Heterostructured α-Bi2O3/BiOCl Nanosheet for Photocatalytic Applications
Abstract
1. Introduction
2. Experimental
2.1. Preparation of α-Bi2O3
2.2. Preparation of BiOCl and B2O3/BiOCl from Adsorbed Cl−
2.3. Characterizations
2.4. Photocatalytic Degradation Measurement
3. Results and Discussion
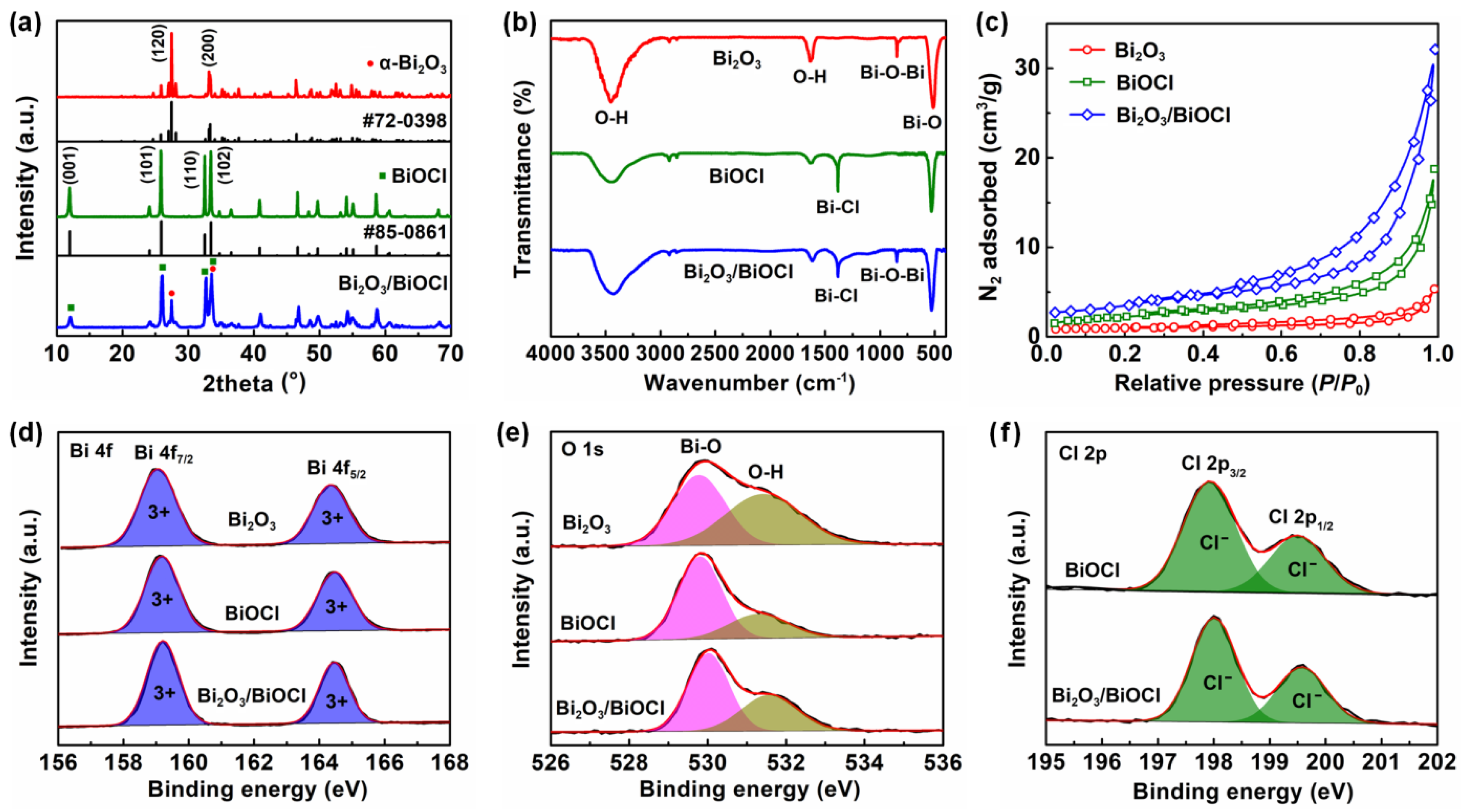
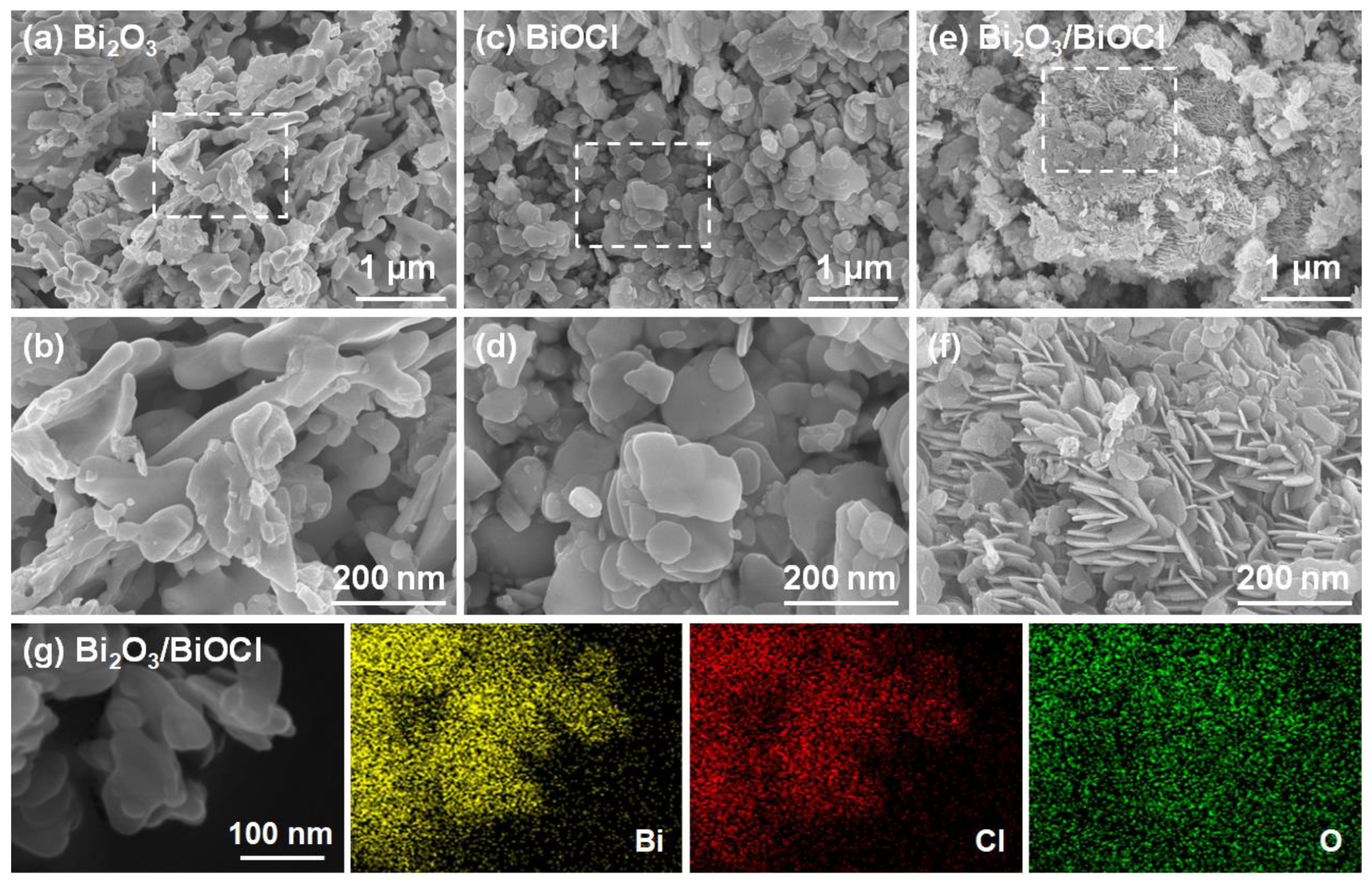
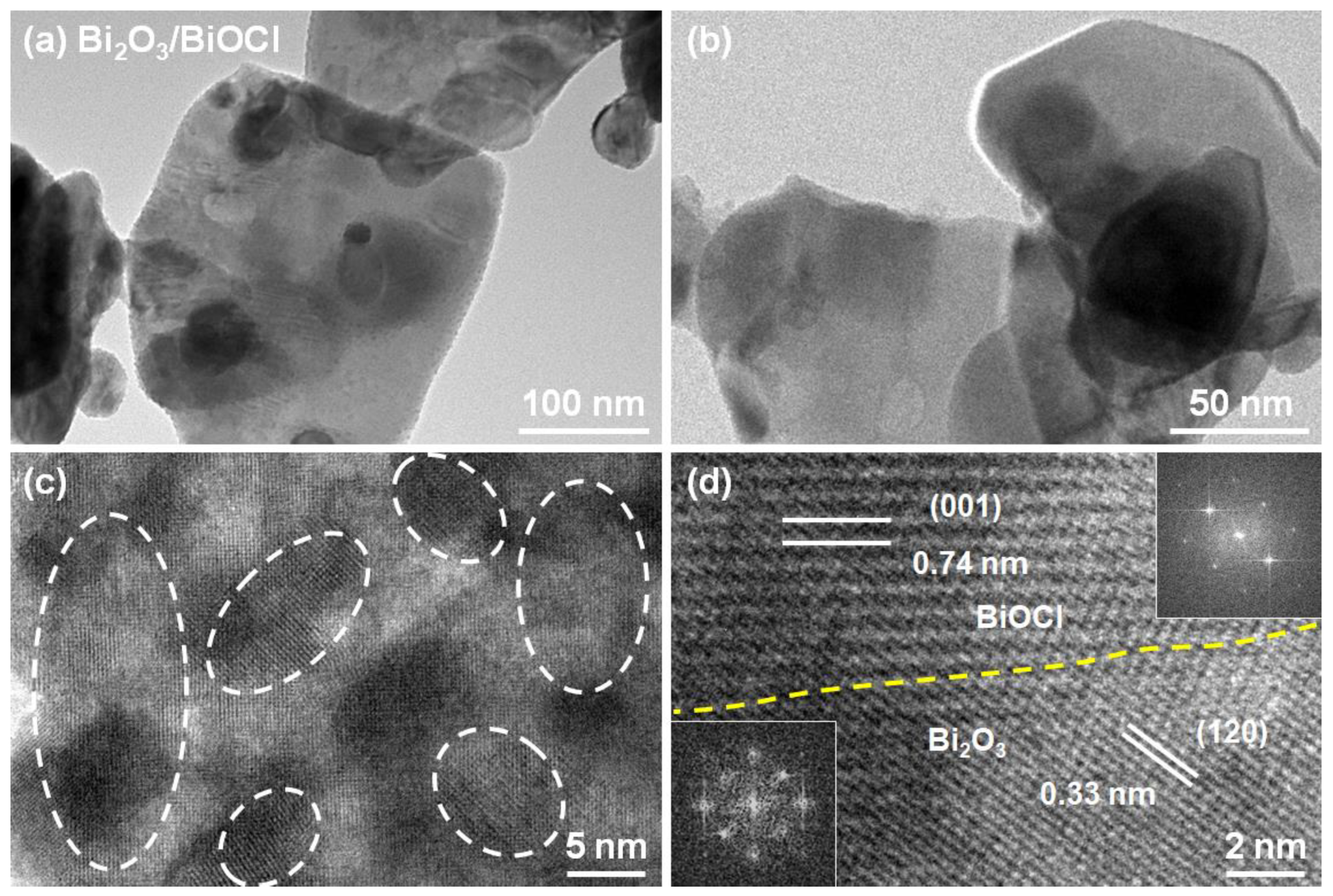
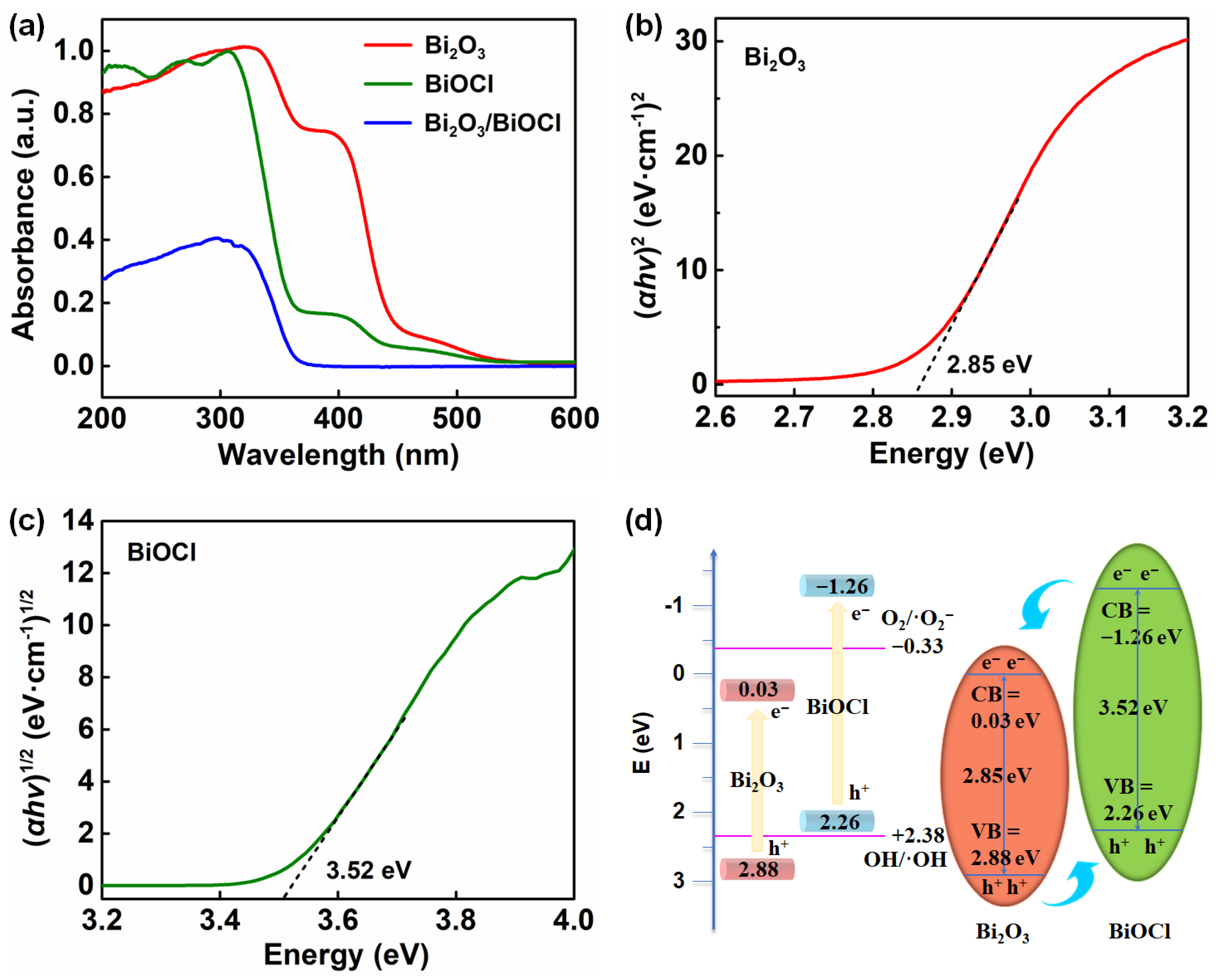
3.1. Physical Properties of Materials
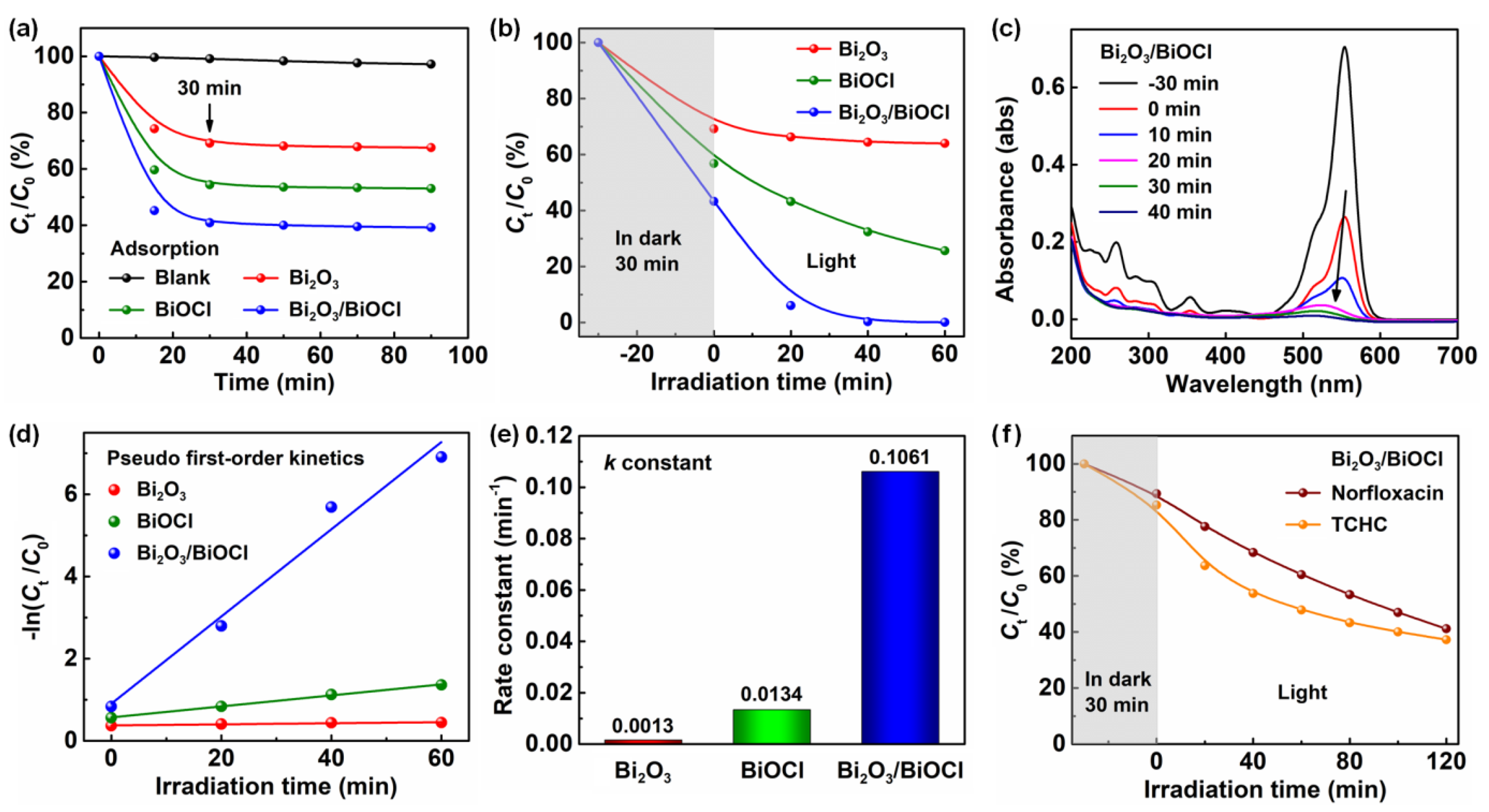
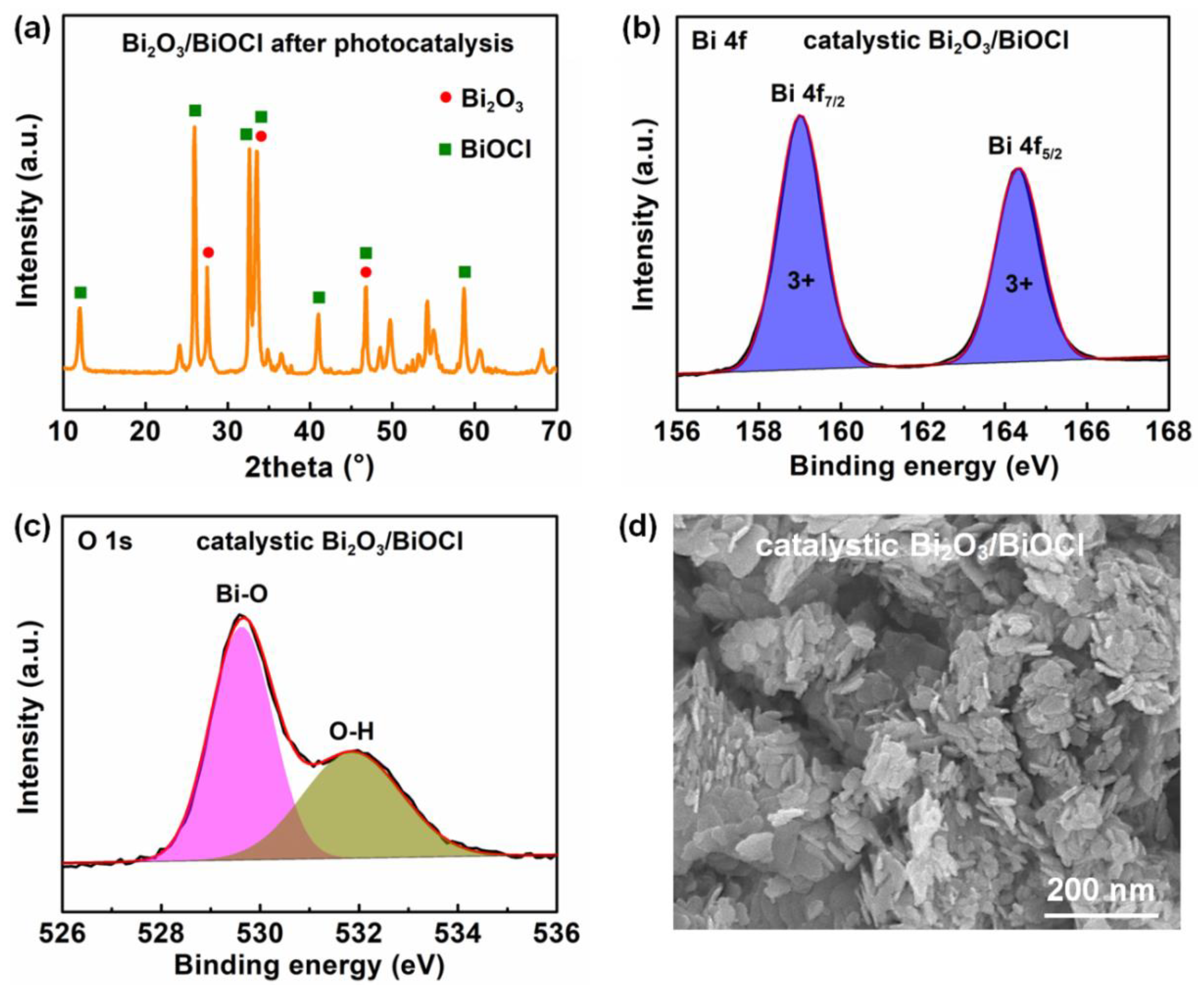
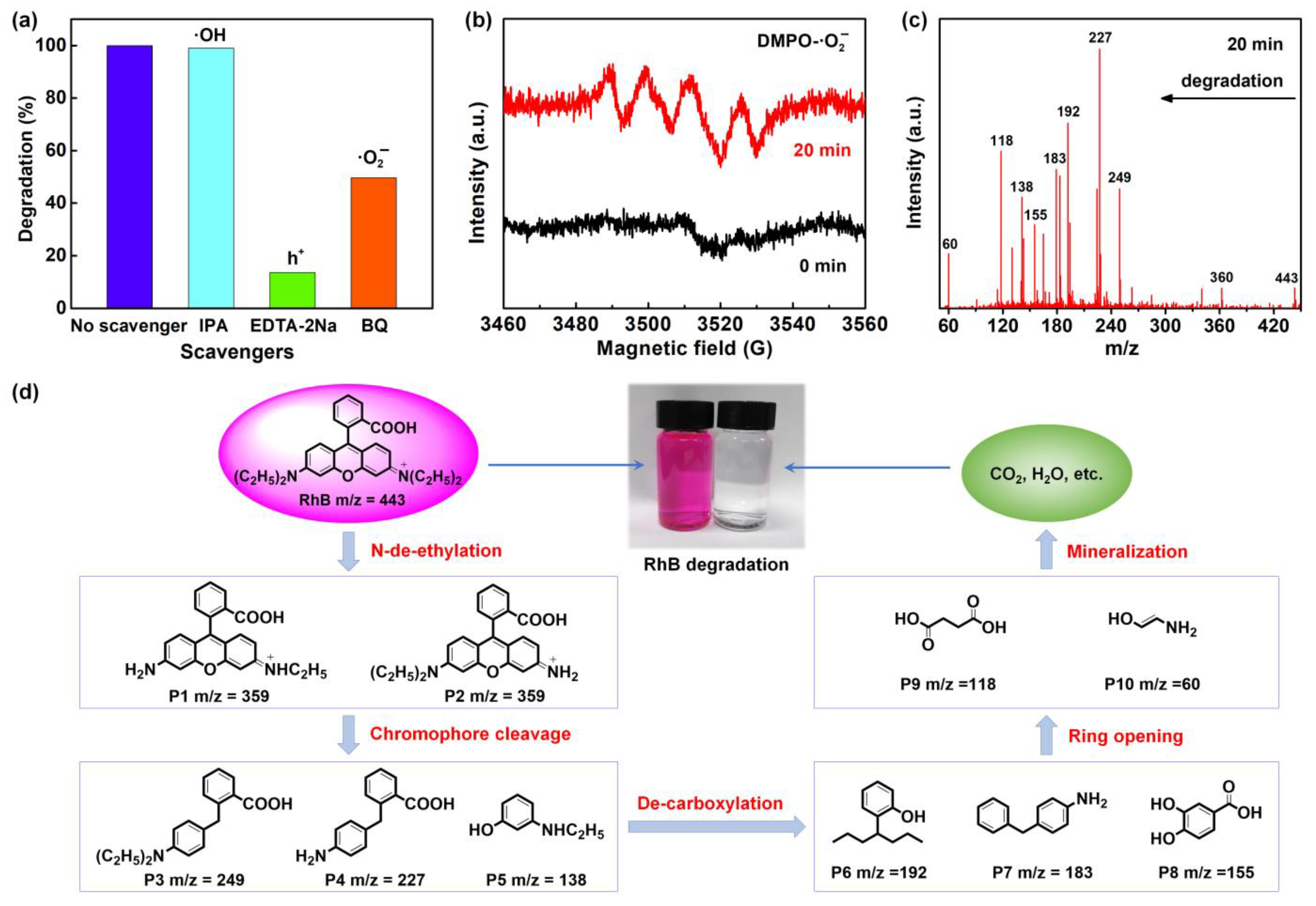
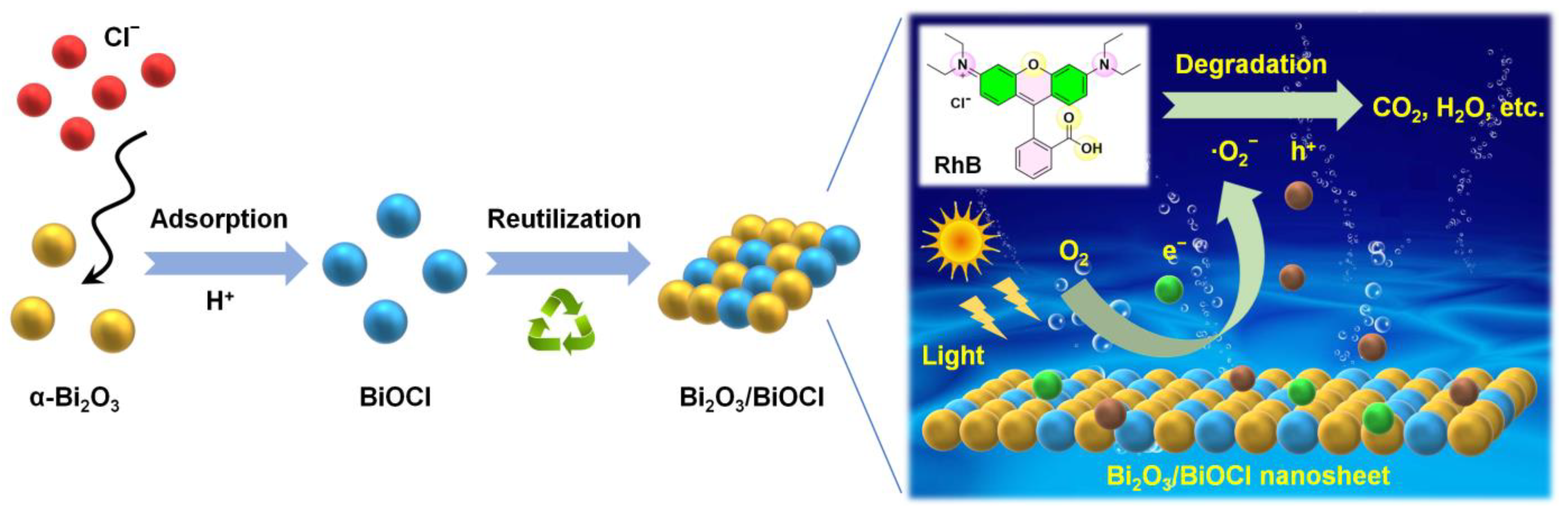
3.2. Photocatalytic Performances
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lim, S.; Shi, J.L.; Gunten, U.; McCurry, D.L. Ozonation of organic compounds in water and wastewater: A critical review. Water Res. 2022, 213, 118053. [Google Scholar] [CrossRef]
- Li, K.; He, Y.; Xu, Y.; Wang, Y.; Jia, J. Degradation of Rhodamine B using an unconventional graded photoelectrode with wedge structure. Environ. Sci. Technol. 2011, 45, 7401–7407. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Feng, Q.; Liu, K.; Li, Z.; Wang, H. Fabrication of magnetic Fe3O4/silica nanofiber composites with enhanced Fenton-like catalytic performance for Rhodamine B degradation. J. Mater. Sci. 2018, 53, 369–384. [Google Scholar] [CrossRef]
- Liu, S.; Wang, Y.; Wang, B.; Huang, J.; Deng, S.; Yu, G. Regeneration of Rhodamine B saturated activated carbon by an electro-peroxone process. J. Clean. Prod. 2017, 168, 584–594. [Google Scholar] [CrossRef]
- Al-Gheethi, A.A.; Azhar, Q.M.; Kumar, P.S.; Yusuf, A.A.; Al-Buriahi, A.K.; Mohamed, R.M.S.R.; Al-shaibani, M.M. Sustainable approaches for removing Rhodamine B dye using agricultural waste adsorbents: A review. Chemosphere 2022, 287, 132080. [Google Scholar] [CrossRef]
- Saruchi; Kumar, V.; Kaith, B.S.; Jindal, R. Synthesis of hybrid ion exchanger for Rhodamine B dye removal: Equilibrium, kinetic and thermodynamic studies. Ind. Eng. Chem. Res. 2016, 55, 10492–10499. [Google Scholar] [CrossRef]
- Ma, J.; Liu, Q.; Zhu, L.; Zou, J.; Wang, K.; Yang, M.; Komarneni, S. Visible light photocatalytic activity enhancement of Ag3PO4 dispersed on exfoliated bentonite for degradation of rhodamine B. Appl. Catal. B Environ. 2016, 182, 26–32. [Google Scholar] [CrossRef]
- Rosa, A.L.D.; Carissimi, E.; Dotto, G.L.; Sander, H.L.; Feris, A. Biosorption of rhodamine B dye from dyeing stones effluents using the green microalgae Chlorella pyrenoidosa. J. Clean. Prod. 2018, 198, 1302–1310. [Google Scholar] [CrossRef]
- Zhang, S.; Li, B.; Wang, X.; Zhao, G.; Hu, B.; Lu, Z.; Wen, T.; Chen, J.; Wang, X. Recent developments of two-dimensional graphene-based composites in visible-light photocatalysis for eliminating persistent organic pollutants from wastewater. Chem. Eng. J. 2020, 390, 124642. [Google Scholar] [CrossRef]
- Liu, G.; Wang, G.; Hu, Z.; Su, Y.; Zhao, L. Ag2O nanoparticles decorated TiO2 nanofibers as a p-n heterojunction for enhanced photocatalytic decomposition of RhB under visible light irradiation. Appl. Surf. Sci. 2019, 465, 902–910. [Google Scholar] [CrossRef]
- Song, R.; Chi, H.; Ma, Q.; Li, D.; Wang, X.; Gao, W.; Wang, H.; Wang, X.; Li, Z.; Li, C. Highly efficient degradation of persistent pollutants with 3D nanocone TiO2-based photoelectrocatalysis. J. Am. Chem. Soc. 2021, 143, 13664–13674. [Google Scholar] [PubMed]
- Lange, M.A.; Krysiak, Y.; Hartmann, J.; Dewald, G.; Cerretti, G.; Tahir, M.N.; Panthöfer, M.; Barton, B.; Reich, T.; Zeier, W.G.; et al. Solid state fluorination on the minute scale: Synthesis of WO3−xFx with photocatalytic activity. Adv. Funct. Mater. 2020, 30, 1909051. [Google Scholar] [CrossRef]
- Xu, X.; Randorn, C.; Irvine, P.E.J.T.S. A red metallic oxide photocatalyst. Nat. Mater. 2012, 11, 595–598. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Xiang, Q.; Liao, Y.; Zhang, H. CdS-based photocatalysts. Energy Environ. Sci. 2018, 11, 1362–1391. [Google Scholar] [CrossRef]
- Toe, C.Y.; Tsounis, C.; Zhang, J.; Masood, H.; Gunawan, D.; Scott, J.; Amal, R. Advancing photoreforming of organics: Highlights on photocatalyst and system designs for selective oxidation reactions. Energy Environ. Sci. 2021, 14, 1140–1175. [Google Scholar] [CrossRef]
- Riente, P.; Adams, A.M.; Albero, J.; Palomares, E.; Pericàs, M.A. Light-driven organocatalysis using inexpensive, nontoxic Bi2O3 as the photocatalyst. Angew. Chem. Int. Ed. 2014, 53, 9613–9616. [Google Scholar] [CrossRef]
- Xiao, X.; Hu, R.; Liu, C.; Xing, C.; Qian, C.; Zuo, X.; Nan, J.; Wang, L. Facile large-scale synthesis of β-Bi2O3 nanospheres as a highly efficient photocatalyst for the degradation of acetaminophen under visible light irradiation. Appl. Catal. B Environ. 2013, 140–141, 433–443. [Google Scholar] [CrossRef]
- Brezesinski, K.; Ostermann, R.; Hartmann, P.; Perlich, J.; Brezesinski, T. Exceptional photocatalytic activity of ordered mesoporous β-Bi2O3 thin films and electrospun nanofiber mats. Chem. Mater. 2010, 22, 3079–3085. [Google Scholar] [CrossRef]
- Cheng, H.; Huang, B.; Lu, J.; Wang, Z.; Xu, B.; Qin, X.; Zhang, X.; Dai, Y. Synergistic effect of crystal and electronic structures on the visible-light-driven photocatalytic performances of Bi2O3 polymorphs. Phys. Chem. Chem. Phys. 2010, 12, 15468–15475. [Google Scholar]
- Bera, K.K.; Majumdar, R.; Chakraborty, M.; Bhattacharya, S.K. Phase control synthesis of α, β and α/β Bi2O3 hetero-junction with enhanced and synergistic photocatalytic activity on degradation of a toxic dye, Rhodamine-B under natural sunlight. J. Hazard Mater. 2018, 352, 182–191. [Google Scholar] [CrossRef]
- Jiang, S.; Wang, L.; Hao, W.; Li, W.; Xin, H.; Wang, W.; Wang, T. Visible-light photocatalytic activity of S-doped α-Bi2O3. J. Phys. Chem. C 2015, 119, 14094–14101. [Google Scholar] [CrossRef]
- Freitas, R.R.Q.; Mota, F.D.B.; Rivelino, R.; Castilho, C.M.C.D.; Kakanakova-Georgieva, A.; Gueorguiev, G.K. Spin-orbit-induced gap modification in buckled honeycomb XBi and XBi3 (X = B, Al, Ga, and In) sheets. J. Phys. Condens. Matter 2015, 27, 485306. [Google Scholar] [CrossRef]
- Hanif, M.A.; Akter, J.; Islam, M.A.; Lee, I.; Sapkota, K.P.; Shrestha, S.; Pandey, A.; Gyawali, N.; Hahn, J.R. Enhancement of visible-light photocatalytic activity of ZnO/ZnS/g-C3N4 by decreasing the bandgap and reducing the crystallite size via facile one-step fabrication. J. Photochem. Photobiol. A 2022, 431, 114066. [Google Scholar] [CrossRef]
- Jia, Z.; Lv, R.; Guo, L.J.; Zhang, J.; Li, R.; Liu, J.; Fan, C. Rapid degradation of ciprofloxacin over BiOCl: Insight into the molecular structure transformation and antibacterial activity elimination. Sep. Purif. Technol. 2021, 257, 117872. [Google Scholar] [CrossRef]
- Kakanakova-Georgieva, A.; Giannazzo, F.; Nicotra, G.; Cora, I.; Gueorguiev, G.K.; Persson, P.O.Å.; Pécz, B. Material proposal for 2D indium oxide. Appl. Surf. Sci. 2021, 548, 149275. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, S.; Shi, R.; Waterhouse, G.I.N.; Tang, J.; Zhang, T. Two-dimensional photocatalyst design: A critical review of recent experimental and computational advances. Mater. Today 2020, 34, 78–91. [Google Scholar] [CrossRef]
- Ding, L.; Wei, R.; Chen, H.; Hu, J.; Li, J. Controllable synthesis of highly active BiOCl hierarchical microsphere self-assembled by nanosheets with tunable thickness. Appl. Catal. B Environ. 2015, 172, 91–99. [Google Scholar] [CrossRef]
- Hu, X.; Zhu, F.; Kong, L.; Peng, X. Sulfate radical-based removal of chloride ion from strongly acidic wastewater: Kinetics and mechanism. J. Hazard. Mater. 2021, 410, 124540. [Google Scholar] [CrossRef]
- Peng, X.; Dou, W.; Kong, L.; Hu, X.; Wang, X. Removal of chloride ions from strongly acidic wastewater using Cu(0)/Cu(II): Efficiency enhancement by UV irradiation and the mechanism for chloride ions removal. Environ. Sci. Technol. 2019, 53, 383–389. [Google Scholar] [CrossRef]
- Huang, S.; Li, L.; Zhu, N.; Lou, Z.; Liu, W.; Cheng, J.; Wang, H.; Luo, P.; Wang, H. Removal and recovery of chloride ions in concentrated leachate by Bi(III) containing oxides quantum dots/two-dimensional flakes. J. Hazard. Mater. 2020, 382, 121041. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, L.; Chen, Z.; Hu, J.; Li, S.; Wang, Z.; Liu, J.; Wang, X. Semiconductor heterojunction photocatalysts: Design, construction, and photocatalytic performances. Chem. Soc. Rev. 2014, 43, 5234–5244. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Castillo, A.L.; Hinojosa-Reyes, M.; Camposeco-Solis, R.; Ruiz, F. Photocatalytic Activity of Bi2O3/BiOCl Heterojunctions Under UV and Visible Light Illumination for Degradation of Caffeine. Top. Catal. 2022, 65, 1071–1087. [Google Scholar] [CrossRef]
- Guan, M.; Xiao, C.; Zhang, J.; Fan, S.; An, R.; Cheng, Q.; Xie, J.; Zhou, M.; Ye, B.; Xie, Y. Vacancy associates promoting solar-driven photocatalytic activity of ultrathin bismuth oxychloride nanosheets. J. Am. Chem. Soc. 2013, 135, 10411–10417. [Google Scholar] [CrossRef]
- Zhang, P.; Rao, Y.; Huang, Y.; Chen, M.; Huang, T.; Ho, W.; Lee, S.; Zhong, J.; Cao, J. Transformation of amorphous Bi2O3 to crystal Bi2O2CO3 on Bi nanospheres surface for photocatalytic NOx oxidation: Intensified hot-electron transfer and reactive oxygen species generation. Chem. Eng. J. 2021, 420, 129814. [Google Scholar] [CrossRef]
- Jiang, R.; Lu, G.; Zhou, R.; Dang, T.; Wang, M. Degradation and detoxification of propranolol by a molecular intercalation bismuth oxychloride semiconductor-organic framework. Chem. Eng. J. 2021, 423, 130222. [Google Scholar] [CrossRef]
- Ortiz-Quiñonez, J.L.; Zumeta-Dubé, I.; Díaz, D.; Nava-Etzana, N.; Cruz-Zaragoza, E.; Santiago-Jacinto, P. Bismuth oxide nanoparticles partially substituted with EuIII, MnIV, and SiIV: Structural, spectroscopic, and optical findings. Inorg. Chem. 2017, 56, 3394–3403. [Google Scholar] [CrossRef]
- Zhong, Y.; Wu, C.; Feng, Y.; Chen, D.; Wang, Y.; Hao, D.; Ding, H. Enriched surface oxygen vacancies of BiOCl boosting efficient charge separation, whole visible-light absorption, and photo to thermal conversion. Appl. Surf. Sci. 2022, 585, 152656. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Z.; Fan, M.; Tong, P.; Sun, J.; Dong, S.; Sun, J. Ultra-light and compressible 3D BiOCl/ RGO aerogel with enriched synergistic effect of adsorption and photocatalytic degradation of oxytetracycline. J. Mater. Res. Technol. 2019, 8, 4577–4587. [Google Scholar] [CrossRef]
- Zhao, Q.; Liu, X.; Xing, Y.; Liu, Z.; Du, C. Synthesizing Bi2O3/BiOCl heterojunctions by partial conversion of BiOCl. J. Mater. Sci. 2017, 52, 2117–2130. [Google Scholar] [CrossRef]
- Cheng, G.; Xiong, J.; Stadler, F.J. Facile template-free and fast refluxing synthesis of 3D desertrose-like BiOCl nanoarchitectures with superior photocatalytic activity. New J. Chem. 2013, 37, 3207–3213. [Google Scholar] [CrossRef]
- Deng, F.; Zhang, Q.; Yang, L.; Luo, X.; Wang, A.; Luo, S.; Dionysiou, D.D. Visible-light-responsive graphene-functionalized Bi-bridge Z-scheme black BiOCl/Bi2O3 heterojunction with oxygen vacancy and multiple charge transfer channels for efficient photocatalytic degradation of 2-nitrophenol and industrial wastewater treatment. Appl. Catal. B Environ. 2018, 238, 61–69. [Google Scholar] [CrossRef]
- Nagar, A.; Basu, S. Fabrication of 3D porous peony flower-like β-Bi2O3/BiOCl heterostructure for synergistically boosting the visible-light-driven degradation of organic pollutants. Environ. Technol. Innovation 2021, 24, 101956. [Google Scholar] [CrossRef]
- Song, Z.; Dong, X.; Wang, N.; Zhu, L.; Luo, Z.; Fang, J.; Xiong, C. Efficient photocatalytic defluorination of perfluorooctanoic acid over BiOCl nanosheets via a hole direct oxidation mechanism. Chem. Eng. J. 2017, 317, 925–934. [Google Scholar] [CrossRef]
- Huang, J.; Chen, W.; Yu, X.; Fu, X.; Zhu, Y.; Zhang, Y. Fabrication of a ternary BiOCl/CQDs/rGO photocatalyst: The roles of CQDs and rGO in adsorption-photocatalytic removal of ciprofloxacin. Colloids Surf. A Physicochem. Eng. Asp. 2020, 597, 124758. [Google Scholar] [CrossRef]
- Kong, S.; An, Z.; Zhang, W.; An, Z.; Yuan, M.; Chen, D. Preparation of hollow flower-like microspherical β-Bi2O3/BiOCl heterojunction and high photocatalytic property for tetracycline hydrochloride degradation. Nanomaterials 2020, 10, 57. [Google Scholar] [CrossRef] [PubMed]
- Mushtaq, F.; Guerrero, M.; Sakar, M.S.; Hoop, M.; Lindo, A.M.; Sort, J.; Chen, X.; Nelson, B.J.; Pellicer, E.l.; Pané, S. Magnetically driven Bi2O3/BiOCl-based hybrid microrobots for photocatalytic water remediation. J. Mater. Chem. A 2015, 3, 23670–23676. [Google Scholar] [CrossRef]
- Chai, S.Y.; Kim, Y.J.; Jung, M.H.; Chakraborty, A.K.; Jung, D.; Lee, W.I. Heterojunctioned BiOCl/Bi2O3, a new visible light photocatalyst. J. Catal. 2009, 262, 144–149. [Google Scholar] [CrossRef]
- Priya, B.; Raizada, P.; Singh, N.; Thakur, P.; Singh, P. Adsorptional photocatalytic mineralization of oxytetracycline and ampicillin antibiotics using Bi2O3/BiOCl supported on graphene sand composite and chitosan. J. Colloid Interface Sci. 2016, 479, 271–283. [Google Scholar] [CrossRef]
- Liu, H.; Yang, C.; Jin, X.; Zhong, J.; Li, J. One-pot hydrothermal synthesis of MXene Ti3C2/TiO2/BiOCl ternary heterojunctions with improved separation of photoactivated carries and photocatalytic behavior toward elimination of contaminants. Colloids Surf. A Physicochem. Eng. Asp. 2020, 603, 125239. [Google Scholar] [CrossRef]
- Ma, F.; Wu, Y.; Shao, Y.; Zhong, Y.; Lv, J.; Hao, X. 0D/2D nanocomposite visible light photocatalyst for highly stable and efficient hydrogen generation via recrystallization of CdS on MoS2 nanosheets. Nano Energy 2016, 27, 466–474. [Google Scholar] [CrossRef]
- Nosaka, Y.; Nosaka, A.Y. Generation and detection of reactive oxygen species in photocatalysis. Chem. Rev. 2017, 117, 11302–11336. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Xie, Y.; Wang, W.; Zhang, L.; Zhang, X.; Shi, Y.; Fan, J.; Tang, Z. In-situ construction of step-scheme MoS2/Bi4O5Br2 heterojunction with improved photocatalytic activity of Rhodamine B degradation and disinfection. J. Colloid Interface Sci. 2022, 623, 500–512. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Qin, F.; Zhao, H.; Ding, J.; Liu, Y.; Chen, R. Crystal defect engineering of aurivillius Bi2MoO6 by Ce doping for increased reactive species production in photocatalysis. ACS Catal. 2016, 6, 3180–3192. [Google Scholar] [CrossRef]
- Kang, Z.; Qin, N.I.; Lin, E.; Wu, J.; Yuan, B.; Bao, D. Effect of Bi2WO6 nanosheets on the ultrasonic degradation of organic dyes: Roles of adsorption and piezocatalysis. J. Clean. Prod. 2020, 261, 121125. [Google Scholar] [CrossRef]
- Ghosh, I.; Khamrai, J.; Savateev, A.; Shlapakov, N.; Antonietti, M.; König, B. Organic semiconductor photocatalyst can bifunctionalize arenes and heteroarenes. Science 2019, 365, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Riente, P.; Fianchini, M.; Llanes, P.; Pericàs, M.A.; Noël, T. Shedding light on the nature of the catalytically active species in photocatalytic reactions using Bi2O3 semiconductor. Nat. Commun. 2021, 12, 625. [Google Scholar] [CrossRef] [PubMed]
- Sang, Y.; Cao, X.; Dai, G.; Wang, L.; Peng, Y.; Geng, B. Facile one-pot synthesis of novel hierarchical Bi2O3/Bi2S3 nanoflower photocatalyst with intrinsic p-n junction for efficient photocatalytic removals of RhB and Cr(VI). J. Hazard. Mater. 2020, 381, 120942. [Google Scholar] [CrossRef] [PubMed]
- Nayak, S.; Swain, G.; Parida, K. Enhanced photocatalytic activities of RhB degradation and H2 evolution from in situ formation of the electrostatic heterostructure MoS2/NiFe LDH nanocomposite through the Z-scheme mechanism via p−n heterojunctions. ACS Appl. Mater. Interfaces 2019, 11, 20923–20942. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Q.; Lan, H.; Zhang, M.; Zhu, H.; Bu, M. Preparation of heterostructure g-C3N4/ZnO nanorods for high photocatalytic activity on different pollutants (MB, RhB, Cr(VI) and eosin). Ceram. Int. 2020, 46, 12192–12199. [Google Scholar] [CrossRef]
- Chankhanittha, T.; Nanan, S. Visible-light-driven photocatalytic degradation of ofloxacin (OFL) antibiotic and Rhodamine B (RhB) dye by solvothermally grown ZnO/Bi2MoO6 heterojunction. J. Colloid Interface Sci. 2021, 582, 412–427. [Google Scholar] [CrossRef]
- Zhou, R.; Zhang, D.; Wang, P.; Huang, Y. Regulation of excitons dissociation in AgI/Bi3O4Br for advanced reactive oxygen species generation towards photodegradation. Appl. Catal. B Environ. 2021, 285, 119820. [Google Scholar] [CrossRef]
| Photocatalyst | Dosage (mg/mL) | RhB Concentration (mg/L) | Light Source | Reaction Time (min) | Removal Rate (%) | Ref. |
|---|---|---|---|---|---|---|
| Bi2O3/BiOCl | 20/100 | 20 | 300 W Xe lamp | 60 | 99.9 | This work |
| Bi2O3/Bi2S3 | 50/100 | 20 | 300 W Xe lamp | 90 | 99.7 | [57] |
| Ag2O/TiO2 | 130/100 | 4.8 | visible-light | 80 | 87.7 | [10] |
| MoS2/NiFe | 100/100 | 20 | 300 W Xe lamp | 120 | 90 | [58] |
| C3N4/ZnO | 100/100 | 10 | 300 W Xe lamp | 90 | 98.5 | [59] |
| ZnO/Bi2MoO6 | 25/100 | 10 | 15 W cool daylight lamp | 180 | 99.3 | [60] |
| AgI/Bi3O4Br | 20/100 | 50 | 300 W Xe lamp | 60 | 98 | [61] |
| Ti3C2/TiO2/BiOCl | 100/100 | 10 | 500 W Xe lamp | 120 | 84 | [49] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teng, D.; Qu, J.; Li, P.; Jin, P.; Zhang, J.; Zhang, Y.; Cao, Y. Heterostructured α-Bi2O3/BiOCl Nanosheet for Photocatalytic Applications. Nanomaterials 2022, 12, 3631. https://doi.org/10.3390/nano12203631
Teng D, Qu J, Li P, Jin P, Zhang J, Zhang Y, Cao Y. Heterostructured α-Bi2O3/BiOCl Nanosheet for Photocatalytic Applications. Nanomaterials. 2022; 12(20):3631. https://doi.org/10.3390/nano12203631
Chicago/Turabian StyleTeng, Daoguang, Jie Qu, Peng Li, Peng Jin, Jie Zhang, Ying Zhang, and Yijun Cao. 2022. "Heterostructured α-Bi2O3/BiOCl Nanosheet for Photocatalytic Applications" Nanomaterials 12, no. 20: 3631. https://doi.org/10.3390/nano12203631
APA StyleTeng, D., Qu, J., Li, P., Jin, P., Zhang, J., Zhang, Y., & Cao, Y. (2022). Heterostructured α-Bi2O3/BiOCl Nanosheet for Photocatalytic Applications. Nanomaterials, 12(20), 3631. https://doi.org/10.3390/nano12203631








