A Review on Gallium Oxide Materials from Solution Processes
Abstract
:1. Introduction
2. Basic Properties of Ga2O3
2.1. Crystalline Structure of Ga2O3
2.2. Band Gap and Density of States of Electrons for Ga2O3

2.3. Other Properties
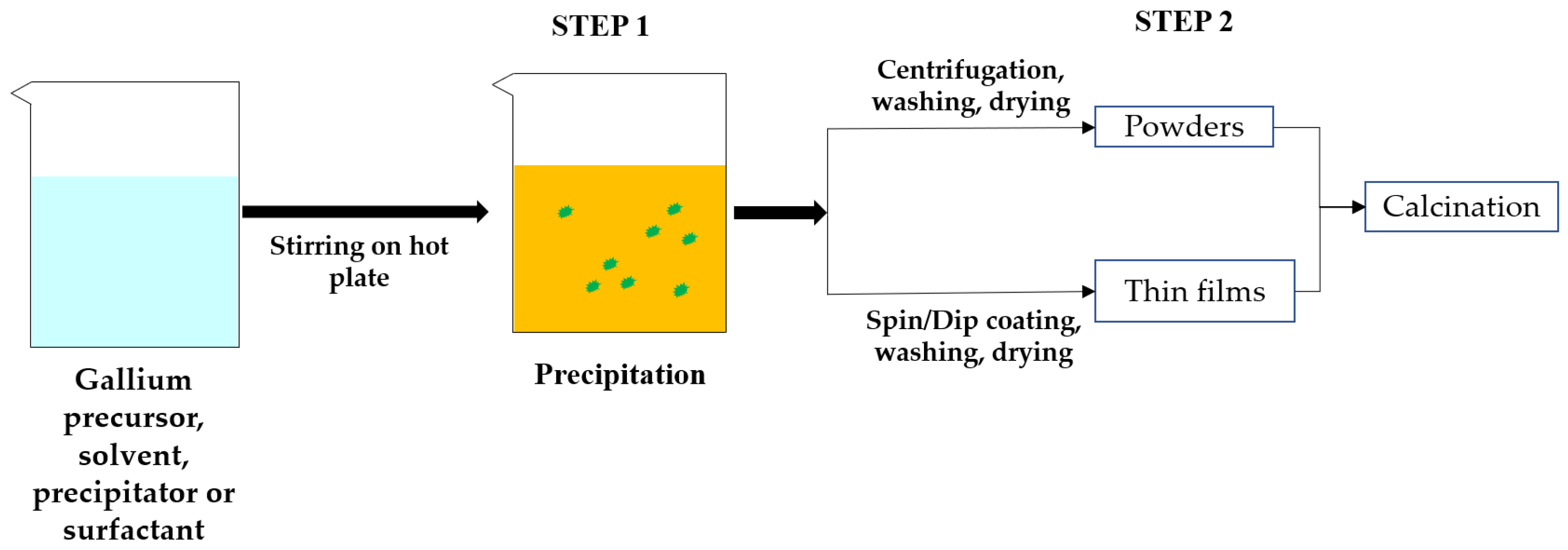
3. Crystal Growth from Solution Process
3.1. Sol-Gel Method
3.2. Hydrothermal Method
3.3. Chemical Bath Deposition
3.4. Other Methods
4. Ga2O3 Materials and Thin Films
4.1. Ga2O3 by Sol-Gel Process
4.2. Ga2O3 by Hydrothermal Process

4.3. Ga2O3 by Chemical Bath Deposition (CBD)
4.4. Other Methods
4.4.1. Solvothermal Method
4.4.2. Forced Hydrolysis Method
4.4.3. Reflux Condensation Method
4.4.4. Electrochemical Deposition
5. Applications
5.1. Deep-Ultraviolet Photodetectors (PD)
5.2. Gas Sensors
5.3. pH Sensors

5.4. Photocatalytic and Photodegradation Applications
5.5. Other Devices
5.6. Limitations and Challenges
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- De Boisbaudran, L. On the chemical and spectroscopic characters of a new metal (gallium). Lond. Edinb. Dublin Philos. Mag. J. Sci. 2009, 50, 414–416. [Google Scholar] [CrossRef]
- Hill, V.G.; Roy, R.; Osborn, E.F. The system alumina-gallia-water. J. Am. Ceram. Soc. 1952, 35, 135–142. [Google Scholar] [CrossRef]
- Roy, R.; Hill, V.G.; Osborn, E.F. Polymorphism of Ga2O3 and the system Ga2O3—H2O. J. Am. Chem. Soc. 1952, 74, 719–722. [Google Scholar] [CrossRef]
- Tippins, H.H. Optical absorption and photoconductivity in band edge of β-Ga2O3. Phys. Rev. 1965, 140, A316–A319. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Shi, J.L.; Qi, D.C.; Chen, L.; Zhang, K.H.L. Recent progress on the electronic structure, defect, and doping properties of Ga2O3. APL Mater. 2020, 8, 35. [Google Scholar] [CrossRef] [Green Version]
- Galazka, Z. β-Ga2O3 for wide-bandgap electronics and optoelectronics. Semicond. Sci. Technol. 2018, 33, 113001. [Google Scholar] [CrossRef]
- Pearton, S.J.; Yang, J.; Cary, P.H.; Ren, F.; Kim, J.; Tadjer, M.J.; Mastro, M.A.A. Review of Ga2O3 materials, processing, and devices. Appl. Phys. Rev. 2018, 5, 011301. [Google Scholar] [CrossRef] [Green Version]
- Shi, F.; Qiao, H. Preparations, properties and applications of gallium oxide nanomaterials–A Review. Nano Select. 2022, 3, 348–373. [Google Scholar] [CrossRef]
- Kim, S.; Kim, J. Highly selective ozone-treated β-Ga2O3 solar-blind deep-UV photodetectors. Appl. Phys. Lett. 2020, 117, 6. [Google Scholar] [CrossRef]
- Hasan, M.N.; Lai, J.Y.; Swinnich, E.; Zheng, Y.X.; Baboukani, B.S.; Nalam, P.C.; Seo, J.H. Investigation of nano-gaps in fractured β-Ga2O3 nanomembranes formed by uniaxial strain. Adv. Electron. Mater. 2021, 7, 9. [Google Scholar] [CrossRef]
- Jedrzejczyk, M.; Zbudniewek, K.; Rynkowski, J.; Keller, V.; Grams, J.; Ruppert, A.M.; Keller, N. Wide band gap Ga2O3 as efficient UV-C photocatalyst for gas-phase degradation applications. Environ. Sci. Pollut. Res. 2017, 24, 26792–26805. [Google Scholar] [CrossRef]
- Minami, T.; Shirai, T.; Nakatani, T.; Miyata, T. Electroluminescent devices with Ga2O3:Mn thin-film emitting layer prepared by sol-gel process. Jpn. J. Appl. Phys. 2000, 39, L524–L526. [Google Scholar] [CrossRef]
- Ji, Z.G.; Du, J.; Fan, J.; Wang, W. Gallium oxide films for filter and solar-blind UV detector. Opt. Mater. 2006, 28, 415–417. [Google Scholar] [CrossRef]
- Kaya, A.; Mao, H.; Gao, J.; Chopdekar, R.V.; Takamura, Y.; Chowdhury, S.; Islam, M.S. An Investigation of Electrical and Dielectric Parameters of Sol–Gel Process Enabled β-Ga2O3 as a Gate Dielectric Material. IEEE Trans. Electron. Devices 2017, 64, 2047–2053. [Google Scholar] [CrossRef]
- Gao, J.; Kaya, A.; Chopdekar, R.V.; Xu, Z.; Takamura, Y.; Islam, M.S.; Chowdhury, S. A Study of Temperature Dependent Current–Voltage (I–V–T) Characteristics in Ni/Sol–Gel β-Ga2O3/n-GaN Structure. J. Mater. Sci. Mater. Electron. 2018, 29, 11265–11270. [Google Scholar] [CrossRef] [Green Version]
- Kaur, D.; Kumar, M.A. Strategic review on gallium oxide based deep-ultraviolet photodetectors: Recent progress and future prospects. Adv. Opt. Mater. 2021, 9, 34. [Google Scholar] [CrossRef]
- Zhao, B.; Wang, F.; Chen, H.Y.; Zheng, L.X.; Su, L.X.; Zhao, D.X.; Fang, X.S. An ultrahigh responsivity (9.7 MA W−1) self-powered solar-blind photodetector based on individual ZnO-Ga2O3 heterostructures. Adv. Funct. Mater. 2017, 27, 8. [Google Scholar] [CrossRef]
- Stepanov, S.I.; Nikolaev, V.I.; Bougrov, V.E.; Romanov, A.E. Gallium Oxide: Properties and Applications-A Review. Rev. Adv. Mater. Sci. 2016, 44, 63–86. [Google Scholar]
- Xu, W.Y.; Chen, L.; Han, S.; Cao, P.J.; Fang, M.; Liu, W.J.; Zhu, D.L.; Lu, Y.M. Aqueous solution-processed boron-doped gallium oxide dielectrics for high-performance thin-film transistors. J. Phys. Chem. C 2020, 124, 8015–8023. [Google Scholar] [CrossRef]
- Huang, C.C.; Yeh, C.S. GaOOH, and β- and γ-Ga2O3 nanowires: Preparation and photoluminescence. New J. Chem. 2010, 34, 103–107. [Google Scholar] [CrossRef]
- Reddy, L.S.; Ko, Y.H.; Yu, J.S. Hydrothermal synthesis and photocatalytic property of β-Ga2O3 nanorods. Nanoscale Res. Lett. 2015, 10, 364. [Google Scholar] [CrossRef]
- Chen, D.Z.; Xu, Y.; An, Z.Y.; Li, Z.; Zhang, C.F. Thin-film transistors based on wide bandgap Ga2O3 films grown by aqueous-solution spin-coating method. Micro Nano Lett. 2019, 14, 1052–1055. [Google Scholar] [CrossRef]
- Jamwal, N.S.; Kiani, A. Gallium Oxide Nanostructures: A Review of Synthesis, Properties and Applications. Nanomaterials 2022, 12, 2061. [Google Scholar] [CrossRef]
- Alhalaili, B.; Mao, H.; Islam, S. Ga2O3 Nanowire Synthesis and Device Applications. In Novel Nanomaterials-Synthesis and Applications; Mao, H., Ed.; InTech: Rijeka, Croatia, 2018; p. Ch. 2; ISBN 978-1-78923-089-5. [Google Scholar]
- Marezio, M.; Remeika, J.P. Bond Lengths in α-Ga2O3 Structure and the high-pressure phase of Ga2-XFexO3. J. Chem. Phys. 1967, 46, 1862–1865. [Google Scholar] [CrossRef]
- Kohn, J.A.; Katz, G.; Broder, J.D. Characterization of β-Ga2O3 and its alumina isomorph, θ-Al2O3. Am. Miner. 1957, 42, 398–407. [Google Scholar]
- He, H.Y.; Blanco, M.A.; Pandey, R. Electronic and thermodynamic properties of β-Ga2O3. Appl. Phys. Lett. 2006, 88, 3. [Google Scholar] [CrossRef] [Green Version]
- He, H.Y.; Orlando, R.; Blanco, M.A.; Pandey, R.; Amzallag, E.; Baraille, I.; Rerat, M. First-principles study of the structural, electronic, and optical properties of Ga2O3 in its monoclinic and hexagonal phases. Phys. Rev. B 2006, 74, 195123. [Google Scholar] [CrossRef] [Green Version]
- Cuong, N.D.; Park, Y.W.; Yoon, S.G. Microstructural and electrical properties of Ga2O3 nanowires grown at various temperatures by vapor-liquid-solid technique. Sens. Actuators B-Chem. 2009, 140, 240–244. [Google Scholar] [CrossRef]
- Saurat, M.; Revcolevschi, A. Preparation by floating zone method, of refractory oxide monocrystals, in particular of gallium oxide, and study of some of their properties. Rev. Int. Hautes Temp. Refract. 1971, 8, 291. [Google Scholar]
- Ahman, J.; Svensson, G.; Albertsson, J.A. Reinvestigation of β-Gallium oxide. Acta Crystallogr. Sect. C-Cryst. Struct. Commun. 1996, 52, 1336–1338. [Google Scholar] [CrossRef] [Green Version]
- Playford, H.Y.; Hannon, A.C.; Barney, E.R.; Walton, R.I. Structures of uncharacterised polymorphs of gallium oxide from total neutron diffraction. Chem.-A Eur. J. 2013, 19, 2803–2813. [Google Scholar] [CrossRef] [PubMed]
- Masataka, H.; Kohei, S.; Hisashi, M.; Yoshinao, K.; Akinori, K.; Akito, K.; Takekazu, M.; Shigenobu, Y. Recent progress in Ga2O3 power devices. Semicond. Sci. Technol. 2016, 31, 34001. [Google Scholar]
- Higashiwaki, M.; Kuramata, A.; Murakami, H.; Kumagai, Y. State-of-the-art technologies of gallium oxide power devices. J. Phys. D: Appl. Phys. 2017, 50, 333002. [Google Scholar] [CrossRef]
- Geller, S. Crystal structure of β-Ga2O3. J. Chem. Phys. 1960, 33, 676–684. [Google Scholar] [CrossRef]
- Playford, H.Y.; Hannon, A.C.; Tucker, M.G.; Dawson, D.M.; Ashbrook, S.E.; Kastiban, R.J.; Sloan, J.; Walton, R.I. Characterization of structural disorder in γ-Ga2O3. J. Phys. Chem. C 2014, 118, 16188–16198. [Google Scholar] [CrossRef] [Green Version]
- Yoshioka, S.; Hayashi, H.; Kuwabara, A.; Oba, F.; Matsunaga, K.; Tanaka, I. Structures and energetics of Ga2O3 polymorphs. J. Phys. Condens. Matter 2007, 19, 11. [Google Scholar] [CrossRef]
- Zhao, Y.; Frost, R.L.; Yang, J.; Martens, W.N. Size and Morphology Control of Gallium Oxide Hydroxide GaO(OH), Nano- to Micro-Sized Particles by Soft-Chemistry Route without Surfactant. J. Phys. Chem. C 2008, 112, 3568–3579. [Google Scholar] [CrossRef]
- Shi, L.; Zhang, J.; Wu, S.; Li, Y.; Jiang, L.; Cui, Q. Phase Evolution of Ga2O3 Produced from Morphology-Controllable α-GaOOH Nanocrystals. J. Am. Ceram. Soc. 2014, 97, 2607–2614. [Google Scholar] [CrossRef]
- Shiba, F.; Yuasa, M.; Okawa, Y. Controlling the shape of wedge-like α-GaOOH particles formed by a hydrolysis process usingsodium acetate as a growth modifier. CrystEngComm 2018, 20, 4910–4915. [Google Scholar] [CrossRef]
- Qian, H.S.; Gunawan, P.; Zhang, Y.X.; Lin, G.F.; Zheng, J.W.; Xu, R. Template-Free Synthesis of Highly Uniform α-GaOOH Spindles and Conversion to α- Ga2O3 and β-Ga2O3. Cryst. Growth Des. 2008, 8, 1282–1287. [Google Scholar] [CrossRef]
- Li, S.J.; Zheng, C.; Lobring, K.C. Refinement of the crystal structure of gallium oxide hydroxide, GaO(OH). Z. Fur Krist.-New Cryst. Struct. 2003, 218, 11–12. [Google Scholar]
- Krehula, S.; Ristić, M.; Kubuki, S.; Iida, Y.; Fabián, M.; Musić, S. The formation and microstructural properties of uniform α-GaOOH particles and their calcination products. J. Alloys Compd. 2015, 620, 217–227. [Google Scholar] [CrossRef]
- Markov, I.V. Crystal Growth for Beginners, 2nd ed.; World Scientific: Singapore, 2003; Volume 75, p. 564. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, Z.; Lin, C.; Lin, J.A. A simple method to synthesize β-Ga2O3 nanorods and their photoluminescence properties. J. Cryst. Growth 2005, 280, 99–106. [Google Scholar] [CrossRef]
- Chikoidze, E.; von Bardeleben, H.J.; Akaiwa, K.; Shigematsu, E.; Kaneko, K.; Fujita, S.; Dumont, Y. Electrical, optical, and magnetic properties of Sn doped α-Ga2O3 thin films. J. Appl. Phys. 2016, 120, 9. [Google Scholar] [CrossRef]
- Xue, H.; He, Q.; Jian, G.; Long, S.; Pang, T.; Liu, M. An Overview of the Ultrawide Bandgap Ga2O3 Semiconductor-Based Schottky Barrier Diode for Power Electronics Application. Nanoscale Res. Lett. 2018, 13, 290. [Google Scholar] [CrossRef] [Green Version]
- Guo, D.; Guo, Q.; Chen, Z.; Wu, Z.; Li, P.; Tang, W. Review of Ga2O3-based optoelectronic devices. Mater. Today Phys. 2019, 11, 100157. [Google Scholar] [CrossRef]
- Cocchi, C.; Zschiesche, H.; Nabok, D.; Mogilatenko, A.; Albrecht, M.; Galazka, Z.; Kirmse, H.; Draxl, C.; Koch, C.T. Atomic signatures of local environment from core-level spectroscopy in β−Ga2O3. Phys. Rev. B 2016, 94, 075147. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Wei, W.; Behrens, M. Synthesis and characterization of α-, β-, and γ-Ga2O3 prepared from aqueous solutions by controlled precipitation. Solid State Sci. 2012, 14, 971–981. [Google Scholar] [CrossRef] [Green Version]
- Zinkevich, M.; Morales, F.M.; Nitsche, H.; Ahrens, M.; Ruhle, M.; Aldinger, F. Microstructural and thermodynamic study of γ-Ga2O3. Z. Fur Met. 2004, 95, 756–762. [Google Scholar] [CrossRef]
- Otero Areán, C.; Bellan, A.L.; Mentruit, M.P.; Delgado, M.R.; Palomino, G.T. Preparation and characterization of mesoporous γ-Ga2O3. Microporous Mesoporous Mater. 2000, 40, 35–42. [Google Scholar] [CrossRef]
- Sharma, A.; Varshney, M.; Saraswat, H.; Chaudhary, S.; Parkash, J.; Shin, H.J.; Chae, K.H.; Won, S.O. Nano-structured phases of gallium oxide (GaOOH, α-Ga2O3, β-Ga2O3, γ-Ga2O3, δ-Ga2O3, and ε-Ga2O3): Fabrication, structural, and electronic structure investigations. Int. Nano Lett. 2020, 10, 71–79. [Google Scholar] [CrossRef]
- Cora, I.; Mezzadri, F.; Boschi, F.; Bosi, M.; Caplovicova, M.; Calestani, G.; Dodony, I.; Pecz, B.; Fornari, R. The real structure of ε-Ga2O3 and its relation to κ-Phase. Crystengcomm 2017, 19, 1509–1516. [Google Scholar] [CrossRef] [Green Version]
- Ollivier, B.; Retoux, R.; Lacorre, P.; Massiot, D.; Ferey, G. Crystal structure of κ-alumina: An X-ray powder diffraction, TEM and NMR study. J. Mater. Chem. 1997, 7, 1049–1056. [Google Scholar] [CrossRef]
- Matsuzaki, K.; Hiramatsu, H.; Nomura, K.; Yanagi, H.; Kamiya, T.; Hirano, M.; Hosono, H. Growth, structure and carrier transport properties of Ga2O3 epitaxial film examined for transparent field-effect transistor. Thin Solid Film. 2006, 496, 37–41. [Google Scholar] [CrossRef]
- Orita, M.; Hiramatsu, H.; Ohta, H.; Hirano, M.; Hosono, H. Preparation of highly conductive, deep ultraviolet transparent β-Ga2O3 thin film at low deposition temperatures. Thin Solid Film. 2002, 411, 134–139. [Google Scholar] [CrossRef]
- Mezzadri, F.; Calestani, G.; Boschi, F.; Delmonte, D.; Bosi, M.; Fornari, R. Crystal Structure and Ferroelectric Properties of ε-Ga2O3 Films Grown on (0001)-Sapphire. Inorg. Chem. 2016, 55, 12079–12084. [Google Scholar] [CrossRef]
- Xia, X.; Chen, Y.; Feng, Q.; Liang, H.; Tao, P.; Xu, M.; Du, G. Hexagonal phase-pure wide band gap ε-Ga2O3 films grown on 6H-SiC substrates by metal organic chemical vapor deposition. Appl. Phys. Lett. 2016, 108, 202103. [Google Scholar] [CrossRef]
- Maccioni, M.B.; Fiorentini, V. Phase diagram and polarization of stable phases of (Ga1-XInx)2O3. Appl. Phys. Express 2016, 9, 4. [Google Scholar] [CrossRef] [Green Version]
- Tadjer, M.J.; Mastro, M.A.; Mahadik, N.A.; Currie, M.; Wheeler, V.D.; Freitas, J.A.; Greenlee, J.D.; Hite, J.K.; Hobart, K.D.; Eddy, C.R.; et al. Structural, Optical, and Electrical Characterization of Monoclinic β-Ga2O3 Grown by MOVPE on Sapphire Substrates. J. Electron. Mater. 2016, 45, 2031–2037. [Google Scholar] [CrossRef]
- Kracht, M.; Karg, A.; Schörmann, J.; Weinhold, M.; Zink, D.; Michel, F.; Rohnke, M.; Schowalter, M.; Gerken, B.; Rosenauer, A.; et al. Tin-Assisted Synthesis of ε-Ga2O3 by Molecular Beam Epitaxy. Phys. Rev. Appl. 2017, 8, 54002. [Google Scholar] [CrossRef]
- Cho, S.B.; Mishra, R. Epitaxial engineering of polar ε-Ga2O3 for tunable two-dimensional electron gas at the heterointerface. Appl. Phys. Lett. 2018, 112, 5. [Google Scholar] [CrossRef] [Green Version]
- Yamaguchi, K. First principles study on electronic structure of β-Ga2O3. Solid State Commun. 2004, 131, 739–744. [Google Scholar] [CrossRef]
- Binet, L.; Gourier, D.; Minot, C. Relation between Electron Band Structure and Magnetic Bistability of Conduction Electrons in β-Ga2O3. J. Solid State Chem. 1994, 113, 420–433. [Google Scholar] [CrossRef]
- Hajnal, Z.; Miro, J.; Kiss, G.; Reti, F.; Deak, P.; Herndon, R.C.; Kuperberg, J.M. Role of oxygen vacancy defect states in the n-type conduction of β-Ga2O3. J. Appl. Phys. 1999, 86, 3792–3796. [Google Scholar] [CrossRef]
- Li, C.; Yan, J.L.; Zhang, L.Y.; Zhao, G. Electronic structures and optical properties of Zn-doped β-Ga2O3 with different doping sites. Chin. Phys. B 2012, 21, 6. [Google Scholar] [CrossRef]
- Ping, L.K.; Mohamed, M.A.; Mondal, A.K.; Taib, M.F.M.; Samat, M.H.; Berhanuddin, D.D.; Menon, P.S.; Bahru, R. First-Principles Studies for Electronic Structure and Optical Properties of Strontium Doped β-Ga2O3. Micromachines 2021, 12, 16. [Google Scholar] [CrossRef]
- Zhao, Y.; Yan, J. First-principles study of n-type tin/fluorine codoped beta-gallium oxides. J. Semicond. 2015, 36, 82004. [Google Scholar] [CrossRef]
- Zhang, L.Y.; Yan, J.L.; Zhang, Y.J.; Li, T.; Ding, X.W. First-principles study on electronic structure and optical properties of N-doped P-type β-Ga2O3. Sci. China-Phys. Mech. Astron. 2012, 55, 19–24. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Yan, J.L.; Zhao, G.; Xie, W.F. First-principles study on electronic structure and optical properties of Sn-doped β-Ga2O3. Phys. B-Condens. Matter 2010, 405, 3899–3903. [Google Scholar] [CrossRef]
- Peelaers, H.; Van deWalle, C.G. Brillouin zone and band structure of β-Ga2O3. Phys. Status Solidi B-Basic Solid State Phys. 2015, 252, 828–832. [Google Scholar] [CrossRef]
- Navarro-Quezada, A.; Alamé, S.; Esser, N.; Furthmüller, J.; Bechstedt, F.; Galazka, Z.; Skuridina, D.; Vogt, P. Near valence-band electronic properties of semiconducting β-Ga2O3 (100) single crystals. Phys. Rev. B 2015, 92, 195306. [Google Scholar] [CrossRef]
- Varley, J.B.; Weber, J.R.; Janotti, A.; Van de Walle, C.G. Oxygen vacancies and donor impurities in β-Ga2O3. Appl. Phys. Lett. 2010, 97, 142106. [Google Scholar] [CrossRef]
- Furthmuller, J.; Bechstedt, F. Quasiparticle bands and spectra of Ga2O3 polymorphs. Phys. Rev. B 2016, 93, 16. [Google Scholar] [CrossRef]
- Dong, L.P.; Jia, R.X.; Xin, B.; Peng, B.; Zhang, Y.M. Effects of oxygen vacancies on the structural and optical properties of β-Ga2O3. Sci. Rep. 2017, 7, 12. [Google Scholar] [CrossRef]
- Ma, X.F.; Zhang, Y.M.; Dong, L.P.; Jia, R.X. First-principles calculations of electronic and optical properties of aluminum-doped β-Ga2O3 with intrinsic defects. Results Phys. 2017, 7, 1582–1589. [Google Scholar] [CrossRef]
- Yan, H.Y.; Guo, Y.R.; Song, Q.G.; Chen, Y.F. First-principles study on electronic structure and optical properties of Cu-doped β-Ga2O3. Phys. B-Condens. Matter 2014, 434, 181–184. [Google Scholar] [CrossRef]
- Orita, M.; Ohta, H.; Hirano, M.; Hosono, H. Deep-ultraviolet transparent conductive β-Ga2O3 thin films. Appl. Phys. Lett. 2000, 77, 4166–4168. [Google Scholar] [CrossRef]
- Litimein, F.; Rached, D.; Khenata, R.; Baltache, H. FPLAPW study of the structural, electronic, and optical properties of Ga2O3: Monoclinic and hexagonal phases. J. Alloys Compd. 2009, 488, 148–156. [Google Scholar] [CrossRef]
- Von Wenckstern, H. Group-III Sesquioxides: Growth, Physical Properties and Devices. Adv. Electron. Mater. 2017, 3, 1600350. [Google Scholar] [CrossRef]
- Higashiwaki, M.; Sasaki, K.; Kuramata, A.; Masui, T.; Yamakoshi, S. Gallium oxide (Ga2O3) metal-semiconductor field-effect transistors on single-crystal β-Ga2O3 (010) substrates. Appl. Phys. Lett. 2012, 100, 13504. [Google Scholar] [CrossRef]
- Sang, L.W.; Liao, M.Y.; Sumiya, M. A Comprehensive Review of Semiconductor Ultraviolet Photodetectors: From Thin Film to One-Dimensional Nanostructures. Sensors 2013, 13, 10482–10518. [Google Scholar] [CrossRef]
- Chen, H.Y.; Liu, K.W.; Hu, L.F.; Al-Ghamdi, A.A.; Fang, X.S. New concept ultraviolet photodetectors. Mater. Today 2015, 18, 493–502. [Google Scholar] [CrossRef]
- Zhao, B.; Wang, F.; Chen, H.Y.; Wang, Y.P.; Jiang, M.M.; Fang, X.S.; Zhao, D.X. Solar-Blind Avalanche Photodetector Based On Single ZnO-Ga2O3 Core-Shell Microwire. Nano Lett. 2015, 15, 3988–3993. [Google Scholar] [CrossRef]
- Suzuki, N.; Ohira, S.; Tanaka, M.; Sugawara, T.; Nakajima, K.; Shishido, T. Fabrication and characterization of transparent conductive Sn-doped β-Ga2O3 single crystal. Phys. Status Solidi C 2007, 4, 2310–2313. [Google Scholar] [CrossRef]
- Zhang, J.G.; Li, B.; Xia, C.T.; Pei, G.Q.; Deng, Q.; Yang, Z.H.; Xu, W.S.; Shi, H.S.; Wu, F.; Wu, Y.Q.; et al. Growth and spectral characterization of β-Ga2O3 single crystals. J. Phys. Chem. Solids 2006, 67, 2448–2451. [Google Scholar] [CrossRef]
- Villora, E.G.; Yamaga, M.; Inoue, T.; Yabasi, S.; Masui, Y.; Sugawara, T.; Fukuda, T. Optical Spectroscopy Study on β-Ga2O3. Jpn. J. Appl. Phys. Part 2-Lett. Express Lett. 2002, 41, L622–L625. [Google Scholar] [CrossRef]
- Ueda, N.; Hosono, H.; Waseda, R.; Kawazoe, H. Synthesis and control of conductivity of ultraviolet transmitting β-Ga2O3 single crystals. Appl. Phys. Lett. 1997, 70, 3561–3563. [Google Scholar] [CrossRef]
- Korhonen, E.; Tuomisto, F.; Gogova, D.; Wagner, G.; Baldini, M.; Galazka, Z.; Schewski, R.; Albrecht, M. Electrical compensation by Ga vacancies in Ga2O3 thin films. Appl. Phys. Lett. 2015, 106, 3. [Google Scholar] [CrossRef] [Green Version]
- Dong, L.P.; Jia, R.X.; Li, C.; Xin, B.; Zhang, Y.M. Ab initio study of N-doped β-Ga2O3 with intrinsic defects: The structural, electronic and optical properties Linpeng The Structural, Electronic and Optical Properties. J. Alloys Compd. 2017, 712, 379–385. [Google Scholar] [CrossRef]
- Galazka, Z.; Irmscher, K.; Uecker, R.; Bertram, R.; Pietsch, M.; Kwasniewski, A.; Naumann, M.; Schulz, T.; Schewski, R.; Klimm, D.; et al. On the bulk β-Ga2O3 single crystals grown by the Czochralski method. J. Cryst. Growth 2014, 404, 184–191. [Google Scholar] [CrossRef]
- Qin, Y.; Long, S.B.; Dong, H.; He, Q.M.; Jian, G.Z.; Zhang, Y.; Hou, X.H.; Tan, P.J.; Zhang, Z.F.; Lv, H.B.; et al. Review of deep ultraviolet photodetector based on gallium oxide. Chin. Phys. B 2019, 28, 17. [Google Scholar] [CrossRef]
- Higashiwaki, M.; Murakami, H.; Kumagai, Y.; Kuramata, A. Current status of Ga2O3 power devices To. Jpn. J. Appl. Phys. 2016, 55, 7. [Google Scholar] [CrossRef]
- Sasaki, K.; Kuramata, A.; Masui, T.; Villora, E.G.; Shimamura, K.; Yamakoshi, S. Device-Quality β-Ga2O3 Epitaxial Films Fabricated by Ozone Molecular Beam Epitaxy. Appl. Phys. Express 2012, 5, 35502. [Google Scholar] [CrossRef]
- Villora, E.G.; Shimamura, K.; Yoshikawa, Y.; Ujiie, T.; Aoki, K. Electrical conductivity and carrier concentration control in β-Ga2O3 by Si doping. Appl. Phys. Lett. 2008, 92, 202120. [Google Scholar] [CrossRef]
- Ma, N.; Tanen, N.; Verma, A.; Guo, Z.; Luo, T.F.; Xing, H.; Jena, D. Intrinsic electron mobility limits in β-Ga2O3. Appl. Phys. Lett. 2016, 109, 212101. [Google Scholar] [CrossRef] [Green Version]
- Baliga, B.J. Power Semiconductor Device Figure of Merit for High-Frequency Applications. IEEE Electron Device Lett. 1989, 10, 455–457. [Google Scholar] [CrossRef]
- Baliga, B.J. Semiconductors for high-voltage, vertical channel field-effect transistors. J. Appl. Phys. 1982, 53, 1759–1764. [Google Scholar] [CrossRef]
- Ueda, N.; Hosono, H.; Waseda, R.; Kawazoe, H. Anisotropy of electrical and optical properties in β-Ga2O3 single crystals. Appl. Phys. Lett. 1997, 71, 933–935. [Google Scholar] [CrossRef]
- Schubert, M.; Korlacki, R.; Knight, S.; Hofmann, T.; Schoche, S.; Darakchieva, V.; Janzen, E.; Monemar, B.; Gogova, D.; Thieu, Q.T.; et al. Anisotropy, phonon modes, and free charge carrier parameters in monoclinic β-gallium oxide single crystals. Phys. Rev. B 2016, 93, 18. [Google Scholar] [CrossRef] [Green Version]
- Ueda, O.; Ikenaga, N.; Koshi, K.; Iizuka, K.; Kuramata, A.; Hanada, K.; Moribayashi, T.; Yamakoshi, S.; Kasu, M. Structural evaluation of defects in β-Ga2O3 single crystals grown by edge-defined film-fed growth process. Jpn. J. Appl. Phys. 2016, 55, 8. [Google Scholar] [CrossRef]
- Guo, Z.; Verma, A.; Wu, X.F.; Sun, F.Y.; Hickman, A.; Masui, T.; Kuramata, A.; Higashiwaki, M.; Jena, D.; Luo, T.F. Anisotropic thermal conductivity in single crystal β-gallium oxide. Appl. Phys. Lett. 2015, 106, 5. [Google Scholar] [CrossRef] [Green Version]
- Mu, W.X.; Jia, Z.T.; Yin, Y.R.; Hu, Q.Q.; Li, Y.; Wu, B.Y.; Zhang, J.; Tao, X.T. High quality crystal growth and anisotropic physical characterization of β-Ga2O3 single crystals grown by EFG method. J. Alloys Compd. 2017, 714, 453–458. [Google Scholar] [CrossRef]
- Hebb, J.P.; Jensen, K.F. The Effect of Patterns on Thermal Stress during Rapid Thermal Processing of Silicon Wafers. IEEE Trans. Semicond. Manuf. 1998, 11, 99–107. [Google Scholar] [CrossRef]
- Heuer, A.H.; Reddy, A.; Hovis, D.B.; Veal, B.; Paulikas, A.; Vlad, A.; Ruhle, M. the effect of surface orientation on oxidation-induced growth strains in single crystal NiAl: An in situ synchrotron study. Scr. Mater. 2006, 54, 1907–1912. [Google Scholar] [CrossRef]
- Ang, K.W.; Chui, K.J.; Bliznetsov, V.; Tung, C.H.; Du, A.; Balasubramanian, N.; Samudra, G.; Li, M.F.; Yeo, Y.C. Lattice strain analysis of transistor structures with silicon-germanium and silicon-carbon source/drain stressors. Appl. Phys. Lett. 2005, 86, 3. [Google Scholar] [CrossRef]
- Toda, A.; Ikarashi, N.; Ono, H.; Ito, S.; Toda, T.; Imai, K. Local lattice strain distribution around a transistor channel in metal-oxide-semiconductor devices. Appl. Phys. Lett. 2001, 79, 4243–4245. [Google Scholar] [CrossRef]
- Villora, E.G.; Shimamura, K.; Ujiie, T.; Aoki, K. Electrical conductivity and lattice expansion of β-Ga2O3 below room temperature. Appl. Phys. Lett. 2008, 92, 3. [Google Scholar] [CrossRef]
- Orlandi, F.; Mezzadri, F.; Calestani, G.; Boschi, F.; Fornari, R. Thermal expansion coefficients of β-Ga2O3 single crystals. Appl. Phys. Express 2015, 8, 3. [Google Scholar] [CrossRef]
- Cheng, Z.Z.; Hanke, M.; Galazka, Z.; Trampert, A. Thermal expansion of single-crystalline β-Ga2O3 from RT to 1200 K studied by synchrotron-based high-resolution x-ray diffraction. Appl. Phys. Lett. 2018, 113, 4. [Google Scholar] [CrossRef] [Green Version]
- Adachi, K.; Ogi, H.; Takeuchi, N.; Nakamura, N.; Watanabe, H.; Ito, T.; Ozaki, Y. Unusual elasticity of monoclinic β-Ga2O3. J. Appl. Phys. 2018, 124, 7. [Google Scholar] [CrossRef]
- Luan, S.Z.; Dong, L.P.; Jia, R.X. Analysis of the structural, anisotropic elastic and electronic properties of β-Ga2O3 with various pressures. J. Cryst. Growth 2019, 505, 74–81. [Google Scholar] [CrossRef]
- Handwerg, M.; Mitdank, R.; Galazka, Z.; Fischer, S.F. Temperature-dependent thermal conductivity and diffusivity of a mg-doped insulating β-Ga2O3 single crystal along [100], [010] and [001]. Semicond. Sci. Technol. 2016, 31, 6. [Google Scholar] [CrossRef] [Green Version]
- Galazka, Z.; Uecker, R.; Irmscher, K.; Albrecht, M.; Klimm, D.; Pietsch, M.; Brutzam, M.; Bertram, R.; Ganschow, S.; Fornari, R. czochralski growth and characterization of β-Ga2O3 single crystals. Cryst. Res. Technol. 2010, 45, 1229–1236. [Google Scholar] [CrossRef]
- Rebien, M.; Henrion, W.; Hong, M.; Mannaerts, J.P.; Fleischer, M. Optical properties of gallium oxide thin films. Appl. Phys. Lett. 2002, 81, 250–252. [Google Scholar] [CrossRef]
- Hoeneisen, B.; Mead, C.A.; Nicolet, M.A. Permittivity of β-Ga2O3 at low frequencies. Solid-State Electron. 1971, 14, 1057–1059. [Google Scholar] [CrossRef]
- Pritula, I.; Sangwal, K. 29-Fundamentals of Crystal Growth from Solutions. In Handbook of Crystal Growth, 2nd ed.; Rudolph, P., Ed.; Elsevier: Boston, MA, USA, 2015; Volume 2, pp. 1185–1227. ISBN 978-0-444-63303-3. [Google Scholar]
- Kashchiev, D.; van Rosmalen, G.M. Review: Nucleation in solutions revisited. Cryst. Res. Technol. 2003, 38, 555–574. [Google Scholar] [CrossRef]
- Charitidis, C.A.; Georgiou, P.; Koklioti, M.A.; Trompeta, A.F.; Markakis, V. Manufacturing nanomaterials: From research to industry. Manuf. Rev. 2014, 1, 11. [Google Scholar] [CrossRef] [Green Version]
- Karatutlu, A.; Barhoum, A.; Sapelkin, A. Chapter 1-Liquid-phase synthesis of nanoparticles and nanostructured materials. In Emerging Applications of Nanoparticles and Architecture Nanostructures: Current Prospects and Future Trends; Barhoum, A., Makhlouf, A.S.H., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1–28. ISBN 978-0-323-51254-1. [Google Scholar]
- Hench, L.L.; West, J.K. The Sol-Gel Process. Chem. Rev. 1990, 90, 33–72. [Google Scholar] [CrossRef]
- Bokov, D.; Jalil, A.T.; Chupradit, S.; Suksatan, W.; Ansari, M.J.; Shewael, I.H.; Valiev, G.H.; Kianfar, E. Nanomaterial by Sol-Gel Method: Synthesis and Application. Adv. Mater. Sci. Eng. 2021, 2021, 21. [Google Scholar] [CrossRef]
- Wang, J.; Li, C.; Xu, B. Basic principle advance and current application situation of Sol-Gel method. Chem. Ind. Eng. 2009, 26, 273–277. [Google Scholar]
- Aggarwal, G.; Nagpal, M. Pharmaceutical Polymer Gels in Drug Delivery. In Polymer Gels; Gels Horizons: From Science to Smart Materials; Thakur, V.K., Thakur, M.K., Voicu, S.I., Eds.; Springer: Singapore, 2018; pp. 249–284. ISBN 978-981-10-6079-3. [Google Scholar]
- Shinde, S.S.; Bhosale, C.H.; Rajpure, K.Y. Photocatalytic degradation of toluene using sprayed N-doped ZnO thin films in aqueous suspension. J. Photochem. Photobiol. B-Biol. 2012, 113, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Shinde, S.S.; Bhosale, C.H.; Rajpure, K.Y. Photoelectrochemical properties of highly mobilized Li-doped ZnO thin film. J. Photochem. Photobiol. B-Biol. 2013, 120, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Shinde, S.S.; Shinde, P.S.; Bhosale, C.H.; Rajpure, K.Y. Zinc oxide mediated heterogeneous photocatalytic degradation of organic species under solar radiation. J. Photochem. Photobiol. B-Biol. 2011, 104, 425–433. [Google Scholar] [CrossRef]
- Jaroenworaluck, A.; Sunsaneeyametha, W.; Kosachan, N.; Stevens, R. Characteristics of silica-coated TiO2 and its UV absorption for sunscreen cosmetic applications. Surf. Interface Anal. 2006, 38, 473–477. [Google Scholar] [CrossRef]
- Vijayalakshmi, R.; Rajendran, V. Synthesis and characterization of nano-TiO2 via different methods. Arch. Appl. Sci. Res. 2012, 4, 1183–1190. [Google Scholar]
- Li, B.R.; Wang, X.H.; Yan, M.Y.; Li, L.T. Preparation and characterization of nano-TiO2 powder. Mater. Chem. Phys. 2003, 78, 184–188. [Google Scholar] [CrossRef]
- Verma, R.; Mantri, B.; Kumar Srivastava, A. Shape control synthesis, characterizations, mechanisms and optical properties of large scaled metal oxide nanostructures of ZnO and TiO2. Adv. Mater. Lett. 2015, 6, 324–333. [Google Scholar] [CrossRef]
- Kandiel, T.A.; Robben, L.; Alkaim, A.; Bahnemann, D. Brookite versus anatase TiO2 photocatalysts: Phase transformations and photocatalytic activities. Photochem. Photobiol. Sci. 2013, 12, 602–609. [Google Scholar] [CrossRef] [Green Version]
- Li, H.X.; Bian, Z.F.; Zhu, J.; Zhang, D.Q.; Li, G.S.; Huo, Y.N.; Li, H.; Lu, Y.F. Mesoporous Titania Spheres with Tunable Chamber Stucture and Enhanced Photocatalytic Activity. J. Am. Chem. Soc. 2007, 129, 8406–8407. [Google Scholar] [CrossRef]
- Verma, R.; Awasthi, A.; Singh, P.; Srivastava, R.; Sheng, H.P.; Wen, J.G.; Miller, D.J.; Srivastava, A.K. Interactions of titania based nanoparticles with silica and green-tea: Photo-degradation and -luminescence. J. Colloid Interface Sci. 2016, 475, 82–95. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.J.; Menendez-Flores, V.M.; Murakami, N.; Ohno, T. Improvement of photocatalytic activity of brookite titanium dioxide nanorods by surface modification using chemical etching. Appl. Surf. Sci. 2012, 258, 5803–5809. [Google Scholar] [CrossRef]
- Collinson, M.M.; Wang, H.M.; Makote, R.; Khramov, A. The effects of drying time and relative humidity on the stability of sol-gel derived silicate films in solution. J. Electroanal. Chem. 2002, 519, 65–71. [Google Scholar] [CrossRef]
- Kajihara, K. Recent advances in sol–gel synthesis of monolithic silica and silica-based glasses. J. Asian Ceram. Soc. 2013, 1, 121–133. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; White, T.; Lim, S.H. Structure Control and Its Influence on Photoactivity and Phase Transformation of TiO2 Nano-Particles. Rev. Adv. Mater. Sci. 2003, 5, 211–215. [Google Scholar]
- Niederberger, M.; Pinna, N. Metal Oxide Nanoparticles in Organic Solvents: Synthesis, Formation, Assembly and Application; Springer Science & Business Media: New York, NY, USA, 2009; ISBN 1848826710. [Google Scholar]
- Modan, E.M.; PlĂIaȘU, A.G. Advantages and Disadvantages of Chemical Methods in the Elaboration of Nanomaterials. Ann. “Dunarea De Jos” Univ. Galati. Fascicle IX Metall. Mater. Sci. 2020, 43, 53–60. [Google Scholar] [CrossRef]
- Byrappa, K.; Yoshimura, M.; McGuire, G.E.; Rossnagel, S.M.; Bunshah, R.F. Hydrothermal Technology—Principles and Applications. In Handbook of hydrothermal technology: A Technology for Crystal Growth and Materials Processing; William Andrew: New York, NY, USA, 2012; pp. 1–52. ISBN 1437778364. [Google Scholar]
- Byrappa, K.; Adschiri, T. Hydrothermal technology for nanotechnology. Prog. Cryst. Growth Charact. Mater. 2007, 53, 117–166. [Google Scholar] [CrossRef] [Green Version]
- Yoshimura, M.; Byrappa, K. Hydrothermal processing of materials: Past, present and future. J. Mater. Sci. 2008, 43, 2085–2103. [Google Scholar] [CrossRef]
- Zheng, H.; Yu, T.; Qu, C.; Li, W.; Wang, Y. Basic Characteristics and Application Progress of Supercritical Water. IOP Conf. Ser. Earth Environ. Sci. 2020, 555, 12036. [Google Scholar] [CrossRef]
- Lester, E.; Blood, P.; Denyer, J.; Giddings, D.; Azzopardi, B.; Poliakoff, M. Reaction engineering: The supercritical water hydrothermal synthesis of nano-particles. J. Supercrit. Fluids 2006, 37, 209–214. [Google Scholar] [CrossRef]
- Hayashi, H.; Hakuta, Y. Hydrothermal Synthesis of Metal Oxide Nanoparticles in Supercritical Water. Materials 2010, 3, 3794–3817. [Google Scholar] [CrossRef] [Green Version]
- Brunner, G.; Kiran, E. Properties of Pure Water In Hydrothermal and Supercritical Water Processes; Elsevier: Amsterdam, The Netherlands, 2014; Volume 5, p. 10. ISBN 0444594183. [Google Scholar]
- Prakasam, M.; Viraphong, O.; Cambon, O.; Largeteau, A. Hydrothermal Crystal Growth and Applications. In Advances in Solid Oxide Fuel Cells and Electronic Ceramics; Bansal, N.P., Kusnezoff, M., Shimamura, K., Eds.; Wiley: Hoboken, NJ, USA, 2015; pp. 151–156. [Google Scholar]
- Demazeau, G.; Largeteau, A. Hydrothermal/Solvothermal Crystal Growth: An Old but Adaptable Process. J. Inorg. Gen. Chem. 2015, 641, 159–163. [Google Scholar] [CrossRef]
- McMillen, C.D.; Kolis, J.W. Bulk single crystal growth from hydrothermal solutions. Philos. Mag. 2012, 92, 2686–2711. [Google Scholar] [CrossRef]
- Xu, H.Y.; Wang, H.; Jin, T.N.; Yan, H. Rapid fabrication of luminescent Eu: YVO4 films by microwave-assisted chemical solution deposition. Nanotechnology 2005, 16, 65–69. [Google Scholar] [CrossRef]
- Xu, H.Y.; Le Xu, S.; Li, X.D.; Wang, H.; Yan, H. chemical bath deposition of hausmannite Mn3O4 thin films. Appl. Surf. Sci. 2006, 252, 4091–4096. [Google Scholar] [CrossRef]
- Niesen, T.P.; De Guire, M.R. Review: Deposition of ceramic thin films at low temperatures from aqueous solutions. J. Electroceramics 2001, 6, 169–207. [Google Scholar] [CrossRef]
- Mane, R.S.; Lokhande, C.D. Chemical deposition method for metal chalcogenide thin films. Mater. Chem. Phys. 2000, 65, 1–31. [Google Scholar] [CrossRef]
- Cheng, A.; Fan, D.B.; Wang, H.; Liu, B.W.; Zhang, Y.C.; Yan, H. chemical bath deposition of crystalline ZnS thin films. Semicond. Sci. Technol. 2003, 18, 676–679. [Google Scholar] [CrossRef]
- Varkey, A.J.; Fort, A.F. Some optical properties of silver peroxide (AgO) and silver oxide (Ag2O) films produced by chemical-bath deposition. Sol. Energy Mater. Sol. Cells 1993, 29, 253–259. [Google Scholar] [CrossRef]
- Varkey, A.J.; Fort, A.F. Solution growth technique for deposition of nickel oxide thin films. Thin Solid Film. 1993, 235, 47–50. [Google Scholar] [CrossRef]
- Saeed, T.; Obrien, P. Deposition and characterisation of ZnO thin films grown by chemical bath deposition. Thin Solid Film. 1995, 271, 35–38. [Google Scholar] [CrossRef]
- Zang, L. Lecture 10: Homogeneous Nucleation. University of Utah. Available online: https://my.eng.utah.edu/~lzang/teaching/index.html (accessed on 1 May 2022).
- Zang, L. Lecture 12: Heterogeneous Nucleation–A Surface Catalyzed Process. University of Utah. Available online: https://my.eng.utah.edu/~lzang/teaching/index.html (accessed on 6 May 2022).
- Nucleation. Encyclopaedia Britannica. Available online: https://www.britannica.com/science/nucleation (accessed on 11 May 2022).
- Nair, P.K.; Nair, M.T.S.; Garcia, V.M.; Arenas, O.L.; Pena, Y.; Castillo, A.; Ayala, I.T.; Gomezdaza, O.; Sanchez, A.; Campos, J.; et al. Semiconductor thin films by chemical bath deposition for solar energy related applications. Sol. Energy Mater. Sol. Cells 1998, 52, 313–344. [Google Scholar] [CrossRef]
- Choi, J.Y.; Kim, K.J.; Yoo, J.B.; Kim, D.H. Properties of Cadmium Sulfide Thin Films Deposited by Chemical Bath Deposition with Ultrasonication. Sol. Energy 1998, 64, 41–47. [Google Scholar] [CrossRef]
- Nichols, L. 1.4K: Reflux. In Organic Chemistry Lab Techniques; Nichols, L., Ed.; LibreTexts: Oroville, CA, USA, 2017; p. 6. [Google Scholar]
- Matijevic, E. Monodispersed Metal (Hydrous) Oxides-A Fascinating Field of Colloid Science. Acc. Chem. Res. 1981, 14, 22–29. [Google Scholar] [CrossRef]
- Cao, G.; Wang, Y. Zero-dimensional nanostructures: Nanoparticles. In Nanostructures and Nanomaterials: Synthesis, Properties, and Applications; Brock, S.L., Ed.; Imperial College Press: London, UK, 2004; pp. 51–109. [Google Scholar]
- Jiang, Y. Forced hydrolysis and chemical co-precipitation. In Handbook of Nanophase and Nanostructured Materials Vol. 1 Synthesis; Wang, Z.L., Liu, Y., Zhang, Z., Eds.; Springer: Boston, MA, USA, 2003; Volume 1, pp. 55–71. ISBN 0306467372. [Google Scholar]
- Ring, T.A.; Kudo, Y. Fundamentals of Forced Hydrolysis of Indium Hydroxide. In Fine Particles Science and Technology: From Micro to Nanoparticles; Pelizzetti, E., Ed.; Springer: Dordrecht, The Netherlands, 1996; Volume 12, pp. 141–159. ISBN 978-94-009-0259-6. [Google Scholar]
- Kandori, K.; Shigetomi, T.; Ishikawa, T. study on forced hydrolysis reaction of acidic Fe2(SO4)3 solution-structure and properties of precipitates. Colloids Surf. a-Physicochem. Eng. Asp. 2004, 232, 19–28. [Google Scholar] [CrossRef]
- Gattorno, G.R.; Oskam, G. Forced Hydrolysis vs Self-Hydrolysis of Zinc Acetate in Ethanol and Iso-butanol. ECS Trans. 2006, 3, 23–28. [Google Scholar] [CrossRef]
- Pathan, H.M.; Lokhande, C.D. Deposition of metal chalcogenide thin films by successive ionic layer adsorption and reaction (SILAR) method. Bull. Mater. Sci. 2004, 27, 85–111. [Google Scholar] [CrossRef] [Green Version]
- Sartale, S.D.; Lokhande, C.D. Growth of copper sulphide thin films by successive ionic layer adsorption and reaction (SILAR) method. Mater. Chem. Phys. 2000, 65, 63–67. [Google Scholar] [CrossRef]
- Ghosh, B.; Das, M.; Banerjee, P.; Das, S. Fabrication and optical properties of SnS thin films by SILAR method. Appl. Surf. Sci. 2008, 254, 6436–6440. [Google Scholar] [CrossRef]
- Ratnayake, S.P.; Ren, J.W.; Colusso, E.; Guglielmi, M.; Martucci, A.; DellaGaspera, E. SILAR Deposition of Metal Oxide Nanostructured Films. Small 2021, 17, 32. [Google Scholar] [CrossRef]
- Mageshwari, K.; Sathyamoorthy, R. Physical properties of nanocrystalline CuO thin films prepared by the SILAR method. Mater. Sci. Semicond. Process. 2013, 16, 337–343. [Google Scholar] [CrossRef]
- Rodriguez, C.A.D.; Tremiliosi-Filho, G. Electrochemical Deposition. In Encyclopedia of Tribology; Wang, Q.J., Chung, Y.W., Eds.; Springer: Boston, MA, USA, 2013; pp. 918–922. ISBN 978-0-387-92897-5. [Google Scholar]
- Therese, G.H.A.; Kamath, P.V. Electrochemical Synthesis of Metal Oxides and Hydroxides. Chem. Mater. 2000, 12, 1195–1204. [Google Scholar] [CrossRef]
- Li, Y.; Trinchi, A.; Wlodarski, W.; Galatsis, K.; Kalantar-Zadeh, K. investigation of the oxygen gas sensing performance of Ga2O3 thin films with different dopants. Sens. Actuators B Chem. 2003, 93, 431–434. [Google Scholar] [CrossRef]
- Ristić, M.; Popović, S.; Musić, S. Application of Sol–Gel Method in the Synthesis of Gallium(III)-Oxide. Mater. Lett. 2005, 59, 1227–1233. [Google Scholar] [CrossRef]
- Kokubun, Y.; Miura, K.; Endo, F.; Nakagomi, S. Sol-gel prepared β-Ga2O3 thin films for ultraviolet photodetectors. Appl. Phys. Lett. 2007, 90, 2–4. [Google Scholar] [CrossRef]
- Suzuki, R.; Nakagomi, S.; Kokubun, Y. Solar-blind photodiodes composed of a Au Schottky contact and a β-Ga2O3 single crystal with a high resistivity cap layer. Appl. Phys. Lett. 2011, 98, 13–15. [Google Scholar] [CrossRef]
- Gopal, R.; Goyal, A.; Saini, A.; Nagar, M.; Sharma, N.; Gupta, D.K.; Dhayal, V. Sol- gel synthesis of Ga2O3 nanorods and effect of precursor chemistry on their structural and morphological properties. Ceram. Int. 2018, 44, 19099–19105. [Google Scholar] [CrossRef]
- Sinha, G.; Ganguli, D.; Chaudhuri, S. Crystallization and optical properties of finite sized β-Ga2O3 in sol-gel derived Ga2O3: SiO2 nanocomposites. J. Phys. Condens. Matter 2006, 18, 11167–11176. [Google Scholar] [CrossRef]
- Sinha, G.; Datta, A.; Panda, S.K.; Chavan, P.G.; More, M.A.; Joag, D.S.; Patra, A. Self-catalytic growth and field-emission properties of Ga2O3 nanowires. J. Phys. D Appl. Phys. 2009, 42, 185409. [Google Scholar] [CrossRef]
- Cheng, B.; Samulski, E.T. Fabrication and characterization of nanotubular semiconductor oxides In2O3 and Ga2O3. J. Mater. Chem. 2001, 11, 2901–2902. [Google Scholar] [CrossRef]
- Mohammadi, M.R.; Fray, D.J. Semiconductor TiO2-Ga2O3 thin film gas sensors derived from particulate sol-gel route. Acta Mater. 2007, 55, 4455–4466. [Google Scholar] [CrossRef]
- Shen, H.; Yin, Y.; Tian, K.; Baskaran, K.; Duan, L.; Zhao, X.; Tiwari, A. Growth and characterization of β-Ga2O3 thin films by sol-gel method for fast-response solar-blind ultraviolet photodetectors. J. Alloys Compd. 2018, 766, 601–608. [Google Scholar] [CrossRef]
- Yu, M.; Lv, C.; Yu, J.; Shen, Y.; Yuan, L.; Hu, J.; Zhang, S.; Cheng, H.; Zhang, Y.; Jia, R. High-performance photodetector based on sol–gel epitaxially grown α/β Ga2O3 thin films. Mater. Today Commun. 2020, 25, 101532. [Google Scholar] [CrossRef]
- Yu, S.; Zhang, G.; Carloni, D.; Wu, Y. fabrication, microstructure and optical properties of Ga2O3 transparent ceramics. Ceram. Int. 2020, 46, 21757–21761. [Google Scholar] [CrossRef]
- Zhu, Y.; Xiu, X.; Cheng, F.; Li, Y.; Xie, Z.; Tao, T.; Chen, P.; Liu, B.; Zhang, R.; Zheng, Y.D. Growth and nitridation of β-Ga2O3 thin films by sol-gel spin-coating epitaxy with post-annealing process. J. Sol-Gel Sci. Technol. 2021, 100, 183–191. [Google Scholar] [CrossRef]
- Sinha, G.; Adhikary, K.; Chaudhuri, S. Effect of annealing temperature on structural transformation of gallium based nanocrystalline oxide thin films and their optical properties. Opt. Mater. 2007, 29, 718–722. [Google Scholar] [CrossRef]
- Fleischer, M.; Hanrieder, W.; Meixner, H. Stability of semiconducting gallium oxide thin films. Thin Solid Film. 1990, 190, 93–102. [Google Scholar] [CrossRef]
- Battiston, G.A.; Gerbasi, R.; Porchia, M.; Bertoncello, R.; Caccavale, F. Chemical vapour deposition and characterization of gallium oxide thin films. Thin Solid Film. 1996, 279, 115–118. [Google Scholar] [CrossRef]
- Mayo, M.J.; Suresh, A.; Porter, W.D. Thermodynamics for nanosystems: Grain and particle-size dependent phase diagrams. Rev. Adv. Mater. Sci. 2003, 5, 100–109. [Google Scholar]
- Binet, L.; Gourier, D. Origin of the blue luminescence of β-Ga2O3. J. Phys. Chem. Solids 1998, 59, 1241–1249. [Google Scholar] [CrossRef]
- Yao, Y.; Okur, S.; Lyle, L.A.M.; Tompa, G.S.; Salagaj, T.; Sbrockey, N.; Davis, R.F.; Porter, L.M. Growth and characterization of α-, β-, and ε-phases of Ga2O3 using MOCVD and HVPE techniques. Mater. Res. Lett. 2018, 6, 268–275. [Google Scholar] [CrossRef] [Green Version]
- Nakagomi, S.; Kokubun, Y. Cross-sectional TEM imaging of β-Ga2O3 thin films formed on c-plane and a -plane sapphire substrates. Phys. Status Solidi A Appl. Mater. Sci. 2013, 210, 1738–1744. [Google Scholar] [CrossRef]
- Xu, W.; Shi, J.; Li, Y.; Xiu, X.; Ding, S.; Xie, Z.; Tao, T.; Chen, P.; Liu, B.; Zhang, R.; et al. Study of β-Ga2O3 films hetero-epitaxially grown on off-angled sapphire substrates by halide vapor phase epitaxy. Mater. Lett. 2021, 289, 129411. [Google Scholar] [CrossRef]
- Li, Y.; Xiu, X.; Xu, W.; Zhang, L.; Xie, Z.; Tao, T.; Chen, P.; Liu, B.; Zhang, R.; Zheng, Y. Microstructural analysis of heteroepitaxial β-Ga2O3 films grown on (0001) sapphire by halide vapor phase epitaxy. J. Phys. D Appl. Phys. 2021, 54, 014003. [Google Scholar] [CrossRef]
- Suman, S.; Mukurala, N.; Kushwaha, A.K. Annealing induced surface restructuring in hydrothermally synthesized gallium oxide nano-cuboids. J. Cryst. Growth 2021, 554, 125946. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, G. Mesoporous mixed-phase Ga2O3: Green synthesis and enhanced photocatalytic activity. Mater. Res. Bull. 2015, 68, 254–259. [Google Scholar] [CrossRef]
- Zhao, Y.; Frost, R.L.; Martens, W.N. Synthesis and Characterization of Gallium Oxide Nanostructures via a Soft-Chemistry Route. J. Phys. Chem. C 2007, 111, 16290–16299. [Google Scholar] [CrossRef]
- Quan, Y.; Fang, D.; Zhang, X.; Liu, S.; Huang, K. Synthesis and Characterization of Gallium Oxide Nanowires via a Hydrothermal Method. Mater. Chem. Phys. 2010, 121, 142–146. [Google Scholar] [CrossRef]
- Wang, J.; Li, Q.; Qiu, X.; He, Y.; Liu, W. Soft-Template Mediated Synthesis of GaOOH Nanorod-Shelled Microspheres and Thermal Conversion to β-Ga2O3. J. Nanosci. Nanotechnol. 2010, 10, 4308–4311. [Google Scholar] [CrossRef]
- Muruganandham, M.; Amutha, R.; Wahed, M.S.M.A.; Ahmmad, B.; Kuroda, Y.; Suri, R.P.S.; Wu, J.J.; Sillanpää, M.E.T. Controlled Fabrication of α-GaOOH and α-Ga2O3 Self-Assembly and Its Superior Photocatalytic Activity. J. Phys. Chem. C 2012, 116, 44–53. [Google Scholar] [CrossRef]
- Li, D.; Duan, X.; Qin, Q.; Fan, H.; Zheng, W. Ionic liquid-assisted synthesis of mesoporous α-Ga2O3 hierarchical structures with enhanced photocatalytic activity. J. Mater. Chem. A 2013, 1, 12417. [Google Scholar] [CrossRef]
- Arul Prakasam, B.; Lahtinen, M.; Muruganandham, M.; Sillanpää, M. Synthesis of self-assembled α-GaOOH microrods and 3d hierarchical architectures with flower like morphology and their conversion to α-Ga2O3. Mater. Lett. 2015, 158, 370–372. [Google Scholar] [CrossRef]
- Kang, B.K.; Mang, S.R.; Song, K.M.; Lee, K.S.; Yoon, D.H. Hydrothermal synthesis and characterization of uniform β-Ga2O3 hollow nanostructures by carbon nanospheres. J. Ceram. Process. Res. 2014, 15, 200–203. [Google Scholar]
- Shi, F.; Qiao, H. Influence of hydrothermal reaction time on crystal qualities and photoluminescence properties of β-Ga2O3 nanorods. J. Mater. Sci. Mater. Electron. 2020, 31, 20223–20231. [Google Scholar] [CrossRef]
- Cui, L.; Wang, H.; Xin, B.; Mao, G. One-step rapid synthesis of ultrafine γ-Ga2O3 nanocrystals by microwave hydrothermal method in ammonium hydroxide medium. Appl. Phys. A Mater. Sci. Process. 2017, 123, 634. [Google Scholar] [CrossRef]
- Zhao, W.; Yang, Y.; Hao, R.; Liu, F.; Wang, Y.; Tan, M.; Tang, J.; Ren, D.; Zhao, D. Synthesis of mesoporous β-Ga2O3 nanorods using PEG as template: Preparation, characterization and photocatalytic properties. J. Hazard. Mater. 2011, 192, 1548–1554. [Google Scholar] [CrossRef]
- Wang, Y.; Li, N.; Duan, P.; Sun, X.; Chu, B.; He, Q. Properties and Photocatalytic Activity of β-Ga2O3 Nanorods under Simulated Solar Irradiation. J. Nanomater. 2015, 2015, 5. [Google Scholar] [CrossRef] [Green Version]
- Fujihara, S.; Shibata, Y.; Hosono, E. Chemical Deposition of Rodlike GaOOH and β-Ga2O3 Films Using Simple Aqueous Solutions. J. Electrochem. Soc. 2005, 152, C764. [Google Scholar] [CrossRef]
- Bae, H.J.; Yoo, T.H.; Yoon, Y.; Lee, I.G.; Kim, J.P.; Cho, B.J.; Hwang, W.S. High-Aspect Ratio β-Ga2O3 Nanorods via Hydrothermal Synthesis. Nanomaterials 2018, 8, 594. [Google Scholar] [CrossRef] [Green Version]
- Pilliadugula, R.; Krishnan, N.G. Gas sensing performance of GaOOH and β-Ga2O3 synthesized by hydrothermal method: A comparison. Mater. Res. Express 2019, 6, 025027. [Google Scholar] [CrossRef]
- Pilliadugula, R.; Krishnan, N.G. Effect of pH dependent morphology on room temperature nh3 sensing performances of β-Ga2O3. Mater. Sci. Semicond. Process. 2020, 112, 105007. [Google Scholar] [CrossRef]
- Shi, F.; Qiao, H. Effects of hydrothermal temperatures on crystalline quality and photoluminescence properties of β-Ga2O3 microspheres using ammonia as a precipitator. CrystEngComm 2021, 23, 492–498. [Google Scholar] [CrossRef]
- Lin, H.J.; Baltrus, J.P.; Gao, H.; Ding, Y.; Nam, C.Y.; Ohodnicki, P.; Gao, P.X. Perovskite Nanoparticle-Sensitized Ga2O3 Nanorod Arrays for CO Detection at High Temperature. ACS Appl. Mater. Interfaces 2016, 8, 8880–8887. [Google Scholar] [CrossRef]
- Zhang, B.; Lin, H.J.; Gao, H.; Lu, X.; Nam, C.Y.; Gao, P.X. Perovskite-sensitized β-Ga2O3 nanorod arrays for highly selective and sensitive NO2 detection at high temperature. J. Mater. Chem. A 2020, 8, 10845–10854. [Google Scholar] [CrossRef]
- Zhang, J.; Jiao, S.; Wan, Y.; Gao, S.; Wang, D.; Wang, J.Z. A well-grown β-Ga2O3 microrod array formed from GaOOH on a Si (100) substrate and growth mechanism study. CrystEngComm 2018, 20, 4329–4335. [Google Scholar] [CrossRef]
- Atilgan, A.; Yildiz, A.; Harmanci, U.; Gulluoglu, M.T.; Salimi, K. β-Ga2O3 nanoflakes/p-Si heterojunction self-powered photodiodes. Mater. Today Commun. 2020, 24, 101105. [Google Scholar] [CrossRef]
- Guo, D.Y.; Chen, K.; Wang, S.L.; Wu, F.M.; Liu, A.P.; Li, C.R.; Li, P.G.; Tan, C.K.; Tang, W.H. Self-Powered Solar-Blind Photodetectors Based on α/β Phase Junction of Ga2O3. Phys. Rev. Appl. 2020, 13, 024051. [Google Scholar] [CrossRef]
- Wu, C.; He, C.; Guo, D.; Zhang, F.; Li, P.; Wang, S.; Liu, A.; Wu, F.; Tang, W. Vertical α/β-Ga2O3 phase junction nanorods array with graphene-silver nanowire hybrid conductive electrode for high-performance self-powered solar-blind photodetectors. Mater. Today Phys. 2020, 12, 100193. [Google Scholar] [CrossRef]
- He, C.; Guo, D.; Chen, K.; Wang, S.; Shen, J.; Zhao, N.; Liu, A.; Zheng, Y.; Li, P.; Wu, Z.; et al. α-Ga2O3 Nanorod Array-Cu2O Microsphere p-n Junctions for Self-Powered Spectrum-Distinguishable Photodetectors. ACS Appl. Nano Mater. 2019, 2, 4095–4103. [Google Scholar] [CrossRef]
- Wang, S.; Chen, K.; Zhao, H.; He, C.; Wu, C.; Guo, D.; Zhao, N.; Ungar, G.; Shen, J.; Chu, X.; et al. β-Ga2O3 nanorod arrays with high light-to-electron conversion for solar-blind deep ultraviolet photodetection. RSC Adv. 2019, 9, 6064–6069. [Google Scholar] [CrossRef]
- Zhang, J.; Jiao, S.; Wang, D.; Gao, S.; Wang, J.; Zhao, L. Nano tree-like branched structure with α-Ga2O3 covered by γ-Al2O3 for highly efficient detection of solar-blind ultraviolet light using self-powered photoelectrochemical method. Appl. Surf. Sci. 2021, 541, 148380. [Google Scholar] [CrossRef]
- Ryou, H.; Yoo, T.H.; Yoon, Y.; Lee, I.G.; Shin, M.; Cho, J.; Cho, B.J.; Hwang, W.S. Hydrothermal Synthesis and Photocatalytic Property of Sn-Doped β-Ga2O3 Nanostructure. ECS J. Solid State Sci. Technol. 2020, 9, 045009. [Google Scholar] [CrossRef]
- Pilliadugula, R.; Gopalakrishnan, N. Room temperature ammonia sensing performances of pure and sn doped β-Ga2O3. Mater. Sci. Semicond. Process. 2021, 135, 106086. [Google Scholar] [CrossRef]
- Wang, J.; Jiang, S.; Liu, H.; Wang, S.; Pan, Q.; Yin, Y.; Zhang, G. P- type gas-sensing behavior of Ga2O3/Al2O3 nanocomposite with high sensitivity to NOx at room temperature. J. Alloys Compd. 2020, 814, 152284. [Google Scholar] [CrossRef]
- Kim, S.; Ryou, H.; Lee, I.G.; Shin, M.; Cho, B.J.; Hwang, W.S. Impact of Al doping on a hydrothermally synthesized β-Ga2O3 nanostructure for photocatalysis applications. RSC Adv. 2021, 11, 7338–7346. [Google Scholar] [CrossRef]
- Shi, Y.; Li, H.; Chen, L.; Huang, X. Obtaining ultra-long copper nanowires via a hydrothermal process. Sci. Technol. Adv. Mater. 2005, 6, 761–765. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Li, S.; Yang, Y.; Peng, S.; Hu, Z.; Qian, Y. Complex-Surfactant-Assisted Hydrothermal Route to Ferromagnetic Nickel Nanobelts. Adv. Mater. 2003, 15, 1946–1948. [Google Scholar] [CrossRef]
- Sun, Y.; Xia, Y. Large-Scale Synthesis of Uniform Silver Nanowires Through a Soft, Self-Seeding, Polyol Process. Adv. Mater. 2002, 14, 833–837. [Google Scholar] [CrossRef]
- Harwig, T.; Kellendonk, F. Some Observations on the Photoluminescence of Doped β-Galliumsesquioxide. J. Solid State Chem. 1978, 24, 255–263. [Google Scholar] [CrossRef]
- Xu, H.; Wang, W. Template Synthesis of Multishelled Cu2O Hollow Spheres with a Single-Crystalline Shell Wall. Angew. Chem.-Int. Ed. 2007, 46, 1489–1492. [Google Scholar] [CrossRef]
- Firouzabadi, H.; Iranpoor, N.; Gholinejad, M. One-Pot Thioetherification of Aryl Halides Using Thiourea and Alkyl Bromides Catalyzed by Copper(I) Iodide Free from Foul-Smelling Thiols in Wet Polyethylene Glycol (PEG 200). Adv. Synth. Catal. 2010, 352, 119–124. [Google Scholar] [CrossRef]
- Hou, Y.; Wang, X.; Wu, L.; Ding, Z.; Fu, X. Efficient Decomposition of Benzene over a β-Ga2O3 Photocatalyst under Ambient Conditions. Environ. Sci. Technol. 2006, 40, 5799–5803. [Google Scholar] [CrossRef] [PubMed]
- Cüneyt Taş, A.; Majewski, P.J.; Aldinger, F. Synthesis of Gallium Oxide Hydroxide Crystals in Aqueous Solutions with or without Urea and Their Calcination Behavior. J. Am. Ceram. Soc. 2002, 85, 1421–1429. [Google Scholar] [CrossRef] [Green Version]
- Jolivet, J.; Henry, M.; Livage, J. Metal Oxide Chemistry and Synthesis: From Solution to Solid State; John Wiley: Hoboken, NJ, USA, 2000; p. 344. ISBN 978-0-471-97056-9. [Google Scholar]
- Hassan, M.A.; Waseem, A.; Johar, M.A.; Bagal, I.V.; Ha, J.S.; Ryu, S.W. Single-Step fabrication of 3d hierarchical ZnO/ZnS heterojunction branched nanowires by MOCVD for enhanced photoelectrochemical water splitting. J. Mater. Chem. A 2020, 8, 8300–8312. [Google Scholar] [CrossRef]
- Oshima, T.; Kato, Y.; Magome, E.; Kobayashi, E.; Takahashi, K. Characterization of pseudomorphic γ-Ga2O3 and γ-Al2O3 films on MgAl2O4 substrates and the band-alignment at the coherent γ-Ga2O3/Al2O3 heterojunction interface. Jpn. J. Appl. Phys. 2019, 58, 060910. [Google Scholar] [CrossRef]
- Edwards, D.D.; Mason, T.O. Subsolidus Phase Relations in the Ga2O3–In2O3–SnO2 System. J. Am. Ceram. Soc. 1998, 81, 3285–3292. [Google Scholar] [CrossRef]
- Pedley, J.B.; Marshall, E.M. Thermochemical Data for Gaseous Monoxides. J. Phys. Chem. Ref. Data 1983, 12, 967–1031. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Q.; Ju, D.; Deng, X.; Huang, J.; Cao, B.; Xu, X. Morphology-Modulation of SnO2 Hierarchical Architectures by Zn Doping for Glycol Gas Sensing and Photocatalytic Applications. Sci. Rep. 2015, 5, 2–10. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Mu, Z.; Hu, J.; Cui, Z. Chemical gas sensing characteristics of composite NiO/Al2O3 for 2-chloroethanol at low temperature. Sens. Actuators B. Chem. 2016, 232, 143–149. [Google Scholar] [CrossRef]
- Peelaers, H.; Varley, J.B.; Speck, J.S.; Van DeWalle, C.G. Structural and electronic properties of Ga2O3-Al2O3 alloys. Appl. Phys. Lett. 2018, 112, 242101. [Google Scholar] [CrossRef]
- Li, G.; Peng, C.; Li, C.; Yang, P.; Hou, Z.; Fan, Y.; Cheng, Z.; Lin, J. Shape-Controllable Synthesis and Morphology-Dependent Luminescence Properties of GaOOH:Dy3+ and β-Ga2O3:Dy3+. Inorg. Chem. 2010, 49, 1449–1457. [Google Scholar] [CrossRef]
- Mizunashi, T.; Fujihara, S. Elaboration and Tunable Photoluminescence of β-Ga2O3: Eu3+ Films by Urea-Assisted Aqueous Chemical Bath Deposition. Electrochem. Solid-State Lett. 2008, 11, 43–46. [Google Scholar] [CrossRef]
- Yeh, C.Y.; Zhao, Y.M.; Li, H.; Yu, F.P.; Zhang, S.; Wuu, D.S. Growth and Photocatalytic Properties of Gallium Oxide Films Using Chemical Bath Deposition. Crystals 2019, 9, 564. [Google Scholar] [CrossRef] [Green Version]
- Hector, G.; Appert, E.; Sarigiannidou, E.; Matheret, E.; Roussel, H.; Chaix-Pluchery, O.; Consonni, V. Chemical Synthesis of β-Ga2O3 microrods on Silicon and Its Dependence on the Gallium Nitrate Concentration. Inorg. Chem. 2020, 59, 15696–15706. [Google Scholar] [CrossRef]
- Chiang, J.L.; Shang, Y.G.; Yadlapalli, B.K.; Yu, F.P.; Wuu, D.S. Ga2O3 nanorod-based extended-gate field-effect transistors for pH sensing. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2022, 276, 115542. [Google Scholar] [CrossRef]
- Sinha, G.; Patra, A. Generation of green, red and white light from rare-earth doped Ga2O3 nanoparticles. Chem. Phys. Lett. 2009, 473, 151–154. [Google Scholar] [CrossRef]
- Sinha, G.; Chaudhuri, S. Controlled solvothermal synthesis of β-Ga2O3 3D microstructures and their optical properties. Mater. Chem. Phys. 2009, 114, 644–649. [Google Scholar] [CrossRef]
- Muruganandham, M.; Suri, R.; Abdel, M.S.M.; Sillanpää, M.; Ahmmad, B.; Lee, G.; Wu, J.J. Solvothermal synthesis of mesoporous α-GaOOH semi-nanospheres. Mater. Lett. 2013, 111, 137–139. [Google Scholar] [CrossRef]
- Zhang, W.; Naidu, B.S.; Ou, J.Z.; O’Mullane, A.P.; Chrimes, A.F.; Carey, B.J.; Wang, Y.; Tang, S.; Sivan, V.; Mitchell, A.; et al. Liquid Metal/Metal Oxide Frameworks with Incorporated Ga2O3 for Photocatalysis. ACS Appl. Mater. Interfaces 2015, 7, 1943–1948. [Google Scholar] [CrossRef] [Green Version]
- Kang, B.K.; Lim, H.D.; Mang, S.R.; Song, K.M.; Jung, M.K.; Kim, S.; Yoon, D.H. Synthesis and Characterization of Monodispersed β-Ga2O3 Nanospheres via Morphology Controlled Ga4(OH)10SO4 Precursors. Langmuir 2015, 31, 833–838. [Google Scholar] [CrossRef]
- Kang, B.K.; Lim, G.H.; Lim, B.; Yoon, D.H. Morphology controllable synthesis and characterization of gallium compound hierarchical structures via forced-hydrolysis method. J. Alloys Compd. 2016, 675, 57–63. [Google Scholar] [CrossRef]
- Huang, E.; Li, J.; Wu, G.; Dai, W.; Guan, N.; Li, L. A simple synthesis of Ga2O3 and GaN nanocrystals. RSC Adv. 2017, 7, 47898–47903. [Google Scholar] [CrossRef] [Green Version]
- Girija, K.; Thirumalairajan, S.; Patra, A.K.; Mangalaraj, D.; Ponpandian, N.; Viswanathan, C. Enhanced photocatalytic performance of novel self-assembled floral β-Ga2O3 nanorods. Curr. Appl. Phys. 2013, 13, 652–658. [Google Scholar] [CrossRef]
- Girija, K.; Thirumalairajan, S.; Mangalaraj, D. Morphology controllable synthesis of parallely arranged single-crystalline β-Ga2O3 nanorods for photocatalytic and antimicrobial activities. Chem. Eng. J. 2014, 236, 181–190. [Google Scholar] [CrossRef]
- Vequizo, J.J.M.; Ichimura, M. Electrodeposition of Ga-O Thin Films from Aqueous Gallium Sulfate Solutions. Jpn. J. Appl. Phys. 2013, 52, 075503. [Google Scholar] [CrossRef]
- Ghazali, N.M.; Mahmood, M.R.; Yasui, K.; Hashim, A.M. Electrochemically deposited gallium oxide nanostructures on silicon substrates. Nanoscale Res. Lett. 2014, 9, 120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- ISO 21348; Space Environment (Natural and Artificial)—Process for Determining Solar Irradiances. 1st ed. ISO: Geneva, Switzerland, 2007.
- Chen, X.; Ren, F.; Gu, S.; Ye, J. Review of Gallium-Oxide-Based Solar-Blind Ultraviolet Photodetectors. Photonics Res. 2019, 7, 381. [Google Scholar] [CrossRef]
- Osamura, K.; Nakajima, K.; Murakami, Y.; Shingu, P.H.; Ohtsuki, A. Fundamental absorption edge in GaN, InN and their alloys. Solid State Commun. 1972, 11, 617–621. [Google Scholar] [CrossRef]
- Ye, J.D.; Gu, S.L.; Zhu, S.M.; Liu, S.M.; Zheng, Y.D.; Zhang, R.; Shi, Y.; Yu, H.Q.; Ye, Y.D. Gallium doping dependence of single-crystal n-type Zno grown by metal organic chemical vapor deposition. J. Cryst. Growth 2005, 283, 279–285. [Google Scholar] [CrossRef]
- Persson, C.; Platzer-Björkman, C.; Malmström, J.; Törndahl, T.; Edoff, M. Strong Valence-Band Offset Bowing of ZnO1-XSx Enhances p-Type Nitrogen Doping of ZnO-like Alloys. Phys. Rev. Lett. 2006, 97, 1–4. [Google Scholar] [CrossRef]
- Vigué, F.; Tournié, E.; Faurie, J.P. Evaluation of the potential of ZnSe and Zn(Mg)BeSe compounds for ultraviolet photodetection. IEEE J. Quantum Electron. 2001, 37, 1146–1152. [Google Scholar] [CrossRef]
- Morkoç, H.; Strite, S.; Gao, G.B.; Lin, M.E.; Sverdlov, B.; Burns, M. Large-band-gap SiC, III-V nitride, and II-VI ZnSe-based semiconductor device technologies. J. Appl. Phys. 1994, 76, 1363–1398. [Google Scholar] [CrossRef]
- Chen, X.; Zhu, H.; Cai, J.; Wu, Z. High-performance 4H-SiC-based ultraviolet p-i-n photodetector. J. Appl. Phys. 2007, 102, 024505. [Google Scholar] [CrossRef]
- Yim, W.M.; Stofko, E.J.; Zanzucchi, P.J.; Pankove, J.I.; Ettenberg, M.; Gilbert, S.L. Epitaxially grown AlN and its optical band gap. J. Appl. Phys. 1973, 44, 292–296. [Google Scholar] [CrossRef]
- Salvatori, S.; Rossi, M.C.; Galluzzi, F.; Pace, E. Solar-blind UV-photodetector based on polycrystalline diamond films: Basic design principle and comparison with experimental results. Mater. Sci. Eng. B 1997, 46, 105–111. [Google Scholar] [CrossRef]
- Chen, Y.C.; Lu, Y.J.; Lin, C.N.; Tian, Y.Z.; Gao, C.J.; Dong, L.; Shan, C.X. Self-powered diamond/β-Ga2O3 photodetectors for solar-blind imaging. J. Mater. Chem. C 2018, 6, 5727–5732. [Google Scholar] [CrossRef]
- Zheng, W.; Lin, R.; Zhang, Z.; Huang, F. Vacuum-Ultraviolet Photodetection in Few-Layered h-BN. ACS Appl. Mater. Interfaces 2018, 10, 27116–27123. [Google Scholar] [CrossRef]
- Onuma, T.; Saito, S.; Sasaki, K.; Masui, T.; Yamaguchi, T.; Honda, T.; Higashiwaki, M. Valence Band Ordering in β-Ga2O3 Studied by Polarized Transmittance and Reflec. Jpn. J. Appl. Phys. 2015, 54, 112601. [Google Scholar] [CrossRef]
- Akaiwa, K.; Fujita, S. Electrical Conductive Corundum-Structured α-Ga2O3 Thin Films on Sapphire with Tin-Doping Grown by Sprayassisted Mist Chemical Vapor Deposition. Jpn. J. Appl. Phys. 2012, 51, 070203. [Google Scholar] [CrossRef]
- Li, D.; Jiang, K.; Sun, X.; Guo, C. AlGaN photonics: Recent advances in materials and ultraviolet devices. Adv. Opt. Photonics 2018, 10, 43. [Google Scholar] [CrossRef]
- Fujita, S.; Oda, M.; Kaneko, K.; Hitora, T. Evolution of corundum-structured iii-oxide semiconductors: Growth, properties, and devices. Jpn. J. Appl. Phys. 2016, 55, 1202A3. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.; Seo, J.H.; Singisetti, U.; Ma, Z. Recent advances in free-standing single crystalline wide band-gap semiconductors and their applications: GaN, SiC, ZnO, β-Ga2O3, and diamond. J. Mater. Chem. C 2017, 5, 8338–8354. [Google Scholar] [CrossRef]
- Tsao, J.Y.; Chowdhury, S.; Hollis, M.A.; Jena, D.; Johnson, N.M.; Jones, K.A.; Kaplar, R.J.; Rajan, S.; Van deWalle, C.G.; Bellotti, E.; et al. Ultrawide-Bandgap Semiconductors: Research Opportunities and Challenges. Adv. Electron. Mater. 2018, 4, 1600501. [Google Scholar] [CrossRef] [Green Version]
- Trinchi, A.; Wlodarski, W.; Li, Y.X. Hydrogen sensitive Ga2O3 schottky diode sensor based on SiC. Sens. Actuators B Chem. 2004, 100, 94–98. [Google Scholar] [CrossRef]
- Wu, Y.-L.; Chang, S.-P.; Chang, S.-J.; Weng, W.Y.; Lin, Y.H.A. Novel PH Sensor Using Extended-Gate Field-Effect Transistors with Ga2O3 Nanowires Fabricated on SiO2/Si Template. Sci. Adv. Mater. 2015, 7, 475–478. [Google Scholar] [CrossRef]
- Manjakkal, L.; Szwagierczak, D.; Dahiya, R. Metal oxides based electrochemical pH sensors: Current progress and future perspectives. Prog. Mater. Sci. 2020, 109, 100635. [Google Scholar] [CrossRef]
- Tan, L.L.; Ong, W.J.; Chai, S.P.; Goh, B.T.; Mohamed, A.R. Visible-light-active oxygen-rich TiO2 decorated 2D graphene oxide with enhanced photocatalytic activity toward carbon dioxide reduction. Appl. Catal. B Environ. 2015, 179, 160–170. [Google Scholar] [CrossRef]
- Liu, L.; Gao, F.; Zhao, H.; Li, Y. Tailoring Cu valence and oxygen vacancy in Cu/TiO2 catalysts for enhanced CO2 photoreduction efficiency. Appl. Catal. B Environ. 2013, 134–135, 349–358. [Google Scholar] [CrossRef]
- Núñez, J.; De La Peña O’Shea, V.A.; Jana, P.; Coronado, J.M.; Serrano, D.P. Effect of copper on the performance of ZnO and ZnO1-XNx oxides as CO2 photoreduction catalysts. Catal. Today 2013, 209, 21–27. [Google Scholar] [CrossRef]
- Pan, Y.X.; Sun, Z.Q.; Cong, H.P.; Men, Y.L.; Xin, S.; Song, J.; Yu, S.H. Photocatalytic CO2 reduction highly enhanced by oxygen vacancies on Pt-nanoparticle-dispersed gallium oxide. Nano Res. 2016, 9, 1689–1700. [Google Scholar] [CrossRef]
- Maeda, K.; Domen, K. Chapter 12 Nano-particulate photocatalysts for overall water splitting under visible light. In Theoretical and Computational Chemistry; Elsevier: Amsterdam, The Netherlands, 2007; Volume 18, pp. 301–315. [Google Scholar]
- Nosaka, Y.; Komori, S.; Yawata, K.; Hirakawa, T.; Nosaka, A.Y. Photocatalytic •OH radical formation in TiO2 aqueous suspension studied by several detection methods. Phys. Chem. Chem. Phys. 2003, 5, 4731–4735. [Google Scholar] [CrossRef]
- Zhou, W.; Fu, H. Defect-mediated electron-hole separation in semiconductor photocatalysis. Inorg. Chem. Front. 2018, 5, 1240–1254. [Google Scholar] [CrossRef]
- Yan, Y.; Yin, W.J.; Wu, Y.; Shi, T.; Paudel, N.R.; Li, C.; Poplawsky, J.; Wang, Z.; Moseley, J.; Guthrey, H.; et al. Physics of grain boundaries in polycrystalline photovoltaic semiconductors. J. Appl. Phys. 2015, 117, 112807. [Google Scholar] [CrossRef]
- Liang, H.; Cui, S.; Su, R.; Guan, P.; He, Y.; Yang, L.; Chen, L.; Zhang, Y.; Mei, Z.; Du, X. Flexible X-Ray Detectors Based on Amorphous Ga2O3 Thin Films. ACS Photonics 2019, 6, 351–359. [Google Scholar] [CrossRef]
- Sasaki, K.; Higashiwaki, M.; Kuramata, A.; Masui, T.; Yamakoshi, S. Si-Ion Implantation Doping in β-Ga2O3 and Its Application to Fabrication of Low-Resistance Ohmic Contacts. Appl. Phys. Express 2013, 6, 2–6. [Google Scholar] [CrossRef]
- Fleischer, M.; Meixner, H. Oxygen Sensing with Long-Term Stable Ga2O3 Thin Films. Sens. Actuators B Chem. 1991, 5, 115–119. [Google Scholar] [CrossRef]
































| Polymorph Type | Crystal Structure | Space Group | Lattice Parameters | Ref. |
|---|---|---|---|---|
| α | Hexagonal or rhombohedral | R-3c | a = b = 4.98 Å, c = 13.43 Å, α = β = 90°, γ = 120° | [25] |
| β | Monoclinic | C2/m | a = 12.23 Å, b = 3.04 Å, c = 5.80 Å, α = γ = 90°, β = 103.7° | [35] |
| γ | Cubic | Fd-3m | a = b = c = 8.24 Å, α = β = γ = 90° | [36] |
| δ | Cubic | Ia3 | a = b = c = 9.402 Å, α = β = γ = 90° | [37] |
| ε | Hexagonal | P63mc | a = b = 2.90 Å, c = 9.26 Å, α = β = 90°, γ = 120° | [32] |
| Property | Value | Ref. |
|---|---|---|
| Band gap (eV) | 4.8~4.9 | [82] |
| breakdown electric field (MV/cm) | ~8 | [47] |
| Saturation velocity (107 cm/s) | 1.8~2.0 | [7] |
| Melting point (°C) | 1793 | [48] |
| Specific heat (J·g−1·K−1) | 0.56 | [92] |
| Thermal conductivity (W·m−1·K−1) | 10.9 ± 1.0 along [100] 27.0 ± 2.0 along [010] 15.0 along [001] | [103] |
| Thermal diffusivity (mm2·s−1) | 3.7 ± 0.4 along [100] 9.6 ± 0.5 along [010] 7.1 ± 0.4 along [001] | [114] |
| Thermal expansion (K−1) | 1.54 × 10−6 along [100] 3.37 × 10−6 along [010] 3.15 × 10−6 along [001] 2.23 × 10−5 for β angle | [110] |
| Cleavage plane | (100), (001) | [115] |
| Absorption edge (nm) | 270~275 | [86,87,88,89] |
| Emission bands at RT (eV) | UV (3.2–3.6) blue (2.8–3.0) green (2.4) | [18] |
| Refractive index (in the visible spectrum) | 1.98~2.1 | [6] |
| High-frequency dielectric constant | 3.57 | [116] |
| Low-frequency (or static) dielectric constant | 10.2 ± 0.3 | [117] |
| Method | Substrate/ Template | Precursor | Synthesis Conditions | Properties | Application | Ref. |
|---|---|---|---|---|---|---|
| Sol-gel dip coating | BaTiO3 (Thickness-0.2 mm) | Gallium acethylacetonate (Ga(C5H7O2)3)—A) + manganese chloride (MnCl2) + methanol (CH3OH)—(B). Molar ratio = ( = 0.08, Mn content: 0.3 at.% | Room Temperature (RT) stirring for 30 min, then added HCl and H2O. pH of solution: 3.4, Stirring at 50 °C for 5 h under N2 ambient, Dip coated films dried at RT for 5 min, Pre-heat: 10 min, 600–100 °C. Process repetition: 25 times, Calcination: 850–1070 °C for 1 h in Ar ambient | Amorphous thin film of Ga2O3: Mn. Thickness: 2 µm | Green-Light emitting TFEL device | [12] |
| Sol-gel spin coating | Sapphire transducers | Gallium isopropoxide (0.15 M) + i. Ce doping—cerium isopropoxide, or ii. W doping—tungsten ethoxide, or iii. Sb doping—antimony butoxide, or iv. Zn doping—zinc acetyleacetonate hydrate. Each element doping level—3 mol% | Solutions prepared in dry N2 ambient, Ultra sonication of mixed solution: 1 h, Aging time: 24 h, Spin coating at 3000 rpm for 30 s, dried at RT for 24 h, Calcination: 600 °C for 1 h | Ga2O3 thin film doped with Ce, Sb, W and Zn. Thickness: 200 nm | O2 gas sensor | [179] |
| Sol-gel | Gallium(III)-isopropoxide (2.714 g) + 2-Propanol—200 mL + Hot water—200 mL (90—100 °C) + 25% w/w aqueous TMAH solution (2 mL) | Ultra-speed centrifuge: 20,000 rpm, washed with ethanol and water then dried, Calcination: 500 °C for 4 h, 900 °C for 2 h | Amorphous α and β-Ga2O3 at 500 °C, β-Ga2O3 at 900 °C (sub-micrometer size) | [180] | ||
| Gallium(III)-isopropoxide (1.796 g) + Twice distilled water—50 mL (20 °C) | Ultrasonication for 1 h, liquid phase evaporation at 70 °C for 24 h. Calcination: 500 °C for 4 h, 900 °C for 2 h | (10–20 nm) sized particles of β-Ga2O3 at 500 °C, sub-micromere size particles of β-Ga2O3 at 900 °C | ||||
| GaCl3 aqueous solution—25 mL (0.284 M) + Twice distilled water—175 mL (20 °C) + 25 % w/w TMAH aqueous solution | pH: 7.82, Ageing time: 2 h, Calcination: 500 °C for 4 h, 900 °C for 2 h | Elongated and uniform sub-micron sized particles of α-Ga2O3 at 500 °C, big agglomerates of β-Ga2O3 with less size than that of α-Ga2O3 | ||||
| Sol-gel spin coating | Sapphire (0001) | (Gallium isopropoxide (A) + (2-Methoxyethanol + monoethanolamine (B)))—0.4 mol/L Molar ratio (Cm ratio): ( = 1) | Solution stirred for 1 h at 60 °C till transparent sol appear, Spin coating at 3000 rpm then dried at 90 °C for 10 min, Pre heat at 300 °C for 20 min, 6 times process repetition Calcination: 1000 °C for 1 h | Thickness: 150–200 nm The band gap of β-Ga2O3 thin films increased due to Al doping into Ga2O3 at 900 °C | Solar-blind Photo-detector | [181] |
| Sol-gel spin coating | β-Ga2O3 (100) substrate(Thickness: 0.4 mm) | (Gallium isopropoxide (A) + (2-Methoxyethanol + monoethanolamine (B)))—0.4 mol/L Molar ratio Cm ratio: ( = 1) | Solution stirred for 1 h at 60 °C till transparent Sol appear, Spin coating at 3000 rpm then dried at 90 °C for 10 min, Pre heat at 300 °C for 20 min, 6 times process repetition Calcination: 1000 °C for 1 h | β-Ga2O3 thin film grown epitaxially on β-Ga2O3 substrate, Thickness: 120 nm | Solar-blind Photo-diode | [182] |
| Sol-gel | (Gallium (III) isopropoxide (Ga (Opri)3) (2 g) + anhydrous iso-propanol (20 mL)) + 2 drops of water-isopropanol | Pre-stirring for 2 h, aged for 2 days with continuous stirring, dried in oven at 100 °C then washed with acetone & water Calcination: 600 °C for 6 h | β-Ga2O3 mono-crystalline nanorods, Length = 100 nm | [183] | ||
| N-phenylsalicylaldimine modified gallium (III) isopropoxide (2 g) + anhydrous iso-propanol (20 mL)) + 2 drops of water-isopropanol | Pre-stirring for 2 h, aged for 2 days with continuous stirring, dried in oven at 100 °C then washed with acetone & water Calcination: 600 °C for 6 h | γ-Ga2O3 polycrystalline nanoparticles, Size = 10 nm | ||||
| Sol-gel | Solution part 1: Gallium metal + HNO3—(Total pH: 1–2), Solution part 2: Tetraethyl orthosilicate + (ethanol-water mixture) + Few drops of 0.1 N HCL then stirred for 1 h | Molar ratio: Ga2O3 to SiO2—10:90, 20:80 and 30:70. Both solutions mixed (pH: 1–2) then stirred for 1 h, Heated at 70 °C for 3.5 h, died at 200 °C for 4 h. Calcination: 400 °C for 11 h, 500 °C for 5 h and 900 °C for 8 h | Ga2O3:SiO2 composite nanoparticles, β-Ga2O3 phase formation at a low temp of 400 °C for each molar ratio | [184] | ||
| Sol-gel dip coating | Amorphous quartz or Silicon | ((Gallium metal + HCl) + dry ethanol)—0.075 mol/L + few drops of acetic acid | Solution stirred until it is appearing clear, Dip coated films dried at 100 °C for 5 min, Calcination: 700 °C for 1 h | β-Ga2O3 thin film | [185] | |
| Sol-gel | Porous alumina Template (Pore size: 200 nm) | Gallium nitrate hydrate (0.4 M) + ethanol (5 M) + concentrated aqueous ammonia diluted in ethanol (50% vol.) (0.25 M) added drop wise | Precipitates separated centrifugally then washed with DI water then peptized in nitric acid to form stable sol, Template immersed in solution for 5 s at RT then dried in air for 30 min, Calcination: 500 °C for 12 h | Hollow nano-tubes of Ga2O3, Length 50 µm, Inside diameter: 100 nm and outside diameter: 200 nm | [186] | |
| Sol-gel dip coating | Quartz and alumina substrate transducers | Solution part 1: Titanium tetraisopropoxide (A) + HCl (B) + H2O (C). Cm ratio: (A:B:C=0.4:0.2:48.8) Solution part 2: Gallium (III) nitrate hydrate + hydroxypropyl cellulose (1.5 g/100 mL) +DI water | Ti:Ga atomic ratios (at.%/at.%) = 100:00, 75:25, 50:50 and 25:75. Part 1 peptized at 70 °C for 2 h. Part 2 stirred for 30 min, Mixed sol stirred for 2 h at 70 °C, dip coated films dried at 150 °C for 1 h Calcination: 600, 800 and 1000 °C for 1 h | Ga2O3 retards the anatase to rutile formation of TiO2, High SSA for Ti:Ga = 50:50 annealed at 600 °C | CO and NO2 gas sensor | [187] |
| Sol-gel drop casting | (100) p-Si wafer doped with boron (Thickness: 280 µm) | Gallium nitrate hydrate + ethanol | After drop casting on substrate, films spun at 3000 rpm for 30 s, Heated at 100 °C for 30 min to evaporate ethanol, Calcination: 800 °C for 2 h in Ar ambience | β-Ga2O3 thin film | MOS capacitor | [14] |
| Sol-gel drop casting | MOCVD grown GaN on sapphire | Gallium nitrate hydrate + ethanol | After drop casting on substrate, films spun at 3000 rpm for 30 s, Heated at 100 °C for 30 min to evaporate ethanol, Calcination: 800 °C for 2 h in Ar ambience | β-Ga2O3 thin film | MOS structure | [15] |
| Sol-gel spin coating | Sapphire (0001) | (Gallium nitrate hydrate (A) + (2-Methoxyethanol + monoethanolamine (B)))—0.5 mol/L Molar ratio Cm ratio: ( = 1) | Solution stirred for 1 h at 60 °C till transparent sol appear, aged at RT for 36 h, after spin coating film kept on hot plate for 10 min, Pre heat at 500 °C for 15 min, Process repetition: 6 times, Calcination: 500 °C to 1100 °C for 2 h | Crystalline β-Ga2O3 thin film at 700 °C and higher, Thickness: 150–200 nm | Solar-blind ultra-violet photo-detectors | [188] |
| Sol-gel spin coating | Sapphire (0001) | (Gallium nitrate hydrate + ethanol)—0.6 mol/L | Solution stirred at 60 °C for 90 min and aged at RT for 36 h, spin coated substrates were heated on hot plate at 100 °C for 15 min, Pre heated at 300 °C for 25 min, Process repetition: 4 times, Calcination: 500 °C–900 °C for 2 h and later at 800 °C in O2, N2 and N2-O2 environments for 2 h | At 600 °C—low intensity β-Ga2O3. At 700–800 °C—α/β polycrystalline Ga2O3 thin film, At 900 °C—polycrystalline β-Ga2O3. Thickness: 240–280 nm | Photo-detector | [189] |
| Citrate sol-gel | Gallium nitrate + (citric acid (C6H8O7)—1.5 mole times than cations) | Stirred in water bath at 90 °C, transparent Sol dried at 200 °C, Calcination: 500 °C for 4 h in O2 ambience | Ga2O3 sub-micro powders | [190] | ||
| Sol-gel spin coating | Sapphire (0001) | (Gallium nitrate(A) + ethanol)—0.4 mol/L) + monoethanolamine (B), Cm ratio: ( = 1) | Spin coated at 1000 rpm for 50 s, Pre-heating of films at 100–500 °C in O2 ambient for 10 min, Calcination: 1000 °C in O2 ambient for 2 h | β-Ga2O3 thin films | [191] |
| Method | Substrate/ Template | Precursor | Synthesis Conditions | Properties | Applications | Ref. | |||
|---|---|---|---|---|---|---|---|---|---|
| hydrothermal method | Gallium acetylacetonate (0.1 M) + DI water | NH4OH added to the transparent solution dropwise till pH-10, Solution stirred at 65 °C for 5 h continuously, Reaction at 140 °C for 10 h, dried at 70 °C for 6 h, Calcination: 600 °C, 800 °C, 850 °C, 950 °C and 1000 °C | Cuboid shape β-Ga2O3 starting from 800–850 °C, Pore size was maximum at 950 °C | [201] | |||||
| Gallium chloride (0.1 M) + DI water | Rice like morphology β-Ga2O3 | ||||||||
| Gallium nitrate (0.1 M) + DI water | Rice like morphology β-Ga2O3 | ||||||||
| hydrothermal method | (Commercial Ga2O3 + HCl) + DI water-35 mL | NaOH solution added till pH = 6–8 Reaction in autoclave at 180 °C for 24 h, hydrothermal crystals filtered, washed and dried at 60 °C for 6 h, Calcination: 900 °C | -At pH 6, β-Ga2O3 regular quadrilateral nanorods (width: 200–300 nm) -At pH 8 spindle like β-Ga2O3 nanorod arrays formed | [45] | |||||
| (Commercial Ga2O3 + HCl) + DI water—35 mL + solvent of diethylene glycol (DEG) and water (1:1) | |||||||||
| hydrothermal method | (GaCl3 aqueous solution + water) + (TMAH (CH3)4NOH) aqueous solution (25% w/w)) | -pH was adjusted to 5, 7 and 9 by adding TMAH gradually, shaken for 5 min, ✓ Reaction at 60 °C (Aging time: pH-5: 5–7 days, pH-7: 1 day and pH-9: 2 days) or ✓ Reaction at 160 °C for 2 h. Calcination: 500 and 900 °C for 2 h | Uniform submicron particles of different shapes (rhombic rods, rhombic prisms, hierarchical structures) of α-Ga2O3 at 500 °C and β-Ga2O3 at 900 °C | [43] | |||||
| hydrothermal method | Gallium metal—0.2 g + DI water—60 mL | Reaction in autoclave at 160 °C for 12 h, precipitate collected by centrifugation then dried at 70 °C, Calcination: Pre heat at 400 °C for 5 h then at 600–800 °C for 1.5 h | Rod-like morphology mixed phase of α-Ga2O3 and β-Ga2O3 at 700 °C | Photocatalyst | [202] | ||||
| hydrothermal method | (((Ga(NO3)3 + water)—0.012 mol/L) + NaOH solution (1.5 M) + 0.036 mol NaOH), kept in shaking bath (100 rmp) at 80 °C for 2 h | PEO or CTAB (0.0048 mol) added | Stirring at RT for 2 h. Reaction at 100 °C for 48 h. Dried at 80 °C | Calcination: 900 °C for 2 h. | PEO-β-Ga2O3 quadrilateral rods length—2.56 µm, CTAB-β-Ga2O3 quadrilateral prisms length—2.56 µm CTAB sample has larger pore size than PEO sample. | [203] | |||
| ((0.003 mol Ga(NO3)3 + water (10 mL)) + (0.015 mol NaNO3 + water—10 mL)), kept in shaking bath (100 rmp) at 80 °C for 2 h | PEO or CTAB (0.0025 mol) added | HNO3 added dropwise at 80 °C to adjust pH = 9.5 | No calcination | Amorphous agglomerates and nanotubes of γ = 0.8–3 nm), More number of nanotubes with PEO than CTAB | |||||
| hydrothermal method | ((Ga(NO3)3·nH2O (A) + DI water)-0.01 mol/L) + SDBS (B), Cm ratio: ( = ) | Reaction in autoclave at 140 °C for 10 h, Products separated, washed with DI water then dried in atmosphere ambient, Calcination: 600 °C or 900 °C for 5 h | Brush-like particles composed with the nanowires | [204] | |||||
| ((Ga(NO3)3.nH2O (A) + DI water)-0.01 mol/L) + SA (B), Cm ratio: ( = ) | Cuboid-like particles | ||||||||
| ((Ga(NO3)3·nH2O + DI water)— 0.01 mol/L) | Spindle like particles | ||||||||
| hydrothermal method | (((Ga(NO3)3·xH2O + DI water—8 mL +NaOH—207 mg) + oleic acid—1.68 mL) + 1.5 mL of oleic acid + 6 mL of ethanol) + Na2S·9H2O—96 mg | Reaction in autoclave at 140 °C for 4, 6, 8 and 16 h. Products collected, washed then dried under vacuum at 70 °C for 8 h. Calcination: 1000 °C for 10 h | β-Ga2O3 microspheres with hollow interior | [205] | |||||
| hydrothermal method | (50 mL of Ga(NO3)3—0.0508 mol/L + 50 mL of oxalic acid (C2H2O4) (0.155–0.666 mol/L)) using water as solvent | Rigorous stirring on a hot plate at 90 °C, Reaction in autoclave at temperatures varying from (175–225 °C) for 10 h. Calcination: 450 °C for 3 h | Calcination of GaOOH prepared at reaction temp 200 °C were resulted into α-Ga2O3 microspheres | Photocatalyst | [206] | ||||
| hydrothermal method | (Ga(NO3)3·xH2O—0.01 g) + water—20 mL + C3H7NO—5 mL + C8H16N2O ([Bmin][OH])—0.05 g | Precipitates collected, washed and dried at 60 °C, Reaction in autoclave at 180 °C for 24 h. Calcination: 450 °C for 3 h | Mesomorphs α-Ga2O3 hierarchical structures | Photo degradation | [207] | ||||
| hydrothermal method | (Ga(NO3)3—2.55 g (10 mmol)) + (biuret—6.18 g (60 mmol)), Each solution prepared using 50 mL of water as solvent | Heated up to boiling, then stirred for 30 min, Reaction in autoclave at 200 °C for 10 h, dried at 120 °C for 2 h, Calcination: 450 °C for 3 h | Mesoporous α-Ga2O3 microrods | [208] | |||||
| (Ga(NO3)3—2.55 g (10 mmol)) + (oxalic acid—7.56 g (60 mmol)), Each solution prepared using 50 mL of water as solvent | Solution heated up to boiling, then stirred for 30 min, Reaction in autoclave at 200 °C for 14 h, dried at 120 °C for 2 h, Calcination: 450 °C for 3 h | Mesoporous α-Ga2O3 micro flowers | |||||||
| hydrothermal method | Carbon spheres as templates | (Ga(NO3)3·xH2O—1.3544 g (6 mmol) + urea—1.8 g (30 mmol) + carbon colloid solution | Reaction in autoclave at 90 °C for 48 h, Dried at RT for 24 h, Calcination: 500 °C (1 °C/min), 600–800 °C (10 °C/min) for 1 h | Uniform β-Ga2O3 hollow nanostructures at 700 °C. | [209] | ||||
| Ga(NO3)3·xH2O—15 mL (0.3 mol/L) + (urea—3.5 g + DI water—15 mL) | Urea solution was kept 90 °C for 1 h then added to solution, Reaction at 140 °C for 1, 3 and 10 h, solution centrifugated, precipitates collected then washed and dried, Calcination: 900 °C for 3 h | β-Ga2O3 nanorods | [210] | ||||||
| Micro-wave hydrothermal method | Ga(NO3)3—10 mL (0.166 mol/L) + (urea (2.0, 2.7, 3.4, 4.1 g) + DI water—10 mL) | Solution stirred at 60 °C for 30 min, Heated to 100 °C in 3 min @ 400 W then to 130–150 °C in 2 min @ 600W and maintained there for 1–4 min, Calcination: 300–700 °C for 2 h | Ultrafine γ-Ga2O3 nanocrystals at 140 °C for 2 min maintaining time, Phase change to β-Ga2O3 at 600 °C | Photo degradation | [211] | ||||
| hydrothermal method | (Ga(NO3)3·9H2O—1.6 g + urea—2.64 g + PEG—20 mL + 70 mL—DI water) | , Reaction in autoclave at 140 °C for 6 h, Precipitates filtered, washed with ethanol, Calcination: 800 °C for 2 h | Mesoporous β-Ga2O3 nanorods | Photocatalyst | [212] | ||||
| (Ga(NO3)3·9H2O (0.01 mol) + urea (0.1 mol) + PEG—200) using DI water as solvent | Stirred at RT for 1 h, Reaction in autoclave at 160 °C for 8 h, Dried at 100 °C for 24 h, Products separated by centrifugation, washed with alcohol then dried at 100 °C for 24 h, Calcination: 800 °C for 10 h | β-Ga2O3 nanorods | Photocatalytic degradation | [213] | |||||
| hydrothermal method | (Ga(NO3)3·nH2O + DI water)— 0.015 mol/L | Reaction in autoclave at 150 °C for 24 h, Solid products collected and washed and dried at RT, Calcination: 700 °C for 1 h. | Rod like morphology polycrystalline β-Ga2O3 films | [214] | |||||
| Ga(NO3)3·nH2O (0.1 M) + DI water—100 mL | NH4OH added to adjust solution pH-9, Reaction at RT to 95 °C for 5 h, Calcination: 500, 800 and 1000 °C for 3 h | α-Ga2O3 nanorods at 500 °C, β-Ga2O3 nanorods at 800 and 1000 °C | Photocatalytic degradation | [21] | |||||
| Ga(NO3)3·xH2O (0.1M) + DI water—50 mL | NH4OH- (28−30% NH3 in solution) added to make pH-10, Stirred at 60 °C while aging for 10 min–6 h, Reaction at 140 °C for 10 h, dried at 70 °C for 6 h, Calcination: 1000 °C for 5 h | β-Ga2O3 nanorods | FET | [215] | |||||
| Ga(NO3)3—1.02292 g (0.05 M) + DI water—80 mL | NH4OH solution added till pH-9, Reaction in autoclave at 95 °C for 5 h, Precipitates separated by centrifugation then dispersed in 5 mL DI water and dried at 75 °C, Calcination: 150 °C, 400–1000 °C in ambient air for 5 h | α-Ga2O3 nanorods at 400–700 °C, β-Ga2O3 nanorods at 900 °C and 1000 °C | CO2 gas sensing | [216] | |||||
| ((Ga(NO3)3·xH2O (0.05 M) + DI water)—160 mL) | Various amounts (1, 1.5, 2, 3 and 6 mL) of NH4OH was added to get different pH = 5, 7, 9, 11 and 14, Reaction at 100 °C for 5 h, dried overnight at 75 °C, Calcination: 1000 °C for 5 h | β-Ga2O3 nano powder | NH3 gas sensor | [217] | |||||
| Ga(NO3)3·9H2O—15 mL (0.3 moL/L) | NH4OH added dropwise till pH-10, Reaction at 40, 80, 120 and 160 °C for 18 h, Centrifugated, washed and dried, Calcination: 900 °C for 3 h | β-Ga2O3 microspheres | [218] | ||||||
| hydrothermal method | Sputter coated 50 nm thick SnO2 seed layer on 1 µm SiO2/Si (100) | Ga(NO3)3·9H2O—0.6 g + DI water—40 mL | Seed layer annealed at 900 °C for 2 h, Solution pH-2, Substrates incubated in solution at 150 °C for 12 h in autoclave, Calcination: 1000 °C for 4 h | β-Ga2O3 nanorod arrays (NRAs) | CO gas detection | [219] | |||
| Sputter coated 50 nm thick SnO2 seed layer on 1 µm SiO2/Si (100) | Ga(NO3)3·9H2O—0.6 g + DI water—40 mL | Seed layer annealed at 900 °C for 2 h, Solution pH-2, Reaction at 150 °C for 12 h, dried overnight at 80 °C Calcination: 1000 °C for 4 h | β-Ga2O3 nanorod arrays (NRAs) | NO2 gas sensor | [220] | ||||
| Si (100) | Nucleation (solution-1): Ga(NO3)3·9H2O—0.2 M + ethanol—10 mL + DI water—30 mL. Crystal Growth (solution-2): Ga(NO3)3·9H2O—0.2 M + DI water—30 mL. | SiO2 oxide layer eliminated by etching with HF, Nucleation: Reaction in solution-1 at 100 °C for 30 min, Growth: Reaction in solution-2 at 150 °C for 12 h, dried at 50 °C for 60 min, Calcination: 800 °C for 4 h | β-Ga2O3 micro rod arrays (MRAs) | [221] | |||||
| Si (100) | Nucleation (solution-1): Ga(NO3)3·9H2O—0.2 M + ethanol—10 mL + DDI water—30 mL. Crystal Growth (solution-2): Ga(NO3)3·9H2O—0.2 M + DDI water—30 mL. | SiO2 oxide layer eliminated by etching with HF, Nucleation: Reaction in solution-1 at 100 °C for 60 min, Growth: Reaction in solution-2 at 150 °C for 12 h, dried at 70 °C for 2 h, Calcination: 800 °C for 4 h | β-Ga2O3 nanoflakes | β-Ga2O3/p-Si hetero junction self-powered photodiode | [222] | ||||
| FTO glass | (Ga(NO3)3—0.3 g + DI water)—30 mL | Reaction at 150 °C for 12 h, Calcination: Pre-annealed at 400 °C for 4 h then annealed at 700 °C for various times or annealed from 770–830 °C for 20 min | α-Ga2O3 NRAs and α/β-Ga2O3 phase junction NRAs | Solid-state type photodetector, photoelectron-chemical type photodetector | [223] | ||||
| FTO glass | (Ga(NO3)3·9H2O—0.3 g + DI water—30 mL)—0.0239 M | Reaction at 150 °C for a night, dried at 80 °C, Calcination: Pre-annealed 400 °C for 4 then annealed at 700 °C for 20 min | α/β-Ga2O3 phase junction NRAs | Self-powered solar-blind photodetector | [224] | ||||
| A layer of SnO2 on the surface of the FTO glass | (Ga(NO3)3 + DI water)—0.39 mol/L | Hydrothermal reaction at 150 °C for 12 h, dried Calcination: 400 °C for 4 h | Hexagonal prism like α-Ga2O3 NRA, average length—1.62 µm and average diameter 80–200 nm | Self-powered spectrum-distinguish- able α-Ga2O3 NRA/Cu2O microsphere (MS) p-n junction electro-chemical photodetector | [225] | ||||
| Spin coated Ga2O3 seed layered FTO glass | (Ga(NO3)3·9H2O—0.3 g + DI water—30 mL)—0.0239 M | Seed layer annealed at 450 °C for 30 min, Reaction at 150 °C for 12 h, washed with DI water then dried at 80 °C, Calcination: 700 °C for 4 h | β-Ga2O3 NRAs | Solar-blind deep UV photodetector | [226] | ||||
| Spin coated Ga2O3 seed layer on FTO substrate | Ga(NO3)3·9H2O—30 mL (0.03 M) + hexamine (C6H12N4) (0.005 M) | Reaction at 180 °C for 12 h, Calcination: 500 °C for 4 h | α-Ga2O3 nanorod arrays (NRAs) | [227] | |||||
| GaOOH NRAs prepared by hydrothermal method as above | Al(NO3)3·9H2O (0.005 M, 0.01 M, 0.015 M) + C6H12N4 (0.005 M) | α-Ga2O3 nanorod was completely covered by γ-Al2O3 | Ga2O3-Al2O3 heterojunction PEC Self-powered UV detector | ||||||
| hydrothermal method | ((Ga(NO3)3·xH2O (0.1 M) + DI water—50 mL) + SnCl4—0, 0.026, 0.052, 0.130 and 0.260 g) | NH4OH (28% in H2O) added to solution at 60 °C to till pH-10, Reaction at 140 °C for 10 h, dried at 70 °C for 6 h, Calcination:1000 °C in O2 ambient for 6 h | β-Ga2O3 nanostructures | Photocatalyst | [228] | ||||
| Ga2(NO3)3·xH2O (0.05 M) + DI water—80 mL + SnCl2·2H2O (0 mol%, 2 mol%, 4 mol%) | NH4OH added to attain pH-7, Reaction at 100 °C for 5 h, dried overnight at 75 °C, Calcination: 1000 °C for 5 h | β-Ga2O3 nano powder | NH3 gas sensor | [229] | |||||
| hydrothermal method | (Al(NO3)3·9H2O—2.01 g + Ga(NO3)3·9H2O—0.11 g + DI water—40 mL) + methenamine (HMT)-3.2 g | Solution stirred ultrasonically for 1 h, Reaction at 180 °C for 9 h, Calcination: 550 °C for 3 h | Ga2O3/Al2O3 composite materials | NOx gas sensing | [230] | ||||
| Ga(NO3)3·xH2O (0.1 M) + DI water—50 mL + Al (NO3)3·9H2O (0.02, 0.064, 0.110 and 0.210 g) | NH4OH added dropwise till pH-10.34, Reaction at 140 °C for 10 h, dried at 70 °C for 6 h, Calcination: 1000 °C for 6 h in O2 ambient | β-Ga2O3 nanostructures with spindle like morphology to microrod structure | Photocatalyst | [231] | |||||
| Method | Substrate/Template | Precursor | Synthesis Conditions | Properties | Applications | Ref. |
|---|---|---|---|---|---|---|
| Chemical bath method | source]—0.5 mol/L | Stirring at RT for 2 h, then to 90 °C in 1 h, films deposition at 90 °C for 4 h, precipitates centrifugated, washed then dried at 90 °C for 24 h. Calcination: 1000 °C for 4 h | At pH-4, submicrospindles.At pH-9, Self-assembled nanoparticle hierarchical microspheres. | [248] | ||
| Chemical bath method | Quartz glass, β-Ga2O3/Quartz (70 nm) | (Ga(NO3)3·xH2O + DI water)—0.015 mol/L | Substrates put in solution bottles, sealed and kept in oven at 60 °C for 1–48 h, Calcination: 600–900 °C for 1 h | A small number of heterogeneous nucleation GaOOH precipitates | [214] | |
| SnO2, TiO2, MgO coated Quartz glass (Thickness: 70 nm), and FTO glass | The rod like particles of GaOOH were build-up closely and vertically on substrates | |||||
| Chemical bath method | SnO2 coated quartz glass | (Ga(NO3)3·nH2O + Eu(CH3COO)3·4H2O+ DI water)—0.015 mol/L + (urea—0.5 mol/L) concentrations of Eu3+ dopants against Ga3+ (1, 3, 5 and 10 at.%) | Substrates put in solution bottles, sealed and kept in oven at 90 °C for 24 h, Calcination: 900 °C for 1 h | β-Ga2O3:Eu3+ oriented along [111] perpendicular to substrate | [249] | |
| Chemical bath method | Glass | Ga(NO3)3·nH2O (0.025 M, 0.05 M, 0.075 M) + HMT (0.5 M) + DI water —1 L | Solution under stirring while reaction at 95 °C for 5 h, films dried at 70 °C for 5 min, Calcination: 400 °C, 500 °C, or 600 °C for 3 h | α-Ga2O3 film had high crystallinity at 500 °C | Photodegradation | [250] |
| Chemical bath method | Si (001) | (Ga(NO3)3·xH2O + ultra-pure water)—(0.015–0.1 M) | Solution stirred at 70 °C for 12 h, Films deposited at 70 °C for 24 h, Films washed and dried under N2, Calcination: 900 °C for 1 h | 3 types of rod-like morphologies of β-Ga2O3 formed directly on Si | [251] | |
| Chemical bath method | ITO/glass (E-beam deposition) -200 nm | Ga(NO3)3· | A seed layer deposition first 10, 20 and 30 min at 95 °C, Deposition at 95 °C for 2 h, Calcination: 400–600 °C for 1 h | α-Ga2O3 film of thickness 3.5 µm, Film’s crystallinity is high for Cm = 1:1 | pH sensor | [252] |
| Method | Substrate/Template | Precursor | Synthesis Conditions | Properties | Applications | Ref. |
|---|---|---|---|---|---|---|
| Solvothermal | GaCl3 aqueous solution (5.79 mmol) + Eu(NO)3.6H2O (4.06 × 10−2 mmol) + Tb(NO)3.6H2O (2.32 × 10−2 mmol) + (ethylene glycol (EG) and water mixture—2:3) | NaOH (1 mol/L) added dropwise till pH = 10, stirred for 2 h, Reaction in autoclave at 200 °C for 4 h, Products separated by centrifugation then washed and dried at 100 °C in vacuum for 4 h. | PL spectra: Un doped β-Ga2O3—Bright blue emission, 0.01% Tb 3+ doped—Green, 0.01% Eu 3+ doped—Red, Co-doped—White | [253] | ||
| Solvothermal | Si | (Ga metal + HF) + (Solvent: mixture of ethylenediamine (En) and water (volume ratio—80:20, 60:40, 50:50, 40:60 and 30:70)) | Selected amount of precipitate and solvent then stirred for 1 h, Films deposition in autoclave at 200 °C for 4 h, films washed then dried at 100 °C for 1 h | 50:50 sample—2D interconnected nanoflakes structure of flake thickness of 15 nm, 60:40 sample—3D microstructures of order 0.4 µm diameter | [254] | |
| Solvothermal | (Gallium nitrate—0.65 g) + ethanol—50 mL) + (Sodium acetate- 0.6 g + ethanol—50 mL) | Reaction in autoclave at 200 °C for 5h and 10 h, washed with DI water and ethanol then dried in oven at 120 °C for 2 h, No calcination | 5 h—GaOOH morphology was incomplete, 10 h—GaOOH semi nanosphere morphology (size: 100–500 nm) | [255] | ||
| Solvothermal | (Ga(NO3)3·nH2O—0.5 g + HCl—mL + H2O) + ethylene glycol—4.5 mL, (water to EG ratio-2:3) | pH of solution = 10, Reaction in autoclave at 195 °C for 6 h, Precipitates washed with water and ethanol then dried at 80 °C | Ga2O3 nanoparticles size range: 5–10 nm, liquid metal/metal oxide (LM/MO) frameworks with Ga2O3 via ultrasonication | Photocatalyst | [256] | |
| Forced hydrolysis | Solution mixture aged in oil bath at 98 ± 1 °C for 2 h, Precipitates separated by centrifugation and washed then freeze dried at −110 °C, Calcination: 500–1000 °C for 1 h | Uniform polycrystalline β-Ga2O3 Ns (diameter 200 nm) by calcination at 1000 °C @ R = 0.33 | [257] | |||
| Forced hydrolysis | Solution mixture aged in oil bath at 98 ± 1 °C for 2–10 h, Precipitates separated by centrifugation and washed then freeze dried at −110 °C, Calcination: 500−1000 °C for 1 h | Aging time: 2 h—NSs morphology, 10 h- microrods morphology, CL spectra: β-Ga2O3 NSs—UV blue emission peak at 375 nm, β-Ga2O3 microrods—Strong blue emission peak at 416 nm | [258] | |||
| Forced hydrolysis | , = ∞, 3, 2, 1, 0.5, 0.3, 0.2 | Hydrolysis at 90 °C for 9 h, Products centrifuged and washed, Calcination: 450 °C in vacuum | Morphology: At R = ∞—spindle like nanorods having two narrow sides and wide center, R = 1 —dumbbell shape with wide sides and narrow center, R = 0.2—Ga2O3 broken into fragments | [259] | ||
| Reflux condensation | Ga(NO3)3·xH2O—0.01 M + DI water—30 mL + urea—0.1 M | Solution refluxed with continuous stirring at 90 °C for 12 h, Precipitate centrifuged and washed then dried at 100 °C, Calcination: 500 °C and 900 °C for 3 h | Self-assembled pattern of β-Ga2O3 nanorods | Photocatalyst | [260] | |
| Reflux condensation | Ga(NO3)3·xH2O (A)—0.01 M + DI water—30 min + CTAB (B)—stoichiometric amount) Cm ratio: ( = ,, ) | Solution under stirring for 30 min, solution refluxed with continuous stirring at 90 °C for 12 h, precipitate washed and dried at 100 °C, Calcination: 900 °C for 3 h | At optimal Cm ratio = 5/1, uniform and side to side aligned of β-Ga2O3 nanorods (length = 200 nm, diameter = 50 nm) | Photocatalyst | [261] | |
| Electro-deposition | FTO glass | Ga2(SO4)3—20 mM + H2O2 (9.79 M H2O2 solution)—0.13 mL | Applied potential: −1.0 to −1.2, Cathode: FTO glass, Cathode: Pt Deposition time: 2–10 min, Calcination: 500–600 °C | At −1.0 and −1.1 V, deposition rate = 30–60 nm/min, At −1.2 V, deposition rate = 1 µm/min | [262] | |
| Electro-deposition | Si (100) | (Ga2O3 (0.1 M, 0.5 M, 1.M) + HCl—1.5 mL) + DI water—6.5 mL | NH4OH added to vary the pH = 4–10, deposition time = 2 h, Cathode: Pt wire current density = 0.15 A/cm2 | At pH = 4: High density nanodots at 0.1 M, High density nanorods at 1.0 M | [263] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiang, J.-L.; Yadlapalli, B.K.; Chen, M.-I.; Wuu, D.-S. A Review on Gallium Oxide Materials from Solution Processes. Nanomaterials 2022, 12, 3601. https://doi.org/10.3390/nano12203601
Chiang J-L, Yadlapalli BK, Chen M-I, Wuu D-S. A Review on Gallium Oxide Materials from Solution Processes. Nanomaterials. 2022; 12(20):3601. https://doi.org/10.3390/nano12203601
Chicago/Turabian StyleChiang, Jung-Lung, Bharath Kumar Yadlapalli, Mu-I Chen, and Dong-Sing Wuu. 2022. "A Review on Gallium Oxide Materials from Solution Processes" Nanomaterials 12, no. 20: 3601. https://doi.org/10.3390/nano12203601
APA StyleChiang, J. -L., Yadlapalli, B. K., Chen, M. -I., & Wuu, D. -S. (2022). A Review on Gallium Oxide Materials from Solution Processes. Nanomaterials, 12(20), 3601. https://doi.org/10.3390/nano12203601






