Sweep-Out of Tigecycline, Chlortetracycline, Oxytetracycline, and Doxycycline from Water by Carbon Nanoparticles Derived from Tissue Waste
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation of CRNPs
2.3. Characterization of CRNPs
2.4. Adsorption Studies
2.5. Application of CRNPs to Natural Water Treatment
3. Results and Discussion
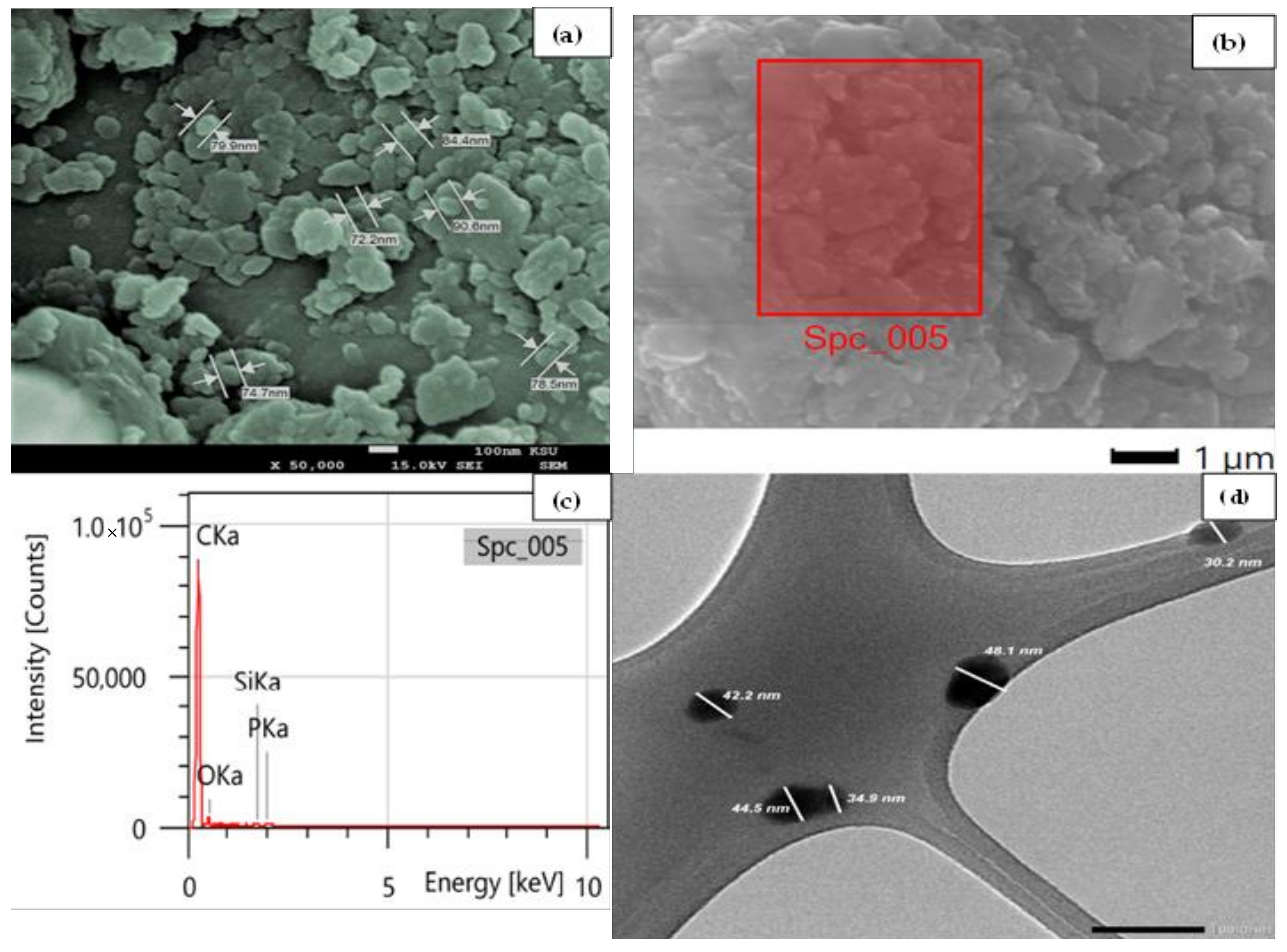
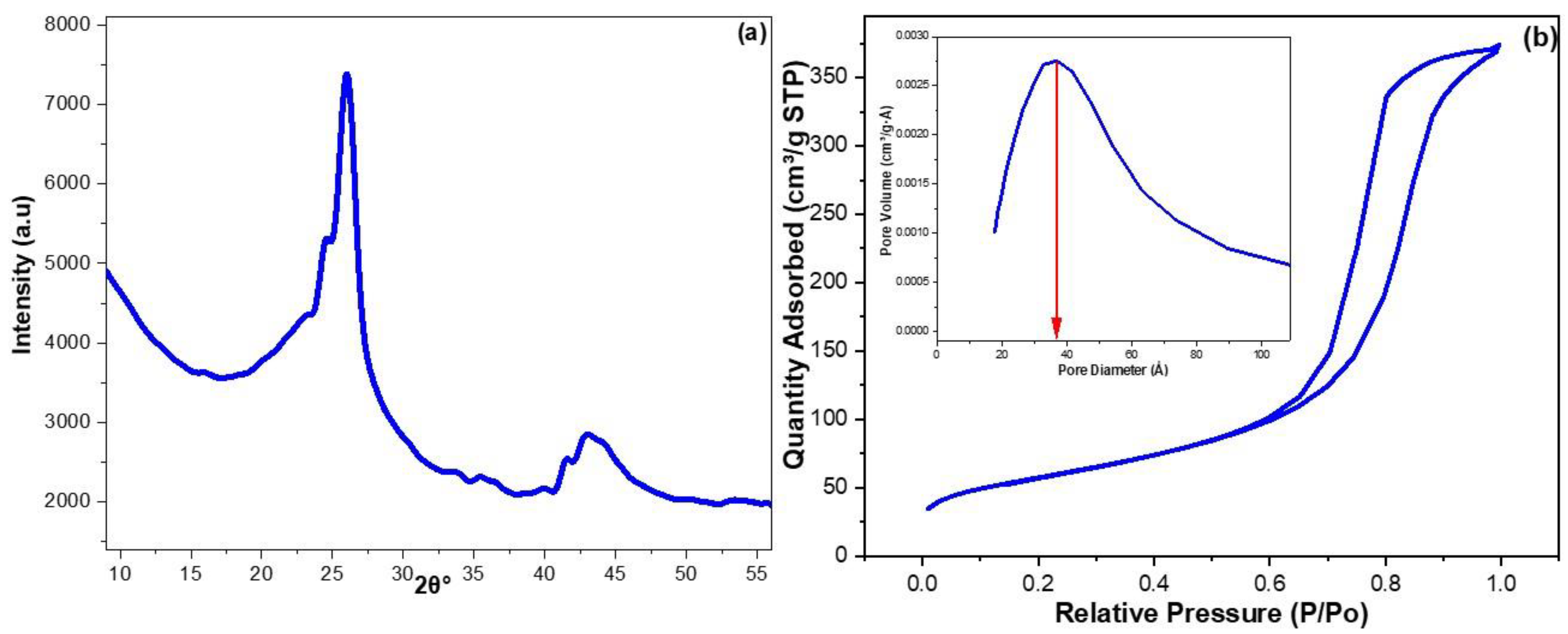
3.1. Characterization of CRNPs
3.2. Adsorption of Pharmaceutical Pollutants by CRNPs
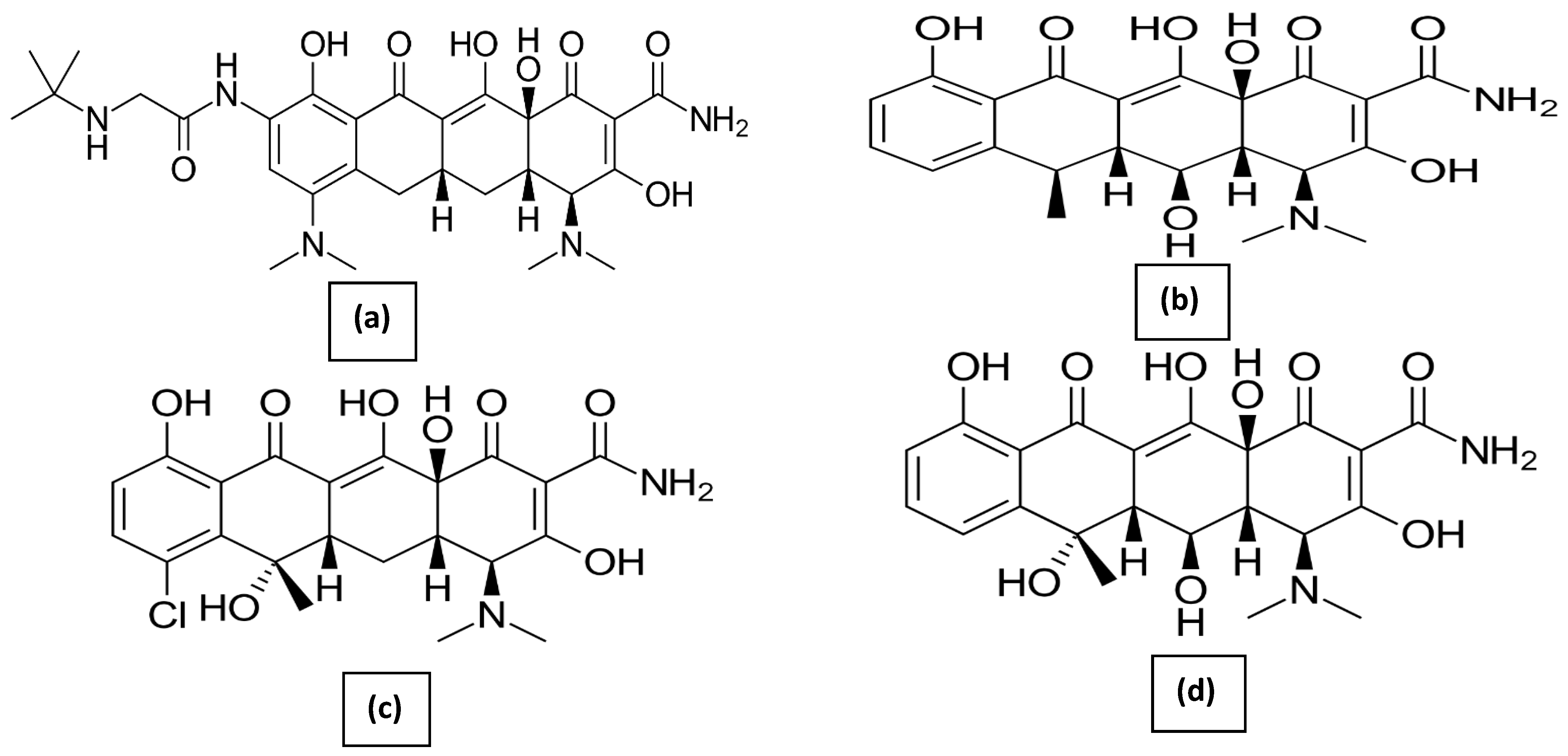
3.3. Possible Adsorption Mechanism
3.4. Adsorption Kinetics
3.5. Adsorption Isotherms
3.6. Adsorption Thermodynamics
3.7. Natural Water Treatment and Regeneration Investigations
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Gleick, P.H.; Palaniappan, M. Peak water limits to freshwater withdrawal and use. Proc. Natl. Acad. Sci. USA 2010, 107, 11155–11162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stumm-Zollinger, E.; Fair, G.M. Biodegradation of steroid hormones. J. Water Pollut. Control Fed. 1965, 37, 1506–1510. [Google Scholar] [PubMed]
- Daughton, C.G.; Ternes, T.A. Pharmaceuticals and personal care products in the environment: Agents of subtle change? Environ. Health Perspect. 1999, 107, 907–938. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Maurer, C.; Wang, Y.; Xue, S.; Davis, D.L. Water pollution and human health in China. Environ. Health Perspect. 1999, 107, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Chander, V.; Sharma, B.; Negi, V.; Aswal, R.; Singh, P.; Singh, R.; Dobhal, R. Pharmaceutical Compounds in Drinking Water. J. Xenobiotics 2016, 6, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kairigo, P.; Ngumba, E.; Sundberg, L.-R.; Gachanja, A.; Tuhkanen, T. Contamination of Surface Water and River Sediments by Antibiotic and Antiretroviral Drug Cocktails in Low and Middle-Income Countries: Occurrence, Risk and Mitigation Strategies. Water 2020, 12, 1376. [Google Scholar] [CrossRef]
- OECD. Health at a Glance 2011: OECD Indicators; OECD Publishing: Paris, France, 2011; p. 2016. [Google Scholar] [CrossRef]
- Khetan, S.K.; Collins, T.J. Human pharmaceuticals in the aquatic environment: A challenge to green chemistry. Chem. Rev. 2007, 107, 2319–2364. [Google Scholar] [CrossRef] [PubMed]
- Sayadi, M.; Trivedy, R.; Pathak, R. Pollution of pharmaceuticals in environment. J. Control Pollut. 2010, 26, 89–94. [Google Scholar]
- Meng, C.; Meng, M.; Sun, X.; Gu, C.; Zou, H.; Li, X. Rapid Degradation of Chlortetracycline Using Hydrodynamic Cavitation with Hydrogen Peroxide. Int. J. Environ. Res. Public Health 2022, 19, 4167. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Fang, X.; Chen, L.; Tang, B.; Song, F.; Li, W. Effect of Acid–Base Modified Biochar on Chlortetracycline Adsorption by Purple Soil. Sustainability 2022, 14, 5892. [Google Scholar] [CrossRef]
- Kim, J.-P.; Jin, D.R.; Lee, W.; Chae, M.; Park, J. Occurrence and Removal of Veterinary Antibiotics in Livestock Wastewater Treatment Plants, South Korea. Processes 2020, 8, 720. [Google Scholar] [CrossRef]
- Li, C.; Sutherland, C.A.; Nightingale, C.H.; Nicolau, D.P. Quantitation of tigecycline, a novel glycyclcycline, by liquid chromatography. J. Chromatogr. B. Anal. Technol. Biomed. Life Sci. 2004, 811, 225–229. [Google Scholar] [CrossRef]
- Sanderson, H.; Ingerslev, F.; Brain, R.A.; Halling-Sorensen, B.; Bestari, J.K.; Wilson, C.J.; Johnson, D.J.; Solomon, K.R. Dissipation of oxytetracycline, chlortetracycline, tetracycline and doxycycline using HPLC–UV and LC/MS/MS under aquatic semi-field microcosm conditions. Chemosphere 2005, 60, 619–629. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yang, H.; Zhang, C.; Mo, Y.; Lu, X. Determination of oxytetracycline, tetracycline and chloramphenicol antibiotics in animal feeds using subcritical water extraction and high performance liquid chromatography. Anal. Chim. Acta 2008, 619, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Du, F.; Zheng, X.; Sun, L.; Qin, Q.; Guo, L.; Ruan, G. Development and validation of polymerized high internal phase emulsion monoliths coupled with HPLC and fluorescence detection for the determination of trace tetracycline antibiotics in environmental water samples. J. Sep. Sci. 2015, 38, 3774–3780. [Google Scholar] [CrossRef]
- Zhao, N.; Liu, K.; Yan, B.; Zhu, L.; Zhao, C.; Gao, J.; Ruan, J.; Zhang, W.; Qiu, R. Chlortetracycline hydrochloride removal by different biochar/Fe composites: A comparative study. J. Hazard. Mater. 2020, 403, 123889. [Google Scholar] [CrossRef] [PubMed]
- El-Shafie, A.S.; Ahsan, I.; Radhwani, M.; Al-Khangi, M.A.; El-Azazy, M. Synthesis and Application of Cobalt Oxide (Co3O4)-Impregnated Olive Stones Biochar for the Removal of Rifampicin and Tigecycline: Multivariate Controlled Performance. Nanomaterials 2022, 12, 379. [Google Scholar] [CrossRef] [PubMed]
- El-Azazy, M.; El-Shafie, A.S.; Al-Meer, S.; Al-Saad, K.A. Eco-structured Adsorptive Removal of Tigecycline from Wastewater: Date Pits’ Biochar versus the Magnetic Biochar. Nanomaterials 2020, 11, 30. [Google Scholar] [CrossRef]
- Bożęcka, A.M.; Orlof-Naturalna, M.M.; Kopeć, M. Methods of Dyes Removal from Aqueous Environment. J. Ecol. Eng. 2021, 22, 111–118. [Google Scholar] [CrossRef]
- Ho, S. Protection, Removal of Dyes from Wastewater by Adsorption onto Activated Carbon: Mini Review. J. Geosci. Environ. Protect. 2020, 8, 120. [Google Scholar] [CrossRef]
- Elamin, M.R.; Taha, K.K.; Abdulkhair, B.Y.; Khezami, L. Preparation of Large Carbon Nanofibers on a Stainless Steel Surface and Elucidation of their Growth Mechanisms. Zeitschrift für Naturforschung A 2018, 74, 253–258. [Google Scholar] [CrossRef]
- Elamin, M.R.; Elzupir, A.O.; Abdulkhair, B.Y. Synthesis and characterization of functionalized carbon nanofibers for efficient removal of highly water-soluble dextromethorphan and guaifenesin from environmental water samples. Environ. Nanotechnol. Monit. Manag. 2020, 15, 100397. [Google Scholar] [CrossRef]
- Ruhoy, I.S.; Daughton, C.G. Beyond the medicine cabinet: An analysis of where and why medications accumulate. Environ. Int. 2008, 34, 1157–1169. [Google Scholar] [CrossRef]
- Zhao, Y.; Xiao, X. Environmental Antibiotics: Exposure Monitoring and Health Endpoints. In Emerging Chemicals and Human Health; Springer: Berlin/Heidelberg, Germany, 2019; pp. 165–178. [Google Scholar]
- Sreekanth, D.; Sivaramakrishna, D.; Himabindu, V.; Anjaneyulu, Y. Thermophilic treatment of bulk drug pharmaceutical industrial wastewaters by using hybrid up flow anaerobic sludge blanket reactor. Bioresour. Technol. 2009, 100, 2534–2539. [Google Scholar] [CrossRef]
- Chelliapan, S. Treatment of Pharmaceutical Wastewater Containing Macrolide Antibiotics by Up-Flow Anaerobic Stage Reactor (UASR). Ph.D. Thesis, University of Newcastle upon Tyne, Newcastle upon Tyne, UK, 2006. [Google Scholar]
- Gadipelly, C.; Pérez-González, A.; Yadav, G.D.; Ortiz, I.; Ibáñez, R.; Rathod, V.K.; Marathe, K.V. Pharmaceutical Industry Wastewater: Review of the Technologies for Water Treatment and Reuse. Ind. Eng. Chem. Res. 2014, 53, 11571–11592. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, Y.; Yao, B.; Zou, D. Removal of chlortetracycline by nano- micro-electrolysis materials: Application and mechanism. Chemosphere 2019, 238, 124543. [Google Scholar] [CrossRef]
- Chen, H.; Zhou, Y.; Hu, X.; Tian, K.; Zhang, J. Effects of chlortetracycline on biological nutrient removal from wastewater. Sci. Total Environ. 2018, 647, 268–274. [Google Scholar] [CrossRef]
- Ghaemi, M.; Absalan, G. Fast removal and determination of doxycycline in water samples and honey by Fe3O4 magnetic nanoparticles. J. Iran. Chem. Soc. 2015, 12, 1–7. [Google Scholar] [CrossRef]
- Tian, Z.; Liu, R.; Zhang, H.; Yang, M.; Zhang, Y. Developmental dynamics of antibiotic resistome in aerobic biofilm microbiota treating wastewater under stepwise increasing tigecycline concentrations. Environ. Int. 2019, 131, 105008. [Google Scholar] [CrossRef] [PubMed]
- Zahoor, M.; Wahab, M.; Salman, S.M.; Sohail, A.; Ali, E.A.; Ullah, R. Removal of Doxycycline from Water using Dalbergia sissoo Waste Biomass Based Activated Carbon and Magnetic Oxide/Activated Bioinorganic Nanocomposite in Batch Adsorption and Adsorption/Membrane Hybrid Processes. Bioinorg. Chem. Appl. 2022, 2022, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Kolpin, D.W.; Furlong, E.T.; Meyer, M.T.; Thurman, E.M.; Zaugg, S.D.; Barber, L.B.; Buxton, H.T. Pharmaceuticals, hormones, and other organic wastewater contaminants in US streams, 1999–2000: A national reconnaissance. Environ. Sci. Technol. 2002, 36, 1202–1211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pelosato, R.; Bolognino, I.; Fontana, F.; Sora, I.N. Applications of Heterogeneous Photocatalysis to the Degradation of Oxytetracycline in Water: A Review. Molecules 2022, 27, 2743. [Google Scholar] [CrossRef] [PubMed]
- Ata, R.; Töre, G.Y. Characterization and removal of antibiotic residues by NFC-doped photocatalytic oxidation from domestic and industrial secondary treated wastewaters in Meric-Ergene Basin and reuse assessment for irrigation. J. Environ. Manage. 2019, 233, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Sani, O.N.; Fezabady, A.A.N.; Yazdani, M.; Taghavi, M. Catalytic ozonation of ciprofloxacin using γ-Al2O3 nanoparticles in synthetic and real wastewaters. J. Water Process Eng. 2019, 32, 100894. [Google Scholar] [CrossRef]
- Wang, Z.; Muhammad, Y.; Tang, R.; Lu, C.; Yu, S.; Song, R.; Tong, Z.; Han, B.; Zhang, H. Dually organic modified bentonite with enhanced adsorption and desorption of tetracycline and ciprofloxacine. Sep. Purif. Technol. 2021, 274, 119059. [Google Scholar] [CrossRef]
- Pankey, G.A. Tigecycline. J. Antimicrob. Chemoter. 2005, 56, 470–480. [Google Scholar] [CrossRef]
- Topare, N.S.; Bokil, S.A. Adsorption of textile industry effluent in a fixed bed column using activated carbon prepared from agro-waste materials. Mater. Today Proc. 2021, 43, 530–534. [Google Scholar] [CrossRef]
- Yurtsever, A.; Basaran, E.; Ucar, D.; Sahinkaya, E. Self-forming dynamic membrane bioreactor for textile industry wastewater treatment. Sci. Total Environ. 2021, 751, 141572. [Google Scholar] [CrossRef]
- Feng, Q.; Gao, B.; Yue, Q.; Guo, K. Flocculation performance of papermaking sludge-based flocculants in different dye wastewater treatment: Comparison with commercial lignin and coagulants. Chemosphere 2020, 262, 128416. [Google Scholar] [CrossRef]
- Othman, M.H.D.; Ridhwan, A.; Kamaludin, R.; Ismail, N.J.; Rahman, M.A.; Jaafar, J. Advanced Membrane Technology for Textile Wastewater Treatment. In Membrane Technology Enhancement for Environmental Protection and Sustainable Industrial Growth; Springer: Berlin/Heidelberg, Germany, 2021; pp. 91–108. [Google Scholar]
- Chowdhury, M.F.; Khandaker, S.; Sarker, F.; Islam, A.; Rahman, M.T.; Awual, M.R. Current treatment technologies and mechanisms for removal of indigo carmine dyes from wastewater: A review. J. Mol. Liq. 2020, 318, 114061. [Google Scholar] [CrossRef]
- Harrache, Z.; Abbas, M.; Aksil, T.; Trari, M. Thermodynamic and kinetics studies on adsorption of Indigo Carmine from aqueous solution by activated carbon. Microchem. J. 2018, 144, 180–189. [Google Scholar] [CrossRef]
- Oberoi, A.S.; Jia, Y.; Zhang, H.; Khanal, S.K.; Lu, H. Insights into the Fate and Removal of Antibiotics in Engineered Biological Treatment Systems: A Critical Review. Environ. Sci. Technol. 2019, 53, 7234–7264. [Google Scholar] [CrossRef] [PubMed]
- Jones, O.; Lester, J.N.; Voulvoulis, N. Pharmaceuticals: A threat to drinking water? Trends Biotechnol. 2005, 23, 163–167. [Google Scholar] [CrossRef]
- Hussin, F.; Aroua, M.K.; Kassim, M.A.; Ali, U.F. Transforming Plastic Waste into Porous Carbon for Capturing Carbon Dioxide: A Review. Energies 2021, 14, 8421. [Google Scholar] [CrossRef]
- Abdulkhair, B.Y.; Elamin, M.R. Low-Cost Carbon Nanoparticles for Removing Hazardous Organic Pollutants from Water: Complete Remediation Study and Multi-Use Investigation. Inorganics 2022, 10, 136. [Google Scholar] [CrossRef]
- Almufarij, R.S.; Abdulkhair, B.Y.; Salih, M.; Aldosari, H.; Aldayel, N.W. Optimization, Nature, and Mechanism Investigations for the Adsorption of Ciprofloxacin and Malachite Green onto Carbon Nanoparticles Derived from Low-Cost Precursor via a Green Route. Molecules 2022, 27, 4577. [Google Scholar] [CrossRef] [PubMed]
- Elamin, M.R.; Abdulkhair, B.Y.; Elzupir, A.O. Removal of ciprofloxacin and indigo carmine from water by carbon nanotubes fabricated from a low-cost precursor: Solution parameters and recyclability. Ain Shams Eng. J. 2022, 14, 101844. [Google Scholar] [CrossRef]
- Elamin, M.R.; Ibnaouf, K.H.; Elamin, N.Y.; Adam, F.A.; Alolayan, A.H.; Abdulkhair, B.Y. Spontaneous Adsorption and Efficient Photodegradation of Indigo Carmine under Visible Light by Bismuth Oxyiodide Nanoparticles Fabricated Entirely at Room Temperature. Inorganics 2022, 10, 65. [Google Scholar] [CrossRef]
- Ghoniem, M.G.; Ali, F.A.M.; Abdulkhair, B.Y.; Elamin, M.R.A.; Alqahtani, A.M.; Rahali, S.; Ben Aissa, M.A. Highly Selective Removal of Cationic Dyes from Wastewater by MgO Nanorods. Nanomaterials 2022, 12, 1023. [Google Scholar] [CrossRef]
- Tang, Y.; Huang, H.; Xue, W.; Chang, Y.; Li, Y.; Guo, X.; Zhong, C. Rigidifying induced fluorescence enhancement in 2D porous covalent triazine framework nanosheets for the simultaneously luminous detection and adsorption removal of antibiotics. Chem. Eng. J. 2020, 384, 123382. [Google Scholar] [CrossRef]
- Sabzehmeidani, M.M.; Mahnaee, S.; Ghaedi, M.; Heidari, H.; Roy, V.A.L. Carbon based materials: A review of adsorbents for inorganic and organic compounds. Mater. Adv. 2021, 2, 598–627. [Google Scholar] [CrossRef]
- Jjagwe, J.; Olupot, P.W.; Menya, E.; Kalibbala, H.M. Synthesis and Application of Granular Activated Carbon from Biomass Waste Materials for Water Treatment: A Review. J. Bioresour. Bioprod. 2021, 6, 292–322. [Google Scholar] [CrossRef]
- Kafil, M.; Nasab, S.B.; Moazed, H.; Jokiniemi, J.; Lähde, A.; Bhatnagar, A. Efficient removal of azo dyes from water with chitosan/carbon nanoflower as a novel nanocomposite synthesized by pyrolysis technique. Desalination Water Treat. 2019, 142, 308–320. [Google Scholar] [CrossRef] [Green Version]
- Wan, S.; Bi, H.; Sun, L. Graphene and carbon-based nanomaterials as highly efficient adsorbents for oils and organic solvents. Nanotechnol. Rev. 2016, 5, 3–22. [Google Scholar] [CrossRef]
- Tavakkoli, M.; Flahaut, E.; Peljo, P.; Sainio, J.; Davodi, F.; Lobiak, E.V.; Mustonen, K.; Kauppinen, E.I. Mesoporous Single-Atom-Doped Graphene–Carbon Nanotube Hybrid: Synthesis and Tunable Electrocatalytic Activity for Oxygen Evolution and Reduction Reactions. ACS Catal. 2020, 10, 4647–4658. [Google Scholar] [CrossRef] [Green Version]
- Xu, W.; Dong, X.; Wang, Y.; Zheng, N.; Zheng, B.; Lin, Q.; Zhao, Y. Controllable Synthesis of MoS2/Carbon Nanotube Hybrids with Enlarged Interlayer Spacings for Efficient Electrocatalytic Hydrogen Evolution. ChemistrySelect 2020, 5, 13603–13608. [Google Scholar] [CrossRef]
- Fu, S.; Chen, X.; Liu, P. Preparation of CNTs/Cu composites with good electrical conductivity and excellent mechanical properties. Mater. Sci. Eng. A 2019, 771, 138656. [Google Scholar] [CrossRef]
- Verma, B.; Goel, S.; Balomajumder, C. Multiwalled CNTs for Cr (VI) removal from industrial wastewater: An advanced study on adsorption, kinetics, thermodynamics for the comparison between the embedded and non-embedded carboxyl group. Can. J. Chem. Eng. 2021, 99, 281–293. [Google Scholar] [CrossRef]
- Elamin, M.R.; Abdulkhair, B.Y.; Elzupir, A.O. Insight to aspirin sorption behavior on carbon nanotubes from aqueous solution: Thermodynamics, kinetics, influence of functionalization and solution parameters. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Mote, V.; Purushotham, Y.; Dole, B. Williamson-Hall analysis in estimation of lattice strain in nanometer-sized ZnO particles. J. Theor. Appl. Phys. 2012, 6, 1–8. [Google Scholar] [CrossRef]
- Rosly, H.N.; Rahman, K.S.; Abdullah, S.F.; Harif, M.N.; Doroody, C.; Chelvanathan, P.; Misran, H.; Sopian, K.; Amin, N. The Role of Deposition Temperature in the Photovoltaic Properties of RF-Sputtered CdSe Thin Films. Crystals 2021, 11, 73. [Google Scholar] [CrossRef]
- Ilyas, S.; Heryanto; Abdullah, B.; Tahir, D. X-ray diffraction analysis of nanocomposite Fe3O4/activated carbon by Williamson–Hall and size-strain plot methods. Nano-Struct. Nano-Objects 2019, 20, 100396. [Google Scholar] [CrossRef]
- Brito, S.M.D.O.; Andrade, H.M.C.; Soares, L.F.; de Azevedo, R.P. Brazil nut shells as a new biosorbent to remove methylene blue and indigo carmine from aqueous solutions. J. Hazard. Mater. 2010, 174, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Supriya, S.; Sriram, G.; Ngaini, Z.; Kavitha, C.; Kurkuri, M.; De Padova, I.P.; Hegde, G. The Role of Temperature on Physical–Chemical Properties of Green Synthesized Porous Carbon Nanoparticles. Waste Biomass- Valorization 2019, 11, 3821–3831. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Rouquerol, J.; Avnir, D.; Fairbridge, C.W.; Everett, D.H.; Haynes, J.M.; Pernicone, N.; Ramsay, J.D.F.; Sing, K.S.W.; Unger, K.K. Recommendations for the characterization of porous solids (Technical Report). Pure Appl. Chem. 1994, 66, 1739–1758. [Google Scholar] [CrossRef]
- Sun, S.; Liang, F.; Tang, L.; Wu, J.; Ma, C. Microstructural investigation of gas shale in Longmaxi Formation, Lower Silurian, NE Sichuan Basin, China. Energy Explor. Exploit. 2017, 35, 406–429. [Google Scholar] [CrossRef]
- Suhdi; Wang, S.-C. Fine Activated Carbon from Rubber Fruit Shell Prepared by Using ZnCl2 and KOH Activation. Appl. Sci. 2021, 11, 3994. [Google Scholar] [CrossRef]
- Bandura, L.; Panek, R.; Madej, J.; Franus, W. Synthesis of zeolite-carbon composites using high-carbon fly ash and their adsorption abilities towards petroleum substances. Fuel 2020, 283, 119173. [Google Scholar] [CrossRef]
- Yang, J.; Wang, X.; Dai, W.; Lian, X.; Cui, X.; Zhang, W.; Zhang, K.; Lin, M.; Zou, R.; Loh, K.P.; et al. From micropores to ultra-micropores inside hard carbon: Toward enhanced capacity in room-/low-temperature sodium-ion storage. Nano-MicroLetters 2021, 13, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Lu, Y.; Zheng, F.; Xue, X.; Li, N.; Liu, D. Adsorption behavior and mechanisms of norfloxacin onto porous resins and carbon nanotube. Chem. Eng. J. 2012, 179, 112–118. [Google Scholar] [CrossRef]
- Lin, P.; Zhang, Y.; Zhang, X.; Chen, C.; Xie, Y.; Suffet, I.H. The influence of chlorinated aromatics’ structure on their adsorption characteristics on activated carbon to tackle chemical spills in drinking water source. Front. Environ. Sci. Eng. 2015, 9, 138–146. [Google Scholar] [CrossRef]
- Naghizadeh, A.; Karimi, A.; Derkhshani, E.; Esform, A. Single-walled carbon nanotubes (SWCNTs) as an efficient adsorbent for removal of reactive dyes from water solution: Equilibrium, kinetic, and thermodynamic. Environ. Qual. Manag. 2021, 31, 133–140. [Google Scholar] [CrossRef]
- An, B. Cu(II) and As(V) Adsorption Kinetic Characteristic of the Multifunctional Amino Groups in Chitosan. Processes 2020, 8, 1194. [Google Scholar] [CrossRef]
- Khayyun, T.S.; Mseer, A.H. Comparison of the experimental results with the Langmuir and Freundlich models for copper removal on limestone adsorbent. Appl. Water Sci. 2019, 9, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Acharya, J.; Sahu, J.N.; Mohanty, C.; Meikap, B. Removal of lead(II) from wastewater by activated carbon developed from Tamarind wood by zinc chloride activation. Chem. Eng. J. 2009, 149, 249–262. [Google Scholar] [CrossRef]
- Salam, M.A.; Al-Zhrani, G.; Kosa, S.A. Removal of heavy metal ions from aqueous solution by multi-walled carbon nanotubes modified with 8-hydroxyquinoline: Kinetic study. J. Ind. Eng. Chem. 2014, 20, 572–580. [Google Scholar] [CrossRef]
- Largitte, L.; Pasquier, R. A review of the kinetics adsorption models and their application to the adsorption of lead by an activated carbon. Chem. Eng. Res. Des. 2016, 109, 495–504. [Google Scholar] [CrossRef]
- Letshwenyo, M.W.; Mokgosi, S. Investigation of water treatment sludge from drinking water treated with Zetafloc 553I coagulant for phosphorus removal from wastewater. J. Environ. Manage. 2021, 282, 111909. [Google Scholar] [CrossRef] [PubMed]
- Demirbas, O.; Calimli, M.H.; Kuyuldar, E.; Alma, M.H.; Nas, M.S.; Sen, F. Equilibrium, Kinetics, and Thermodynamic of Adsorption of Enzymes on Diatomite Clay Materials. BioNanoScience 2019, 9, 474–482. [Google Scholar] [CrossRef]
- Aarab, N.; Hsini, A.; Essekri, A.; Laabd, M.; Lakhmiri, R.; Albourine, A. Removal of an emerging pharmaceutical pollutant (metronidazole) using PPY-PANi copolymer: Kinetics, equilibrium and DFT identification of adsorption mechanism. Groundw. Sustain. Dev. 2020, 11, 100416. [Google Scholar] [CrossRef]
- Vasudevan, S.; Lakshmi, J. Studies relating to an electrochemically assisted coagulation for the removal of chromium from water using zinc anode. Water Supply 2011, 11, 142–150. [Google Scholar] [CrossRef]
- Inglezakis, V.J.; Zorpas, A.A. Heat of adsorption, adsorption energy and activation energy in adsorption and ion exchange systems. Desalination Water Treat. 2012, 39, 149–157. [Google Scholar] [CrossRef]
- Wang, N.; Han, Y.; Li, S. Adsorption characteristic of Cr (VI) onto different activated coal fly ashes: Kinetics, thermodynamic, application feasibility, and error analysis. Water Air Soil Pollut. 2019, 230, 1–13. [Google Scholar] [CrossRef]






| Adsorption Kinetic | ||||||||
|---|---|---|---|---|---|---|---|---|
| Adsorption rate order | ||||||||
| PSFOM | PSSOM | |||||||
| Parameter | TGCN | DXCN | CTCN | OTCN | TGCN | DXCN | CTCN | OTCN |
| qe exp. (mg g−1) | 82.80 | 122.87 | 117.21 | 138.44 | 82.80 | 122.87 | 117.21 | 138.44 |
| qe cal. (mg g−1) | 18.437 | 29.714 | 34.765 | 42.360 | 81.037 | 114.286 | 111.111 | 130.039 |
| R2 | 0.951 | 0.910 | 0.981 | 0.974 | 0.930 | 0.809 | 0.904 | 0.912 |
| Rate constant | 0.065 | 0.034 | 0.049 | 0.042 | 0.012 | 0.009 | 0.009 | 0.008 |
| Adsorption mechanism | ||||||||
| Pollutant | IDM | LDM | ||||||
| KIP (mg g−1 min0.5) | C (mg g−1) | R2 | KLF (min−1) | R2 | ||||
| TGCN | 1.632 | 30.916 | 0.884 | 0.068 | 0.976 | |||
| DXCN | 2.043 | 44.514 | 0.887 | 0.064 | 0.818 | |||
| CTCN | 2.828 | 38.692 | 0.942 | 0.071 | 0.913 | |||
| OTCN | 3.466 | 44.019 | 0.932 | 0.068 | 0.877 | |||
| Adsorption Isotherms | ||||||
|---|---|---|---|---|---|---|
| Isotherm model | LIM | FIM | ||||
| Drug ↓ | R2 (a.u.) | KL (L mg−1) | qm (mg g−1) | R2 (a.u.) | Kf (L mg−1) | n−1 (a.u.) |
| TGCN | 0.974 | 106.925 | 0.684 | 0.972 | 0.022 | 0.238 |
| DXCN | 0.944 | 184.213 | 0.236 | 0.848 | 0.326 | 0.311 |
| CTCN | 0.937 | 138.692 | 0.243 | 0.943 | 0.029 | 0.407 |
| OTCN | 0.973 | 157.557 | 0.397 | 0.949 | 0.021 | 0.372 |
| Thermodynamic parameters | ||||||
| Initial conc. (mg L−1) | ΔH° (k Jmol−1) | ΔS° (kJ mol−1) | ΔG° (kJ mol−1) 298 K | ΔG° (kJ mol−1) 308 K | ΔG° (kJ mol−1) 318 K | |
| TGCN | ||||||
| 20 | 38.451 | 0.152 | −6.931 | −9.215 | −11.499 | |
| 40 | 76.793 | 0.271 | −3.949 | −8.014 | −12.078 | |
| 80 | 38.448 | 0.131 | −0.676 | −2.646 | −4.615 | |
| 100 | 29.776 | 0.098 | 0.700 | −0.764 | −2.227 | |
| DXCN | ||||||
| 20 | 34.002 | 0.136 | −6.498 | −8.536 | −10.575 | |
| 40 | 69.313 | 0.255 | −6.642 | −10.465 | −14.288 | |
| 80 | 43.464 | 0.189 | −12.751 | −15.581 | −18.410 | |
| 100 | 31.822 | 0.110 | −0.910 | −2.558 | −4.205 | |
| CTCN | ||||||
| 20 | 102.717 | 0.356 | −3.276 | −8.611 | −13.947 | |
| 40 | 24.608 | 0.088 | −1.556 | −2.873 | −4.190 | |
| 80 | 34.168 | 0.116 | −0.501 | −2.246 | −3.991 | |
| 100 | 36.517 | 0.125 | −0.743 | −2.619 | −4.494 | |
| OTCN | ||||||
| 20 | 78.631 | 0.280 | −4.673 | −8.866 | −13.059 | |
| 40 | 90.231 | 0.316 | −4.015 | −8.758 | −13.502 | |
| 80 | 35.365 | 0.125 | −1.819 | −3.691 | −5.562 | |
| 100 | 27.247 | 0.096 | −1.353 | −2.792 | −4.232 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almufarij, R.S.; Abdulkhair, B.Y.; Salih, M.; Alhamdan, N.M. Sweep-Out of Tigecycline, Chlortetracycline, Oxytetracycline, and Doxycycline from Water by Carbon Nanoparticles Derived from Tissue Waste. Nanomaterials 2022, 12, 3617. https://doi.org/10.3390/nano12203617
Almufarij RS, Abdulkhair BY, Salih M, Alhamdan NM. Sweep-Out of Tigecycline, Chlortetracycline, Oxytetracycline, and Doxycycline from Water by Carbon Nanoparticles Derived from Tissue Waste. Nanomaterials. 2022; 12(20):3617. https://doi.org/10.3390/nano12203617
Chicago/Turabian StyleAlmufarij, Rasmiah S., Babiker Y. Abdulkhair, Mutaz Salih, and Nujud M. Alhamdan. 2022. "Sweep-Out of Tigecycline, Chlortetracycline, Oxytetracycline, and Doxycycline from Water by Carbon Nanoparticles Derived from Tissue Waste" Nanomaterials 12, no. 20: 3617. https://doi.org/10.3390/nano12203617
APA StyleAlmufarij, R. S., Abdulkhair, B. Y., Salih, M., & Alhamdan, N. M. (2022). Sweep-Out of Tigecycline, Chlortetracycline, Oxytetracycline, and Doxycycline from Water by Carbon Nanoparticles Derived from Tissue Waste. Nanomaterials, 12(20), 3617. https://doi.org/10.3390/nano12203617






