Synthesis, Characterization and Photocatalytic Activity of CoFe2O4/Fe2O3 Dispersed in Mesoporous KIT-6
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of KIT-6 Support
2.2. Synthesis of Catalysts
2.3. Characterizations
2.4. Photocatalytic Tests
3. Results and Discussion
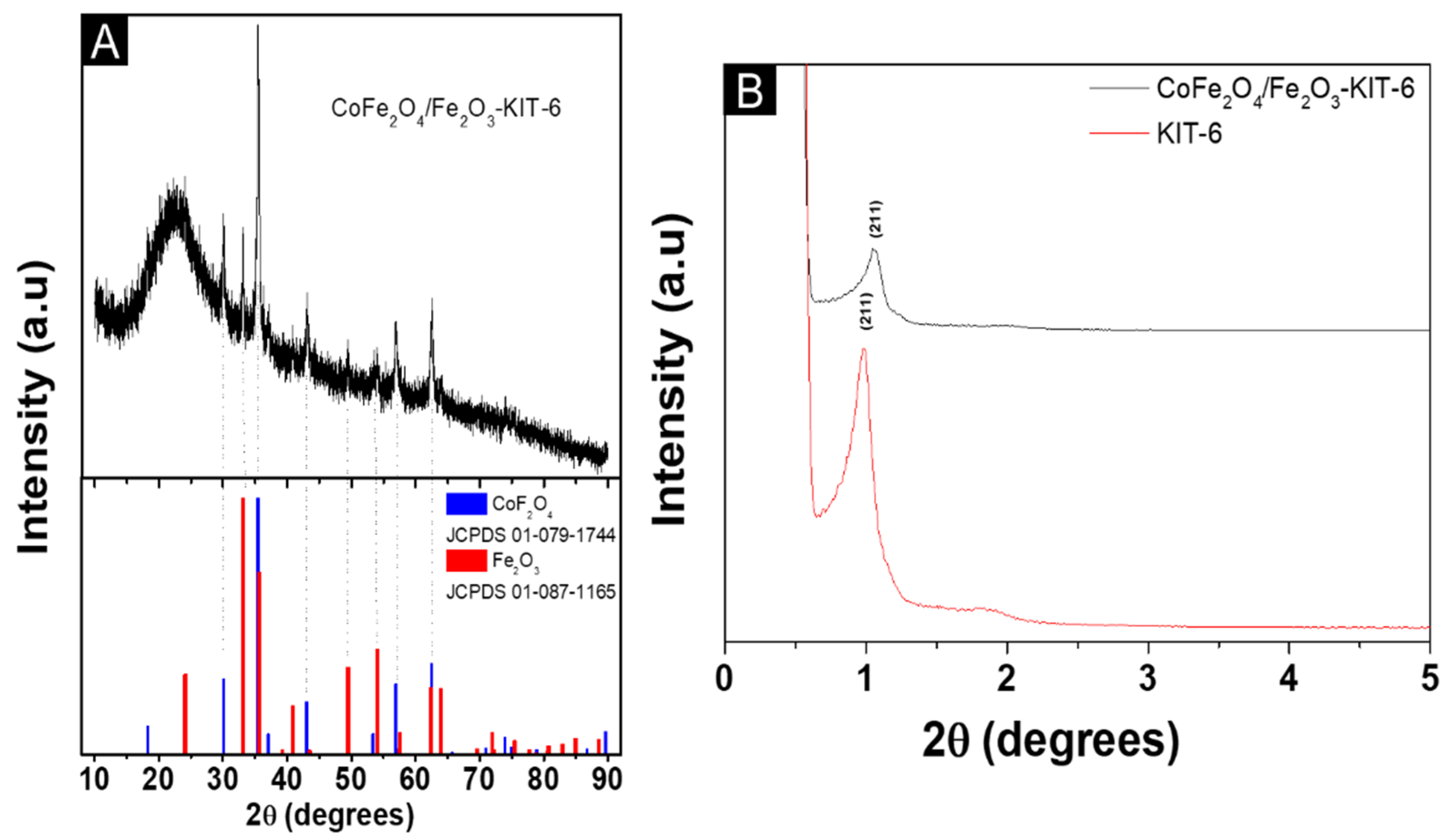
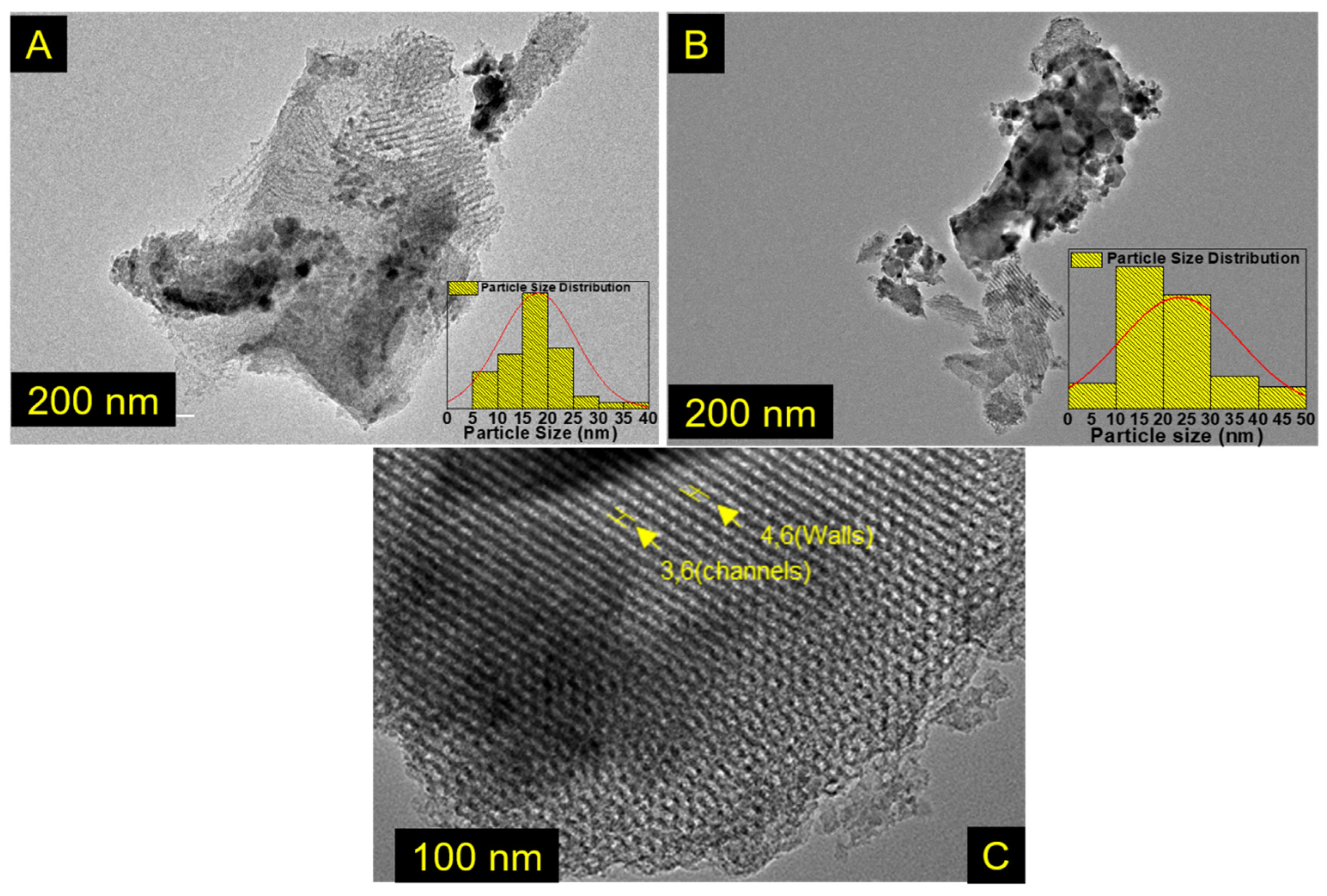
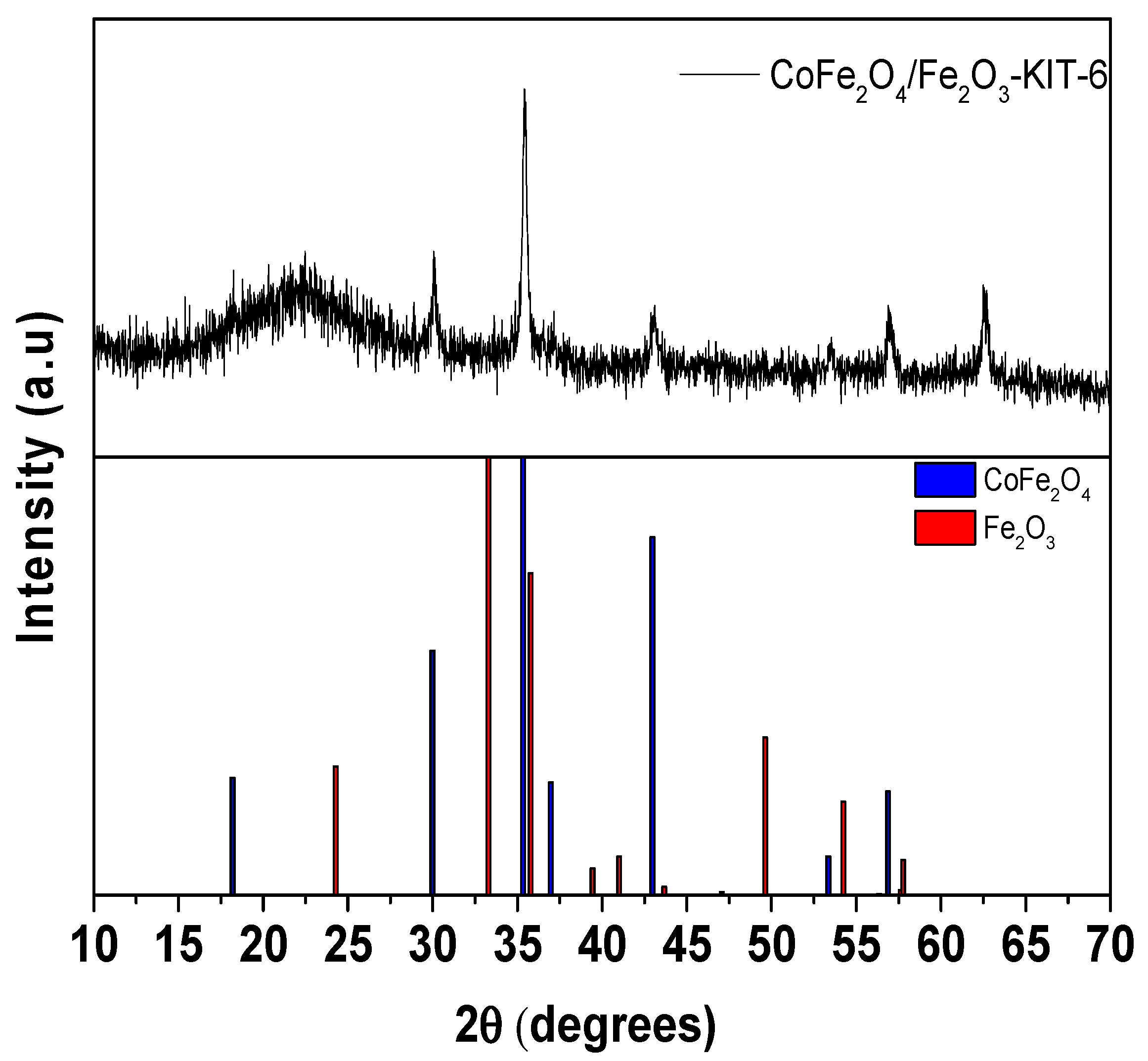
3.1. Structural and Morphological Characterization
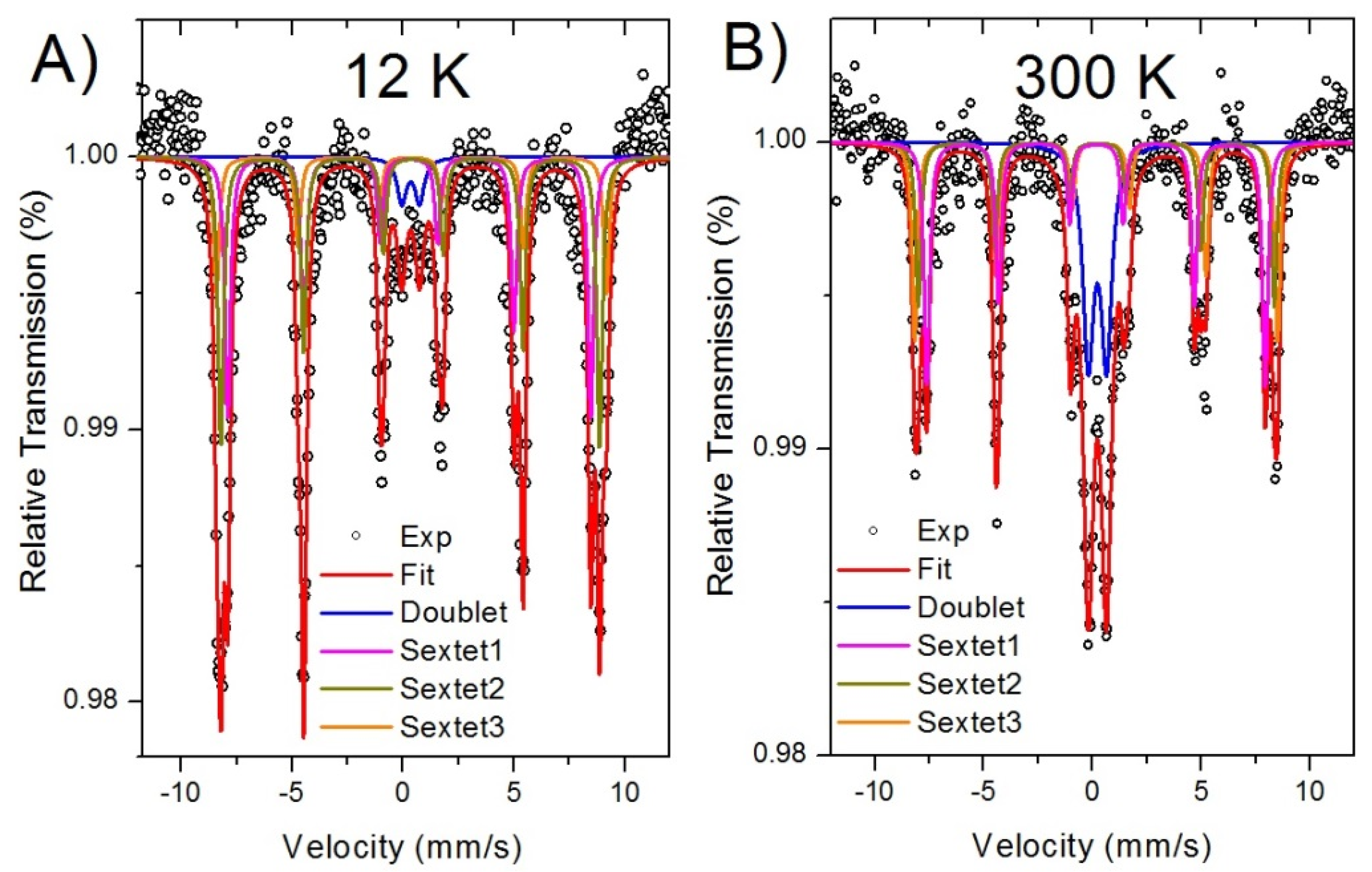
3.2. Chemical and Electronic Characterization
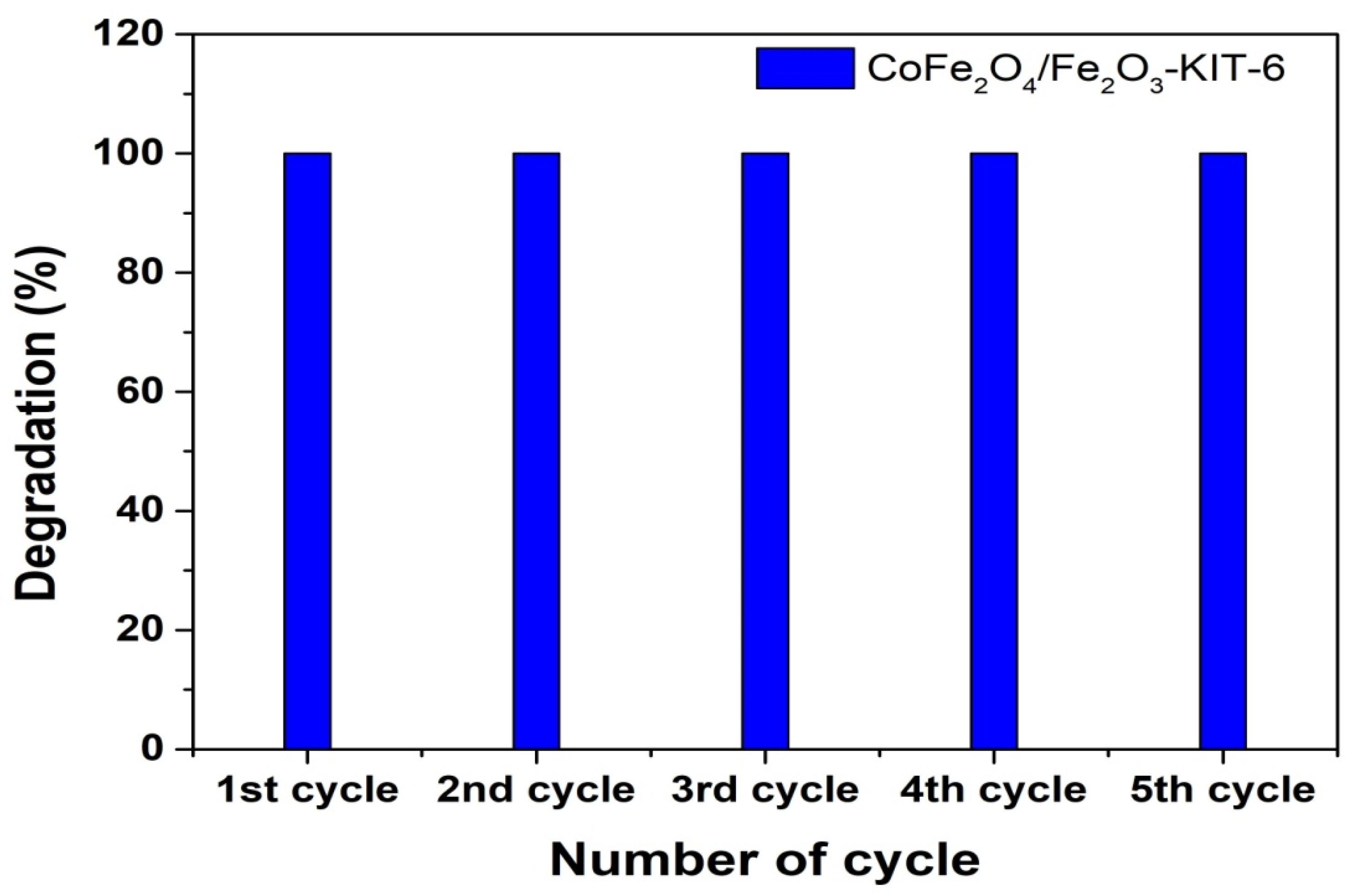
3.3. Application in Dye Degradation
3.4. Degradation Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Konicki, W.; Helminak, A.; Arbczyk, W.; Mijowska, E. Removal of anionic dyes using magnetic Fe@graphite core-shell nanocomposite as an adsorbent from aqueous solutions. J. Colloid Interface Sci. 2017, 494, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Soufi, A.; Hajjaoui, H.; Elmoubarki, R.; Abdennouri, M.; Qourzal, S.; Barka, N. Spinel ferrites nanoparticles: Synthesis methods and application in heterogeneous Fenton oxidation of organic pollutants—A review. Appl. Surf. Sci. Adv. 2021, 6, 100145. [Google Scholar] [CrossRef]
- Moghaddam, A.Z.; Ghiamati, E.; Pourashuri, A.; Allahresani, A. Modified nickel ferrite nanocomposite/functionalized chitosan as a novel adsorbent for the removal of acidic dyes. Int. J. Biol. Macromol. 2018, 120, 1714–1725. [Google Scholar] [CrossRef]
- Silva, M.C.; Corrêa, A.D.; Torres, J.A.; Amorim, M.T.S.P. Descoloração de corantes industriais e efluentes têxteis simulados por peroxidase de nabo (Brassica campestre). Quím. Nova 2012, 35, 889–894. [Google Scholar] [CrossRef] [Green Version]
- BRASIL. Resolução no 357 de 17 de Março de 2005 do Conselho Nacional do Meio Ambiente. Available online: http://www2.mma.gov.br/port/conama/res/res05/res35705.pdf (accessed on 8 September 2022).
- Kunz, A.; Peralta-Zamora, P.; De Moraes, S.G.; Durán, N. Novas tendências no tratamento de efluentes têxteis. Quím. Nova 2002, 25, 78–82. [Google Scholar] [CrossRef]
- Islam, T.; Hyder, A.G.; Saenz-Arana, R.; Hernandez, C.; Guinto, T.; Ahsan, A.; Alvarado-Tenorio, B.; Noveron, J.C. Removal of methylene blue and tetracycline from water using peanut shell derived adsorbent prepared by sulfuric acid reflux. J. Environ. Chem. Eng. 2019, 7, 102816. [Google Scholar] [CrossRef]
- Rivera-Utrilla, J.; Sánchez-Polo, M.; Ferro-García, M.Á.; Prados-Joya, G.; Ocampo-Perez, R. Pharmaceuticals as emerging contaminants and their removal from water. A review. Chemosphere 2013, 93, 1268–1287. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, M.T.P.; Villarroel-Rocha, J.; Toncón-Leal, C.F.; Barbosa, F.F.; Miranda, M.O.; Torres, M.A.M.; Sapag, K.; Pergher, S.B.; Braga, T.P. Textural and photocatalytic characteristics of iron-cobalt based nanocomposites supported on SBA-15: Synergistic effect between Fe2+ and Fe0 on photoactivity. Microporous Mesoporous Mater. 2021, 310, 110582. [Google Scholar] [CrossRef]
- Da Silva, M.T.P.; Barbosa, F.F.; Torre, M.A.M.; Villarroel-Rocha, J.; Sapag, K.; Pergher, S.B.C.; Braga, T.P. Synthesis of Fe2SiO4-Fe7Co3 Nanocomposite Dispersed in the Mesoporous SBA-15: Application as Magnetically Separable Adsorbent. Molecules 2020, 25, 1016. [Google Scholar] [CrossRef] [Green Version]
- Cao, J.; Xu, B.; Lin, H.; Luo, B.; Chen, S. Chemical etching preparation of BiOI/BiOBr heterostructures with enhanced photocatalytic properties for organic dye removal. Chem. Eng. J. 2012, 185–186, 91–99. [Google Scholar] [CrossRef]
- Natarajan, S.; Bajaj, H.C.; Tayade, R.J. Recent advances based on the synergetic effect of adsorption for removal of dyes from waste water using photocatalytic process. J. Environ. Sci. 2018, 65, 201–222. [Google Scholar] [CrossRef] [PubMed]
- Kheirabadi, M.; Samadi, M.; Asadian, E.; Zhou, Y.; Dong, C.; Zhang, J.; Moshfegh, A.Z. Well-designed Ag/ZnO/3D graphene structure for dye removal: Adsorption, photocatalysis and physical separation capabilities. J. Colloid Interface Sci. 2019, 537, 66–78. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, J.A.; dos Santos, G.B.; Madeira, V.S.; de Almeida Ramalho, M.L.; Brasileiro, I.L.; Cahino, A.M. Use of Fe2O3-TiO2 in solar photo-Fenton process for the phenol degradation. Engevista 2018, 20, 757–771. [Google Scholar]
- Hossain, M.A.; Kayes, N.; Hossain, M. Removal of Remazol Red RR from Aqueous Solution by Glass Supported Films of Synthesized ZnO Nanoparticles. ICRRD Qual. Index Res. J. 2021, 2, 109–119. [Google Scholar] [CrossRef]
- El-Dossoki, F.I.; Atwee, T.M.; Hamada, A.M.; El-Bindary, A.A. Photocatalytic degradation of Remazol Red B and Rhodamine B dyes using TiO2 nanomaterial: Estimation of the effective operating parameters. Desalination Water Treat. 2021, 233, 319–330. [Google Scholar] [CrossRef]
- Pipil, H.; Yadav, S.; Chawla, H.; Taneja, S.; Verma, M.; Singla, N.; Haritash, A.K. Comparison of TiO2 catalysis and Fenton’s treatment for rapid degradation of Remazol Red Dye in textile industry effluent. Rend. Lince 2022, 33, 105–114. [Google Scholar] [CrossRef]
- Soares, M.D.C.B.; Barbosa, F.F.; Torres, M.A.M.; Valentini, A.; Albuquerque, A.D.R.; Sambrano, J.R.; Pergher, S.B.C.; Essayem, N.; Braga, T.P. Oxidative dehydrogenation of ethylbenzene to styrene over the CoFe2O4–MCM-41 catalyst: Preferential adsorption on the O2−Fe3+O2− sites located at octahedral positions. Catal. Sci. Technol. 2019, 9, 2469–2484. [Google Scholar] [CrossRef]
- Diodati, S.; Walton, R.I.; Mascotto, S.; Gross, S. Low-temperature wet chemistry synthetic approaches towards ferrites. Inorg. Chem. Front. 2020, 7, 3282–3314. [Google Scholar] [CrossRef]
- Sonu; Dutta, V.; Sharma, S.; Raizada, P.; Hosseini-Bandegharaei, A.; Gupta, V.K.; Singh, P. Review on augmentation in photocatalytic activity of CoFe2O4 via heterojunction formation for photocatalysis of organic pollutants in water. J. Saudi Chem. Soc. 2019, 23, 1119–1136. [Google Scholar] [CrossRef]
- Du, Y.; Ma, W.; Liu, P.; Zou, B.; Ma, J. Magnetic CoFe2O4 nanoparticles supported on titanate nanotubes (CoFe2O4/TNTs) as a novel heterogeneous catalyst for peroxymonosulfate activation and degradation of organic pollutants. J. Hazard. Mater. 2016, 308, 58–66. [Google Scholar] [CrossRef]
- Ciocarlan, R.-G.; Seftel, E.M.; Mertens, M.; Pui, A.; Mazaj, M.; Tusar, N.N.; Cool, P. Novel magnetic nanocomposites containing quaternary ferrites systems Co0.5Zn0.25M0.25Fe2O4 (M = Ni, Cu, Mn, Mg) and TiO2-anatase phase as photocatalysts for wastewater remediation under solar light irradiation. Mater. Sci. Eng. B 2018, 230, 1–7. [Google Scholar] [CrossRef]
- Haw, C.; Chiu, W.; Rahman, S.A.; Khiew, P.; Radiman, S.; Shukor, R.A.; Hamid, M.A.A.; Ghazali, N. The design of new magnetic-photocatalyst nanocomposites (CoFe2O4–TiO2) as smart nanomaterials for recyclable-photocatalysis applications. New J. Chem. 2016, 40, 1124–1136. [Google Scholar] [CrossRef]
- Shih, Y.-J.; Su, C.-C.; Chen, C.-W.; Dong, C.-D. Synthesis of magnetically recoverable ferrite (MFe2O4, M Co, Ni and Fe)-supported TiO2 photocatalysts for decolorization of methylene blue. Catal. Commun. 2015, 72, 127–132. [Google Scholar] [CrossRef]
- Casbeer, E.; Sharma, V.K.; Li, X.-Z. Synthesis and photocatalytic activity of ferrites under visible light: A review. Sep. Purif. Technol. 2012, 87, 1–14. [Google Scholar] [CrossRef]
- Mandal, B.; Panda, J.; Paul, P.K.; Sarkar, R.; Tudu, B. MnFe2O4 decorated reduced graphene oxide heterostructures: Nanophotocatalyst for methylene blue dye degradation. Vacuum 2019, 173, 109150. [Google Scholar] [CrossRef]
- Delgado, A.D.; Alvarez-Contreras, L.; Beltrán, K.A.; Cárdenas, P.S.; Leyva-Porras, C.; Aguilar-Elguezabal, A. Green synthesis of KIT-6 from water-glass as support for hydrodesulfurization catalysts, and its comparison with KIT-6 synthesized from TEOS. Microporous Mesoporous Mater. 2020, 306, 110436. [Google Scholar] [CrossRef]
- Kleitz, F.; Choi, S.H.; Ryoo, R. Cubic Ia3d large mesoporous silica: Synthesis and replication to platinum nanowires, carbon nanorods and carbon nanotubes Electronic supplementary information (ESI) available: TEM images of mesoporous cubic silica and Pt networks, XRD patterns during formation of the cubic phase. Chem. Commun. 2003, 17, 2136–2137. [Google Scholar] [CrossRef]
- Boulaoued, A.; Fechete, I.; Donnio, B.; Bernard, M.; Turek, P.; Garin, F. Mo/KIT-6, Fe/KIT-6 and Mo–Fe/KIT-6 as new types of heterogeneous catalysts for the conversion of MCP. Microporous Mesoporous Mater. 2012, 155, 131–142. [Google Scholar] [CrossRef]
- Jakhar, S.; Duhan, S.; Nain, S. Novel one step hydrothermal synthesis of cubic Ia3d large pore 3D mesoporous In2O3/KIT-6 hybrid nanocomposite with humidity sensing applications. J. Porous Mater. 2020, 27, 1253–1263. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Júnior, A.V.D.M.; Araújo, A.M.D.M.; Fernandes, V.J.; dos Santos, N.A.; Santos, A.G.D.; Gondim, A.D. Comparative study of the thermo-catalytic degradation of waste frying and Pachira aquatica Aubl. oil in the presence of Mo/KIT-6. J. Therm. Anal. 2019, 139, 535–544. [Google Scholar] [CrossRef]
- Arelaro, A.D.; Rossi, L.M.; Rechenberg, H.R. In-field Mössbauer characterization of MFe2O4(M = Fe, Co, Ni) nanoparticles. J. Phys. Conf. Ser. 2010, 217, 1–4. [Google Scholar] [CrossRef]
- Gallo-Cordova, A.; Espinosa, A.; Serrano, A.; Gutiérrez, L.; Menéndez, N.; Morales, M.D.P.; Mazarío, E. New insights into the structural analysis of maghemite and (MFe2O4, M = Co, Zn) ferrite nanoparticles synthesized by a microwave-assisted polyol process. Mater. Chem. Front. 2020, 4, 3063–3073. [Google Scholar] [CrossRef]
- Ahmad, W.R.W.; Mamat, M.H.; Zoolfakar, A.S.; Khusaimi, Z.; Rusop, M. A review on hematite α-Fe2O3 focusing on nanostructures, synthesis methods and applications. In Proceedings of the 2016 IEEE Student Conference on Research and Development (SCOReD), Kuala Lumpur, Malaysia, 13–14 December 2016; pp. 1–6. [Google Scholar] [CrossRef]
- Wang, L.; Yang, C.; Zhang, L.; Hu, Y.; Li, J.; Xu, S.; Li, H. The exchange coupling interaction in CoFe2O4/Fe3O4 hard and soft magnetic nanocomposites. Vacuum 2020, 181, 109751. [Google Scholar] [CrossRef]
- Hähsler, M.; Landers, J.; Nowack, T.; Salamon, S.; Zimmermann, M.; Heißler, S.; Wende, H.; Behrens, S. Magnetic Properties and Mössbauer Spectroscopy of Fe3O4/CoFe2O4 Nanorods. Inorg. Chem. 2020, 59, 3677–3685. [Google Scholar] [CrossRef] [PubMed]
- Gütlich, P.; Bill, E.; Trautwein, A.X. (Eds.) Mössbauer Spectroscopy and Transition Metal Chemistry: Fundamentals and Applications; Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- Verziu, M.; Cojocaru, B.; Hu, J.; Richards, R.; Ciuculescu, C.; Filip, P.; Parvulescu, V.I. Transesterificação de óleo de girassol e colza para biodiesel sobre diferentes catalisadores nanocristalinos de MgO. Química Verde. Soc. Real Quím. RSC 2008, 10, 373–381. [Google Scholar] [CrossRef]
- Liu, Y.; Shi, J.; Chen, J.; Ye, Q.; Pan, H.; Shao, Z.; Shi, Y. Dynamic performance of CO2 adsorption with tetraethylenepentamine-loaded KIT-6. Microporous Mesoporous Mater. 2010, 134, 16–21. [Google Scholar] [CrossRef]
- Mathew, T.; Malwadkar, S.; Pai, S.; Sharanappa, N.; Sebastian, C.; Satyanarayana, C.; Bokade, V. Oxidative Dehydrogenation of Ethylbenzene over Cu1-xCoxFe2O4Catalyst System: Influence of Acid–Base Property. Catal. Lett. 2003, 91, 217–224. [Google Scholar] [CrossRef]
- Chandekar, K.V.; Shkir, M.; Alfaify, S. Tuning the optical band gap and magnetization of oleic acid coated CoFe2O4 NPs synthesized by facile hydrothermal route. Mater. Sci. Eng. B 2020, 259, 114603. [Google Scholar] [CrossRef]
- Valero-Luna, C.; Palomares-Sanchéz, S.A.; Ruíz, F. Catalytic activity of the barium hexaferrite with H2O2/visible light irradiation for degradation of Methylene Blue. Catal. Today 2016, 266, 110–119. [Google Scholar] [CrossRef]
- Singh, M.; Goyal, M.; Devlal, K. Size and shape effects on the band gap of semiconductor compound nanomaterials. J. Taibah Univ. Sci. 2018, 12, 470–475. [Google Scholar] [CrossRef] [Green Version]
- Souza, F.L. Sunlight-driven water splitting using hematite nanorod photoelectrodes. An. Acad. Bras. Cienc. 2018, 90, 745–762. [Google Scholar] [CrossRef] [PubMed]
- Gholami, M.; Amin, M.H.; Tardio, J. Estudos sobre a adsorção de fosfato usando KIT-6 funcionalizado com lantanídeos. Microporous Mesoporous Mater. 2019, 286, 77–83. [Google Scholar] [CrossRef]
- Zielińska-Jurek, A.; Bielan, Z.; Dudziak, S.; Wolak, I.; Sobczak, Z.; Klimczuk, T.; Nowaczyk, G.; Hupka, J. Design and Application of Magnetic Photocatalysts for Water Treatment. The Effect of Particle Charge on Surface Functionality. Catalysts 2017, 7, 360. [Google Scholar] [CrossRef] [Green Version]
- Sanpo, N.; Tharajak, J.; Li, Y.; Berndt, C.C.; Wen, C.; Wang, J. Biocompatibilidade de nanopartículas de ferrita de cobalto substituídas por metal de transição. J. Nanoparticle Res. 2014, 16, 2510. [Google Scholar] [CrossRef]
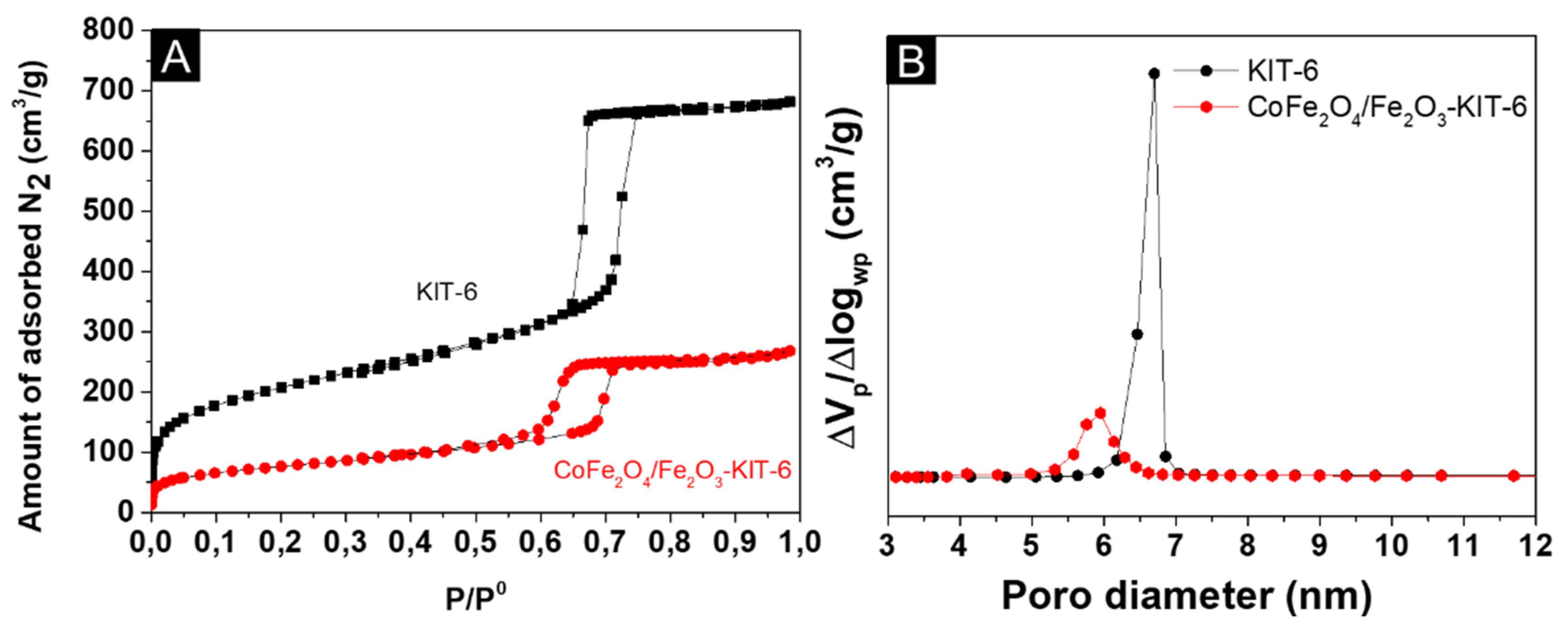
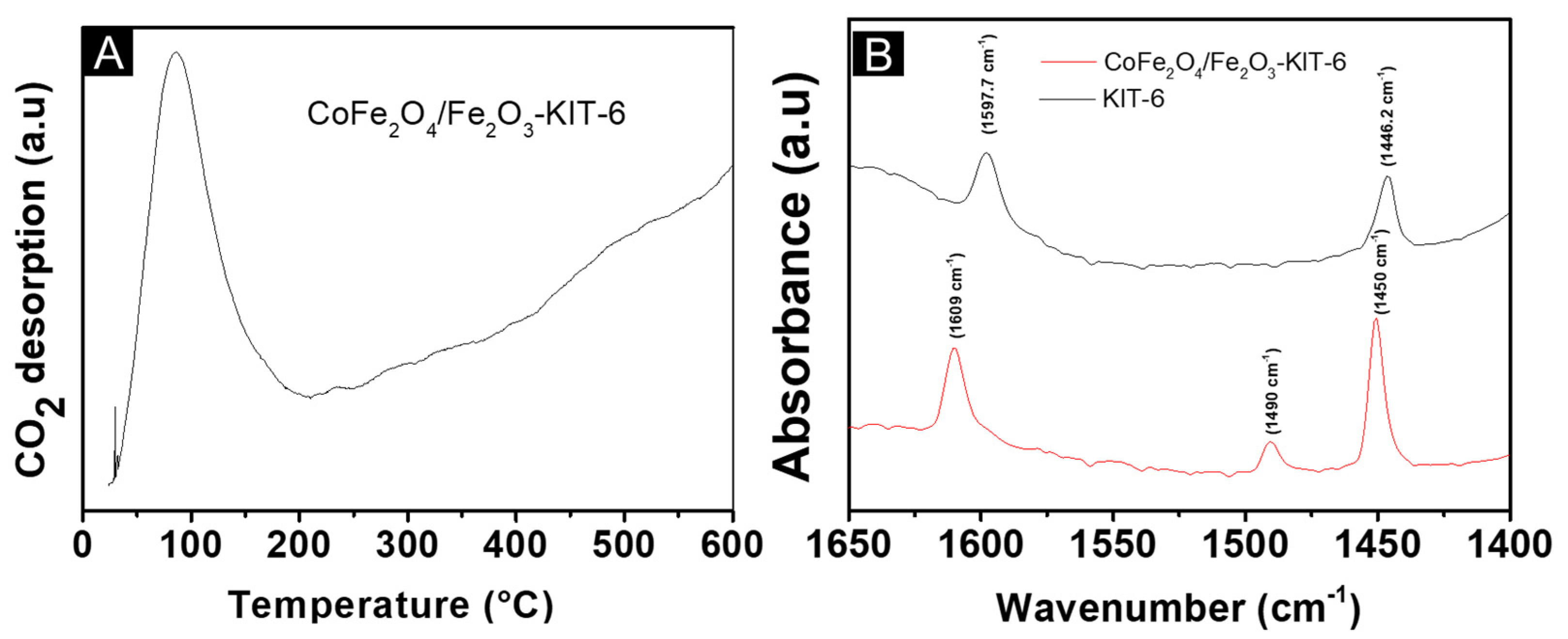
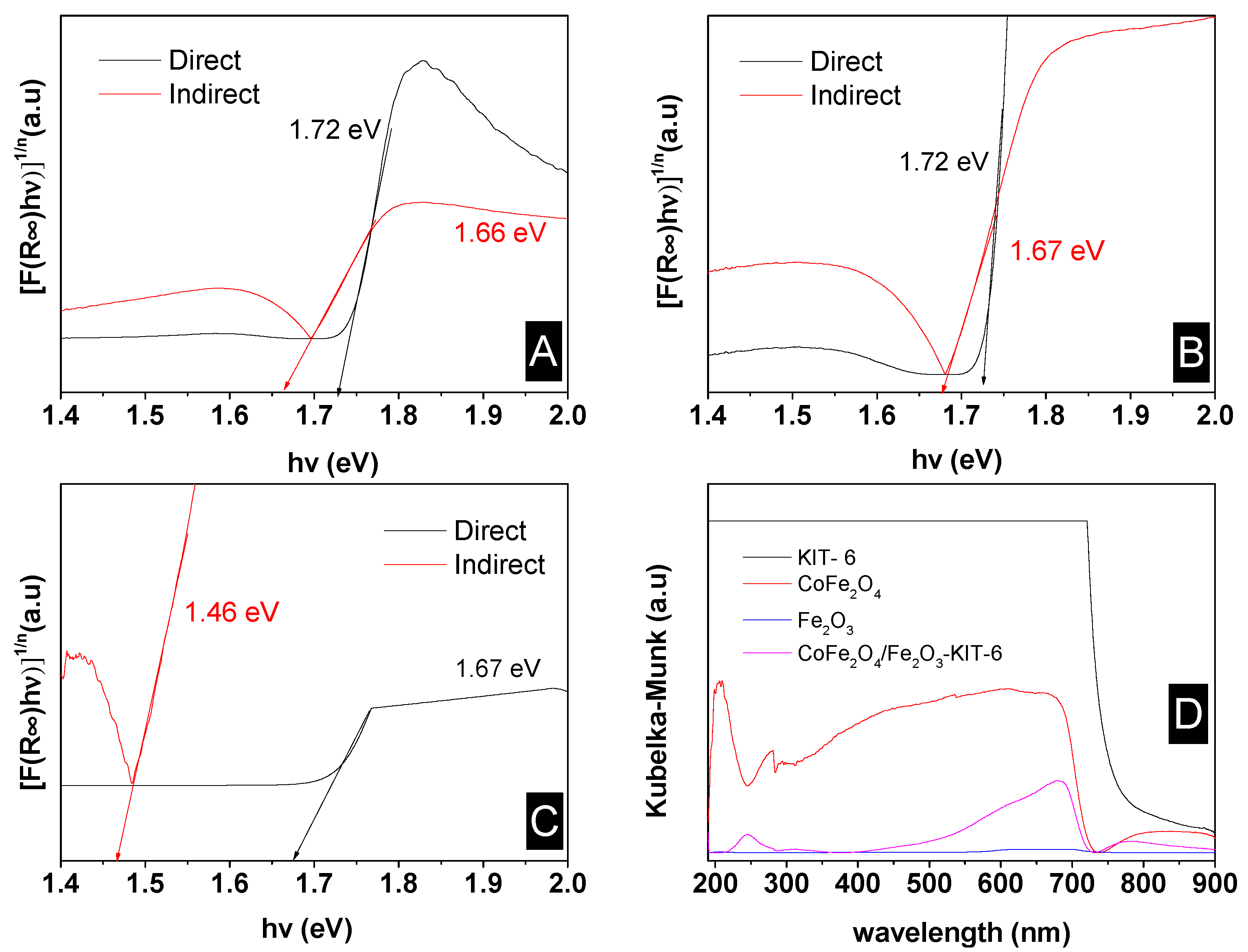
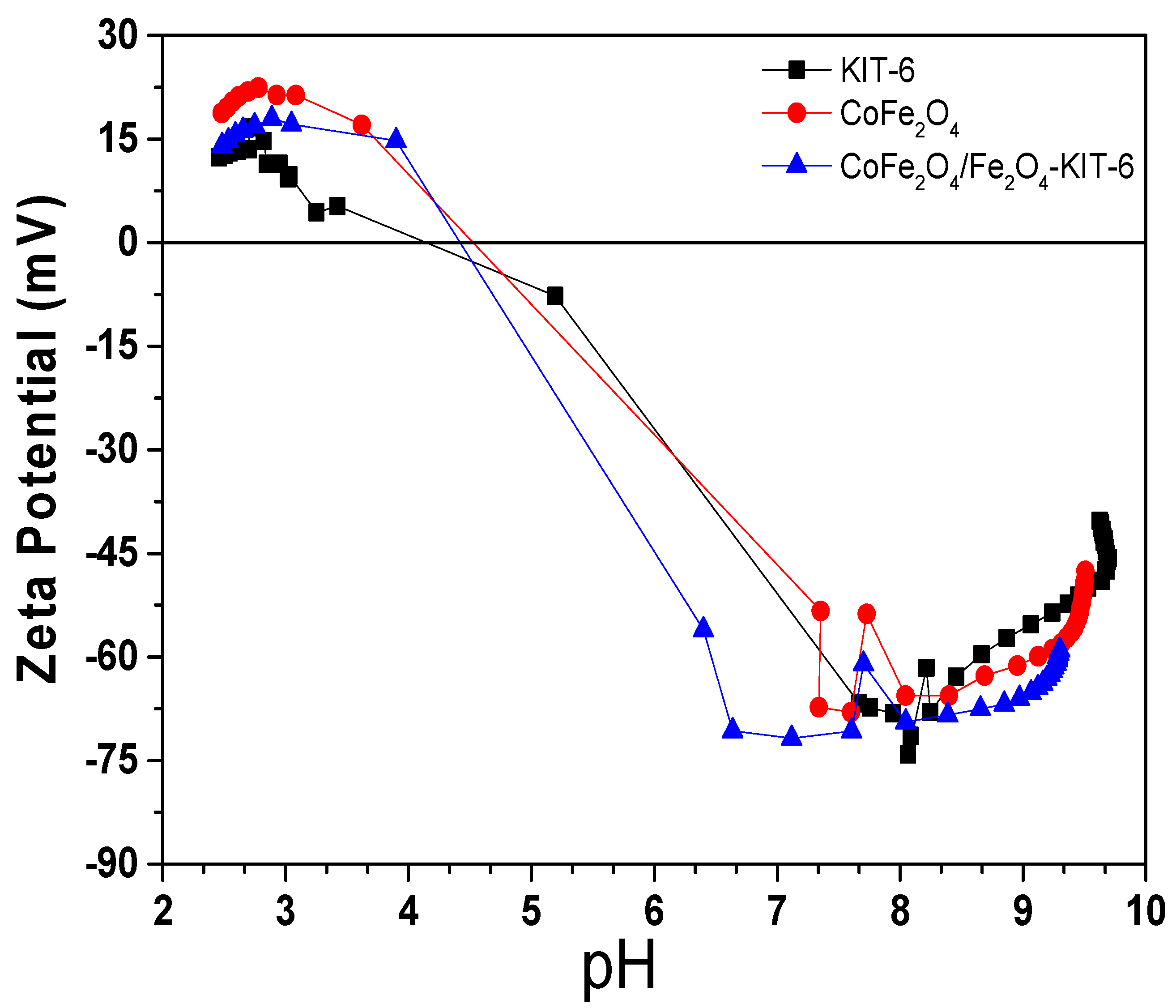

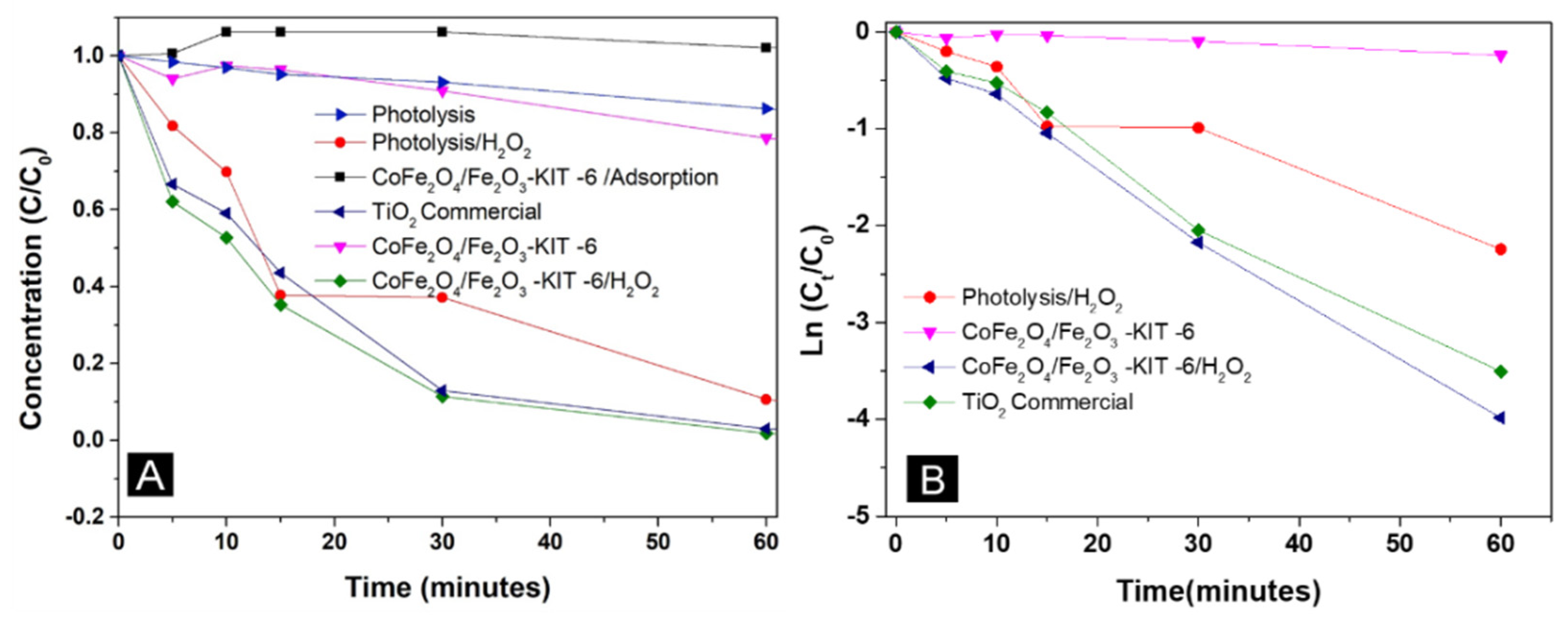

| Sample | SBET (m2/g) | Vp (cm3/g) | Dp (nm) |
|---|---|---|---|
| KIT-6 | 746 | 1.05 | 6.7 |
| CoFe2O4/Fe2O3-KIT-6 | 275 | 0.41 | 6.0 |
| Temperature | Component | IS (mm/s) | QS (mm/s) | Bhf (T) | Area (%) |
|---|---|---|---|---|---|
| 12 K | Sextet 1 | 0.273 | 0.017 | - | 35 |
| Sextet 2 | 0.394 | −0.137 | 50.8 | 39 | |
| Sextet 3 | 0.363 | 0.036 | 52.9 | 18 | |
| Doublet | 0.341 | 0.881 | 54.5 | 7 | |
| 300 K | Sextet 1 | 0.278 | 0.019 | 48.2 | 23 |
| Sextet 2 | 0.313 | −0.071 | 50.7 | 18 | |
| Sextet 3 | 0.382 | −0.216 | 51.7 | 19 | |
| Doublet | 0.331 | 0.837 | - | 40 |
| Test | Degradation Rate (%) |
|---|---|
| Photolysis without H2O2 | 14 |
| Photolysis with H2O2 | 89 |
| CoFe2O4/Fe2O3 –KIT-6 without H2O2 | 21 |
| CoFe2O4/Fe2O3 –KIT-6 with H2O2 | 98 |
| TiO2 commercial without H2O2 | 96 |
| Test | R2 | k` (min−1) | t1/2 (min) |
|---|---|---|---|
| Photolysis without H2O2 | 0.98 | 0.0023 | 291.2 |
| Photolysis with H2O2 | 0.94 | 0.03596 | 19.3 |
| CoFe2O4/Fe2O3 –KIT-6 without H2O2 | 0.97 | 0.0041 | 169.0 |
| CoFe2O4/Fe2O3 –KIT-6 with H2O2 | 0.99 | 0.06624 | 10.5 |
| TiO2 commercial without H2O2 | 0.98 | 0.05925 | 11.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soares, J.d.O.; Cavalcanti, W.E.C.; Torres, M.A.M.; Pergher, S.B.C.; De Oliveira, F.J.V.E.; Braga, T.P. Synthesis, Characterization and Photocatalytic Activity of CoFe2O4/Fe2O3 Dispersed in Mesoporous KIT-6. Nanomaterials 2022, 12, 3566. https://doi.org/10.3390/nano12203566
Soares JdO, Cavalcanti WEC, Torres MAM, Pergher SBC, De Oliveira FJVE, Braga TP. Synthesis, Characterization and Photocatalytic Activity of CoFe2O4/Fe2O3 Dispersed in Mesoporous KIT-6. Nanomaterials. 2022; 12(20):3566. https://doi.org/10.3390/nano12203566
Chicago/Turabian StyleSoares, Johnatan de Oliveira, Wesley Eulálio Cabral Cavalcanti, Marco Antonio Morales Torres, Sibele Berenice Castella Pergher, Fernando José Volpi Eusébio De Oliveira, and Tiago Pinheiro Braga. 2022. "Synthesis, Characterization and Photocatalytic Activity of CoFe2O4/Fe2O3 Dispersed in Mesoporous KIT-6" Nanomaterials 12, no. 20: 3566. https://doi.org/10.3390/nano12203566
APA StyleSoares, J. d. O., Cavalcanti, W. E. C., Torres, M. A. M., Pergher, S. B. C., De Oliveira, F. J. V. E., & Braga, T. P. (2022). Synthesis, Characterization and Photocatalytic Activity of CoFe2O4/Fe2O3 Dispersed in Mesoporous KIT-6. Nanomaterials, 12(20), 3566. https://doi.org/10.3390/nano12203566








