Prospects of Polymeric Nanocomposite Membranes for Water Purification and Scalability and their Health and Environmental Impacts: A Review
Abstract
:1. Introduction
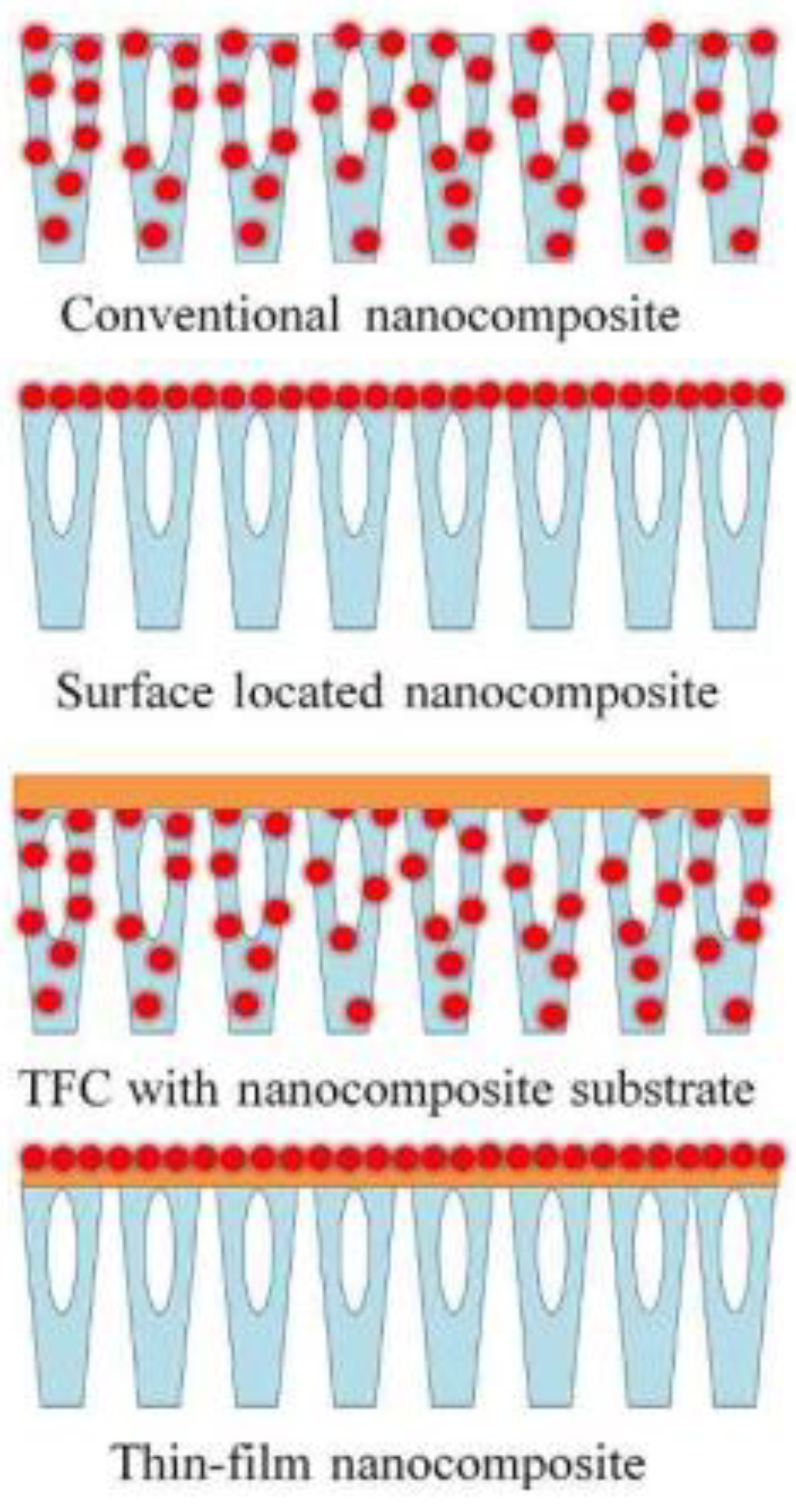
2. Nano-Enhanced and Nanostructured Membranes
3. Nanoparticles
4. Membranes Made of Polymeric Nanocomposite (PNC)
5. Methods of Membrane Preparation
5.1. Traditional Methods for the Preparation of Nanocomposite Membranes
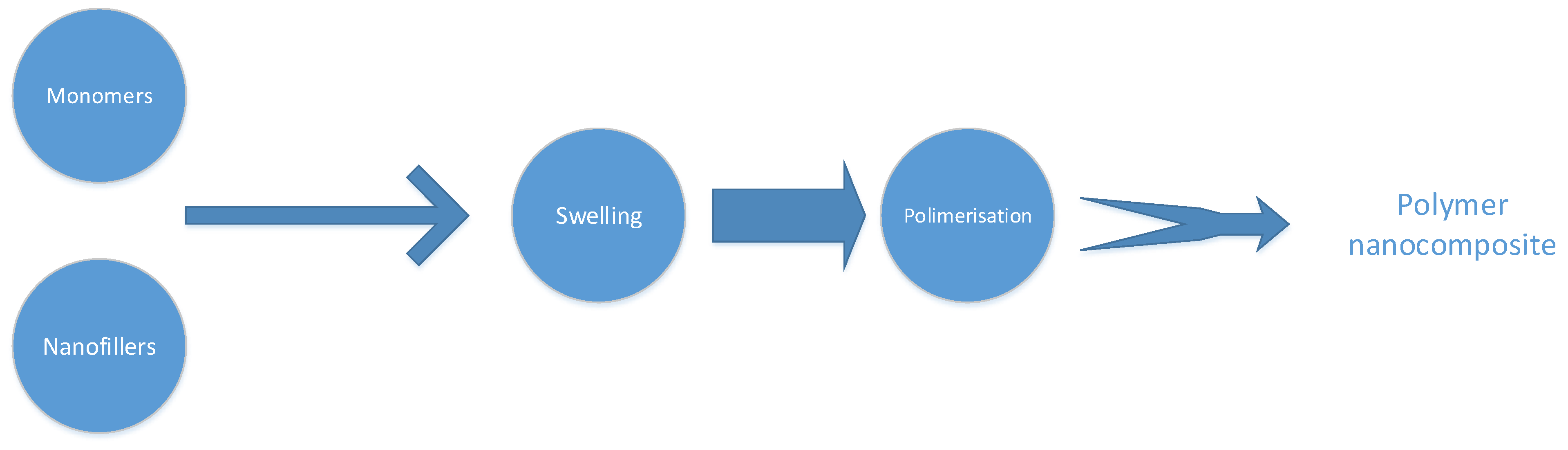
5.1.1. Located Polymerisation
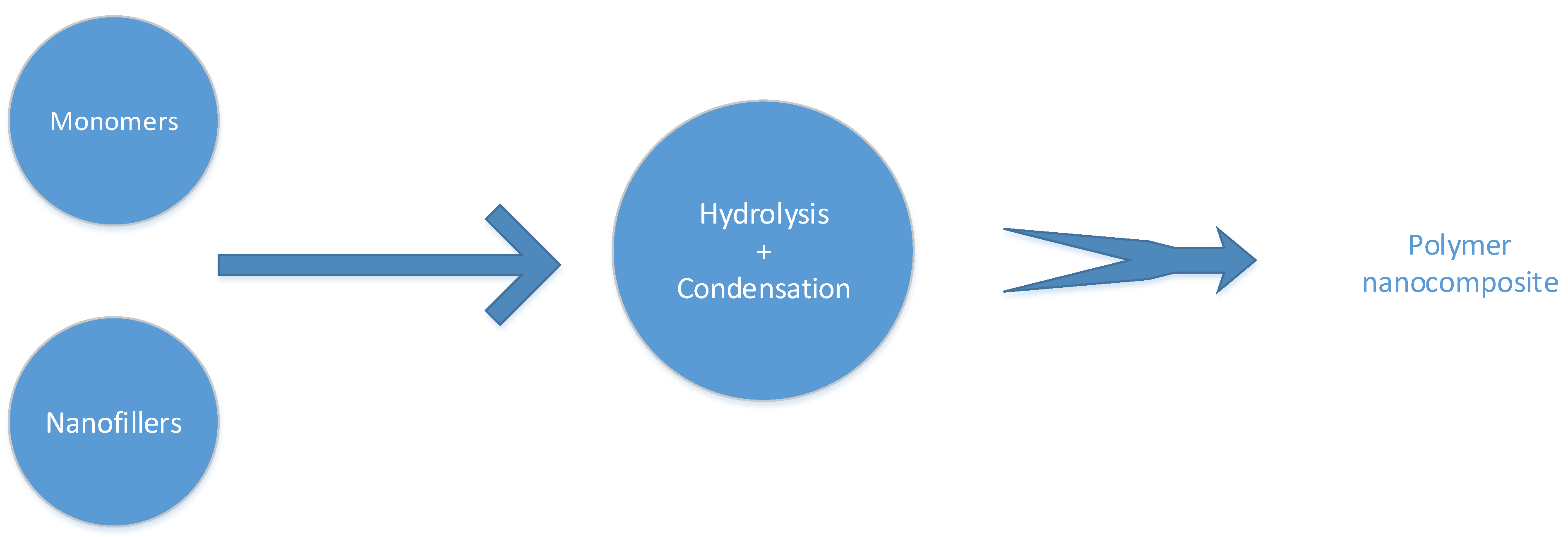
5.1.2. Sol-Gel
5.1.3. Physical Combining
Solution Blending
Melt Blending
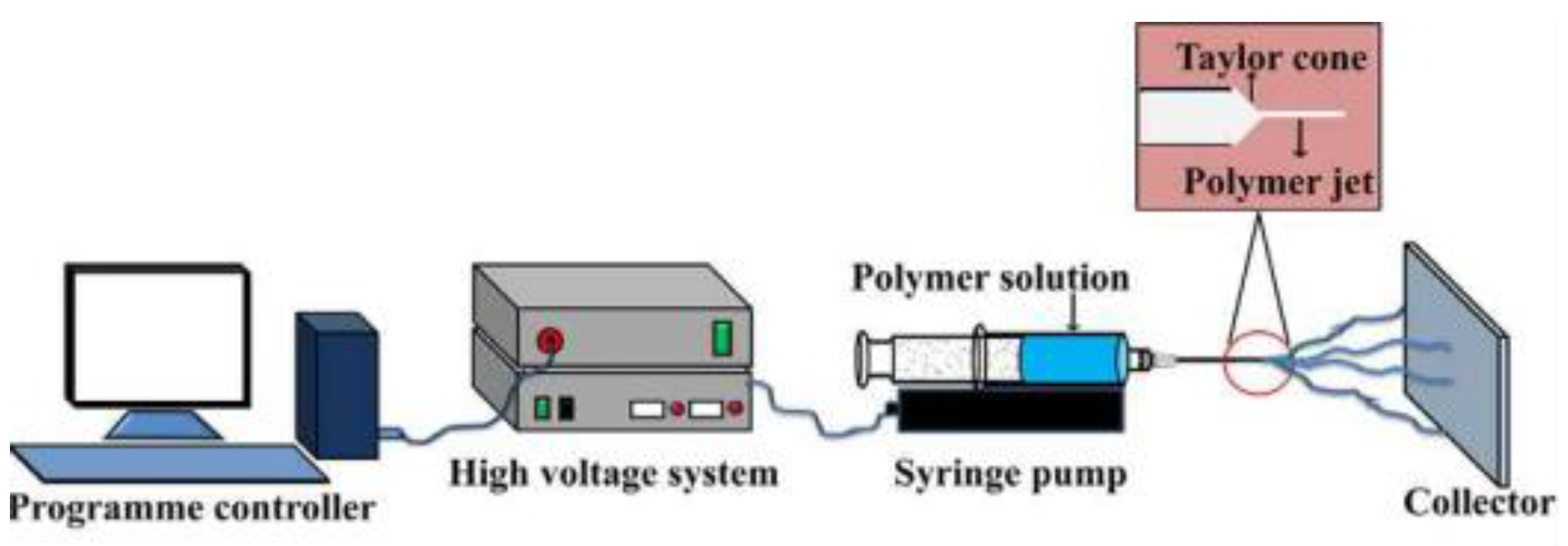
5.2. Electrospinning
5.2.1. Instrumentation and Conceptual Framework
5.2.2. Parameters of Control in an Electrospinning Process
5.2.3. Membrane Electrospinning of Composites
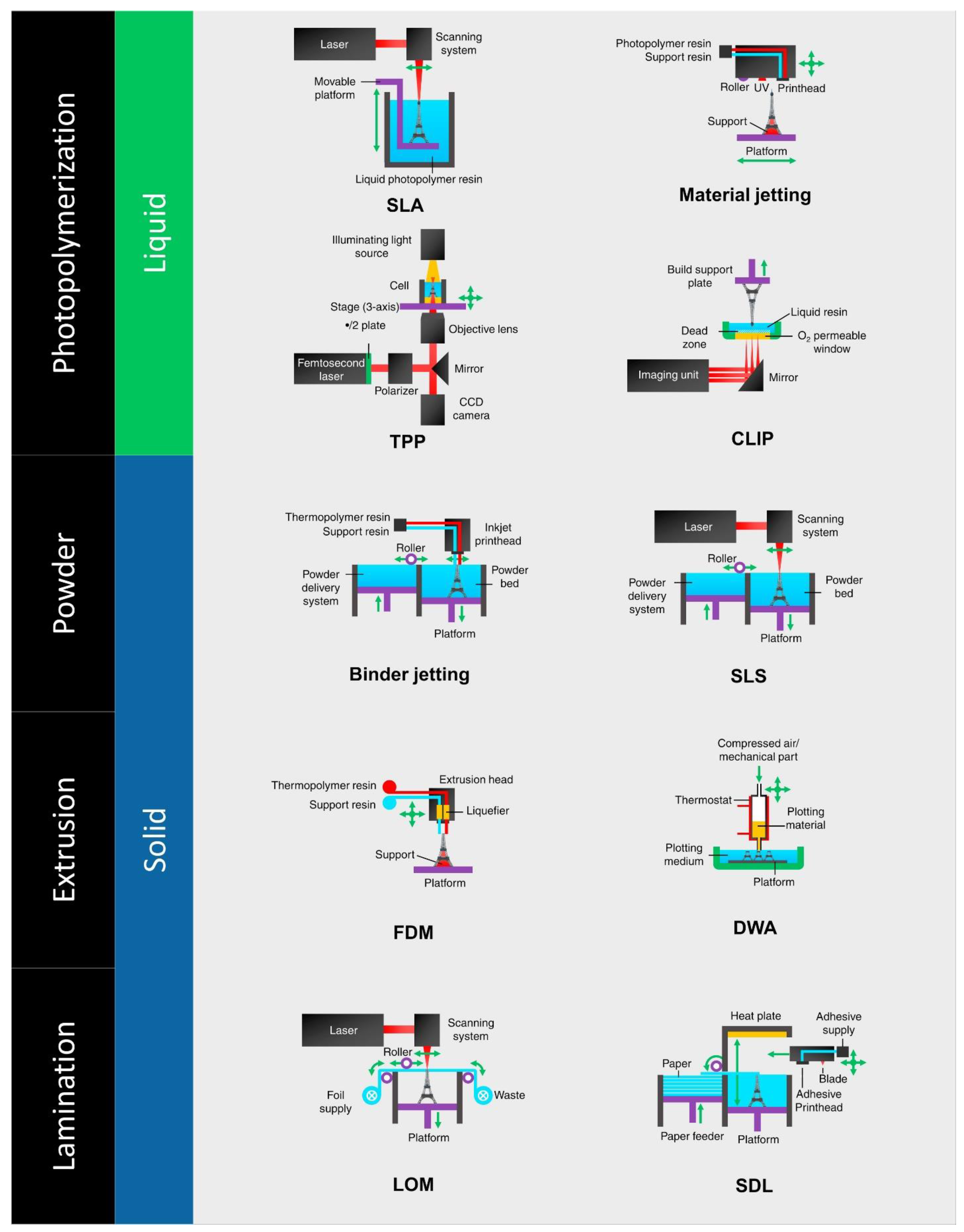
5.3. The 3D Printing Innovation
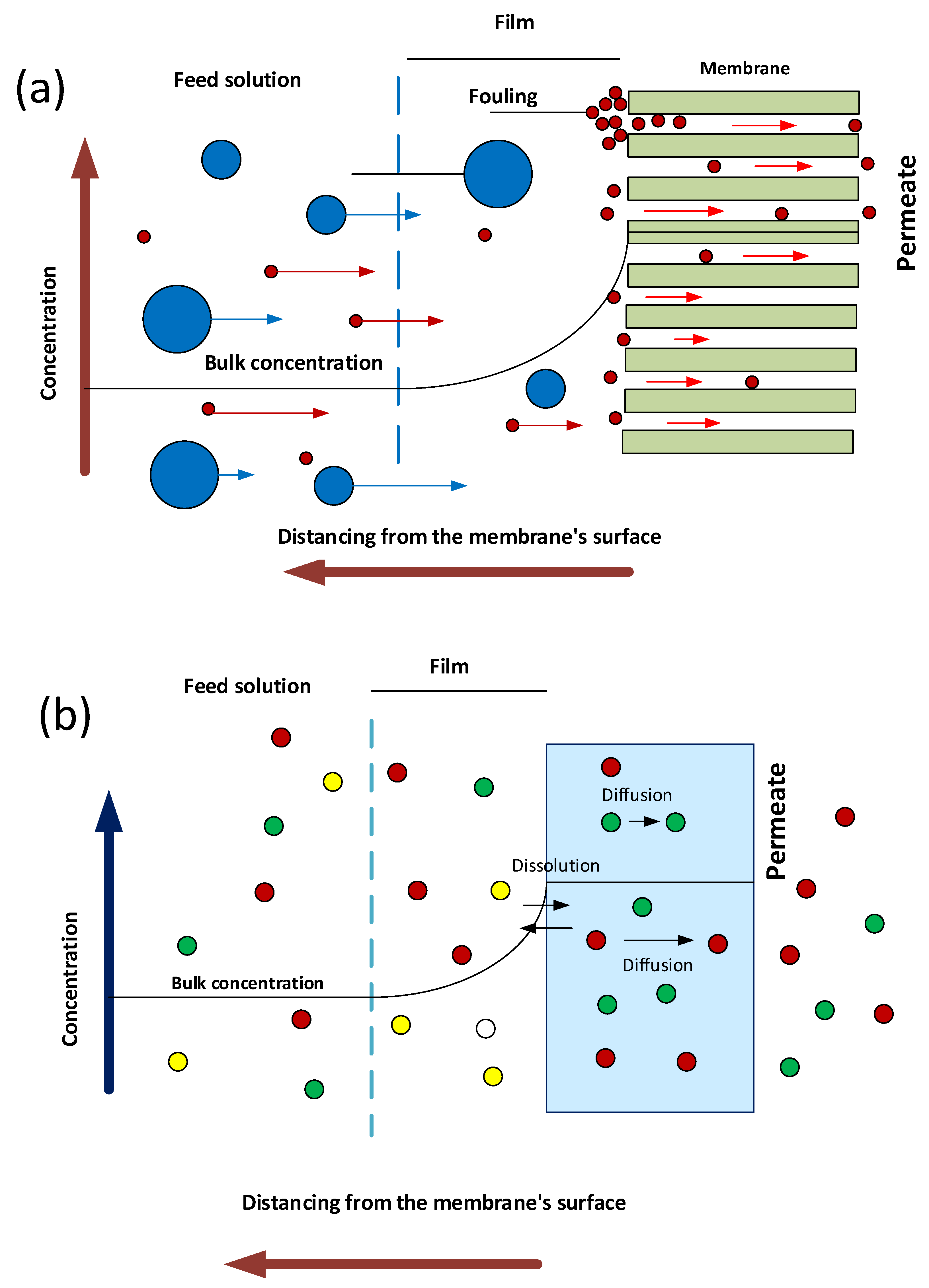
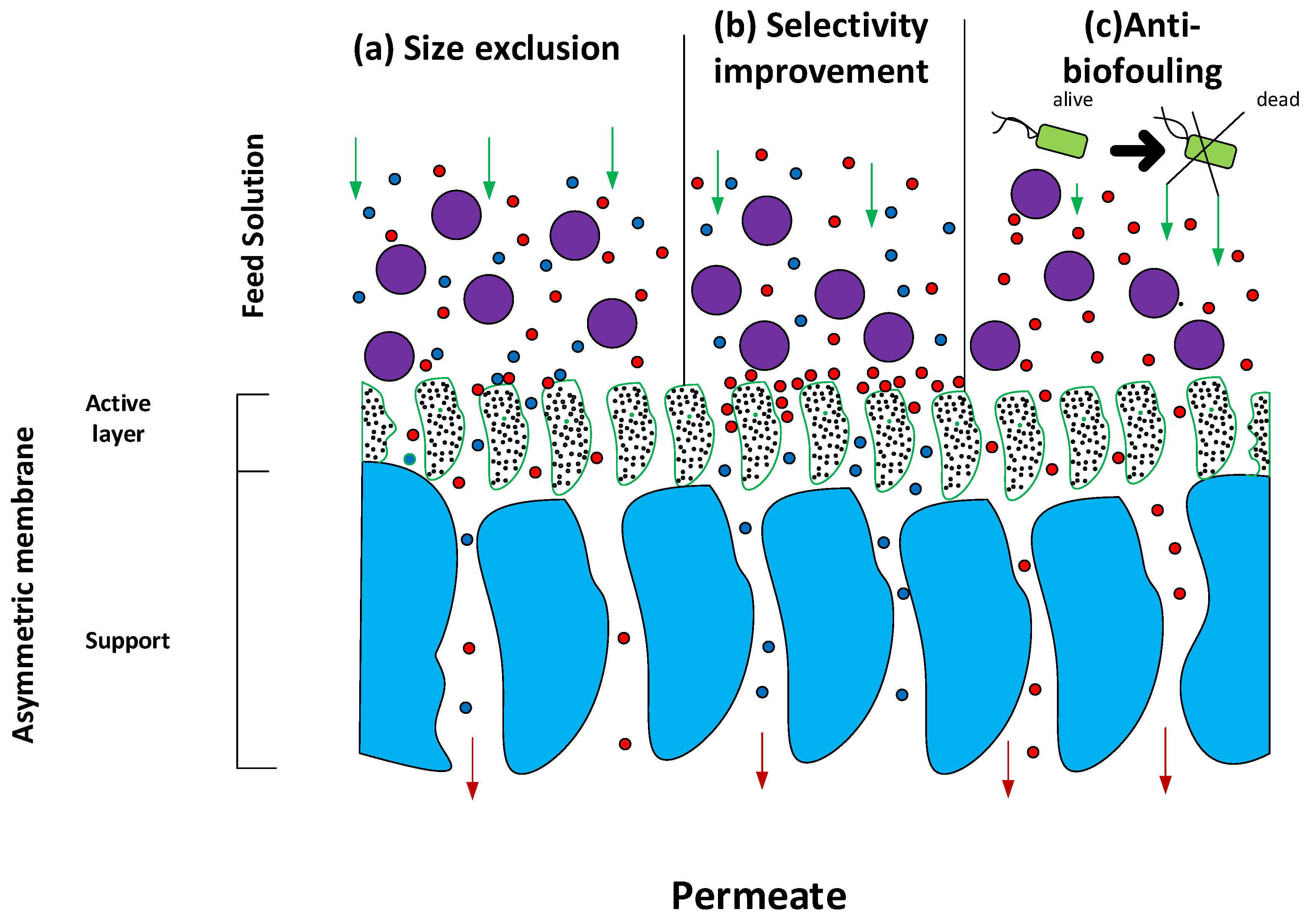
6. Mechanism of Separation of PNC
6.1. Impact on the Size Exclusion Mechanism
6.2. Influence on the Dissolution–Diffusion Mechanism
7. Nanocomposite Membrane Stability
8. Membrane Hydrophobicity/Hydrophilicity
9. Aspects of Nanocomposite Membranes in Water Purification That Present Difficulties
9.1. Scalability
- Methods of synthesis examined to obtain a better result.
- Engineering suitability studies and economic analyses, including the structuring of scalable models.
- Effective quality control and applicable analytic techniques across all process phases to ensure quality.
- Comparison of the purity profiles of the initial and final materials.
- Production of large, homogenous amounts of well-characterised nanocomposite membranes for key prototype phase testing, and hence industrial evaluation.
- Excellent record of technology transfer techniques.
9.2. Stability
- Using analytical technologies such as atomic absorption spectroscopy (SAA) and inductively coupled plasma emission (ICP) [257] to investigate the leaching behaviour of nanocomposite membranes into the aqueous medium.
- The excellent stability of nanocomposite membranes creates a double-paned window of enticing possibilities for their industrial uses. Reusability and regeneration for extended cycles are ideal examples.
9.3. Recyclability and Renewal
9.4. Interruptions
9.5. Cost Efficiency
- (i)
- Time delay: The commercialisation of nanocomposite-based membrane technology must not exceed three to five years [273].
- (ii)
- Funding for research prototype development: There is a significant gap between obtaining funding for commercialisation and prototyping and a favourable research outcome. Compared to research costs, commercialisation expenses are high. Scientists do not focus on the actual application of their research, but businesses want a return on their investments.
- (iii)
- A lack of necessary equipment: Research that is based on nanotechnology is very costly and requires very expensive fabrication equipment. The inability to promote the goods is hampered by a lack of equipment.
- (iv)
- The lack of an assessment standard: The lack of performance evaluation criteria is a significant obstacle in nanoparticle preparation.
- (v)
- Lack of financing: The commercialisation of nanoparticle-based membranes requires substantial expenditures that small- and medium-sized businesses cannot undertake.
- (vi)
- Lack of qualified experts: Sufficiently qualified scientists, researchers, engineers, and technicians are desperately needed in this discipline.
- (vii)
- Support from the general public: The general public is looking forward to novel scientific ideas such as nanotechnology. Therefore, companies who are interested in investing in this sector to deliver high-efficiency output obtain a stronger brand image, but sole proprietorships and small firms that now control a major share of membrane technology do not [274,275,276,277].
9.6. Persistence and Toxicity
10. Prospective Studies
11. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Drinking-Water. Available online: https://www.who.int/news-room/fact-sheets/detail/drinking-water (accessed on 31 July 2022).
- Pendergast, M.M.; Hoek, E.M.V. A review of water treatment membrane nanotechnologies. Energy Environ. Sci. 2011, 4, 1946–1971. [Google Scholar] [CrossRef] [Green Version]
- Arias-Estévez, M.; López-Periago, E.; Martínez-Carballo, E.; Simal-Gándara, J.; Mejuto, J.C.; García-Río, L. The mobility and degradation of pesticides in soils and the pollution of groundwater resources. Agric. Ecosyst. Environ. 2008, 123, 247–260. [Google Scholar] [CrossRef]
- Lin, W.C.; Li, Z.; Burns, M.A. A Drinking Water Sensor for Lead and Other Heavy Metals. Anal. Chem. 2017, 89, 8748–8756. [Google Scholar] [CrossRef] [PubMed]
- Hunt, H.; Briscoe, H.T. The electrical conductivity of organic acids in water, alcohols, and acetone, and the electronic structures of the acids. J. Phys. Chem. 1929, 33, 1495–1513. [Google Scholar] [CrossRef]
- Kremser, U.; Schnug, E. Impact of fertilizers on aquatic ecosystems and protection of water bodies from mineral nutrients. Landbauforsch. Volkenrode 2002, 52, 81–90. [Google Scholar]
- Naim, M.M.; Al-Harby, N.F.; El Batouti, M.; Elewa, M.M. Macro-Reticular Ion Exchange Resins for Recovery of Direct Dyes from Spent Dyeing and Soaping Liquors. Molecules 2022, 27, 1593. [Google Scholar] [CrossRef] [PubMed]
- Nabeela, F.; Azizullah, A.; Bibi, R.; Uzma, S.; Murad, W.; Shakir, S.K.; Ullah, W.; Qasim, M.; Häder, D.P. Microbial contamination of drinking water in Pakistan—A review. Environ. Sci. Pollut. Res. 2014, 21, 13929–13942. [Google Scholar] [CrossRef]
- El Batouti, M.; Al-Harby, N.F.; Elewa, M.M. A Review on Promising Membrane Technology Approaches for Heavy Metal Removal from Water and Wastewater to Solve Water Crisis. Water 2021, 13, 3241. [Google Scholar] [CrossRef]
- Ali, S.; Rehman, S.A.U.; Shah, I.A.; Farid, M.U.; An, A.K.; Huang, H. Efficient removal of zinc from water and wastewater effluents by hydroxylated and carboxylated carbon nanotube membranes: Behaviors and mechanisms of dynamic filtration. J. Hazard. Mater. 2019, 365, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Masood, N.; Farooqi, A.; Zafar, M.I. Health risk assessment of arsenic and other potentially toxic elements in drinking water from an industrial zone of Gujrat, Pakistan: A case study. Environ. Monit. Assess. 2019, 191, 95. [Google Scholar] [CrossRef]
- Pandey, N.; Shukla, S.K.; Singh, N.B. Water purification by polymer nanocomposites: An overview. Nanocomposites 2017, 3, 47–66. [Google Scholar] [CrossRef]
- Sharma, A.; Tulsyan, A.; Motamarri, S. A comparative study of chromium (VI) removal using sawdust and eucalyptus bark. Water Sci. Technol. Water Supply 2009, 9, 343–347. [Google Scholar] [CrossRef]
- Yin, J.; Deng, B. Polymer-matrix nanocomposite membranes for water treatment. J. Membr. Sci. 2015, 479, 256–275. [Google Scholar] [CrossRef]
- Shannon, M.A.; Bohn, P.W.; Elimelech, M.; Georgiadis, J.G.; Mariñas, B.J.; Mayes, A.M.; Marĩas, B.J.; Mayes, A.M. Science and technology for water purification in the coming decades. Nature 2008, 452, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Qadir, D.; Mukhtar, H.; Keong, L.K. Mixed Matrix Membranes for Water Purification Applications. Sep. Purif. Rev. 2017, 46, 62–80. [Google Scholar] [CrossRef]
- Baig, N.; Salhi, B.; Sajid, M.; Aljundi, I.H. Recent Progress in Microfiltration/Ultrafiltration Membranes for Separation of Oil and Water Emulsions. Chem. Rec. 2022, 22, e202100320. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Ma, B.; Yang, C.; Duan, X.; Tang, Q. Fabrication of anti-fouling and photocleaning PVDF microfiltration membranes embedded with N-TiO2 photocatalysts. Sep. Purif. Technol. 2022, 298, 121673. [Google Scholar] [CrossRef]
- Antolín-Cerón, V.H.; González-López, F.J.; Astudillo-Sánchez, P.D.; Barrera-Rivera, K.A.; Martínez-Richa, A. High-Performance Polyurethane Nanocomposite Membranes Containing Cellulose Nanocrystals for Protein Separation. Polymer 2022, 14, 831. [Google Scholar] [CrossRef]
- Yousef, S.; Eimontas, J.; Striūgas, N.; Mohamed, A.; Ali Abdelnaby, M. Pyrolysis kinetic behavior and TG-FTIR-GC–MS analysis of end-life ultrafiltration polymer nanocomposite membranes. Chem. Eng. J. 2022, 428, 131181. [Google Scholar] [CrossRef]
- Naim, M.M.; El-Shafei, A.A.; Moneer, A.A.; Elewa, M.M. Ultrafiltration by a super-hydrophilic regenerated cellulose membrane. Water Pract. Technol. 2015, 10, 337–346. [Google Scholar] [CrossRef]
- Khoerunnisa, F.; Nurhayati, M.; Annisa, N.A.A.; Fatimah, S.; Nashrah, N.; Hendrawan, H.; Ko, Y.G.; Ng, E.P.; Opaprakasit, P. Effects of Benzalkonium Chloride Contents on Structures, Properties, and Ultrafiltration Performances of Chitosan-Based Nanocomposite Membranes. Membranes 2022, 12, 268. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Yin, Y.; Liu, S.; Li, H.; Su, B.; Han, L.; Gao, X.; Gao, C. Interlayered thin-film nanocomposite membrane with synergetic effect of COFs interlayer and GQDs incorporation for organic solvent nanofiltration. J. Membr. Sci. 2022, 662, 120930. [Google Scholar] [CrossRef]
- Dashtbozorg, A.; Saljoughi, E.; Mousavi, S.M.; Kiani, S. High-performance and robust polysulfone nanocomposite membrane containing 2D functionalized MXene nanosheets for the nanofiltration of salt and dye solutions. Desalination 2022, 527, 115600. [Google Scholar] [CrossRef]
- Zhang, W.; Xu, H.; Xie, F.; Ma, X.; Niu, B.; Chen, M.; Zhang, H.; Zhang, Y.; Long, D. General synthesis of ultrafine metal oxide/reduced graphene oxide nanocomposites for ultrahigh-flux nanofiltration membrane. Nat. Commun. 2022, 13, 471. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, X.-P.; Zong, Z.-A.; Lin, R.; Zhang, X.-Y.; Chen, F.-S.; Ding, W.-D.; Zhang, L.-L.; Meng, X.-M.; Hou, J. Thin film nanocomposite membrane incorporated with 2D-MOF nanosheets for highly efficient reverse osmosis desalination. J. Membr. Sci. 2022, 653, 120520. [Google Scholar] [CrossRef]
- Bian, S.; Wang, Y.; Xiao, F.; Tong, Y.; Gao, C.; Zhu, G. Fabrication of polyamide thin-film nanocomposite reverse osmosis membrane with improved permeability and antibacterial performances using silver immobilized hollow polymer nanospheres. Desalination 2022, 539, 115953. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhao, D.L.; Feng, F.; Chung, T.S.; Chen, S.B. Thin-film nanocomposite reverse osmosis membranes incorporated with citrate-modified layered double hydroxides (LDHs) for brackish water desalination and boron removal. Desalination 2022, 527, 115583. [Google Scholar] [CrossRef]
- Burts, K.; Plisko, T.; Dmitrenko, M.; Zolotarev, A.; Kuzminova, A.; Bildyukevich, A.; Ermakov, S.; Penkova, A. Novel Thin Film Nanocomposite Membranes Based on Chitosan Succinate Modified with Fe-BTC for Enhanced Pervaporation Dehydration of Isopropanol. Membranes 2022, 12, 653. [Google Scholar] [CrossRef] [PubMed]
- Elewa, M.M.; El-Shafei, A.A.; Moneer, A.A.; Naim, M.M. Effect of cell hydrodynamics in desalination of saline water by sweeping air pervaporation technique using innovated membrane. Desalin. Water Treat. 2016, 57, 23293–23307. [Google Scholar] [CrossRef]
- Naim, M.; Elewa, M.; El-Shafei, A.; Moneer, A. Desalination of simulated seawater by purge-air pervaporation using an innovative fabricated membrane. Water Sci. Technol. 2015, 72, 785–793. [Google Scholar] [CrossRef] [PubMed]
- Elsheniti, M.B.; Elbessomy, M.O.; Wagdy, K.; Elsamni, O.A.; Elewa, M.M. Augmenting the distillate water flux of sweeping gas membrane distillation using turbulators: A numerical investigation. Case Stud. Therm. Eng. 2021, 26, 101180. [Google Scholar] [CrossRef]
- Yadav, A.; Mandal, J.R.; Panda, A.B.; Shahi, V.K. Structural tailoring of ceria nanoparticles for fabricating fouling resistant nanocomposite membranes with high flux distillation. Colloids Surf. A Physicochem. Eng. Asp. 2022, 633, 127858. [Google Scholar] [CrossRef]
- Gontarek-Castro, E.; Castro-Muñoz, R.; Lieder, M. New insights of nanomaterials usage toward superhydrophobic membranes for water desalination via membrane distillation: A review. Crit. Rev. Environ. Sci. Technol. 2022, 52, 2104–2149. [Google Scholar] [CrossRef]
- Yassari, M.; Shakeri, A.; Salehi, H.; Razavi, S.R. Enhancement in forward osmosis performance of thin-film nanocomposite membrane using tannic acid-functionalized graphene oxide. J. Polym. Res. 2022, 29, 43. [Google Scholar] [CrossRef]
- Meier-Haack, J. Special Issue: New Challenges in Thin-Film Nanocomposite Membranes. Coatings 2022, 12, 1169. [Google Scholar] [CrossRef]
- Wei, X.; Liu, Y.; Zheng, J.; Wang, X.; Xia, S.; Van der Bruggen, B. A critical review on thin-film nanocomposite membranes enabled by nanomaterials incorporated in different positions and with diverse dimensions: Performance comparison and mechanisms. J. Membr. Sci. 2022, 661, 120952. [Google Scholar] [CrossRef]
- Bernardo, P.; Drioli, E.; Golemme, G. Membrane gas separation: A review/state of the art. Ind. Eng. Chem. Res. 2009, 48, 4638–4663. [Google Scholar] [CrossRef]
- Ulbricht, M. Advanced functional polymer membranes. Polymer 2006, 47, 2217–2262. [Google Scholar] [CrossRef] [Green Version]
- Goh, P.S.; Ismail, A.F. A review on inorganic membranes for desalination and wastewater treatment. Desalination 2018, 434, 60–80. [Google Scholar] [CrossRef]
- Ng, L.Y.; Mohammad, A.W.; Leo, C.P.; Hilal, N. Polymeric membranes incorporated with metal/metal oxide nanoparticles: A comprehensive review. Desalination 2013, 308, 15–33. [Google Scholar] [CrossRef]
- Lee, A.; Elam, J.W.; Darling, S.B. Membrane materials for water purification: Design, development, and application. Environ. Sci. Water Res. Technol. 2016, 2, 17–42. [Google Scholar] [CrossRef]
- Lee, J.; Jeong, S.; Liu, Z. Progress and challenges of carbon nanotube membrane in water treatment. Crit. Rev. Environ. Sci. Technol. 2016, 46, 999–1046. [Google Scholar] [CrossRef]
- Lee, J.; Ye, Y.; Ward, A.J.; Zhou, C.; Chen, V.; Minett, A.I.; Lee, S.; Liu, Z.; Chae, S.R.; Shi, J. High flux and high selectivity carbon nanotube composite membranes for natural organic matter removal. Sep. Purif. Technol. 2016, 163, 109–119. [Google Scholar] [CrossRef]
- El Batouti, M.; Alharby, N.F.; Elewa, M.M. Review of New Approaches for Fouling Mitigation in Membrane Separation Processes in Water Treatment Applications. Separations 2021, 9, 1. [Google Scholar] [CrossRef]
- Raval, H.D.; Trivedi, J.J.; Joshi, S.V.; Devmurari, C.V. Flux enhancement of thin film composite RO membrane by controlled chlorine treatment. Desalination 2010, 250, 945–949. [Google Scholar] [CrossRef]
- Guo, W.; Ngo, H.-H.H.; Li, J. A mini-review on membrane fouling. Bioresour. Technol. 2012, 122, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Hegab, H.M.; Zou, L. Graphene oxide-assisted membranes: Fabrication and potential applications in desalination and water purification. J. Membr. Sci. 2015, 484, 95–106. [Google Scholar] [CrossRef]
- Li, X.; Janke, A.; Formanek, P.; Fery, A.; Stamm, M.; Tripathi, B.P. One pot preparation of polysulfone-amino functionalized SiO2 nanoparticle ultrafiltration membranes for water purification. J. Environ. Chem. Eng. 2018, 6, 4598–4604. [Google Scholar] [CrossRef]
- Esfahani, M.R.; Tyler, J.L.; Stretz, H.A.; Wells, M.J.M. Effects of a dual nanofiller, nano-TiO2 and MWCNT, for polysulfone-based nanocomposite membranes for water purification. Desalination 2015, 372, 47–56. [Google Scholar] [CrossRef]
- Zhang, L.; Shan, C.; Jiang, X.; Li, X.; Yu, L. High hydrophilic antifouling membrane modified with capsaicin-mimic moieties via microwave assistance (MWA) for efficient water purification. Chem. Eng. J. 2018, 338, 688–699. [Google Scholar] [CrossRef]
- Vatanpour, V.; Jouyandeh, M.; Akhi, H.; Mousavi Khadem, S.S.; Ganjali, M.R.; Moradi, H.; Mirsadeghi, S.; Badiei, A.; Esmaeili, A.; Rabiee, N.; et al. Hyperbranched polyethylenimine functionalized silica/polysulfone nanocomposite membranes for water purification. Chemosphere 2022, 290, 133363. [Google Scholar] [CrossRef]
- Barzegar, T.; Hassanajili, S. Fabrication and characterization of dual layer PEBAX-SiO2/polyethersulfone nanocomposite membranes for separation of CO2/CH4 gases. J. Appl. Polym. Sci. 2022, 139, 51624. [Google Scholar] [CrossRef]
- Yoon, K.; Hsiao, B.S.; Chu, B. High flux ultrafiltration nanofibrous membranes based on polyacrylonitrile electrospun scaffolds and crosslinked polyvinyl alcohol coating. J. Membr. Sci. 2009, 338, 145–152. [Google Scholar] [CrossRef]
- Iranpoury, A.; Mehrnia, M.R.; Jafari, S.H.; Najmi, M. Improvement of fouling resistance and mechanical reinforcement of polyacrylonitrile membranes by amino-functionalized multiwalled carbon nanotubes for membrane bioreactors applications. J. Appl. Polym. Sci. 2022, 139, e52733. [Google Scholar] [CrossRef]
- Tan, Z.; Chen, S.; Peng, X.; Zhang, L.; Gao, C. Polyamide membranes with nanoscale Turing structures for water purification. Science 2018, 360, 518–521. [Google Scholar] [CrossRef] [Green Version]
- Vatanpour, V.; Iranpour Boroujeni, N.; Pasaoglu, M.E.; Mahmodi, G.; Mohammadikish, M.; Kazemi-Andalib, F.; Koyuncu, I. Novel infinite coordination polymer (ICP) modified thin-film polyamide nanocomposite membranes for simultaneous enhancement of antifouling and chlorine-resistance performance. J. Membr. Sci. 2022, 647, 120305. [Google Scholar] [CrossRef]
- Muhammad, S.; Niazi, J.H.; Shawuti, S.; Qureshi, A. Functional POSS based polyimide nanocomposite for enhanced structural, thermal, antifouling and antibacterial properties. Mater. Today Commun. 2022, 31, 103287. [Google Scholar] [CrossRef]
- Li, E.; Chen, Z.; Duan, C.; Yuan, B.; Yan, S.; Luo, X.; Pan, F.; Jiang, Z. Enhanced CO2-capture performance of polyimide-based mixed matrix membranes by incorporating ZnO@MOF nanocomposites. Sep. Purif. Technol. 2022, 289, 120714. [Google Scholar] [CrossRef]
- Nouri, M.; Marjani, A.; Tajdari, M.; Heidary, F.; Salimi, M. Preparation of cellulose acetate membrane coated by PVA/Fe3O4 nanocomposite thin film: An in situ procedure. Colloid Polym. Sci. 2018, 296, 1213–1223. [Google Scholar] [CrossRef]
- Bhanthumnavin, W.; Wanichapichart, P.; Taweepreeda, W.; Sirijarukula, S.; Paosawatyanyong, B. Surface modification of bacterial cellulose membrane by oxygen plasma treatment. Surf. Coat. Technol. 2016, 306, 272–278. [Google Scholar] [CrossRef]
- Keskin, B.; Naziri Mehrabani, S.A.; Arefi-Oskoui, S.; Vatanpour, V.; Orhun Teber, O.; Khataee, A.; Orooji, Y.; Koyuncu, I. Development of Ti2AlN MAX phase/cellulose acetate nanocomposite membrane for removal of dye, protein and lead ions. Carbohydr. Polym. 2022, 296, 119913. [Google Scholar] [CrossRef]
- Elbadawi, N.A.; Ramadan, A.R.; Esawi, A.M.K. Studying the Effect of Shortening Carbon Nanotubes via Ball Milling on Cellulose Acetate Nanocomposite Membranes for Desalination Applications. Membranes 2022, 12, 474. [Google Scholar] [CrossRef] [PubMed]
- Gao, A.; Liu, F.; Xue, L. Preparation and evaluation of heparin-immobilized poly (lactic acid) (PLA) membrane for hemodialysis. J. Membr. Sci. 2014, 452, 390–399. [Google Scholar] [CrossRef]
- Vatanpour, V.; Dehqan, A.; Paziresh, S.; Zinadini, S.; Zinatizadeh, A.A.; Koyuncu, I. Polylactic acid in the fabrication of separation membranes: A review. Sep. Purif. Technol. 2022, 296, 121433. [Google Scholar] [CrossRef]
- Yakdoumi, F.Z.; Hadj-Hamou, A.S.; Rahoui, N.; Rahman, M.M.; Abetz, V. Polylactic acid nanocomposites containing functionalized multiwalled carbon nanotubes as antimicrobial packaging materials. Int. J. Biol. Macromol. 2022, 213, 55–69. [Google Scholar] [CrossRef]
- He, Y.; Yan, J.; He, X.; Weng, W.; Cheng, K. PLLA/Graphene Nanocomposites Membranes with Improved Biocompatibility and Mechanical Properties. Coatings 2022, 12, 718. [Google Scholar] [CrossRef]
- Svang-Ariyaskul, A.; Huang, R.Y.M.; Douglas, P.L.; Pal, R.; Feng, X.; Chen, P.; Liu, L. Blended chitosan and polyvinyl alcohol membranes for the pervaporation dehydration of isopropanol. J. Membr. Sci. 2006, 280, 815–823. [Google Scholar] [CrossRef]
- Ahmad, S.; Jahan, Z.; Sher, F.; Niazi, M.B.K.; Noor, T.; Hou, H.; Azhar, O.; Sher, E.K. Polyvinyl alcohol and aminated cellulose nanocrystal membranes with improved interfacial compatibility for environmental applications. Environ. Res. 2022, 214, 113793. [Google Scholar] [CrossRef]
- Asadpour, S.; Raeisi vanani, A.; Kooravand, M.; Asfaram, A. A review on zinc oxide/poly(vinyl alcohol) nanocomposites: Synthesis, characterization and applications. J. Clean. Prod. 2022, 362, 132297. [Google Scholar] [CrossRef]
- Liu, F.; Hashim, N.A.; Liu, Y.; Abed, M.R.M.; Li, K. Progress in the production and modification of PVDF membranes. J. Membr. Sci. 2011, 375, 1–27. [Google Scholar] [CrossRef]
- Tomaszewska, M. Preparation and properties of flat-sheet membranes from poly(vinylidene fluoride) for membrane distillation. Desalination 1996, 104, 1–11. [Google Scholar] [CrossRef]
- Pagliero, M.; Alloisio, M.; Costa, C.; Firpo, R.; Mideksa, E.A.; Comite, A. Carbon Black/Polyvinylidene Fluoride Nanocomposite Membranes for Direct Solar Distillation. Energies 2022, 15, 740. [Google Scholar] [CrossRef]
- Kumar, A.; Ghosh, U.K. Polyvinylidene fluoride/boehmite nanocomposite membrane for effective removal of arsenate ion from water. J. Water Process Eng. 2022, 47, 102652. [Google Scholar] [CrossRef]
- Chai, J.; Wang, G.; Zhang, A.; Dong, G.; Li, S.; Zhao, J.; Zhao, G. Microcellular injection molded lightweight and tough poly (L-lactic acid)/in-situ polytetrafluoroethylene nanocomposite foams with enhanced surface quality and thermally-insulating performance. Int. J. Biol. Macromol. 2022, 215, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Asadi, A.; Gholami, F.; Nazari, S.; Dolatshah, M. Improved filtration performance of polyvinylidene fluoride nanocomposite membranes embedded with deep eutectic solvent: Application towards MBR. Desalination 2022, 543, 116088. [Google Scholar] [CrossRef]
- Sun, D.; Zhou, T.; Lu, Y.; Yan, Y.; Liu, C.; Che, G. Ion-imprinted antifouling nanocomposite membrane for separation of lithium ion. Korean J. Chem. Eng. 2022, 39, 2482–2490. [Google Scholar] [CrossRef]
- Observatory Nano. Nanoenhanced Membranes for Improved Water Treatment Briefing No.16 Environment; Observatory Nano: Edinburgh, UK, 2011. [Google Scholar]
- Buonomenna, M.G. Nano-enhanced reverse osmosis membranes. Desalination 2013, 314, 73–88. [Google Scholar] [CrossRef]
- Chen, X.; Gao, X.; Fu, K.; Qiu, M.; Xiong, F.; Ding, D.; Cui, Z.; Wang, Z.; Fan, Y.; Drioli, E. Tubular hydrophobic ceramic membrane with asymmetric structure for water desalination via vacuum membrane distillation process. Desalination 2018, 443, 212–220. [Google Scholar] [CrossRef]
- Castro-Muñoz, R.; Galiano, F.; Fíla, V.; Drioli, E.; Figoli, A. Matrimid®5218 dense membrane for the separation of azeotropic MeOH-MTBE mixtures by pervaporation. Sep. Purif. Technol. 2018, 199, 27–36. [Google Scholar] [CrossRef]
- Ursino, C.; Castro-Muñoz, R.; Drioli, E.; Gzara, L.; Albeirutty, M.H.; Figoli, A. Progress of nanocomposite membranes for water treatment. Membranes 2018, 8, 18. [Google Scholar] [CrossRef] [Green Version]
- Zoromba, M.S.; Ismail, M.I.M.; Bassyouni, M.I.; Abdel-Aziz, M.H.; Salah, N.; Alshahrie, A.; Memic, A. Fabrication and characterization of poly (aniline-co-o-anthranilic acid)/magnetite nanocomposites and their application in wastewater treatment. Colloids Surf. A Physicochem. Eng. Asp. 2017, 520, 121–130. [Google Scholar] [CrossRef]
- Misdan, N.; Ismail, A.F.; Hilal, N. Recent advances in the development of (bio) fouling resistant thin film composite membranes for desalination. Desalination 2016, 380, 105–111. [Google Scholar] [CrossRef] [Green Version]
- Cui, L.; Chen, P.; Chen, S.; Yuan, Z.; Yu, C.; Ren, B.; Zhang, K. In situ study of the antibacterial activity and mechanism of action of silver nanoparticles by surface-enhanced raman spectroscopy. Anal. Chem. 2013, 85, 5436–5443. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, G.; Shi, Z.; Yang, M.; Luck, R.L. Two hydrogen-bond-cross-linked molybdenum (VI) network polymers: Synthesis, crystal structures and cyclooctene epoxidation with H2O2. Struct. Chem. 2009, 20, 869–876, Erratum in Struct. Chem. 2009, 20, 1115. [Google Scholar] [CrossRef]
- Marambio-Jones, C.; Hoek, E.M.V. A review of the antibacterial effects of silver nanomaterials and potential implications for human health and the environment. J. Nanopart. Res. 2010, 12, 1531–1551. [Google Scholar] [CrossRef]
- Naim, M.M.; El-Shafei, A.A.; Elewa, M.M.; Moneer, A.A. Application of silver-, iron-, and chitosan- nanoparticles in wastewater treatment. Desalin. Water Treat. 2017, 73, 268–280. [Google Scholar] [CrossRef]
- Li, X.; Sotto, A.; Li, J.; Van der Bruggen, B. Progress and perspectives for synthesis of sustainable antifouling composite membranes containing in situ generated nanoparticles. J. Membr. Sci. 2017, 524, 502–528. [Google Scholar] [CrossRef]
- Gholami, A.; Moghadassi, A.R.; Hosseini, S.M.; Shabani, S.; Gholami, F. Preparation and characterization of polyvinyl chloride based nanocomposite nanofiltration-membrane modified by iron oxide nanoparticles for lead removal from water. J. Ind. Eng. Chem. 2014, 20, 1517–1522. [Google Scholar] [CrossRef]
- Hernandez, M.; Medioni, G.; Hu, Z.; Sadda, S. Multimodal registration of multiple retinal images based on line structures. In Proceedings of the 2015 IEEE Winter Conference on Applications of Computer Vision, WACV, Waikoloa, HI, USA, 5–9 January 2015; pp. 907–914. [Google Scholar]
- Huang, Q.; Shi, X.; Pinto, R.A.; Petersen, E.J.; Weber, W.J. Tunable synthesis and immobilization of zero-valent iron nanoparticles for environmental applications. Environ. Sci. Technol. 2008, 42, 8884–8889. [Google Scholar] [CrossRef]
- Ahmad, R.; Sharma, N.; Mishra, C.; Singh, N.J.; Rawat, G.S.; Bhatnagar, Y.V. Security, size, or sociality: What makes markhor (Capra falconeri) sexually segregate? J. Mammal. 2018, 99, 55–63. [Google Scholar] [CrossRef]
- Mastropietro, T.F.; Drioli, E.; Poerio, T. Low temperature synthesis of nanosized NaY zeolite crystals from organic-free gel by using supported seeds. RSC Adv. 2014, 4, 21951–21957. [Google Scholar] [CrossRef]
- Gutub, S.A.; Bassyouni, M.; Abdel-Hamid, S.M.S. Dissolved solids adsorption of freshwater using synthesized bio-foam composite. Life Sci. J. 2013, 10, 464–471. [Google Scholar]
- Dasgupta, J.; Chakraborty, S.; Sikder, J.; Kumar, R.; Pal, D.; Curcio, S.; Drioli, E. The effects of thermally stable titanium silicon oxide nanoparticles on structure and performance of cellulose acetate ultrafiltration membranes. Sep. Purif. Technol. 2014, 133, 55–68. [Google Scholar] [CrossRef]
- Alsalhy, Q.F.; Salih, H.A.; Simone, S.; Zablouk, M.; Drioli, E.; Figoli, A. Poly (ether sulfone) (PES) hollow-fiber membranes prepared from various spinning parameters. Desalination 2014, 345, 21–35. [Google Scholar] [CrossRef]
- Rizzuto, C.; Pugliese, G.; Bahattab, M.A.; Aljlil, S.A.; Drioli, E.; Tocci, E. Multiwalled carbon nanotube membranes for water purification. Sep. Purif. Technol. 2018, 193, 378–385. [Google Scholar] [CrossRef]
- Chen, B.; Sun, W.; Wang, C.; Guo, X. Size-dependent impact of inorganic nanoparticles on sulfamethoxazole adsorption by carbon nanotubes. Chem. Eng. J. 2017, 316, 160–170. [Google Scholar] [CrossRef]
- Kaur, H.; Bulasara, V.K.; Gupta, R.K. Effect of carbonates composition on the permeation characteristics of low-cost ceramic membrane supports. J. Ind. Eng. Chem. 2016, 44, 185–194. [Google Scholar] [CrossRef]
- Azizi, S.; Ahmad, M.B.; Ibrahim, N.A.; Hussein, M.Z.; Namvar, F. Cellulose nanocrystals/ZnO as a bifunctional reinforcing nanocomposite for poly(vinyl alcohol)/chitosan blend films: Fabrication, characterization and properties. Int. J. Mol. Sci. 2014, 15, 11040–11053. [Google Scholar] [CrossRef] [Green Version]
- Poyraz, B.; Tozluoğlu, A.; Candan, Z.; Demir, A.; Yavuz, M. Influence of PVA and silica on chemical, thermo-mechanical and electrical properties of Celluclast-treated nanofibrillated cellulose composites. Int. J. Biol. Macromol. 2017, 104, 384–392. [Google Scholar] [CrossRef]
- Niazi, M.B.K.; Jahan, Z.; Berg, S.S.; Gregersen, Ø.W. Mechanical, thermal and swelling properties of phosphorylated nanocellulose fibrils/PVA nanocomposite membranes. Carbohydr. Polym. 2017, 177, 258–268. [Google Scholar] [CrossRef]
- Jahan, Z.; Niazi, M.B.K.; Gregersen, Ø.W. Mechanical, thermal and swelling properties of cellulose nanocrystals/PVA nanocomposites membranes. J. Ind. Eng. Chem. 2018, 57, 113–124. [Google Scholar] [CrossRef]
- Sigwadi, R.; Dhlamini, M.S.; Mokrani, T.; Nemavhola, F. Enhancing the mechanical properties of zirconia/Nafion® nanocomposite membrane through carbon nanotubes for fuel cell application. Heliyon 2019, 5, e02112. [Google Scholar] [CrossRef] [PubMed]
- Sigwadi, R.; Dhlamini, M.S.; Mokrani, T.; Ṋemavhola, F.; Nonjola, P.F.; Msomi, P.F. The proton conductivity and mechanical properties of Nafion®/ZrP nanocomposite membrane. Heliyon 2019, 5, e02240. [Google Scholar] [CrossRef] [Green Version]
- Naim, M.M.; Batouti, M.E.; Elewa, M.M. Novel heterogeneous cellulose-based ion-exchange membranes for electrodialysis. Polym. Bull. 2021, 79, 9753–9777. [Google Scholar] [CrossRef]
- Csetneki, I.; Filipcsei, G.; Zrínyi, M. Smart nanocomposite polymer membranes with on/off switching control. Macromolecules 2006, 39, 1939–1942. [Google Scholar] [CrossRef]
- Shemshadi, R.; Ghafarian, R.; Gorji, M.; Avazverdi, E. A smart thermoregulatory nanocomposite membrane with improved thermal properties: Simultaneous use of graphene family and micro-encapsulated phase change material. Text. Res. J. 2018, 0040517517750644. [Google Scholar] [CrossRef]
- Lee, C.T.; Wang, Y.S. High-performance room temperature NH3 gas sensors based on polyaniline-reduced graphene oxide nanocomposite sensitive membrane. J. Alloys Compd. 2019, 789, 693–696. [Google Scholar] [CrossRef]
- Prasad, B.; Gill, F.S.; Panwar, V.; Anoop, G. Development of strain sensor using conductive poly(vinylidene fluoride) (PVDF) nanocomposite membrane reinforced with ionic liquid (IL) & carbon nanofiber (CNF). Compos. Part B Eng. 2019, 173, 106990. [Google Scholar] [CrossRef]
- Hittini, W.; Abu-Hani, A.F.; Reddy, N.; Mahmoud, S.T. Cellulose-Copper Oxide hybrid nanocomposites membranes for H2S gas detection at low temperatures. Sci. Rep. 2020, 10, 2940. [Google Scholar] [CrossRef] [Green Version]
- Inukai, S.; Cruz-Silva, R.; Ortiz-Medina, J.; Morelos-Gomez, A.; Takeuchi, K.; Hayashi, T.; Tanioka, A.; Araki, T.; Tejima, S.; Noguchi, T.; et al. High-performance multi-functional reverse osmosis membranes obtained by carbon nanotube·polyamide nanocomposite. Sci. Rep. 2015, 5, 13562. [Google Scholar] [CrossRef] [Green Version]
- Emami, N.; Razmjou, A.; Noorisafa, F.; Korayem, A.H.; Zarrabi, A.; Ji, C. Fabrication of smart magnetic nanocomposite asymmetric membrane capsules for the controlled release of nitrate. Environ. Nanotechnol. Monit. Manag. 2017, 8, 233–243. [Google Scholar] [CrossRef]
- Ghaee, A.; Zerafat, M.M.; Askari, P.; Sabbaghi, S.; Sadatnia, B. Fabrication of polyamide thin-film nanocomposite membranes with enhanced surface charge for nitrate ion removal from water resources. Environ. Technol. 2017, 38, 772–781. [Google Scholar] [CrossRef]
- Shukla, A.K.; Alam, J.; Ansari, M.A.; Alhoshan, M.; Ali, F.A.A. Antimicrobial and antifouling properties of versatile PPSU/carboxylated GO nanocomposite membrane against Gram-positive and Gram-negative bacteria and protein. Environ. Sci. Pollut. Res. 2018, 25, 34103–34113. [Google Scholar] [CrossRef]
- Wang, W.; Li, Y.; Wang, W.; Gao, B.; Wang, Z. Palygorskite/silver nanoparticles incorporated polyamide thin film nanocomposite membranes with enhanced water permeating, antifouling and antimicrobial performance. Chemosphere 2019, 236, 124396. [Google Scholar] [CrossRef]
- Shakeri, A.; Salehi, H.; Ghorbani, F.; Amini, M.; Naslhajian, H. Polyoxometalate based thin film nanocomposite forward osmosis membrane: Superhydrophilic, anti-fouling, and high water permeable. J. Colloid Interface Sci. 2019, 536, 328–338. [Google Scholar] [CrossRef]
- Wen, Y.; Yuan, J.; Ma, X.; Wang, S.; Liu, Y. Polymeric nanocomposite membranes for water treatment: A review. Environ. Chem. Lett. 2019, 17, 1539–1551. [Google Scholar] [CrossRef]
- Palencia, M.; Córdoba, A.; Vera, M. Membrane Technology and Chemistry. Nanostruct. Polym. Membr. 2016, 1, 27–54. [Google Scholar] [CrossRef]
- Palencia, M.; Martínez-Lara, J.M.; Chate-Galvis, N.G.; Durango-Petro, J.M. Functionality-Structure Relationship into Functional Polymeric Nanocomposite Membranes for Removal and Monitoring of Pollutants in Fluid Phases. In Engineering Materials; Shalan, A.E., Hamdy Makhlouf, A.S., Lanceros-Méndez, S., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 299–330. ISBN 978-3-030-94319-6. [Google Scholar]
- Cong, H.; Radosz, M.; Towler, B.F.; Shen, Y. Polymer-inorganic nanocomposite membranes for gas separation. Sep. Purif. Technol. 2007, 55, 281–291. [Google Scholar] [CrossRef]
- Pourzare, K.; Mansourpanah, Y.; Farhadi, S. Advanced nanocomposite membranes for fuel cell applications: A comprehensive review. Biofuel Res. J. 2016, 3, 496–513. [Google Scholar] [CrossRef] [Green Version]
- Bee, S.L.; Abdullah, M.A.A.; Bee, S.T.; Sin, L.T.; Rahmat, A.R. Polymer nanocomposites based on silylated-montmorillonite: A review. Prog. Polym. Sci. 2018, 85, 57–82. [Google Scholar] [CrossRef]
- Alateyah, A.I.; Dhakal, H.N.; Zhang, Z.Y. Processing, properties, and applications of polymer nanocomposites based on layer silicates: A review. Adv. Polym. Technol. 2013, 32, 21368. [Google Scholar] [CrossRef]
- Paul, D.R.; Robeson, L.M. Polymer nanotechnology: Nanocomposites. Polymer 2008, 49, 3187–3204. [Google Scholar] [CrossRef]
- Sheikholeslami, S.N.; Rafizadeh, M.; Taromi, F.A.; Bouhendi, H. Synthesis and characterization of poly(trimethylene terephthalate)/organoclay nanocomposite via in situ polymerization: Including thermal properties and dyeability. J. Thermoplast. Compos. Mater. 2014, 27, 1530–1552. [Google Scholar] [CrossRef]
- Alexandre, M.; Dubois, P. Polymer-layered silicate nanocomposites: Preparation, properties and uses of a new class of materials. Mater. Sci. Eng. R Rep. 2000, 28, 1–63. [Google Scholar] [CrossRef]
- Pavlidou, S.; Papaspyrides, C.D. A review on polymer-layered silicate nanocomposites. Prog. Polym. Sci. 2008, 33, 1119–1198. [Google Scholar] [CrossRef]
- VanderHart, D.L.; Asano, A.; Gilman, J.W. Solid-state NMR investigation of paramagnetic nylon-6 clay nanocomposites. 2. Measurement of clay dispersion, crystal stratification, and stability of organic modifiers. Chem. Mater. 2001, 13, 3796–3809. [Google Scholar] [CrossRef]
- Gao, F. Clay/polymer composites: The story. Mater. Today 2004, 7, 50–55. [Google Scholar] [CrossRef]
- Panwar, A.; Choudhary, V.; Sharma, D.K. Review: A review: Polystyrene/clay nanocomposites. J. Reinf. Plast. Compos. 2011, 30, 446–459. [Google Scholar] [CrossRef]
- Usuki, A.; Kojima, Y.; Kawasumi, M.; Okada, A.; Fukushima, Y.; Kurauchi, T.; Kamigaito, O. Synthesis of nylon 6-clay hybrid. J. Mater. Res. 1993, 8, 1179–1184. [Google Scholar] [CrossRef]
- Doucouré, A.; Guizard, C.; Durand, J.; Berjoan, R.; Cot, L. Plasma polymerization of fluorinated monomers on mesoporous silica membranes and application to gas permeation. J. Membr. Sci. 1996, 117, 143–150. [Google Scholar] [CrossRef]
- Patel, N.P.; Miller, A.C.; Spontak, R.J. Highly CO2-permeable and selective polymer nanocomposite membranes. Adv. Mater. 2003, 15, 729–733. [Google Scholar] [CrossRef]
- Patel, N.P.; Aberg, C.M.; Sanchez, A.M.; Capracotta, M.D.; Martin, J.D.; Spontak, R.J. Morphological, mechanical and gas-transport characteristics of crosslinked poly(propylene glycol): Homopolymers, nanocomposites and blends. Polymer 2004, 45, 5941–5950. [Google Scholar] [CrossRef]
- Nunes, S.P.; Peinemann, K.V.; Ohlrogge, K.; Alpers, A.; Keller, M.; Pires, A.T.N. Membranes of poly(ether imide) and nanodispersed silica. J. Membr. Sci. 1999, 157, 219–226. [Google Scholar] [CrossRef]
- Rubio, L.R.; Teijido, R.; Veloso-Fernández, A.; Pérez-Yáñez, S.; Vilas-Vilela, J.L. Polymeric Nanocomposite Membranes for Water Remediation: From Classic Approaches to 3D Printing. In Engineering Materials; Shalan, A.E., Hamdy Makhlouf, A.S., Lanceros-Méndez, S., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 191–243. ISBN 978-3-030-94319-6. [Google Scholar]
- Bounor-Legaré, V.; Cassagnau, P. In situ synthesis of organic-inorganic hybrids or nanocomposites from sol-gel chemistry in molten polymers. Prog. Polym. Sci. 2014, 39, 1473–1497. [Google Scholar] [CrossRef]
- Brzesowsky, R.H.; De With, G.; Van Den Cruijsem, S.; Snijkers-Hendrickx, I.J.M.; Wolter, W.A.M.; Van Lierop, J.G. Glass strengthening by silica particle reinforced organic-inorganic coatings. J. Non-Cryst. Solids 1998, 241, 27–37. [Google Scholar] [CrossRef]
- Livage, J.; Sanchez, C. Sol-gel chemistry. J. Non-Cryst. Solids 1992, 145, 11–19. [Google Scholar] [CrossRef]
- Kioul, A.; Mascia, L. Compatibility of polyimide-silicate ceramers induced by alkoxysilane silane coupling agents. J. Non-Cryst. Solids 1994, 175, 169–186. [Google Scholar] [CrossRef]
- Smaïhi, M.; Jermoumi, T.; Marignan, J.; Noble, R.D. Organic-inorganic gas separation membranes: Preparation and characterization. J. Membr. Sci. 1996, 116, 211–220. [Google Scholar] [CrossRef]
- Day, V.W.; Eberspacher, T.A.; Chen, Y.; Hao, J.; Klemperer, W.G. Low-nuclearity titanium oxoalkoxides: The trititanates [Ti3O](OPri)10 and [Ti3O](OPri)9(OMe). Inorg. Chim. Acta 1995, 229, 391–405. [Google Scholar] [CrossRef]
- Livage, J. Basic Principles of Sol-Gel Chemistry. In Sol-Gel Technologies for Glass Producers and Users; Aegerter, M.A., Mennig, M., Eds.; Springer: Boston, MA, USA, 2004; pp. 3–14. ISBN 978-0-387-88953-5. [Google Scholar]
- Iwata, M.; Adachi, T.; Tomidokoro, M.; Ohta, M.; Kobayashi, T. Hybrid sol-gel membranes of polyacrylonitrile-tetraethoxysilane composites for gas permselectivity. J. Appl. Polym. Sci. 2003, 88, 1752–1759. [Google Scholar] [CrossRef]
- Gomes, D.; Nunes, S.P.; Peinemann, K.V. Membranes for gas separation based on poly(1-trimethylsilyl-1-propyne)- silica nanocomposites. J. Membr. Sci. 2005, 246, 13–25. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, X.; Hu, P.; Peng, X. ZIF-8 coated polyvinylidenefluoride (PVDF) hollow fiber for highly efficient separation of small dye molecules. Appl. Mater. Today 2016, 5, 103–110. [Google Scholar] [CrossRef]
- Stephen, R.; Ranganathaiah, C.; Varghese, S.; Joseph, K.; Thomas, S. Gas transport through nano and micro composites of natural rubber (NR) and their blends with carboxylated styrene butadiene rubber (XSBR) latex membranes. Polymer 2006, 47, 858–870. [Google Scholar] [CrossRef]
- Mascia, L.; Zhang, Z.; Shaw, S.J. Carbon fibre composites based on polyimide/silica ceramers: Aspects of structure-properties relationship. Compos. Part A Appl. Sci. Manuf. 1996, 27, 1211–1221. [Google Scholar] [CrossRef]
- Gacitua, W.; Ballerini, A.; Zhang, J. Polymer Nanocomposites: Synthetic and Natural Fillers a Review. Maderas Cienc. Y Tecnol. 2005, 7, 159–178. [Google Scholar] [CrossRef] [Green Version]
- Ragab, D.; Gomaa, H.G.; Sabouni, R.; Salem, M.; Ren, M.; Zhu, J. Micropollutants removal from water using microfiltration membrane modified with ZIF-8 metal organic frameworks (MOFs). Chem. Eng. J. 2016, 300, 273–279. [Google Scholar] [CrossRef]
- Low, Z.X.; Razmjou, A.; Wang, K.; Gray, S.; Duke, M.; Wang, H. Effect of addition of two-dimensional ZIF-L nanoflakes on the properties of polyethersulfone ultrafiltration membrane. J. Membr. Sci. 2014, 460, 9–17. [Google Scholar] [CrossRef] [Green Version]
- Bhiwankar, N.N.; Weiss, R.A. Melt intercalation/exfoliation of polystyrene-sodium-montmorillonite nanocomposites using sulfonated polystyrene ionomer compatibilizers. Polymer 2006, 47, 6684–6691. [Google Scholar] [CrossRef]
- Yoon, J.T.; Jo, W.H.; Lee, M.S.; Ko, M.B. Effects of comonomers and shear on the melt intercalation of styrenics/clay nanocomposites. Polymer 2001, 42, 329–336. [Google Scholar] [CrossRef]
- Motamedi, P.; Bagheri, R. Investigation of the nanostructure and mechanical properties of polypropylene/polyamide 6/layered silicate ternary nanocomposites. Mater. Des. 2010, 31, 1776–1784. [Google Scholar] [CrossRef]
- Huang, Z.M.; Zhang, Y.Z.; Kotaki, M.; Ramakrishna, S. A review on polymer nanofibers by electrospinning and their applications in nanocomposites. Compos. Sci. Technol. 2003, 63, 2223–2253. [Google Scholar] [CrossRef]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and electrospun nanofibers: Methods, materials, and applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Crandall, C.; Sahadevan, R.; Menkhaus, T.J.; Fong, H. Microfiltration performance of electrospun nanofiber membranes with varied fiber diameters and different membrane porosities and thicknesses. Polymer 2017, 114, 64–72. [Google Scholar] [CrossRef]
- Dolina, J.; Jiříček, T.; Lederer, T. Biocide modification of ultrafiltration membranes using nanofiber structures. Desalin. Water Treat. 2015, 56, 3252–3258. [Google Scholar] [CrossRef]
- Wang, X.; Fang, D.; Hsiao, B.S.; Chu, B. Nanofiltration membranes based on thin-film nanofibrous composites. J. Membr. Sci. 2014, 469, 188–197. [Google Scholar] [CrossRef]
- Wang, X.; Ma, H.; Chu, B.; Hsiao, B.S. Thin-film nanofibrous composite reverse osmosis membranes for desalination. Desalination 2017, 420, 91–98. [Google Scholar] [CrossRef]
- Li, J.J.; Zhu, L.T.; Luo, Z.H. Electrospun fibrous membrane with enhanced swithchable oil/water wettability for oily water separation. Chem. Eng. J. 2016, 287, 474–481. [Google Scholar] [CrossRef]
- Najafi, M.; Frey, M.W. Electrospun nanofibers for chemical separation. Nanomaterials 2020, 10, 982. [Google Scholar] [CrossRef]
- Bhardwaj, N.; Kundu, S.C. Electrospinning: A fascinating fiber fabrication technique. Biotechnol. Adv. 2010, 28, 325–347. [Google Scholar] [CrossRef]
- Hou, D.; Lin, D.; Ding, C.; Wang, D.; Wang, J. Fabrication and characterization of electrospun superhydrophobic PVDF-HFP/SiNPs hybrid membrane for membrane distillation. Sep. Purif. Technol. 2017, 189, 82–89. [Google Scholar] [CrossRef]
- Yar, A.; Haspulat, B.; Üstün, T.; Eskizeybek, V.; Avci, A.; Kamiş, H.; Achour, S. Electrospun TiO2/ZnO/PAN hybrid nanofiber membranes with efficient photocatalytic activity. RSC Adv. 2017, 7, 29806–29814. [Google Scholar] [CrossRef] [Green Version]
- Essalhi, M.; Khayet, M. Self-sustained webs of polyvinylidene fluoride electrospun nano-fibers: Effects of polymer concentration and desalination by direct contact membrane distillation. J. Membr. Sci. 2014, 454, 133–143. [Google Scholar] [CrossRef]
- Ziabari, M.; Mottaghitalab, V.; Haghi, A.K. Application of direct tracking method for measuring electrospun nanofiber diameter. Braz. J. Chem. Eng. 2009, 26, 53–62. [Google Scholar] [CrossRef] [Green Version]
- Biswas, P.; Bandyopadhyaya, R. Biofouling prevention using silver nanoparticle impregnated polyethersulfone (PES) membrane: E. coli cell-killing in a continuous cross-flow membrane module. J. Colloid Interface Sci. 2017, 491, 13–26. [Google Scholar] [CrossRef]
- Zheng, Y.; Gong, R.H.; Zeng, Y. Multijet motion and deviation in electrospinning. RSC Adv. 2015, 5, 48533–48540. [Google Scholar] [CrossRef]
- Li, D.; Xia, Y. Direct fabrication of composite and ceramic hollow nanofibers by electrospinning. Nano Lett. 2004, 4, 933–938. [Google Scholar] [CrossRef]
- Lee, G.H.; Song, J.C.; Yoon, K.B. Controlled wall thickness and porosity of polymeric hollow nanofibers by coaxial electrospinning. Macromol. Res. 2010, 18, 571–576. [Google Scholar] [CrossRef]
- Li, D.; Xia, Y. Fabrication of titania nanofibers by electrospinning. Nano Lett. 2003, 3, 555–560. [Google Scholar] [CrossRef]
- Malwal, D.; Gopinath, P. Fabrication and characterization of poly(ethylene oxide) templated nickel oxide nanofibers for dye degradation. Environ. Sci. Nano 2015, 2, 78–85. [Google Scholar] [CrossRef]
- Ray, S.S.; Chen, S.S.; Li, C.W.; Nguyen, N.C.; Nguyen, H.T. A comprehensive review: Electrospinning technique for fabrication and surface modification of membranes for water treatment application. RSC Adv. 2016, 6, 85495–85514. [Google Scholar] [CrossRef]
- Lee, C.G.; Javed, H.; Zhang, D.; Kim, J.H.; Westerhoff, P.; Li, Q.; Alvarez, P.J.J. Porous Electrospun Fibers Embedding TiO2 for Adsorption and Photocatalytic Degradation of Water Pollutants. Environ. Sci. Technol. 2018, 52, 4285–4293. [Google Scholar] [CrossRef]
- Ognibene, G.; Gangemi, C.M.A.; D’Urso, A.; Purrello, R.; Cicala, G.; Fragalà, M.E. Combined Approach to Remove and Fast Detect Heavy Metals in Water Based on PES-TiO2 Electrospun Mats and Porphyrin Chemosensors. ACS Omega 2018, 3, 7182–7190. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, A.; Osman, T.A.; Toprak, M.S.; Muhammed, M.; Yilmaz, E.; Uheida, A. Visible light photocatalytic reduction of Cr(VI) by surface modified CNT/titanium dioxide composites nanofibers. J. Mol. Catal. A Chem. 2016, 424, 45–53. [Google Scholar] [CrossRef]
- Schiffman, J.D.; Elimelech, M. Antibacterial Activity of Electrospun Polymer Mats with Incorporated Narrow Diameter Single-Walled Carbon Nanotubes. ACS Appl. Mater. Interfaces 2011, 3, 462–468. [Google Scholar] [CrossRef] [PubMed]
- Ford, E.N.J.; Suthiwangcharoen, N.; D’Angelo, P.A.; Nagarajan, R. Role of single-walled carbon nanotubes on ester hydrolysis and topography of electrospun bovine serum albumin/poly(vinyl alcohol) membranes. ACS Appl. Mater. Interfaces 2014, 6, 11741–11748. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Loh, C.H.; Wang, R.; Fane, A.G. Electrospun superhydrophobic membranes with unique structures for membrane distillation. ACS Appl. Mater. Interfaces 2014, 6, 16035–16048. [Google Scholar] [CrossRef] [PubMed]
- Obaid, M.; Ghouri, Z.K.; Fadali, O.A.; Khalil, K.A.; Almajid, A.A.; Barakat, N.A.M. Amorphous SiO2 NP-Incorporated Poly(vinylidene fluoride) Electrospun Nanofiber Membrane for High Flux Forward Osmosis Desalination. ACS Appl. Mater. Interfaces 2016, 8, 4561–4574. [Google Scholar] [CrossRef]
- Li, X.; Yu, X.; Cheng, C.; Deng, L.; Wang, M.; Wang, X. Electrospun Superhydrophobic Organic/Inorganic Composite Nanofibrous Membranes for Membrane Distillation. ACS Appl. Mater. Interfaces 2015, 7, 21919–21930. [Google Scholar] [CrossRef]
- Son, W.K.; Youk, J.H.; Park, W.H. Antimicrobial cellulose acetate nanofibers containing silver nanoparticles. Carbohydr. Polym. 2006, 65, 430–434. [Google Scholar] [CrossRef]
- De Faria, A.F.; Perreault, F.; Shaulsky, E.; Arias Chavez, L.H.; Elimelech, M. Antimicrobial Electrospun Biopolymer Nanofiber Mats Functionalized with Graphene Oxide-Silver Nanocomposites. ACS Appl. Mater. Interfaces 2015, 7, 12751–12759. [Google Scholar] [CrossRef]
- Karagoz, S.; Kiremitler, N.B.; Sakir, M.; Salem, S.; Onses, M.S.; Sahmetlioglu, E.; Ceylan, A.; Yilmaz, E. Synthesis of Ag and TiO2 modified polycaprolactone electrospun nanofibers (PCL/TiO2-Ag NFs) as a multifunctional material for SERS, photocatalysis and antibacterial applications. Ecotoxicol. Environ. Saf. 2020, 188, 109856. [Google Scholar] [CrossRef] [PubMed]
- Kayaci, F.; Ozgit-Akgun, C.; Donmez, I.; Biyikli, N.; Uyar, T. Polymer-inorganic core-shell nanofibers by electrospinning and atomic layer deposition: Flexible nylon-ZnO core-shell nanofiber mats and their photocatalytic activity. ACS Appl. Mater. Interfaces 2012, 4, 6185–6194. [Google Scholar] [CrossRef]
- Kim, J.H.; Joshi, M.K.; Lee, J.; Park, C.H.; Kim, C.S. Polydopamine-assisted immobilization of hierarchical zinc oxide nanostructures on electrospun nanofibrous membrane for photocatalysis and antimicrobial activity. J. Colloid Interface Sci. 2018, 513, 566–574. [Google Scholar] [CrossRef] [PubMed]
- Barhoum, A.; Bechelany, M.; Makhlouf, A.S.H. (Eds.) Handbook of Nanofibers; Springer International Publishing: Cham, Switzerland, 2019; ISBN 978-3-319-53654-5. [Google Scholar]
- Xiao, S.; Shen, M.; Guo, R.; Wang, S.; Shi, X. Immobilization of Zerovalent Iron Nanoparticles into Electrospun Polymer Nanofibers: Synthesis, Characterization, and Potential Environmental Applications. J. Phys. Chem. C 2009, 113, 18062–18068. [Google Scholar] [CrossRef]
- Zhang, D.; Jin, X.Z.; Huang, T.; Zhang, N.; Qi, X.D.; Yang, J.H.; Zhou, Z.W.; Wang, Y. Electrospun Fibrous Membranes with Dual-Scaled Porous Structure: Super Hydrophobicity, Super Lipophilicity, Excellent Water Adhesion, and Anti-Icing for Highly Efficient Oil Adsorption/Separation. ACS Appl. Mater. Interfaces 2019, 11, 5073–5083. [Google Scholar] [CrossRef]
- Letnik, I.; Avrahami, R.; Rokem, J.S.; Greiner, A.; Zussman, E.; Greenblatt, C. Living Composites of Electrospun Yeast Cells for Bioremediation and Ethanol Production. Biomacromolecules 2015, 16, 3322–3328. [Google Scholar] [CrossRef] [PubMed]
- Yap, C.Y.; Chua, C.K.; Dong, Z.L.; Liu, Z.H.; Zhang, D.Q.; Loh, L.E.; Sing, S.L. Review of selective laser melting: Materials and applications. Appl. Phys. Rev. 2015, 2, 041101. [Google Scholar] [CrossRef]
- Fasel, U.; Keidel, D.; Baumann, L.; Cavolina, G.; Eichenhofer, M.; Ermanni, P. Composite additive manufacturing of morphing aerospace structures. Manuf. Lett. 2020, 23, 85–88. [Google Scholar] [CrossRef]
- Lewandowski, J.J.; Seifi, M. Metal Additive Manufacturing: A Review of Mechanical Properties. Annu. Rev. Mater. Res. 2016, 46, 151–186. [Google Scholar] [CrossRef] [Green Version]
- Schmitt, M.; Mehta, R.M.; Kim, I.Y. Additive manufacturing infill optimization for automotive 3D-printed ABS components. Rapid Prototyp. J. 2020, 26, 89–99. [Google Scholar] [CrossRef]
- Lim, C.W.J.; Le, K.Q.; Lu, Q.; Wong, C.H. An Overview of 3-D Printing in Manufacturing, Aerospace, and Automotive Industries. IEEE Potentials 2016, 35, 18–22. [Google Scholar] [CrossRef]
- Tay, Y.W.D.; Panda, B.; Paul, S.C.; Noor Mohamed, N.A.; Tan, M.J.; Leong, K.F. 3D printing trends in building and construction industry: A review. Virtual Phys. Prototyp. 2017, 12, 261–276. [Google Scholar] [CrossRef]
- Lin, K.; Zhang, D.; Macedo, M.H.; Cui, W.; Sarmento, B.; Shen, G. Advanced Collagen-Based Biomaterials for Regenerative Biomedicine. Adv. Funct. Mater. 2019, 29, 1804943. [Google Scholar] [CrossRef]
- Derakhshanfar, S.; Mbeleck, R.; Xu, K.; Zhang, X.; Zhong, W.; Xing, M. 3D bioprinting for biomedical devices and tissue engineering: A review of recent trends and advances. Bioact. Mater. 2018, 3, 144–156. [Google Scholar] [CrossRef]
- Sun, J.; Zhou, W.; Huang, D.; Fuh, J.Y.H.; Hong, G.S. An Overview of 3D Printing Technologies for Food Fabrication. Food Bioprocess Technol. 2015, 8, 1605–1615. [Google Scholar] [CrossRef]
- Lalia, B.S.; Kochkodan, V.; Hashaikeh, R.; Hilal, N. A review on membrane fabrication: Structure, properties and performance relationship. Desalination 2013, 326, 77–95. [Google Scholar] [CrossRef]
- Issac, M.N.; Kandasubramanian, B. Review of manufacturing three-dimensional-printed membranes for water treatment. Environ. Sci. Pollut. Res. 2020, 27, 36091–36108. [Google Scholar] [CrossRef] [PubMed]
- Ngo, T.D.; Kashani, A.; Imbalzano, G.; Nguyen, K.T.Q.; Hui, D. Additive manufacturing (3D printing): A review of materials, methods, applications and challenges. Compos. Part B Eng. 2018, 143, 172–196. [Google Scholar] [CrossRef]
- Low, Z.X.; Chua, Y.T.; Ray, B.M.; Mattia, D.; Metcalfe, I.S.; Patterson, D.A. Perspective on 3D printing of separation membranes and comparison to related unconventional fabrication techniques. J. Membr. Sci. 2017, 523, 596–613. [Google Scholar] [CrossRef] [Green Version]
- Yusuf, A.; Sodiq, A.; Giwa, A.; Eke, J.; Pikuda, O.; De Luca, G.; Di Salvo, J.L.; Chakraborty, S. A review of emerging trends in membrane science and technology for sustainable water treatment. J. Clean. Prod. 2020, 266, 121867. [Google Scholar] [CrossRef]
- Tijing, L.D.; Dizon, J.R.C.; Ibrahim, I.; Nisay, A.R.N.; Shon, H.K.; Advincula, R.C. 3D printing for membrane separation, desalination and water treatment. Appl. Mater. Today 2020, 18, 100486. [Google Scholar] [CrossRef]
- Koh, J.J.; Lim, G.J.H.; Zhou, X.; Zhang, X.; Ding, J.; He, C. 3D-Printed Anti-Fouling Cellulose Mesh for Highly Efficient Oil/Water Separation Applications. ACS Appl. Mater. Interfaces 2019, 11, 13787–13795. [Google Scholar] [CrossRef]
- Sangiorgi, A.; Gonzalez, Z.; Ferrandez-Montero, A.; Yus, J.; Sanchez-Herencia, A.J.; Galassi, C.; Sanson, A.; Ferrari, B. 3D Printing of Photocatalytic Filters Using a Biopolymer to Immobilize TiO2 Nanoparticles. J. Electrochem. Soc. 2019, 166, H3239–H3248. [Google Scholar] [CrossRef]
- Gude, V.G. Emerging Technologies for Sustainable Desalination Handbook; Butterworth-Heinemann: Oxford, UK, 2018; pp. 1–529. [Google Scholar] [CrossRef]
- Kononova, S.V.; Gubanova, G.N.; Korytkova, E.N.; Sapegin, D.A.; Setnickova, K.; Petrychkovych, R.; Uchytil, P. Polymer nanocomposite membranes. Appl. Sci. 2018, 8, 1181. [Google Scholar] [CrossRef] [Green Version]
- Liang, C.Z.; Chung, T.S.; Lai, J.Y. A review of polymeric composite membranes for gas separation and energy production. Prog. Polym. Sci. 2019, 97, 101141. [Google Scholar] [CrossRef]
- Bassyouni, M.; Abdel-Aziz, M.H.; Zoromba, M.S.; Abdel-Hamid, S.M.S.; Drioli, E. A review of polymeric nanocomposite membranes for water purification. J. Ind. Eng. Chem. 2019, 73, 19–46. [Google Scholar] [CrossRef]
- Li, N.N.; Long, R.B.; Henley, E.J. Membrane Separation Processes; PHI Learning: Delhi, India, 1965; Volume 57. [Google Scholar]
- Palencia, M. Fundamental and Methodological Aspects of Porous Membrane Characterization by Hydrodynamic Permeability Test-A review. J. Sci. Technol. Appl. 2019, 7, 17–25. [Google Scholar] [CrossRef]
- Palencia, M. Liquid-phase polymer-based retention: Theory, modeling, and application for the removal of pollutant inorganic ions. J. Chem. 2015, 2015, 965624. [Google Scholar] [CrossRef] [Green Version]
- Ching, C.B.; Hidajat, K.; Uddin, M.S. Evaluation of Equilibrium and Kinetic Parameters of Smaller Molecular Size Amino Acids on KX Zeolite Crystals via Liquid Chromatographic Techniques. Sep. Sci. Technol. 1989, 24, 581–597. [Google Scholar] [CrossRef]
- Lerma, T.A.; Martínez, G.; Palencia, M. Generation of thiolated porous surfaces by interpenetrating polymeric networks: Study of their surface properties. J. Sci. Technol. Appl. 2017, 3, 56–65. [Google Scholar] [CrossRef]
- Palencia, M.S.; Berrio, M.E.; Palencia, S.L. Effect of capping agent and diffusivity of different silver nanoparticles on their antibacterial properties. J. Nanosci. Nanotechnol. 2017, 17, 5197–5204. [Google Scholar] [CrossRef]
- Ambrosio, R.; Carrillo, A.; Mota, M.L.; de la Torre, K.; Torrealba, R.; Moreno, M.; Vazquez, H.; Flores, J.; Vivaldo, I. Polymeric nanocomposites membranes with high permittivity based on PVA-ZnO nanoparticles for potential applications in flexible electronics. Polymers 2018, 10, 1370. [Google Scholar] [CrossRef] [Green Version]
- Nizamuddin, S.; Maryam, S.; Baloch, H.A.; Siddiqui, M.T.H.; Takkalkar, P.; Mubarak, N.M.; Jatoi, A.S.; Abbasi, S.A.; Griffin, G.J.; Qureshi, K.; et al. Electrical properties of sustainable nano-composites containing nano-fillers: Dielectric properties and electrical conductivity. In Sustainable Polymer Composites and Nanocomposites; Inamuddin, Thomas, S., Kumar Mishra, R., Asiri, A.M., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 899–914. ISBN 9783030053994. [Google Scholar]
- Drioli, E.; Giorno, L. Comprehensive Membrane Science and Engineering; Elsevier: Amsterdam, The Netherlands, 2010; Volume 1–4, ISBN 9780080932507. [Google Scholar]
- Rostamzadeh, H.; Namin, A.S.; Ghaebi, H.; Amidpour, M. Performance assessment and optimization of a humidification dehumidification (HDH) system driven by absorption-compression heat pump cycle. Desalination 2018, 447, 84–101. [Google Scholar] [CrossRef]
- Som, C.; Berges, M.; Chaudhry, Q.; Dusinska, M.; Fernandes, T.F.; Olsen, S.I.; Nowack, B. The importance of life cycle concepts for the development of safe nanoproducts. Toxicology 2010, 269, 160–169. [Google Scholar] [CrossRef]
- Zodrow, K.; Brunet, L.; Mahendra, S.; Li, D.; Zhang, A.; Li, Q.; Alvarez, P.J.J. Polysulfone ultrafiltration membranes impregnated with silver nanoparticles show improved biofouling resistance and virus removal. Water Res. 2009, 43, 715–723. [Google Scholar] [CrossRef] [Green Version]
- Kingston, C.; Zepp, R.; Andrady, A.; Boverhof, D.; Fehir, R.; Hawkins, D.; Roberts, J.; Sayre, P.; Shelton, B.; Sultan, Y.; et al. Release characteristics of selected carbon nanotube polymer composites. Carbon N. Y. 2014, 68, 33–57. [Google Scholar] [CrossRef]
- Vilar, G.; Fernández-Rosas, E.; Puntes, V.; Jamier, V.; Aubouy, L.; Vázquez-Campos, S. Monitoring migration and transformation of nanomaterials in polymeric composites during accelerated aging. J. Phys. Conf. Ser. 2013, 429, 012044. [Google Scholar] [CrossRef] [Green Version]
- Hu, M.; Zhong, K.; Liang, Y.; Ehrman, S.H.; Mi, B. Effects of Particle Morphology on the Antibiofouling Performance of Silver Embedded Polysulfone Membranes and Rate of Silver Leaching. Ind. Eng. Chem. Res. 2017, 56, 2240–2246. [Google Scholar] [CrossRef]
- Hu, R.; He, Y.; Zhang, C.; Zhang, R.; Li, J.; Zhu, H. Graphene oxide-embedded polyamide nanofiltration membranes for selective ion separation. J. Mater. Chem. A 2017, 5, 25632–25640. [Google Scholar] [CrossRef]
- Li, X.; Fang, X.; Pang, R.; Li, J.; Sun, X.; Shen, J.; Han, W.; Wang, L. Self-assembly of TiO2 nanoparticles around the pores of PES ultrafiltration membrane for mitigating organic fouling. J. Membr. Sci. 2014, 467, 226–235. [Google Scholar] [CrossRef]
- Wan, H.; Briot, N.J.; Saad, A.; Ormsbee, L.; Bhattacharyya, D. Pore functionalized PVDF membranes with in-situ synthesized metal nanoparticles: Material characterization, and toxic organic degradation. J. Membr. Sci. 2017, 530, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.H.; Tseng, H.H.; Wey, M.Y.; Lin, M. Der Characteristics, morphology, and stabilization mechanism of PAA250K-stabilized bimetal nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2009, 349, 137–144. [Google Scholar] [CrossRef]
- Wu, J.; Yu, C.; Li, Q. Regenerable antimicrobial activity in polyamide thin film nanocomposite membranes. J. Membr. Sci. 2015, 476, 119–127. [Google Scholar] [CrossRef]
- Salas, E.C.; Sun, Z.; Lüttge, A.; Tour, J.M. Reduction of Graphene Oxide via Bacterial Respiration. ACS Nano 2010, 4, 4852–4856. [Google Scholar] [CrossRef]
- Wang, G.; Qian, F.; Saltikov, C.W.; Jiao, Y.; Li, Y. Microbial reduction of graphene oxide by Shewanella. Nano Res. 2011, 4, 563–570. [Google Scholar] [CrossRef]
- Akhavan, O.; Ghaderi, E. Escherichia coli bacteria reduce graphene oxide to bactericidal graphene in a self-limiting manner. Carbon N. Y. 2012, 50, 1853–1860. [Google Scholar] [CrossRef]
- Liu, J.; Gao, Y.; Cao, D.; Zhang, L.; Guo, Z. Nanoparticle dispersion and aggregation in polymer nanocomposites: Insights from molecular dynamics simulation. Langmuir 2011, 27, 7926–7933. [Google Scholar] [CrossRef]
- Yuan, Y.; Lee, T.R. Contact angle and wetting properties. In Surface Science Techniques; Springer Series in Surface Sciences; Springer Nature: Cham, Switzerland, 2013; Volume 51, pp. 3–34. [Google Scholar]
- Kim, S.H.; Kwak, S.Y.; Sohn, B.H.; Park, T.H. Design of TiO2 nanoparticle self-assembled aromatic polyamide thin-film-composite (TFC) membrane as an approach to solve biofouling problem. J. Membr. Sci. 2003, 211, 157–165. [Google Scholar] [CrossRef]
- Kwak, S.Y.; Kim, S.H.; Kim, S.S. Hybrid organic/inorganic reverse osmosis (RO) membrane for bactericidal anti-fouling. 1. Preparation and characterization of TiO2 nanoparticle self-assembled aromatic polyamide thin-film-composite (TFC) membrane. Environ. Sci. Technol. 2001, 35, 2388–2394. [Google Scholar] [CrossRef]
- Han, Y.; Xu, Z.; Gao, C. Ultrathin graphene nanofiltration membrane for water purification. Adv. Funct. Mater. 2013, 23, 3693–3700. [Google Scholar] [CrossRef]
- Brady-Estévez, A.S.; Kang, S.; Elimelech, M. A single-walled-carbon-nanotube filter for removal of viral and bacterial pathogens. Small 2008, 4, 481–484. [Google Scholar] [CrossRef]
- Bae, T.H.; Kim, I.C.; Tak, T.M. Preparation and characterization of fouling-resistant TiO2 self-assembled nanocomposite membranes. J. Membr. Sci. 2006, 275, 1–5. [Google Scholar] [CrossRef]
- Luo, M.L.; Zhao, J.Q.; Tang, W.; Pu, C.S. Hydrophilic modification of poly(ether sulfone) ultrafiltration membrane surface by self-assembly of TiO2 nanoparticles. Appl. Surf. Sci. 2005, 249, 76–84. [Google Scholar] [CrossRef]
- Li, J.H.; Xu, Y.Y.; Zhu, L.P.; Wang, J.H.; Du, C.H. Fabrication and characterization of a novel TiO2 nanoparticle self-assembly membrane with improved fouling resistance. J. Membr. Sci. 2009, 326, 659–666. [Google Scholar] [CrossRef]
- Yang, S.; Gu, J.S.; Yu, H.Y.; Zhou, J.; Li, S.F.; Wu, X.M.; Wang, L. Polypropylene membrane surface modification by RAFT grafting polymerization and TiO2 photocatalysts immobilization for phenol decomposition in a photocatalytic membrane reactor. Sep. Purif. Technol. 2011, 83, 157–165. [Google Scholar] [CrossRef]
- Madaeni, S.S.; Ghaemi, N.; Alizadeh, A.; Joshaghani, M. Influence of photo-induced superhydrophilicity of titanium dioxide nanoparticles on the anti-fouling performance of ultrafiltration membranes. Appl. Surf. Sci. 2011, 257, 6175–6180. [Google Scholar] [CrossRef]
- Fan, Z.; Wang, Z.; Sun, N.; Wang, J.; Wang, S. Performance improvement of polysulfone ultrafiltration membrane by blending with polyaniline nanofibers. J. Membr. Sci. 2008, 320, 363–371. [Google Scholar] [CrossRef]
- Tiraferri, A.; Kang, Y.; Giannelis, E.P.; Elimelech, M. Superhydrophilic thin-film composite forward osmosis membranes for organic fouling control: Fouling behavior and antifouling mechanisms. Environ. Sci. Technol. 2012, 46, 11135–11144. [Google Scholar] [CrossRef]
- Wang, Z.; Ma, H.; Hsiao, B.S.; Chu, B. Nanofibrous ultrafiltration membranes containing cross-linked poly(ethylene glycol) and cellulose nanofiber composite barrier layer. Polymer 2014, 55, 366–372. [Google Scholar] [CrossRef]
- Rasheed, I.A.; Rehan, Z.A.; Khalid, T.; Zahid, M.; Ahmad, H. Prospects of nanocomposite membranes in commercial scale. In Nanocomposite Membranes for Water and Gas Separation; Sadrzadeh, M., Mohammadi, T., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 457–473. ISBN 9780128167106. [Google Scholar]
- Zahid, M.; Rashid, A.; Akram, S.; Rehan, Z.A.; Razzaq, W. A Comprehensive Review on Polymeric Nano-Composite Membranes for Water Treatment. J. Membr. Sci. Technol. 2018, 8, 1–20. [Google Scholar] [CrossRef]
- Tiraferri, A.; Vecitis, C.D.; Elimelech, M. Covalent binding of single-walled carbon nanotubes to polyamide membranes for antimicrobial surface properties. ACS Appl. Mater. Interfaces 2011, 3, 2869–2877. [Google Scholar] [CrossRef] [PubMed]
- Kang, G.-D.; Cao, Y.-M. Development of antifouling reverse osmosis membranes for water treatment: A review. Water Res. 2012, 46, 584–600. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Zhang, X.; Zhang, Y.; Liu, J.; Zhang, H. Development of a hydrophilic PES ultrafiltration membrane containing SiO2@N-Halamine nanoparticles with both organic antifouling and antibacterial properties. Desalination 2013, 326, 69–76. [Google Scholar] [CrossRef]
- Chkirida, S.; Zari, N.; Achour, R.; Hassoune, H.; Lachehab, A.; Qaiss, A.e.k.; Bouhfid, R. Highly synergic adsorption/photocatalytic efficiency of Alginate/Bentonite impregnated TiO2 beads for wastewater treatment. J. Photochem. Photobiol. A Chem. 2021, 412, 113215. [Google Scholar] [CrossRef]
- Bilal, M.; Ihsanullah, I.; Younas, M.; Ul Hassan Shah, M. Recent advances in applications of low-cost adsorbents for the removal of heavy metals from water: A critical review. Sep. Purif. Technol. 2022, 278, 119510. [Google Scholar] [CrossRef]
- Pang, Y.; Yu, J.; Tang, L.; Zeng, G.; Zhu, C.; Wei, X. Magnetic Nanohybrid Materials for Water-Pollutant Removal. In Nanohybrid and Nanoporous Materials for Aquatic Pollution Control; Tang, L., Deng, Y., Wang, J., Wang, J., Zeng, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1–30. ISBN 9780128141557. [Google Scholar]
- Fan, C.; Li, K.; Wang, Y.; Qian, X.; Jia, J. The stability of magnetic chitosan beads in the adsorption of Cu2+. RSC Adv. 2016, 6, 2678–2686. [Google Scholar] [CrossRef]
- Salvador, F.; Martin-Sanchez, N.; Sanchez-Montero, M.J.; Montero, J.; Izquierdo, C. Regeneration of activated carbons contaminated by phenol using supercritical water. J. Supercrit. Fluids 2013, 74, 1–7. [Google Scholar] [CrossRef]
- Sun, Z.; Li, C.; Wu, D. Removal of methylene blue from aqueous solution by adsorption onto zeolite synthesized from coal fly ash and its thermal regeneration. J. Chem. Technol. Biotechnol. 2010, 85, 845–850. [Google Scholar] [CrossRef]
- Shah, I.K.; Pre, P.; Alappat, B.J. Steam Regeneration of Adsorbents: An Experimental and Technical Review. Chem. Sci. Trans. 2013, 2, 1078–1088. [Google Scholar] [CrossRef] [Green Version]
- Chkirida, S.; Zari, N.; Bouhfid, R.; Qaiss, A.e.k. Insight into the bionanocomposite applications on wastewater decontamination: Review. J. Water Process Eng. 2021, 43, 102198. [Google Scholar] [CrossRef]
- Hlongwane, G.N.; Sekoai, P.T.; Meyyappan, M.; Moothi, K. Simultaneous removal of pollutants from water using nanoparticles: A shift from single pollutant control to multiple pollutant control. Sci. Total Environ. 2019, 656, 808–833. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Deng, W.; Zhou, W.; Luo, J. Novel magnetic polysaccharide/graphene oxide @Fe3O4 gel beads for adsorbing heavy metal ions. Carbohydr. Polym. 2019, 216, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Shchipunov, Y. Bionanocomposites: Green sustainable materials for the near future. Pure Appl. Chem. 2012, 84, 2579–2607. [Google Scholar] [CrossRef]
- Darder, M.; Aranda, P.; Ferrer, M.L.; Gutiérrez, M.C.; Del Monte, F.; Ruiz-Hitzky, E. Progress in bionanocomposite and bioinspired foams. Adv. Mater. 2011, 23, 5262–5267. [Google Scholar] [CrossRef] [PubMed]
- Gupta, O.; Roy, S. Recent progress in the development of nanocomposite membranes. In Nanocomposite Membranes for Water and Gas Separation; Sadrzadeh, M., Mohammadi, T., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 29–67. ISBN 9780128167106. [Google Scholar]
- Baten, R.; Stummeyer, K. How sustainable can desalination be? Desalin. Water Treat. 2013, 51, 44–52. [Google Scholar] [CrossRef]
- Miller, S.; Shemer, H.; Semiat, R. Energy and environmental issues in desalination. Desalination 2015, 366, 2–8. [Google Scholar] [CrossRef]
- Haddad, B.M. A case for an ecological-economic research program for desalination. Desalination 2013, 324, 72–78. [Google Scholar] [CrossRef]
- Beer, C.; Foldbjerg, R.; Hayashi, Y.; Sutherland, D.S.; Autrup, H. Toxicity of silver nanoparticles-Nanoparticle or silver ion? Toxicol. Lett. 2012, 208, 286–292. [Google Scholar] [CrossRef]
- Naseri, R.; Davoodi, R. Commercialization of Nanotechnology in Developing Countries. In Proceedings of the 3rd International Conference on Information and Financial Engineering (ICIFE 2011), Shanghai, China, 19–21 August 2011; Volume 12, pp. 385–389. [Google Scholar]
- Mazumder, S.; Sarkar, D.; Puri, I.K. Nanotechnology Commercialization: Prospects in India. J. Mater. Sci. Nanotechnol. 2014, 1, 201. [Google Scholar]
- Aithal, P.S.; Aithal, S. Nanotechnological innovations & business environment for Indian automobile sector: A futuristic approach. J. Sci. Res. Mod. 2020, 1, 296–307. [Google Scholar]
- Aithal, S. Nanotechnology Innovations & Business Opportunities: A Review. Int. J. Manag. IT Eng. 2016, 6, 182–204. [Google Scholar]
- Karthikeyan, P.; Vigneshwaran, S.; Meenakshi, S. Al3+ incorporated chitosan-gelatin hybrid microspheres and their use for toxic ions removal: Assessment of its sustainability metrics. Environ. Chem. Ecotoxicol. 2020, 2, 97–106. [Google Scholar] [CrossRef]
- Buzea, C.; Pacheco, I.I.; Robbie, K. Nanomaterials and nanoparticles: Sources and toxicity. Biointerphases 2007, 2, MR17–MR71. [Google Scholar] [CrossRef] [Green Version]
- Pacheco, I.; Buzea, C. Nanomaterials and Nanocomposites: Classification and Toxicity. In Handbook of Nanomaterials and Nanocomposites for Energy and Environmental Applications; Kharissova, O.V., Torres-Martínez, L.M., Kharisov, B.I., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 3–39. ISBN 978-3-030-36268-3. [Google Scholar]
- Sarin, H. Physiologic upper limits of pore size of different blood capillary types and another perspective on the dual pore theory of microvascular permeability. J. Angiogenes. Res. 2010, 2, 1–19. [Google Scholar] [CrossRef]
- Wu, T.; Tang, M. Review of the effects of manufactured nanoparticles on mammalian target organs. J. Appl. Toxicol. 2018, 38, 25–40. [Google Scholar] [CrossRef] [PubMed]
- Karmakar, A.; Zhang, Q.; Zhang, Y. Neurotoxicity of nanoscale materials. J. Food Drug Anal. 2014, 22, 147–160. [Google Scholar] [CrossRef] [Green Version]
- Schrand, A.M.; Rahman, M.F.; Hussain, S.M.; Schlager, J.J.; Smith, D.A.; Syed, A.F. Metal-based nanoparticles and their toxicity assessment. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2010, 2, 544–568. [Google Scholar] [CrossRef] [Green Version]
- Castranova, V.; Schulte, P.A.; Zumwalde, R.D. Occupational nanosafety considerations for carbon nanotubes and carbon nanofibers. Acc. Chem. Res. 2013, 46, 642–649. [Google Scholar] [CrossRef] [Green Version]
- Dahm, M.M.; Schubauer-Berigan, M.K.; Evans, D.E.; Birch, M.E.; Fernback, J.E.; Deddens, J.A. Carbon nanotube and nanofiber exposure assessments: An analysis of 14 site visits. Ann. Occup. Hyg. 2015, 59, 705–723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, Y.; Kuhlbusch, T.A.J.; Van Tongeren, M.; Jiménez, A.S.; Tuinman, I.; Chen, R.; Alvarez, I.L.; Mikolajczyk, U.; Nickel, C.; Meyer, J.; et al. Airborne engineered nanomaterials in the workplace—A review of release and worker exposure during nanomaterial production and handling processes. J. Hazard. Mater. 2017, 322, 17–28. [Google Scholar] [CrossRef] [Green Version]
- Kuijpers, E.; Bekker, C.; Brouwer, D.; Le Feber, M.; Fransman, W. Understanding workers’ exposure: Systematic review and data-analysis of emission potential for NOAA. J. Occup. Environ. Hyg. 2017, 14, 349–359. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, F.S. Nano-zerovalent iron contained porous carbons developed from waste biomass for the adsorption and dechlorination of PCBs. Bioresour. Technol. 2010, 101, 2562–2564. [Google Scholar] [CrossRef] [PubMed]
- Varanasi, P.; Fullana, A.; Sidhu, S. Remediation of PCB contaminated soils using iron nano-particles. Chemosphere 2007, 66, 1031–1038. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Wang, Q.; Choi, H.C.; Kim, Y.H. Encapsulation of iron nanoparticles with PVP nanofibrous membranes to maintain their catalytic activity. J. Membr. Sci. 2010, 348, 231–237. [Google Scholar] [CrossRef]
- Tong, M.; Yuan, S.; Long, H.; Zheng, M.; Wang, L.; Chen, J. Reduction of nitrobenzene in groundwater by iron nanoparticles immobilized in PEG/nylon membrane. J. Contam. Hydrol. 2011, 122, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Dong, T.; Luo, H.; Wang, Y.; Hu, B.; Chen, H. Stabilization of Fe-Pd bimetallic nanoparticles with sodium carboxymethyl cellulose for catalytic reduction of para-nitrochlorobenzene in water. Desalination 2011, 271, 11–19. [Google Scholar] [CrossRef]
- Konstantinou, I.K.; Albanis, T.A. TiO2-assisted photocatalytic degradation of azo dyes in aqueous solution: Kinetic and mechanistic investigations: A review. Appl. Catal. B Environ. 2004, 49, 1–14. [Google Scholar] [CrossRef]
- Zhu, H.; Jiang, R.; Xiao, L.; Chang, Y.; Guan, Y.; Li, X.; Zeng, G. Photocatalytic decolorization and degradation of Congo Red on innovative crosslinked chitosan/nano-CdS composite catalyst under visible light irradiation. J. Hazard. Mater. 2009, 169, 933–940. [Google Scholar] [CrossRef]
- Konstantinou, I.K.; Sakellarides, T.M.; Sakkas, V.A.; Albanis, T.A. Photocatalytic degradation of selected s-triazine herbicides and oganophosphorus insecticides over aqueous TiO2 suspensions. Environ. Sci. Technol. 2001, 35, 398–405. [Google Scholar] [CrossRef]
- Song, H.; Carraway, E.R.; Kim, Y.H.; Batchelor, B.; Jeon, B.H.; Kim, J.G. Amendment of hydroxyapatite in reduction of tetrachloroethylene by zero-valent zinc: Its rate enhancing effect and removal of Zn(II). Chemosphere 2008, 73, 1420–1427. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Huang, W.; Fennell, D.E.; Peng, P. Kinetics of reductive dechlorination of 1,2,3,4-TCDD in the presence of zero-valent zinc. Chemosphere 2008, 71, 360–368. [Google Scholar] [CrossRef]
- Mahendra, S.; Li, Q.; Lyon, D.Y.; Brunet, L.; Alvarez, P.J.J. Nanotechnology-Enabled Water Disinfection and Microbial Control: Merits and Limitations. In Nanotechnology Applications for Clean Water; Savage, N., Diallo, M., Duncan, J., Street, A., Sustich, R., Eds.; William Andrew Publishing: Boston, MA, USA, 2009; pp. 157–166. ISBN 9780815515784. [Google Scholar]
- Li, Q.; Mahendra, S.; Lyon, D.Y.; Brunet, L.; Liga, M.V.; Li, D.; Alvarez, P.J.J. Antimicrobial nanomaterials for water disinfection and microbial control: Potential applications and implications. Water Res. 2008, 42, 4591–4602. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.; Pradeep, T. Potential of silver nanoparticle-coated polyurethane foam as an antibacterial water filter. Biotechnol. Bioeng. 2005, 90, 59–63. [Google Scholar] [CrossRef] [Green Version]
- Lv, Y.; Liu, H.; Wang, Z.; Liu, S.; Hao, L.; Sang, Y.; Liu, D.; Wang, J.; Boughton, R.I. Silver nanoparticle-decorated porous ceramic composite for water treatment. J. Membr. Sci. 2009, 331, 50–56. [Google Scholar] [CrossRef]
- Dankovich, T.A.; Gray, D.G. Bactericidal paper impregnated with silver nanoparticles for point-of-use water treatment. Environ. Sci. Technol. 2011, 45, 1992–1998. [Google Scholar] [CrossRef]
- Abebe, L.S.; Smith, J.A.; Narkiewicz, S.; Oyanedel-Craver, V.; Conaway, M.; Singo, A.; Amidou, S.; Mojapelo, P.; Brant, J.; Dillingham, R. Ceramic water filters impregnated with silver nanoparticles as a point-of-use water-treatment intervention for HIV-positive individuals in Limpopo Province, South Africa: A pilot study of technological performance and human health benefits. J. Water Health 2014, 12, 288–300. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Bhalli, J.A.; Ding, W.; Yan, J.; Pearce, M.G.; Sadiq, R.; Cunningham, C.K.; Jones, M.Y.; Monroe, W.A.; Howard, P.C.; et al. Cytotoxicity and genotoxicity assessment of silver nanoparticles in mouse. Nanotoxicology 2014, 8, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Carlson, C.; Hussein, S.M.; Schrand, A.M.; Braydich-Stolle, L.K.; Hess, K.L.; Jones, R.L.; Schlager, J.J. Unique cellular interaction of silver nanoparticles: Size-dependent generation of reactive oxygen species. J. Phys. Chem. B 2008, 112, 13608–13619. [Google Scholar] [CrossRef] [PubMed]
- Trouiller, B.; Reliene, R.; Westbrook, A.; Solaimani, P.; Schiestl, R.H. Titanium dioxide nanoparticles induce DNA damage and genetic instability in vivo in mice. Cancer Res. 2009, 69, 8784–8789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, E.J.; Bae, E.; Yi, J.; Kim, Y.; Choi, K.; Lee, S.H.; Yoon, J.; Lee, B.C.; Park, K. Repeated-dose toxicity and inflammatory responses in mice by oral administration of silver nanoparticles. Environ. Toxicol. Pharmacol. 2010, 30, 162–168. [Google Scholar] [CrossRef]
- Kim, Y.S.; Kim, J.S.; Cho, H.S.; Rha, D.S.; Kim, J.M.; Park, J.D.; Choi, B.S.; Lim, R.; Chang, H.K.; Chung, Y.H.; et al. Twenty-eight-day oral toxicity, genotoxicity, and gender-related tissue distribution of silver nanoparticles in Sprague-Dawley rats. Inhal. Toxicol. 2008, 20, 575–583. [Google Scholar] [CrossRef]
- Adibzadeh, S.; Bazgir, S.; Katbab, A.A. Fabrication and characterization of chitosan/poly(vinyl alcohol) electrospun nanofibrous membranes containing silver nanoparticles for antibacterial water filtration. Iran. Polym. J. 2014, 23, 645–654. [Google Scholar] [CrossRef]
- Westerhoff, P.; Atkinson, A.; Fortner, J.; Wong, M.S.; Zimmerman, J.; Gardea-Torresdey, J.; Ranville, J.; Herckes, P. Low risk posed by engineered and incidental nanoparticles in drinking water. Nat. Nanotechnol. 2018, 13, 661–669. [Google Scholar] [CrossRef]
- Chkirida, S.; Zari, N.; Qaiss, A.E.K.; Bouhfid, R. Nanocomposite Materials Based on TiO2/Clay for Wastewater Treatment. In Nanotechnology in the Life Sciences; Prasad, R., Karchiyappan, T., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 363–380. ISBN 978-3-030-02381-2. [Google Scholar]
- Bundschuh, M.; Filser, J.; Lüderwald, S.; McKee, M.S.; Metreveli, G.; Schaumann, G.E.; Schulz, R.; Wagner, S. Nanoparticles in the environment: Where do we come from, where do we go to? Environ. Sci. Eur. 2018, 30, 6. [Google Scholar] [CrossRef]
- Jabbari, V.; Veleta, J.M.; Zarei-Chaleshtori, M.; Gardea-Torresdey, J.; Villagrán, D. Green synthesis of magnetic MOF@GO and MOF@CNT hybrid nanocomposites with high adsorption capacity towards organic pollutants. Chem. Eng. J. 2016, 304, 774–783. [Google Scholar] [CrossRef] [Green Version]
- Abdi, J.; Vossoughi, M.; Mahmoodi, N.M.; Alemzadeh, I. Synthesis of metal-organic framework hybrid nanocomposites based on GO and CNT with high adsorption capacity for dye removal. Chem. Eng. J. 2017, 326, 1145–1158. [Google Scholar] [CrossRef]
- Torad, N.L.; Hu, M.; Ishihara, S.; Sukegawa, H.; Belik, A.A.; Imura, M.; Ariga, K.; Sakka, Y.; Yamauchi, Y. Direct synthesis of MOF-derived nanoporous carbon with magnetic Co nanoparticles toward efficient water treatment. Small 2014, 10, 2096–2107. [Google Scholar] [CrossRef]




| Type | Polymeric Phase | Nanophase | Functionality | Ref. |
|---|---|---|---|---|
| Type I: Conventional PNC | Polyvinyl alcohol (PVA) and chitosan | Cellulose nanocrystals/ZnO | Enhanced mechanical characteristics | [101] |
| PVA | Nano fibrillated cellulose | The enhancement of mechanical, thermal, and chemical qualities | [102] | |
| Phosphorylated nanocellulose fibrils | Mechanical, thermal, and chemical characteristics are enhanced | [103] | ||
| Cellulose nanocrystals | Enhancement in tensile strength, thermal stability, and swelling capacity | [104] | ||
| Nafion@ | Zirconium phosphates + carbon nanotubes | Enhanced mechanical characteristics | [105] | |
| Zirconium phosphates | [106] | |||
| Type II: Active-bulk phase PNC | PVA crosslinked with glutaraldehyde | Nanostructured Fe3O4/polystyrene core-shell | Adjusting membrane permeability in response to temperature | [108] |
| Poly(acrylamide) | Graphene oxide and reduced graphene oxide | Improve thermal conductivity | [109] | |
| Poly(aniline) | Graphene oxide | Sensitive to NH3 gas | [110] | |
| Conductive poly(vinylidene fluoride) | Carbon nanofiber + ionic liquid | Sensitive to strain | [111] | |
| Cellulose | Copper Oxide | Gaseous compound detection at low temperatures (e.g., H2S) | [112] | |
| Type III: Active-surface PNC | Aromatic polyamide | Carbon nanotubes | Antifouling, increased permeability, and chlorine resistance | [113] |
| Polyethersulfone (PES) | Fe2O3 nanoparticles | Ionic strength sensitive | [114] | |
| Carboxylated graphene | Anti-biofouling properties and protein antifouling | [116] | ||
| Polyamide (PA) | Natural zeolite nanoparticles | Nitrate rejection and permeability increase | [115] | |
| Highly hydrophilic clay mineral + Ag | Increased NaCl rejection and anti-biofouling | [117] | ||
| Silica nanoparticles functionalised with quaternary ammonium groups | Super hydrophilic, antifouling, and very permeable to water | [118] |
| Nanocomponent | Membrane Composite System | Application | Ref. |
|---|---|---|---|
| TiO2 NPs | poly(vinylpyrrolidone) (PVP)-co-poly (vinylidene fluoride) (PVDF)/P25-TiO2 NPs | Organic pollutants are broken down by photocatalysis | [177] |
| poly(ethersulfones) (PES)/TiO2 NPs/water soluble porphyrins | Heavy metals are easy to find and remove | [178] | |
| Carbon Nanotubes (CNT) | poly(acrylonitrile)(PAN)/CNT/TiO2-NH2 NPs | Metal ions can be broken down by photocatalysis | [179] |
| PES/single-walled CNT (SWCNTs) | Membranes with antimicrobial activity | [180] | |
| poly(vinyl alcohol) (PVA)/bovine serum albumin (BSA)/SWNTs | There was more enzyme binding and ester hydrolysis | [181] | |
| SiO2 NPs | PVDF/SiO2 NPs | Increase in flux (24 Lm−2·h−1) in water–oil separations | [182] |
| PVDF/Amorphous SiO2 NPs | Forward osmosis (FO) desalination showed an increase in water flow (83 Lm−2·h−1) | [183] | |
| PVDF/SiO2 NPs | For processes of membrane distillation (MD) | [184] | |
| Ag NPs | Cellulose acetate/Ag NPs | Antimicrobial activity | [185] |
| poly(lactide-co-glycoside) PLGA/chitosan (10%)/graphene-oxide-Ag decorated NPs (GO-Ag NPs) | Antimicrobial activity | [186] | |
| Polycaprolactone (PCL)/TiO2-Ag NPs | Photocatalytic and antibacterial activity membranes | [187] | |
| ZnO | Nylon 6,6/ZnO core-shell nanofibers | Photocatalytic membranes that are very stable and flexible | [188] |
| Polyurethane (PU)/polydopamine/ZnO nanorods | Antifouling membranes that work through photocatalysis | [189] | |
| PAN/ZnO-Ag heterostructure NPs | Antibacterial activity | [190] | |
| Fe NPs | Poly(acrylic acid) (PAA)/polyvinyl alcohol (PVA)/FeCl3 (aq.) | Complexation of Fe3+ ions and creation of new Fe nanoparticles (NPs), dye degradation | [191] |
| Fe2O3 NPs | poly(lactic acid)/γ-Fe2O3 NPs | Oil is absorbed and separated very efficiently | [192] |
| Yeast Cells | Core: PVP/Yeast Cells Sheath: PVDF-co-hexafluoropropylene (HFP)/poly(ethylene glycol) (PEG) | Degradation of phenol | [193] |
| Fabrication Technique | Key Benefits | Drawbacks |
|---|---|---|
| Interfacial polymerisation |
|
|
| Phase inversion |
|
|
| Track-etching |
|
|
| Electrospinning |
|
|
| 3D printing |
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al Harby, N.F.; El-Batouti, M.; Elewa, M.M. Prospects of Polymeric Nanocomposite Membranes for Water Purification and Scalability and their Health and Environmental Impacts: A Review. Nanomaterials 2022, 12, 3637. https://doi.org/10.3390/nano12203637
Al Harby NF, El-Batouti M, Elewa MM. Prospects of Polymeric Nanocomposite Membranes for Water Purification and Scalability and their Health and Environmental Impacts: A Review. Nanomaterials. 2022; 12(20):3637. https://doi.org/10.3390/nano12203637
Chicago/Turabian StyleAl Harby, Nouf F., Mervette El-Batouti, and Mahmoud M. Elewa. 2022. "Prospects of Polymeric Nanocomposite Membranes for Water Purification and Scalability and their Health and Environmental Impacts: A Review" Nanomaterials 12, no. 20: 3637. https://doi.org/10.3390/nano12203637





