Organo-Vermiculites Modified by Aza-Containing Gemini Surfactants: Efficient Uptake of 2-Naphthol and Bromophenol Blue
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Gemini Surfactants and Organo-Vts
2.3. Adsorption Experiment
2.4. Adsorption Kinetics, Isotherms and Thermodynamics
2.5. Theoretical Calculation
3. Results and Discussion
3.1. Characterization
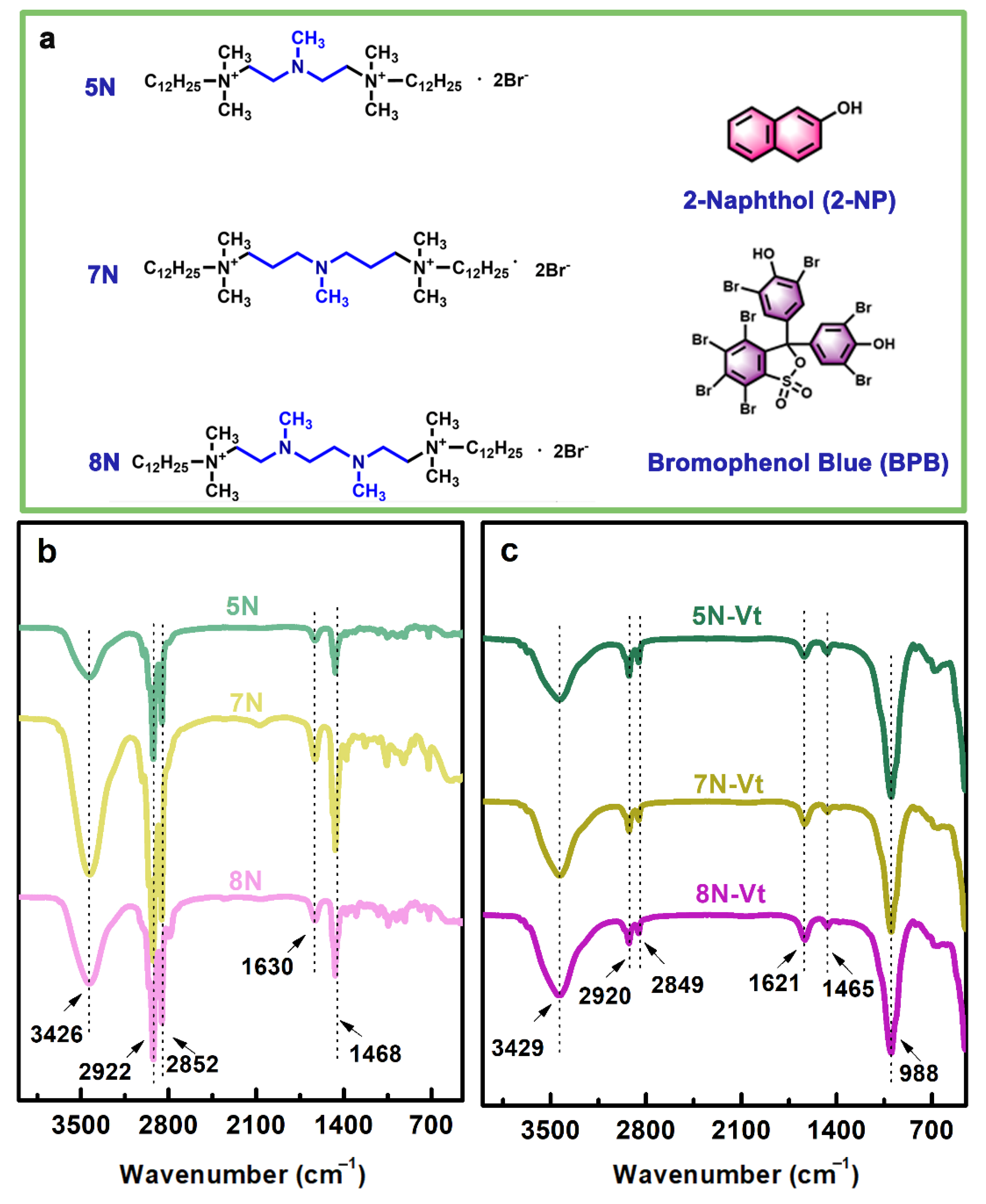
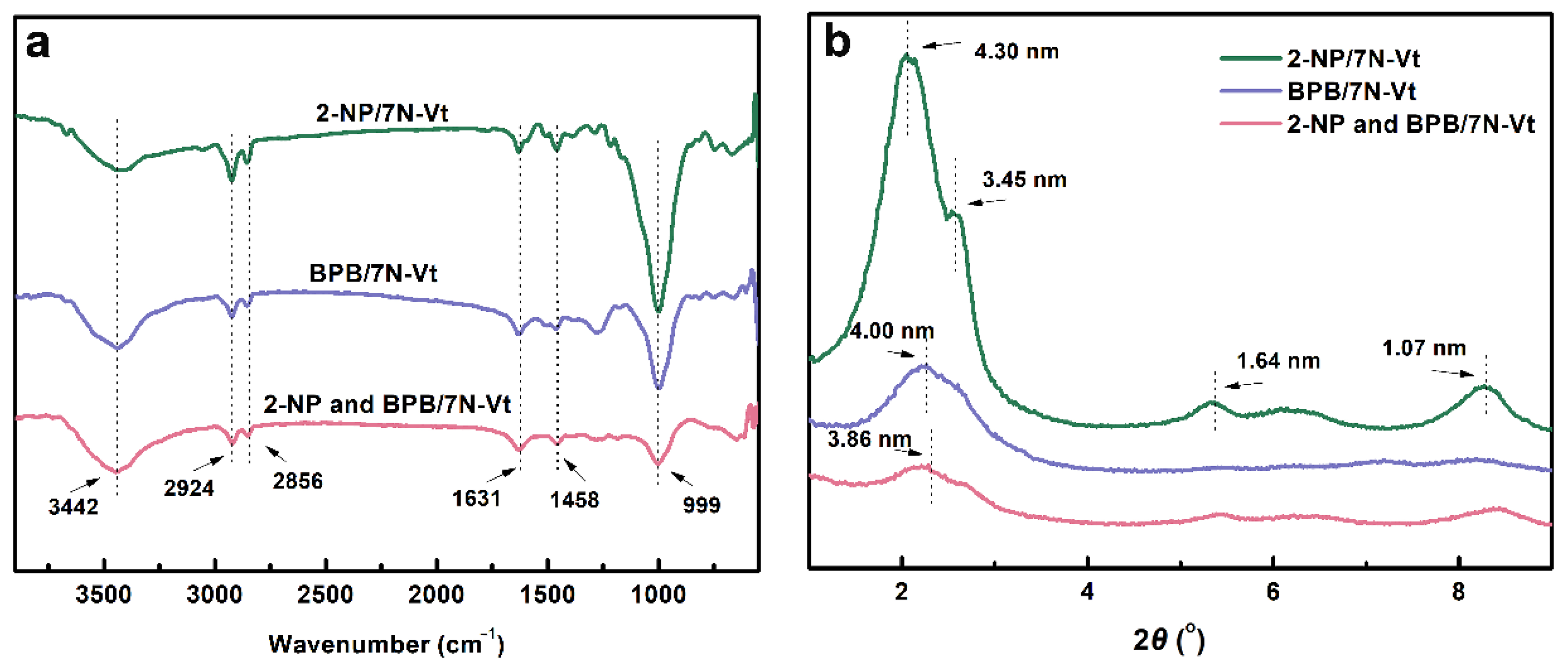
3.1.1. FT-IR Analysis of Surfactants and Organo-Vts
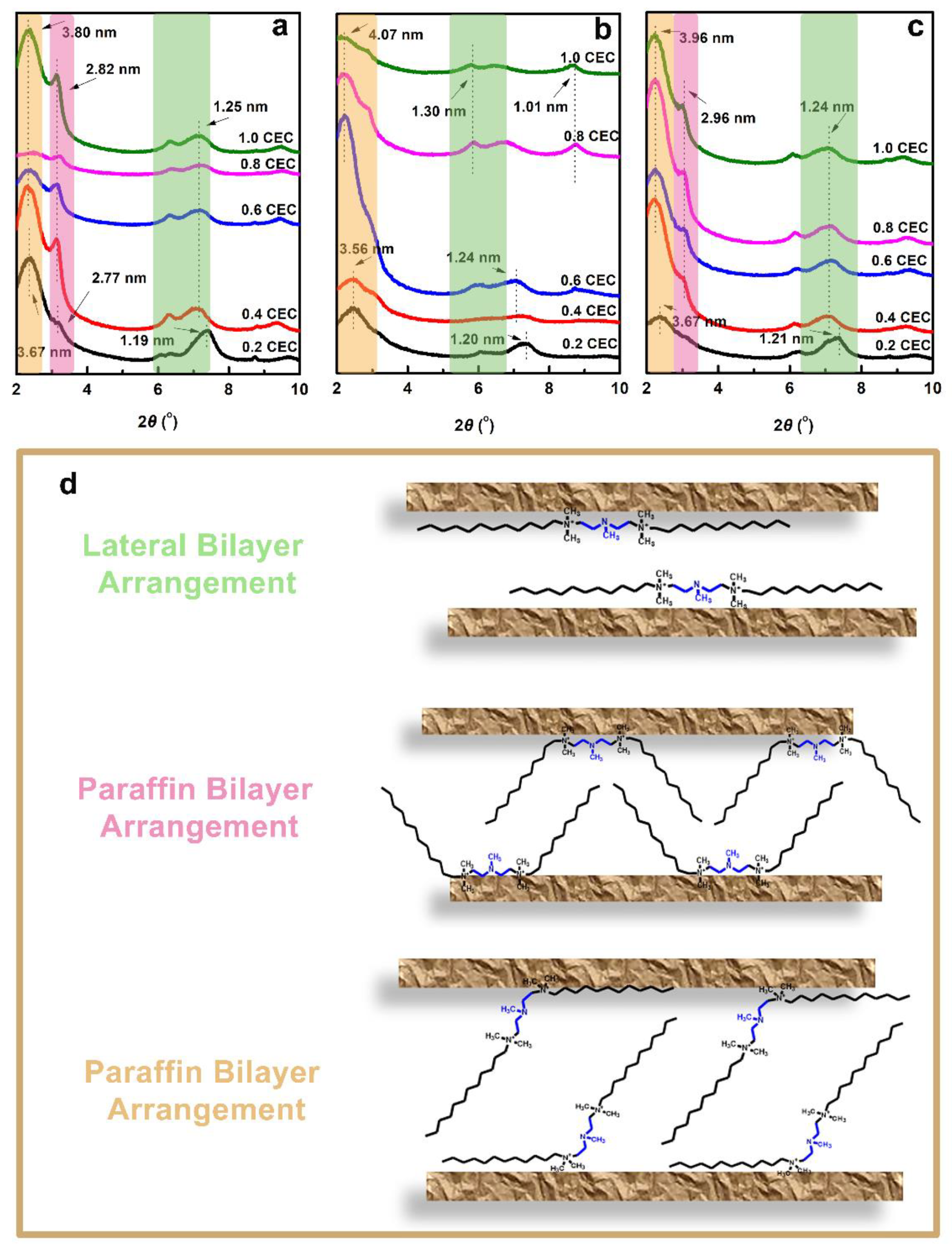
3.1.2. XRD Patterns of Organo-Vts
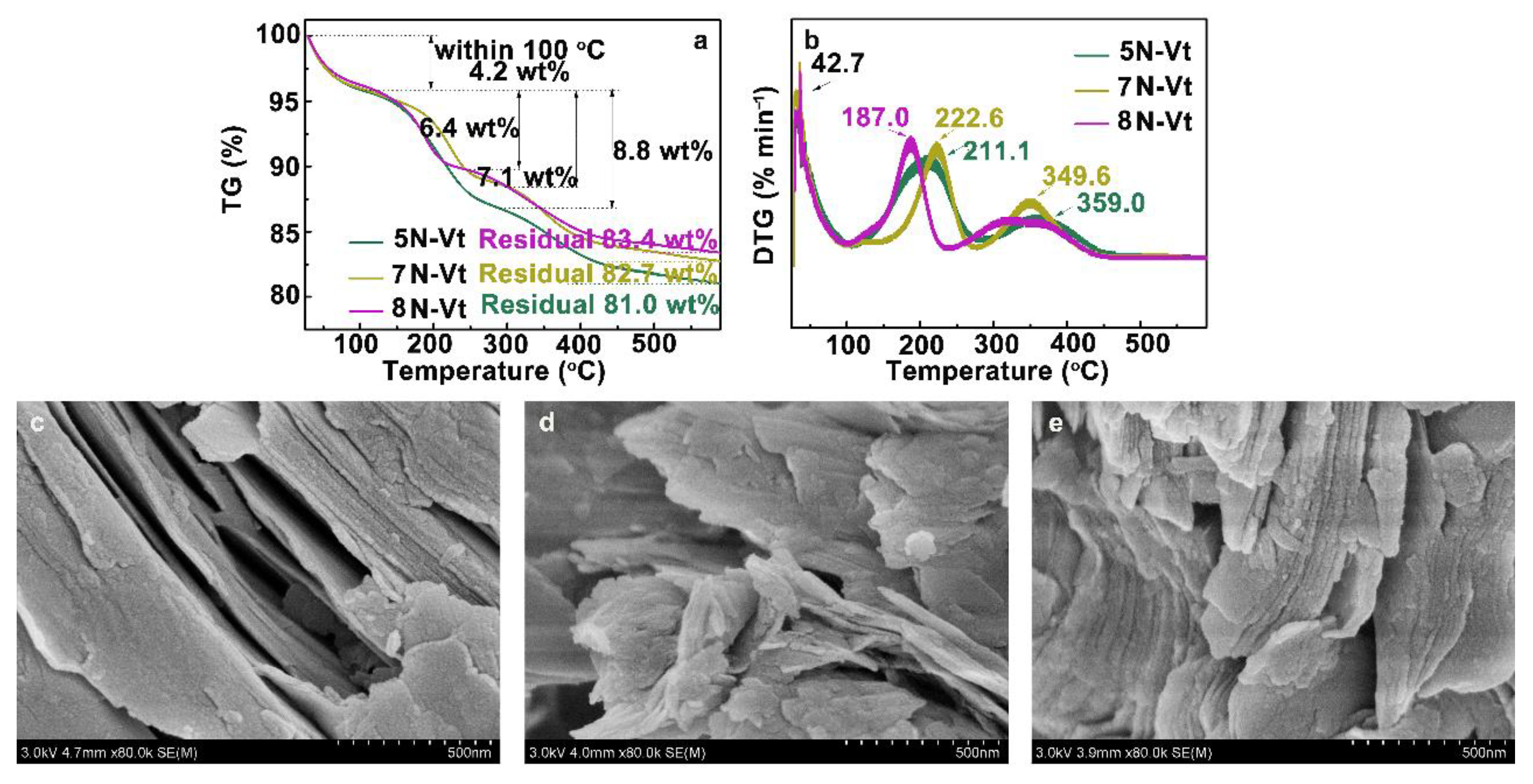
3.1.3. TG-DTG Curves and EA Analysis of Organo-Vts
3.1.4. SEM Images of Organo-Vts
3.2. Adsorption Test
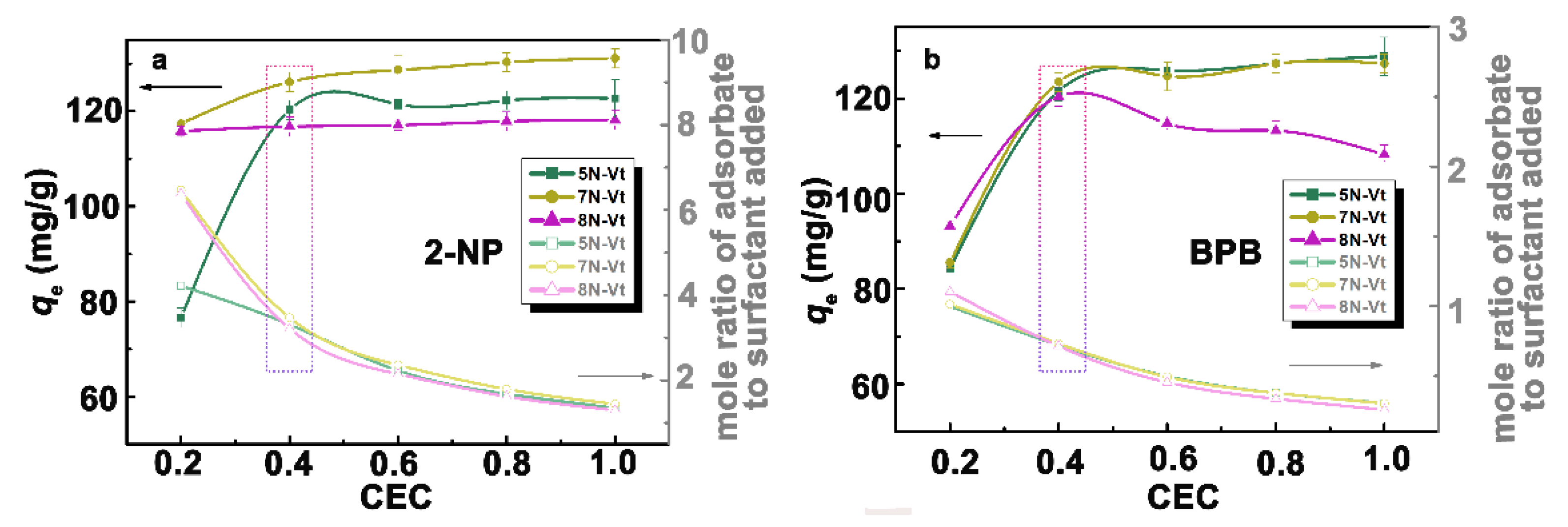
3.2.1. Effect of Modifier Dosage on Adsorption
3.2.2. Effect of Time and Adsorption Kinetics
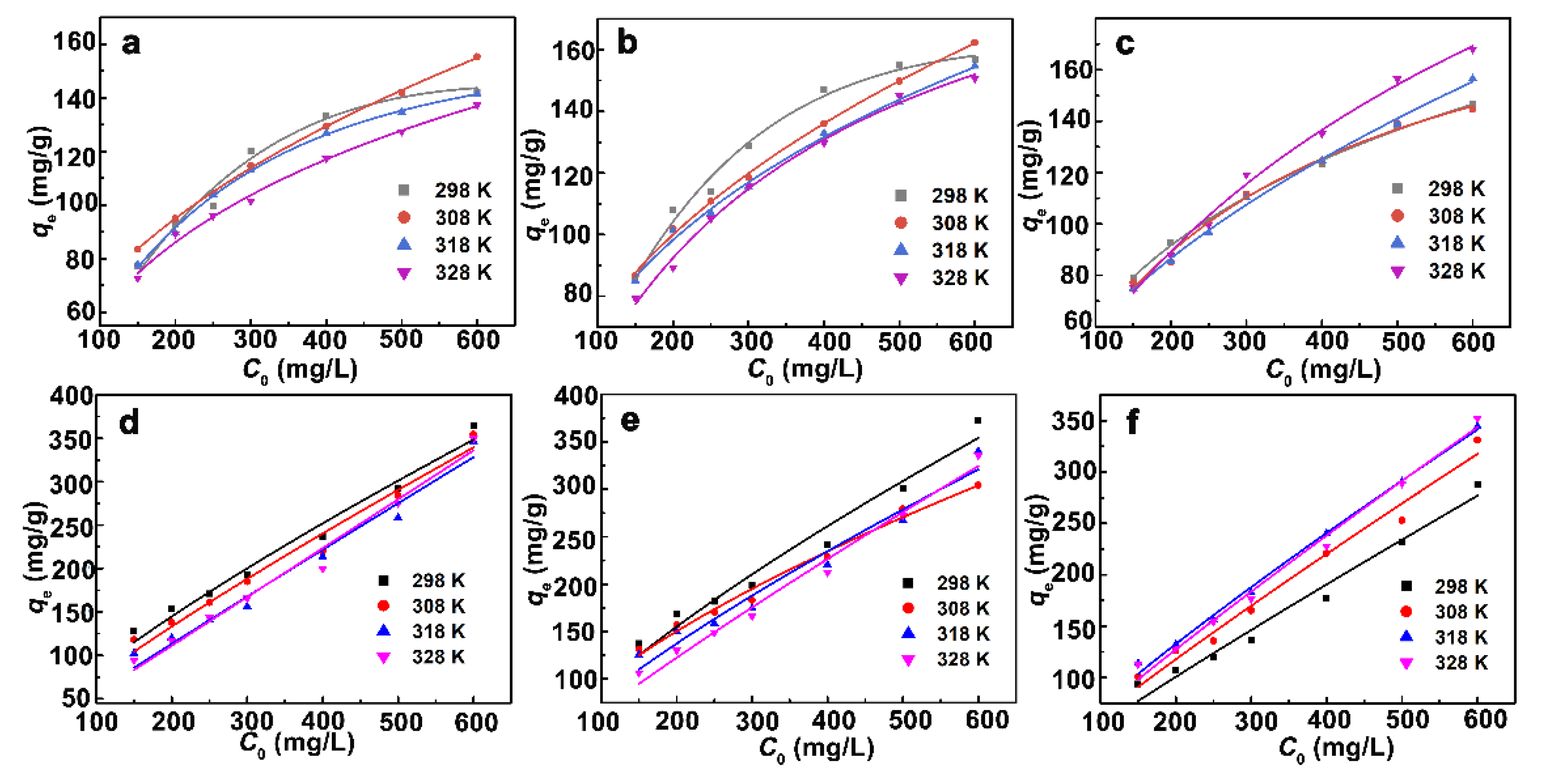
3.2.3. Effect of Concentration and Adsorption Isotherms
3.2.4. Effect of Temperature and Adsorption Thermodynamics
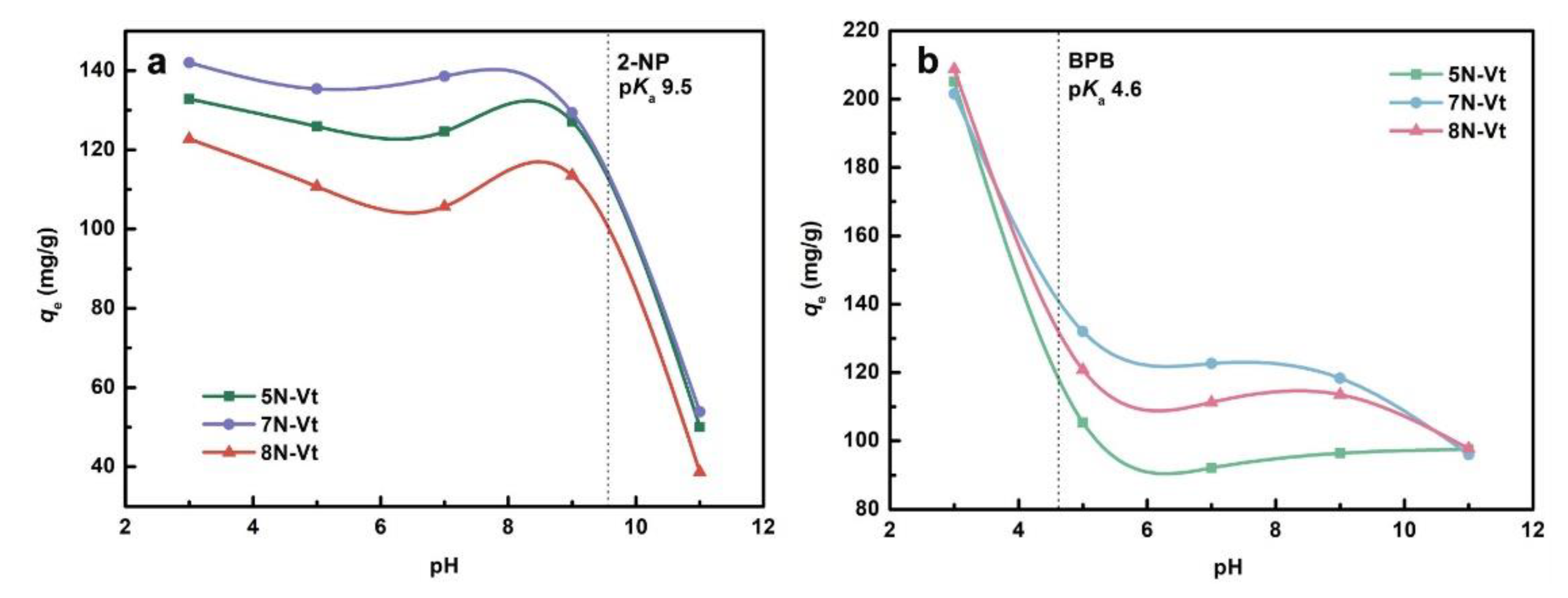
3.2.5. Effect of Solution pH
3.3. Regeneration
3.4. Adsorption Feasibility of Organo-Vts in Binary-Component System
3.5. Adsorption Mechanism
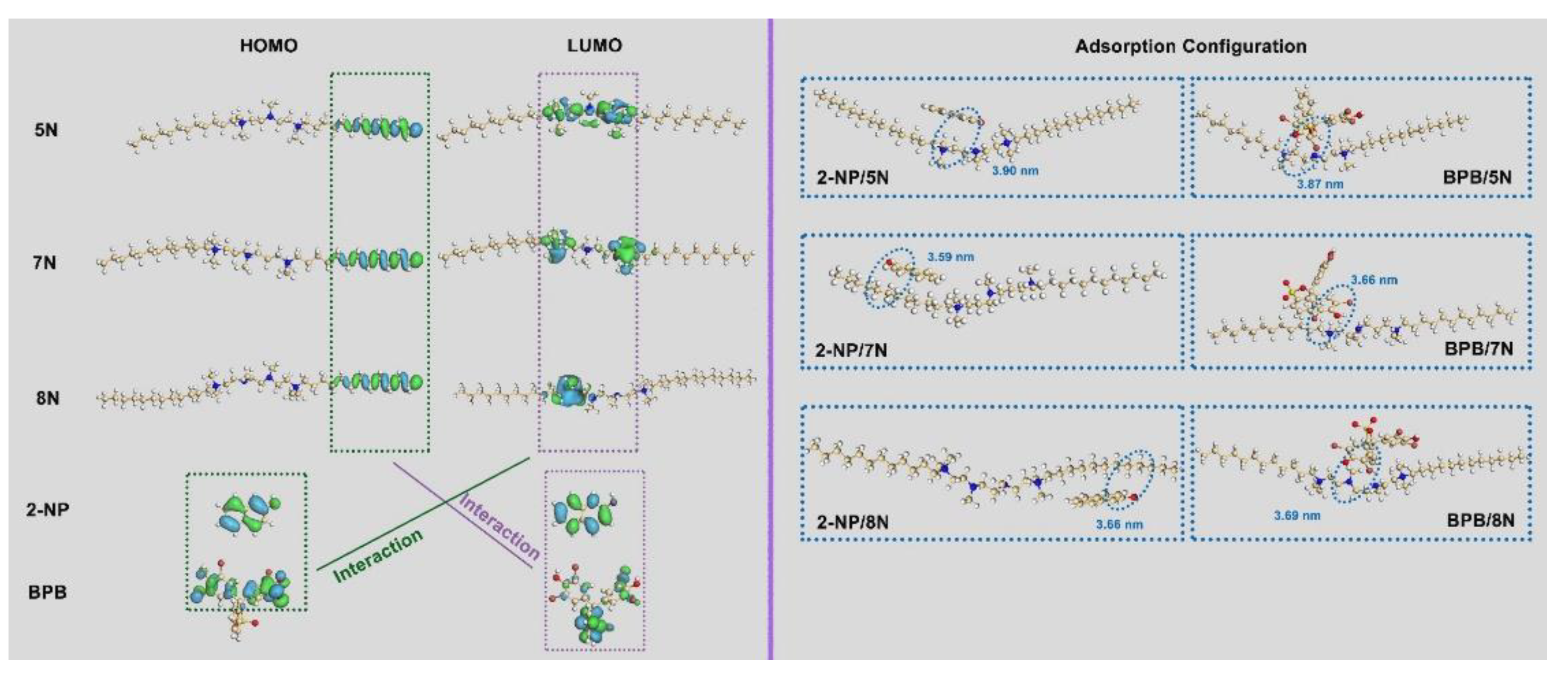
3.5.1. Theoretical Simulation of Molecular Orbitals and Adsorption Configuration
3.5.2. Discussion of Log KOM and Characterization of Spent Organo-Vts
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shen, T.; Gao, M. Gemini surfactant modified organo-clays for removal of organic pollutants from water: A review. Chem. Eng. J. 2019, 375, 121910. [Google Scholar] [CrossRef]
- Kamal, M.; Hussein, I.; Sultan, A. Review on Surfactant Flooding: Phase Behavior, Retention, IFT, and Field Applications. Energy Fuels 2017, 31, 7701–7720. [Google Scholar] [CrossRef]
- Nguyen, V.; Thi, D.; Dao, T.; Tran, H.; Nguyen, D.; Ahn, D. Synthesis of organoclays and their application for the adsorption of phenolic compouds from aqueous solution. J. Ind. Eng. Chem. 2013, 19, 640–644. [Google Scholar] [CrossRef]
- Changchaivong, S.; Khaodhiar, S. Adsorption of naphthalene and phenanthrene on dodecylpyridinium-modified bentonite. Appl. Clay Sci. 2009, 43, 317–321. [Google Scholar] [CrossRef]
- Perelomov, L.; Mandzhieva, S.; Minkina, T.; Atroshchenko, Y.; Perelomova, I.; Bauer, T.; Pinsky, D.; Barakhov, A. The Synthesis of Organoclays Based on Clay Minerals with Different Structural Expansion Capacities. Minerals 2021, 11, 707. [Google Scholar] [CrossRef]
- Wu, C.; Guan, D.; Cui, Y.; Hu, D.; Liu, Y.; Cheng, L.; Pu, X.; Cao, J. The Effect of Vermiculite Loaded with MnO2 on Adsorption of Heavy Metal Pb(II) in Wastewater. In Proceedings of the Chinese Materials Conference (CMC), Yinchuan City, China, 6–12 July 2017. [Google Scholar]
- Fernandez, M.; Fernandez, M. Effect of Organic Modifier and Clay Content on Non-Isothermal Cold Crystallization and Melting Behavior of Polylactide/Organovermiculite Nanocomposites. Polymers 2020, 12, 364. [Google Scholar] [CrossRef] [Green Version]
- Cai, Y. Preparation of Organo-Vermiculite by Modification with Hexadecyltrimethylammonium Bromide Under Microwave Irradiation. Asian J. Chem. 2012, 24, 252–254. [Google Scholar]
- Lv, W.; Shen, T.; Ding, F.; Mao, S.; Ma, Z.; Xie, J.; Gao, M. A novel NH2-rich polymer/graphene oxide/organo-vermiculite adsorbent for the efficient removal of azo dyes. J. Mol. Liq. 2021, 341, 13. [Google Scholar] [CrossRef]
- Zang, W.; Gao, M.; Shen, T.; Ding, F.; Wang, J. Facile modification of homoionic-vermiculites by a gemini surfactant: Comparative adsorption exemplified by methyl orange. Colloids Surf. A 2017, 533, 99–108. [Google Scholar] [CrossRef]
- Ding, F.; Gao, M.; Shen, T.; Zeng, H.; Xiang, Y. Comparative study of organo-vermiculite, organo-montmorillonite and organo-silica nanosheets functionalized by an ether-spacer-containing Gemini surfactant: Congo red adsorption and wettability. Chem. Eng. J. 2018, 349, 388–396. [Google Scholar] [CrossRef]
- Shen, T.; Wang, L.; Zhao, Q.; Guo, S.; Gao, M. Single and simultaneous adsorption of basic dyes by novel organo vermiculite A combined experimental and theoretical study. Colloids Surf. A 2020, 601, 125059. [Google Scholar] [CrossRef]
- Yu, M.; Gao, M.; Shen, T.; Wang, J. Organo-vermiculites modified by low-dosage Gemini surfactants with different spacers for adsorption toward p-nitrophenol. Colloids Surf. A 2018, 553, 601–611. [Google Scholar] [CrossRef]
- Wang, J.; Gao, M.; Shen, T.; Yu, M.; Xiang, Y.; Liu, J. Insights into the efficient adsorption of rhodamine B on tunable organo-vermiculites. J. Hazard. Mater. 2019, 366, 501–511. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Zhu, R.; Zhu, J.; Ge, F.; Yuan, P.; He, H.; Ming, C. Simultaneous sorption of crystal violet and 2-naphthol to bentonite with different CECs. J. Hazard. Mater. 2009, 166, 195–199. [Google Scholar] [CrossRef]
- Zeroual, Y.; Kim, B.; Kim, C.; Blaghen, M.; Lee, K. A comparative study on biosorption characteristics of certain fungi for bromophenol blue dye. Appl. Biochem. Biotechnol. 2006, 134, 51–60. [Google Scholar] [CrossRef]
- Zheng, X.; Yang, L.; Shen, Q.; Zhou, C. Evaluation of Zinc Oxide Nanoparticles-Induced Effects on Nitrogen and Phosphorus Removal from Real and Synthetic Municipal Wastewater. Ind. Eng. Chem. Res. 2019, 58, 7929–7936. [Google Scholar] [CrossRef]
- Wettig, S.; Badea, I.; Donkuru, M.; Verrall, R.; Foldvari, M. Structural and transfection properties of amine-substituted gemini surfactant-based nanoparticles. J. Gene Med. 2007, 9, 649–658. [Google Scholar] [CrossRef]
- Lobato-Aguilar, H.; Uribe-Calderon, J.; Herrera-Kao, W.; Duarte-Aranda, S.; Baas-Lopez, J.; Escobar-Morales, B.; Cauich-Rodríguez, J.V.; Cervantes-Uc, J.M. Synthesis, characterization and chlorhexidine release from either montmorillonite or palygorskite modified organoclays for antibacterial applications. J. Drug Deliv. Sci. Technol. 2018, 46, 452–460. [Google Scholar] [CrossRef]
- Mouacher, L.; Yahiaoui, A.; Hachemaoui, A.; Dehbi, A.; Benkouider, A.; Reguig, A. Synthesis and Characterization of Conducting Poly(2-aminothiazole)/Modified-Clay Nanocomposites. Polym. Sci. Ser. B 2021, 63, 314–321. [Google Scholar] [CrossRef]
- Shen, T.; Han, T.; Zhao, Q.; Ding, F.; Mao, S.; Gao, M. Efficient removal of mefenamic acid and ibuprofen on organo-Vts with a quinoline-containing gemini surfactant: Adsorption studies and model calculations. Chemosphere 2022, 295, 133846. [Google Scholar] [CrossRef]
- Shen, T.; Gao, M.; Ding, F.; Zeng, H.; Yu, M. Organo-vermiculites with biphenyl and dipyridyl gemini surfactants for adsorption of bisphenol A: Structure, mechanism and regeneration. Chemosphere 2018, 207, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Cao, G.; Gao, M.; Shen, T.; Zhao, B.; Zeng, H. Comparison between Asymmetric and Symmetric Gemini Surfactant-Modified Novel Organo-vermiculites for Removal of Phenols. Ind. Eng. Chem. Res. 2019, 58, 12927–12938. [Google Scholar] [CrossRef]
- Fu, L.; Li, J.; Wang, G.; Luan, Y.; Dai, W. Adsorption behavior of organic pollutants on microplastics. Ecotox Environ. Safe 2021, 217, 15. [Google Scholar] [CrossRef]
- Kim, T.; Song, H.; Dar, M.; Lee, H.; Kim, D. Fast adsorption kinetics of highly dispersed ultrafine nickel/carbon nanoparticles for organic dye removal. Appl. Surf. Sci. 2018, 439, 364–370. [Google Scholar] [CrossRef]
- Song, H.; Desmet, G.; Cabooter, D. Assessment of intra-particle diffusion in hydrophilic interaction liquid chromatography and reversed-phase liquid chromatography under conditions of identical packing structure. J. Chromatogr. A 2017, 1523, 204–214. [Google Scholar] [CrossRef]
- Wang, J.; Guo, X. Adsorption isotherm models: Classification, physical meaning, application and solving method. Chemosphere 2020, 258, 25. [Google Scholar] [CrossRef]
- Shukla, P.; Chong, S.; Pan, G.; Wang, S.; Ang, M.; Rudolph, V. Adsorption of phenolic contaminants from water on activated carbon: An insight into single and multicomponent adsorption isotherms. Asia-Pac. J. Chem. Eng. 2019, 14, 14. [Google Scholar] [CrossRef]
- Qin, Y.; Xiong, Y.; Kang, C.; Wang, J.; Liu, H.; He, C.; Lin, X.Y. Comparison on the Adsorption Thermodynamics of Three Typical Petroleum Hydrocarbons in the Single and Mixed Solution on Aquifer Media. In Proceedings of the International Conference on Manufacturing Science and Technology (ICMST 2011), Singapore, 16–18 September 2011. [Google Scholar]
- Zheng, T.; Gan, Q.; Qian, J.; Feng, C. Thermodynamics of Pb2+ Adsorption on Amino Ion Exchange Fiber. In Proceedings of the International Conference on Materials Science and Energy Engineering (CMSEE), Sanya, China, 12–14 December 2014. [Google Scholar]
- Hossen, T.; Sahu, K. Effect of Photoacid Strength on Fluorescence Modulation of 2-Naphthol Derivatives inside beta-Cyclodextrin Cavity: Insights from Fluorescence, Isothermal Calorimetry, and Molecular Dynamics Simulations. J. Phys. Chem. B 2019, 123, 9291–9301. [Google Scholar] [CrossRef]
- Li, J.; Chatterjee, K.; Medek, A.; Shalaev, E.; Zografi, G. Acid-base characteristics of bromophenol blue-citrate buffer systems in the amorphous state. J. Pharm. Sci. 2004, 93, 697–712. [Google Scholar] [CrossRef]
- Evans, P.; Hooshmand, Z.; Rahman, T.; Dowben, P.A. The importance of frontier orbital symmetry in the adsorption of diiodobenzene on MoS2(0001). Surf. Sci. 2020, 702, 8. [Google Scholar] [CrossRef]
- Shen, T.; Mao, S.; Ding, F.; Han, T.; Gao, M.L. Selective adsorption of cationic/anionic tritoluene dyes on functionalized amorphous silica: A mechanistic correlation between the precursor, modifier and adsorbate. Colloid Surf A-Physicochem. Eng Asp. 2021, 618, 11. [Google Scholar] [CrossRef]
- Watts, H.; Tribe, L.; Kubicki, J. Arsenic Adsorption onto Minerals: Connecting Experimental Observations with Density Functional Theory Calculations. Minerals 2014, 4, 208–240. [Google Scholar] [CrossRef] [Green Version]
- Manzhos, S. Comparative density functional theory and density functional tight binding study of 2-anthroic acid on TiO2. Chem. Phys. Lett. 2016, 643, 16–20. [Google Scholar] [CrossRef]
- Sharma, V.; Shahnaz, T.; Subbiah, S.; Narayanasamy, S. New Insights into the Remediation of Water Pollutants using Nanobentonite Incorporated Nanocellulose Chitosan Based Aerogel. J. Polym. Environ. 2020, 28, 2008–2019. [Google Scholar] [CrossRef]

| Element | Na-Vt | 5N-Vt | 7N-Vt | 8N-Vt |
|---|---|---|---|---|
| C | 0.41 | 12.78 | 12.71 | 12.06 |
| H | 0.82 | 2.95 | 2.90 | 2.79 |
| N | 0.13 | 1.33 | 1.19 | 1.47 |
| System | qe,exp | Pseudo-First-Order | Pseudo-Second-Order | Intraparticle Diffusion | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| qe,cal | k1 × 10−2 | R2 | SSE | qe,cal | k2 × 10−2 | k2qe | R2 | SSE × 10−5 | kid | C × 102 | R2 | SSE | ||
| 2NP/5N-Vt | 124.10 | 3.78 | 0.73 | 0.1416 | 5.07 | 123.92 | 9.24 | 11.45 | 0.9999 | 2.68 | 0.40 | 1.21 | 0.4122 | 2.8528 |
| 2NP/7N-Vt | 132.13 | 9.31 | 2.18 | 0.8814 | 1.75 | 132.45 | 1.65 | 2.19 | 0.9999 | 3.18 | 1.16 | 1.22 | 0.8796 | 0.6548 |
| 2NP/8N-Vt | 117.94 | 8.84 | 2.21 | 0.3886 | 17.80 | 118.34 | 1.83 | 2.17 | 0.9999 | 7.21 | 0.94 | 1.11 | 0.9786 | 0.0732 |
| BPB/5N-Vt | 179.95 | 22.74 | 2.41 | 0.3536 | 23.78 | 179.21 | 0.54 | 0.96 | 0.9997 | 26.96 | 5.82 | 1.33 | 0.9834 | 2.15 |
| BPB/7N-Vt | 182.73 | 23.78 | 3.10 | 0.6744 | 12.09 | 183.82 | 0.55 | 1.00 | 0.9999 | 6.11 | 4.26 | 1.50 | 0.9996 | 0.02 |
| BPB/8N-Vt | 179.39 | 40.04 | 2.50 | 0.5243 | 14.07 | 182.82 | 0.25 | 0.45 | 0.9993 | 59.29 | 10.09 | 1.02 | 0.9998 | 0.07 |
| Adsorbent | Temperature | Langmuir | Freundlich | Redlich Peterson | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| qmax | KL | R2 | Kf | n | R2 | a | b | g | R2 | ||
| 5N-Vt | 298 K | 177.14 | 0.010 | 0.9608 | 20.30 | 3.06 | 0.9784 | 1.56 | 4.89 × 10−3 | 1.09 | 0.9534 |
| 308 K | 178.82 | 0.011 | 0.9438 | 20.24 | 3.01 | 0.9970 | 8.21 × 103 | 4.05 × 103 | 0.67 | 0.9962 | |
| 318 K | 168.35 | 0.011 | 0.9931 | 21.07 | 3.18 | 0.9955 | 2.26 | 0.02 | 0.92 | 0.9935 | |
| 328 K | 161.53 | 0.010 | 0.9656 | 17.81 | 3.00 | 0.9881 | 7.94 | 0.32 | 0.71 | 0.9861 | |
| 7N-Vt | 298 K | 185.36 | 0.014 | 0.9758 | 27.97 | 3.44 | 0.9771 | 2.51 | 0.01 | 1.00 | 0.9698 |
| 308 K | 184.67 | 0.012 | 0.9427 | 22.73 | 3.11 | 0.9956 | 7.46 × 103 | 3.28 × 102 | 0.68 | 0.9945 | |
| 318 K | 173.72 | 0.013 | 0.9428 | 24.31 | 3.31 | 0.9913 | 3.77 × 103 | 1.55 × 102 | 0.70 | 0.9891 | |
| 328 K | 185.91 | 0.009 | 0.9788 | 17.56 | 2.81 | 0.9838 | 3.39 | 0.08 | 0.78 | 0.9856 | |
| 8N-Vt | 298 K | 171.38 | 1.06 × 10−2 | 0.9677 | 19.61 | 3.04 | 0.9965 | 17.12 | 0.74 | 0.69 | 0.9959 |
| 308 K | 178.86 | 0.89 × 10−2 | 0.9727 | 16.62 | 2.80 | 0.9808 | 3.62 | 0.10 | 0.76 | 0.9801 | |
| 318 K | 200.84 | 6.59 × 10−2 | 0.9605 | 11.72 | 2.36 | 0.9930 | 4.39 × 103 | 3.74 × 102 | 0.58 | 0.9913 | |
| 328 K | 238.54 | 6.32 × 10−2 | 0.9790 | 9.70 | 2.12 | 0.9875 | 3.80 | 0.19 | 0.63 | 0.9853 | |
| Adsorbent | Temperature | Langmuir | Freundlich | Redlich Peterson | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| qmax | KL | R2 | Kf | n | R2 | a | b | g | R2 | ||
| 5N-Vt | 298 K | 4.74 × 102 | 0.83 × 10−2 | 0.7566 | 19.93 | 1.96 | 0.8660 | 2.02 × 106 | 1.01 × 104 | 0.49 | 0.8325 |
| 308 K | 6.26 × 102 | 0.41 × 10−2 | 0.8242 | 10.11 | 1.60 | 0.8820 | 7.99 × 105 | 7.91 × 103 | 0.37 | 0.8525 | |
| 318 K | 2.51 × 103 | 5.42 × 10−4 | 0.8537 | 2.58 | 1.16 | 0.8658 | 4.56 × 103 | 1.77 × 103 | 0.14 | 0.8322 | |
| 328 K | 4.14 × 104 | 3.05 × 10−5 | 0.8851 | 1.68 | 1.06 | 0.8874 | 5.19 × 102 | 3.09 × 102 | 0.05 | 0.8592 | |
| 7N-Vt | 298 K | 3.61 × 102 | 0.02 | 0.5762 | 40.30 | 2.62 | 0.7882 | 7.83 × 105 | 1.94 × 104 | 0.62 | 0.7352 |
| 308 K | 3.72 × 102 | 0.01 | 0.6425 | 29.04 | 2.36 | 0.8191 | 4.05 × 105 | 1.39 × 104 | 0.58 | 0.7738 | |
| 318 K | 4.13 × 102 | 0.86 × 10−2 | 0.6714 | 20.04 | 2.07 | 0.8097 | 8.65 × 106 | 4.32 × 105 | 0.52 | 0.7621 | |
| 328 K | 8.78 × 102 | 0.20 × 10−2 | 0.8852 | 5.33 | 1.38 | 0.9191 | 2.12 × 104 | 3.98 × 103 | 0.27 | 0.8988 | |
| 8N-Vt | 298 K | 1.57 × 103 | 6.62 × 10−4 | 0.8854 | 2.32 | 1.21 | 0.9047 | 6.06 × 103 | 2.62 × 103 | 0.18 | 0.8809 |
| 308 K | 9.63 × 102 | 1.68 × 10−3 | 0.8945 | 4.21 | 1.31 | 0.9139 | 9.87 × 103 | 2.34 × 103 | 0.24 | 0.8924 | |
| 318 K | 7.84 × 102 | 2.87 × 10−3 | 0.9423 | 7.33 | 1.45 | 0.9699 | 2.33 × 104 | 3.18 × 103 | 0.31 | 0.9624 | |
| 328 K | 1.12 × 103 | 1.69 × 10−3 | 0.9066 | 5.02 | 1.32 | 0.9339 | 1.86 × 104 | 3.70 × 103 | 0.24 | 0.9174 | |
| System | ΔG° (kJ/mol) | ΔS° (J/(mol K)) | ΔH° (kJ/mol) | |||
|---|---|---|---|---|---|---|
| T = 298 K | T = 308 K | T = 318 K | T = 328 K | |||
| 2NP/5N-Vt | 11.41 | 11.55 | 11.92 | 12.56 | −3.76 | 0.10 |
| 2NP/7N-Vt | 10.58 | 11.33 | 11.48 | 12.85 | −6.89 | 0.10 |
| 2NP/8N-Vt | 11.27 | 12.09 | 7.19 | 7.53 | −7.71 | 0.60 |
| BPB/5N-Vt | 11.87 | 14.08 | 19.88 | 28.35 | −5.45 | −0.15 |
| BPB/7N-Vt | 9.69 | 11.79 | 12.57 | 16.95 | −2.23 | −0.06 |
| BPB/8N-Vt | 18.14 | 16.36 | 15.48 | 17.41 | −1.34 | 0.06 |
| Molecules | EHOMO (eV) | ELUMO (eV) | ΔN between 2-NP | ΔN between BPB |
|---|---|---|---|---|
| 5N | −9.094 | −5.29 | 0.46 | 0.62 |
| 7N | −9.016 | −4.681 | 0.39 | 0.53 |
| 8N | −8.973 | −4.547 | 0.37 | 0.51 |
| 2-NP | −5.577 | −2.006 | - | - |
| BPB | −4.465 | −1.212 | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gong, J.; Wang, T.; Zhang, W.; Han, L.; Gao, M.; Chen, T.; Shen, T.; Ji, Y. Organo-Vermiculites Modified by Aza-Containing Gemini Surfactants: Efficient Uptake of 2-Naphthol and Bromophenol Blue. Nanomaterials 2022, 12, 3636. https://doi.org/10.3390/nano12203636
Gong J, Wang T, Zhang W, Han L, Gao M, Chen T, Shen T, Ji Y. Organo-Vermiculites Modified by Aza-Containing Gemini Surfactants: Efficient Uptake of 2-Naphthol and Bromophenol Blue. Nanomaterials. 2022; 12(20):3636. https://doi.org/10.3390/nano12203636
Chicago/Turabian StyleGong, Jianchao, Tingting Wang, Wei Zhang, Lin Han, Mingxiao Gao, Tianen Chen, Tao Shen, and Yaxiong Ji. 2022. "Organo-Vermiculites Modified by Aza-Containing Gemini Surfactants: Efficient Uptake of 2-Naphthol and Bromophenol Blue" Nanomaterials 12, no. 20: 3636. https://doi.org/10.3390/nano12203636
APA StyleGong, J., Wang, T., Zhang, W., Han, L., Gao, M., Chen, T., Shen, T., & Ji, Y. (2022). Organo-Vermiculites Modified by Aza-Containing Gemini Surfactants: Efficient Uptake of 2-Naphthol and Bromophenol Blue. Nanomaterials, 12(20), 3636. https://doi.org/10.3390/nano12203636