn disease: Correlation of findings at contrast-enhanced US with severity at endoscopy. Radiology 2009, 253, 241–248. [Google Scholar] [CrossRef]
- Romanini, L.; Passamonti, M.; Navarria, M.; Lanzarotto, F.; Villanacci, V.; Grazioli, L.; Calliada, F.; Maroldi, R. Quantitative analysis of contrast-enhanced ultrasonography of the bowel wall can predict disease activity in inflammatory bowel disease. Eur. J. Radiol. 2014, 83, 1317–1323. [Google Scholar] [CrossRef]
- Ripolles, T.; Rausell, N.; Paredes, J.M.; Grau, E.; Martinez, M.J.; Vizuete, J. Effectiveness of contrast-enhanced ultrasound for characterisation of intestinal inflammation in Crohn’s disease: A comparison with surgical histopathology analysis. J. Crohn’s Colitis 2013, 7, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Wong, D.D.; Forbes, G.M.; Zelesco, M.; Mason, R.; Pawlik, J.; Mendelson, R.M. Crohn’s disease activity: Quantitative contrast-enhanced ultrasound assessment. Abdom. Imaging 2012, 37, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, S.; Ohtani, H.; Watanabe, Y.; Fukushima, K.; Matsumoto, T.; Kitano, A.; Kobayashi, K.; Nagura, H. In situ expression of the cell adhesion molecules in inflammatory bowel disease. Evidence of immunologic activation of vascular endothelial cells. Lab. Investig. 1993, 69, 77–85. [Google Scholar] [PubMed]
- Schurmann, G.M.; Bishop, A.E.; Facer, P.; Vecchio, M.; Lee, J.C.; Rampton, D.S.; Polak, J.M. Increased expression of cell adhesion molecule P-selectin in active inflammatory bowel disease. Gut 1995, 36, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Gulubova, M.V.; Manolova, I.M.; Vlaykova, T.I.; Prodanova, M.; Jovchev, J.P. Adhesion molecules in chronic ulcerative colitis. Int. J. Colorectal. Dis. 2007, 22, 581–589. [Google Scholar] [CrossRef]
- Wang, H.; Hyvelin, J.M.; Felt, S.A.; Guracar, I.; Vilches-Moure, J.G.; Cherkaoui, S.; Bettinger, T.; Tian, L.; Lutz, A.M.; Willmann, J.K. US Molecular Imaging of Acute Ileitis: Anti-Inflammatory Treatment Response Monitored with Targeted Microbubbles in a Preclinical Model. Radiology 2018, 289, 90–100. [Google Scholar] [CrossRef]
- Wang, H.; Vilches-Moure, J.G.; Cherkaoui, S.; Tardy, I.; Alleaume, C.; Bettinger, T.; Lutz, A.; Paulmurugan, R. Chronic Model of Inflammatory Bowel Disease in IL-10(-/-) Transgenic Mice: Evaluation with Ultrasound Molecular Imaging. Theranostics 2019, 9, 6031–6046. [Google Scholar] [CrossRef]
- Wang, H.; Machtaler, S.; Bettinger, T.; Lutz, A.M.; Luong, R.; Bussat, P.; Gambhir, S.S.; Tranquart, F.; Tian, L.; Willmann, J.K. Molecular imaging of inflammation in inflammatory bowel disease with a clinically translatable dual-selectin-targeted US contrast agent: Comparison with FDG PET/CT in a mouse model. Radiology 2013, 267, 818–829. [Google Scholar] [CrossRef]
- Machtaler, S.; Knieling, F.; Luong, R.; Tian, L.; Willmann, J.K. Assessment of Inflammation in an Acute on Chronic Model of Inflammatory Bowel Disease with Ultrasound Molecular Imaging. Theranostics 2015, 5, 1175–1186. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Felt, S.A.; Machtaler, S.; Guracar, I.; Luong, R.; Bettinger, T.; Tian, L.; Lutz, A.M.; Willmann, J.K. Quantitative Assessment of Inflammation in a Porcine Acute Terminal Ileitis Model: US with a Molecularly Targeted Contrast Agent. Radiology 2015, 276, 809–817. [Google Scholar] [CrossRef]
- Bachmann, C.; Klibanov, A.L.; Olson, T.S.; Sonnenschein, J.R.; Rivera-Nieves, J.; Cominelli, F.; Ley, K.F.; Lindner, J.R.; Pizarro, T.T. Targeting mucosal addressin cellular adhesion molecule (MAdCAM)-1 to noninvasively image experimental Crohn’s disease. Gastroenterology 2006, 130, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Sadlack, B.; Merz, H.; Schorle, H.; Schimpl, A.; Feller, A.C.; Horak, I. Ulcerative colitis-like disease in mice with a disrupted interleukin-2 gene. Cell 1993, 75, 253–261. [Google Scholar] [CrossRef]
- Willerford, D.M.; Chen, J.; Ferry, J.A.; Davidson, L.; Ma, A.; Alt, F.W. Interleukin-2 receptor alpha chain regulates the size and content of the peripheral lymphoid compartment. Immunity 1995, 3, 521–530. [Google Scholar] [CrossRef]
- Bayer, A.L.; Pugliese, A.; Malek, T.R. The IL-2/IL-2R system: From basic science to therapeutic applications to enhance immune regulation. Immunol. Res. 2013, 57, 197–209. [Google Scholar] [CrossRef] [PubMed]
- Poussier, P.; Ning, T.; Chen, J.; Banerjee, D.; Julius, M. Intestinal inflammation observed in IL-2R/IL-2 mutant mice is associated with impaired intestinal T lymphopoiesis. Gastroenterology 2000, 118, 880–891. [Google Scholar] [CrossRef]
- Wakabayashi, K.; Lian, Z.X.; Moritoki, Y.; Lan, R.Y.; Tsuneyama, K.; Chuang, Y.H.; Yang, G.; Ridgway, W.; Ueno, Y.; Ansari, A.A.; et al. IL-2 receptor alpha(-/-) mice and the development of primary biliary cirrhosis. Hepatology 2006, 44, 1240–1249. [Google Scholar] [CrossRef] [PubMed]
- Bettinger, T.; Bussat, P.; Tardy, I.; Pochon, S.; Hyvelin, J.M.; Emmel, P.; Henrioud, S.; Biolluz, N.; Willmann, J.K.; Schneider, M.; et al. Ultrasound molecular imaging contrast agent binding to both E- and P-selectin in different species. Investig. Radiol. 2012, 47, 516–523. [Google Scholar] [CrossRef]
- Deshpande, N.; Lutz, A.M.; Ren, Y.; Foygel, K.; Tian, L.; Schneider, M.; Pai, R.; Pasricha, P.J.; Willmann, J.K. Quantification and monitoring of inflammation in murine inflammatory bowel disease with targeted contrast-enhanced US. Radiology 2012, 262, 172–180. [Google Scholar] [CrossRef]
- Jones-Hall, Y.L.; Grisham, M.B. Immunopathological characterization of selected mouse models of inflammatory bowel disease: Comparison to human disease. Pathophysiology 2014, 21, 267–288. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Kundig, T.M.; Furlonger, C.; Wakeham, A.; Timms, E.; Matsuyama, T.; Schmits, R.; Simard, J.J.L.; Ohashi, P.S.; Griesser, H.; et al. Deregulated T cell activation and autoimmunity in mice lacking interleukin-2 receptor beta. Science 1995, 268, 1472–1476. [Google Scholar] [CrossRef] [PubMed]
- Baumgart, D.C.; Olivier, W.A.; Reya, T.; Peritt, D.; Rombeau, J.L.; Carding, S.R. Mechanisms of intestinal epithelial cell injury and colitis in interleukin 2 (IL2)-deficient mice. Cell Immunol. 1998, 187, 52–66. [Google Scholar] [CrossRef] [PubMed]
- Schultz, M.; Tonkonogy, S.L.; Sellon, R.K.; Veltkamp, C.; Godfrey, V.L.; Kwon, J.; Grenther, W.B.; Balish, E.; Horak, I.; Sartor, R.B. IL-2-deficient mice raised under germfree conditions develop delayed mild focal intestinal inflammation. Am. J. Physiol. 1999, 276, G1461–G1472. [Google Scholar] [CrossRef] [PubMed]
- Mosli, M.H.; Zou, G.; Garg, S.K.; Feagan, S.G.; MacDonald, J.K.; Chande, N.; Sandborn, W.; Feagan, B.G. C-Reactive Protein, Fecal Calprotectin, and Stool Lactoferrin for Detection of Endoscopic Activity in Symptomatic Inflammatory Bowel Disease Patients: A Systematic Review and Meta-Analysis. Am. J. Gastroenterol. 2015, 110, 802–819. [Google Scholar] [CrossRef] [PubMed]
- Barros, L.L.; Farias, A.Q.; Rezaie, A. Gastrointestinal motility and absorptive disorders in patients with inflammatory bowel diseases: Prevalence, diagnosis and treatment. World J. Gastroenterol. 2019, 25, 4414–4426. [Google Scholar] [CrossRef]
- Willmann, J.K.; Bonomo, L.; Carla Testa, A.; Rinaldi, P.; Rindi, G.; Valluru, K.S.; Petrone, G.; Martini, M.; Lutz, A.M.; Gambhir, S.S. Ultrasound Molecular Imaging With BR55 in Patients With Breast and Ovarian Lesions: First-in-Human Results. J. Clin. Oncol. 2017, 35, 2133–2140. [Google Scholar] [CrossRef] [PubMed]





| Mortality | |
|---|---|
| IL-2Rα−/− mice < 6 weeks | 4.9% (2/41) |
| IL-2Rα−/− mice between 6–30 weeks | 26.8% (11/41) |
| Number of mice imaged | |
| Wild-type mice | 6 |
| Male | 3 |
| Female | 3 |
| IL-2Rα−/− mice | 39 |
| Male | 21 |
| Female | 18 |
| Number of USMI acquisitions and mice with positive USMI signals | |
| Total USMI acquisitions of IL-2Rα−/− mice | 387 |
| Male | 201 |
| Female | 186 |
| Total positive acquisitions with low-high USMI signals | 42.9% (166/387) |
| Male | 42.3% (85/201) |
| Female | 43.5% (81/186) |
| Mice with at least one positive USMI imaging acquisition | 100% (39/39) |
| Male | 100% (21/21) |
| Female | 100% (18/18) |
| Onset of colitis | Weeks |
| Earliest | 6 |
| Latest | 14 |
| Mean | 10.5 |
| Median | 11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Vilches-Moure, J.G.; Bettinger, T.; Cherkaoui, S.; Lutz, A.; Paulmurugan, R. Contrast Enhanced Ultrasound Molecular Imaging of Spontaneous Chronic Inflammatory Bowel Disease in an Interleukin-2 Receptor α−/− Transgenic Mouse Model Using Targeted Microbubbles. Nanomaterials 2022, 12, 280. https://doi.org/10.3390/nano12020280
Wang H, Vilches-Moure JG, Bettinger T, Cherkaoui S, Lutz A, Paulmurugan R. Contrast Enhanced Ultrasound Molecular Imaging of Spontaneous Chronic Inflammatory Bowel Disease in an Interleukin-2 Receptor α−/− Transgenic Mouse Model Using Targeted Microbubbles. Nanomaterials. 2022; 12(2):280. https://doi.org/10.3390/nano12020280
Chicago/Turabian StyleWang, Huaijun, Jose G. Vilches-Moure, Thierry Bettinger, Samir Cherkaoui, Amelie Lutz, and Ramasamy Paulmurugan. 2022. "Contrast Enhanced Ultrasound Molecular Imaging of Spontaneous Chronic Inflammatory Bowel Disease in an Interleukin-2 Receptor α−/− Transgenic Mouse Model Using Targeted Microbubbles" Nanomaterials 12, no. 2: 280. https://doi.org/10.3390/nano12020280
APA StyleWang, H., Vilches-Moure, J. G., Bettinger, T., Cherkaoui, S., Lutz, A., & Paulmurugan, R. (2022). Contrast Enhanced Ultrasound Molecular Imaging of Spontaneous Chronic Inflammatory Bowel Disease in an Interleukin-2 Receptor α−/− Transgenic Mouse Model Using Targeted Microbubbles. Nanomaterials, 12(2), 280. https://doi.org/10.3390/nano12020280






