Effect of Ag-Decorated BiVO4 on Photoelectrochemical Water Splitting: An X-ray Absorption Spectroscopic Investigation
Abstract
:1. Introduction
2. Materials and Methods
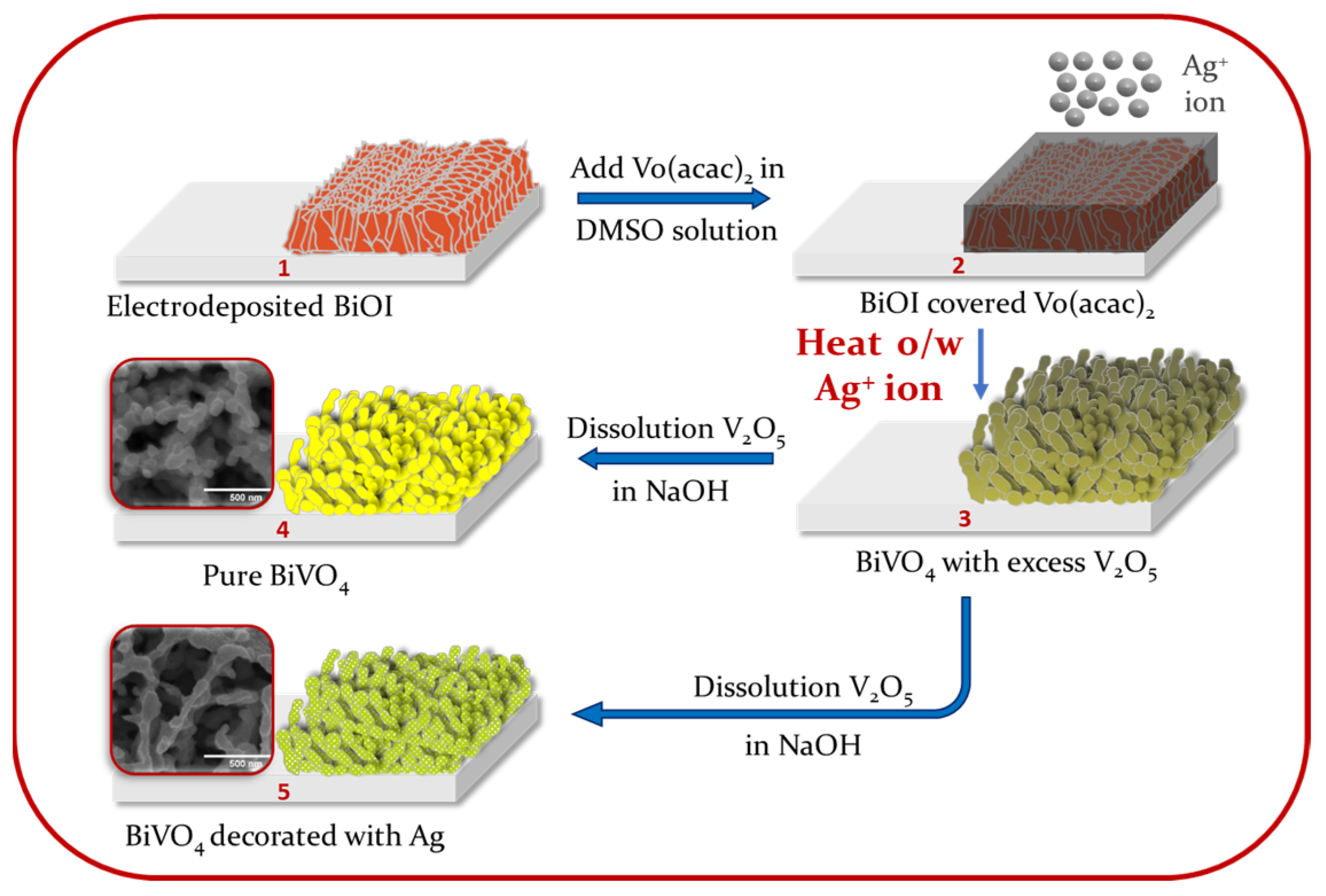
2.1. Synthesis of Pure BiVO4 and Ag–BiVO4
2.2. Photoelectrochemical Measurement
2.3. Characterization
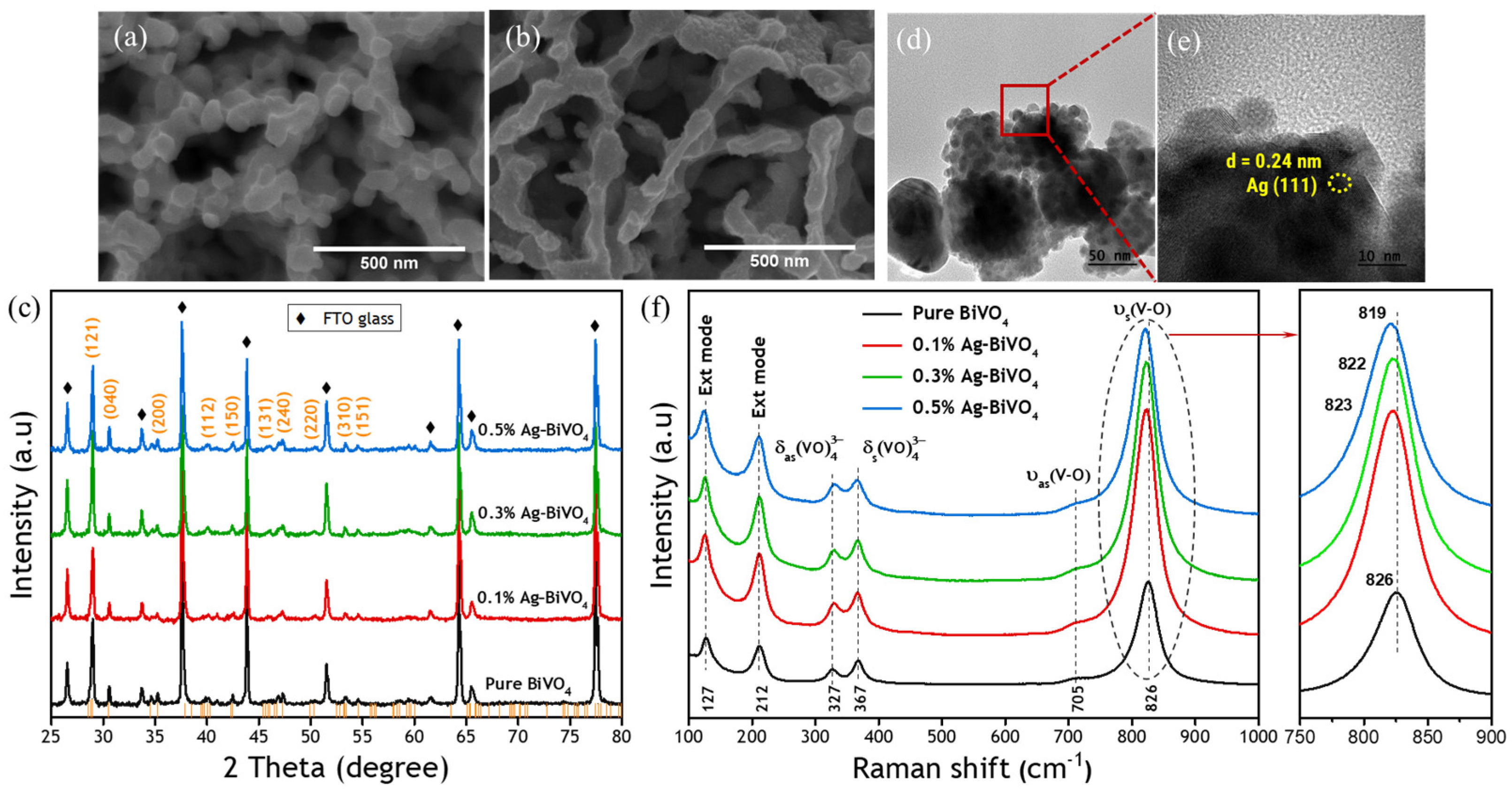
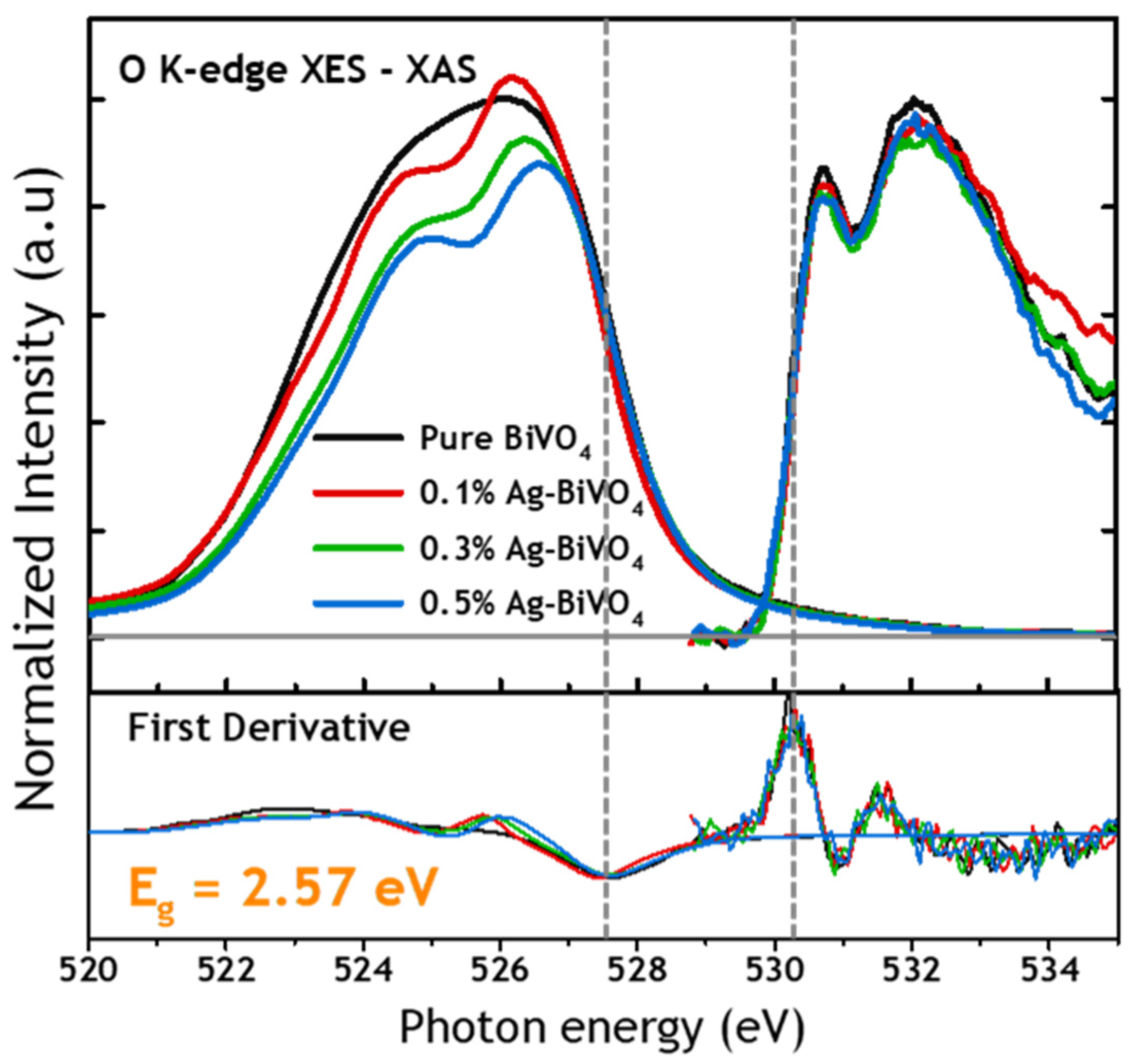
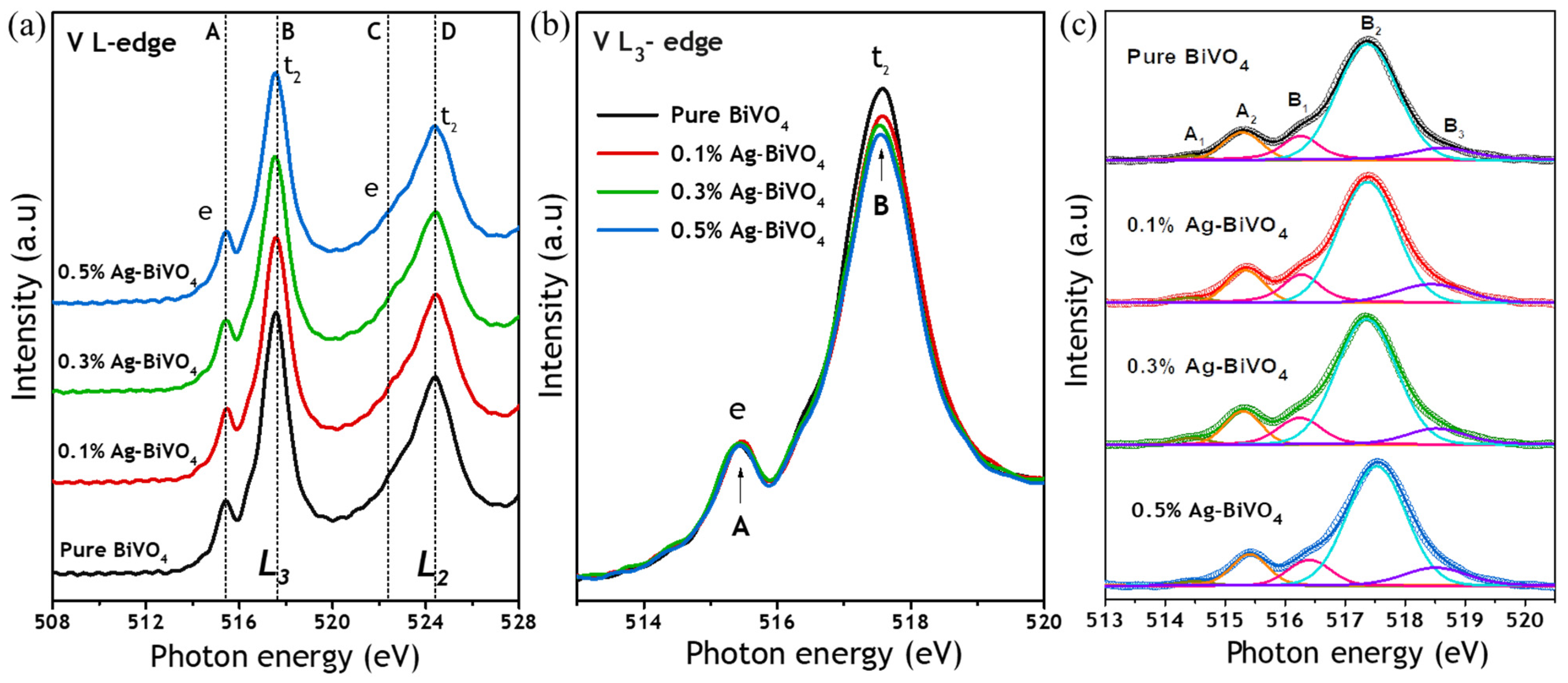
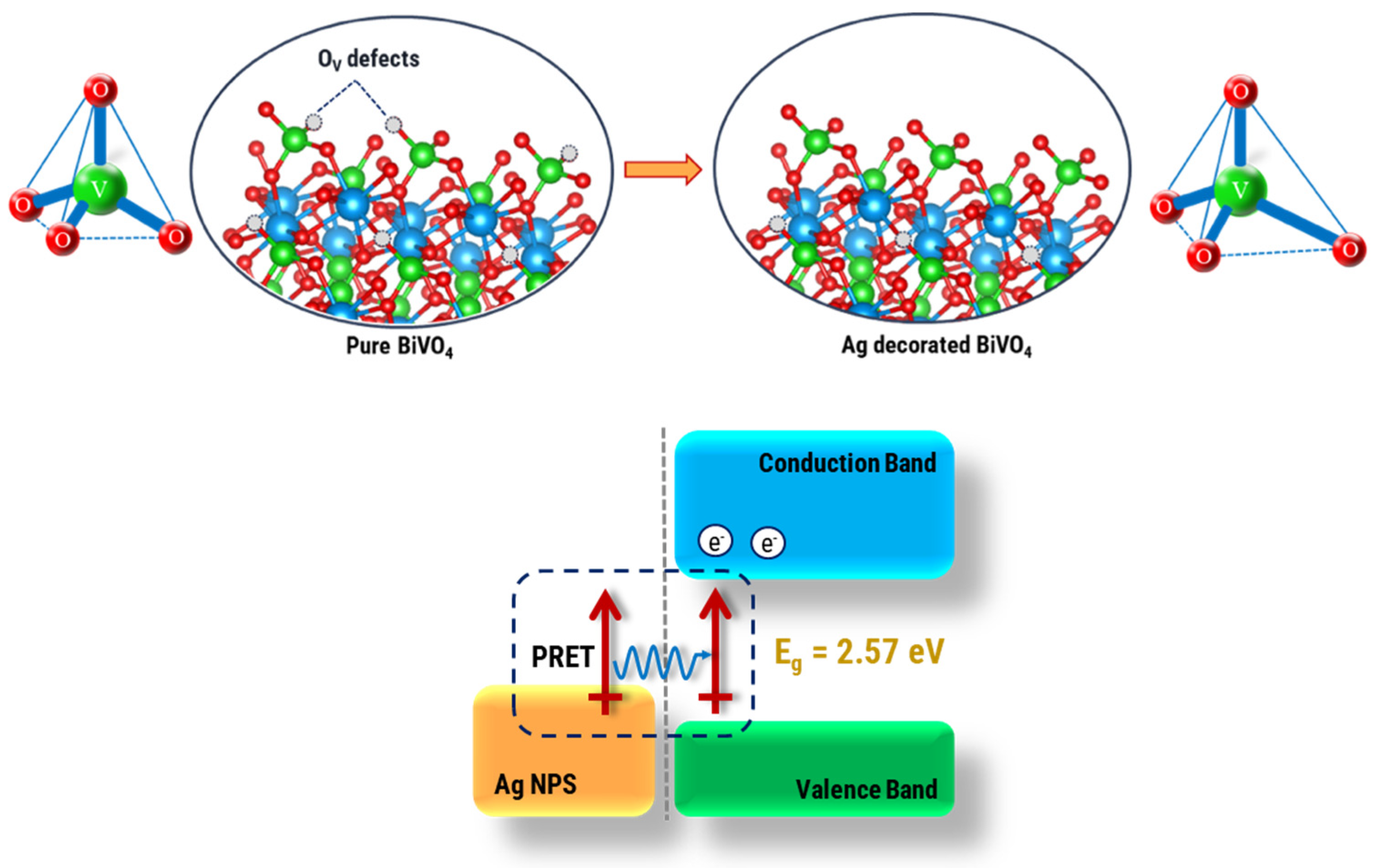
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tran, D.P.; Wong, L.H.; Barber, J.; Loo, J.S.C. Recent advances in hybrid photocatalysts for solar fuel production. Energy Environ. Sci. 2012, 5, 5902–5918. [Google Scholar] [CrossRef]
- Chen, X.; Li, C.; Gratzel, M.; Kostecki, R.; Mao, S.S. Nanomaterials for renewable energy production and storage. Chem. Soc. Rev. 2012, 41, 7909–7937. [Google Scholar] [CrossRef] [PubMed]
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Chen, X.; Liu, L.; Yu, P.Y.; Mao, S.S. Increasing solar absorption for photocatalysis with black hydrogenated titanium dioxide nanocrystals. Science 2011, 331, 746–750. [Google Scholar] [CrossRef] [PubMed]
- Pal, M.; Rakshit, R.; Mandal, K. Facile functionalization of Fe2O3 nanoparticles to induce inherent photoluminescence and excellent photocatalytic activity. Appl. Phys. Lett. 2014, 104, 233110. [Google Scholar] [CrossRef]
- Fàbrega, C.; Murcia-López, S.; Monllor-Satoca, D.; Prades, J.D.; Hernandez-Alonso, M.D.; Penelas, G.; Morante, J.R.; Andreu, T. Efficient WO3 photoanodes fabricated by pulsed laser deposition for photoelectrochemical water splitting with high faradaic efficiency. Appl. Catal. B Environ. 2016, 189, 133–140. [Google Scholar] [CrossRef]
- Djurišić, A.B.; Chen, X.; Leung, Y.H.; Ng, A.M.C. ZnO nanostructures: Growth, properties and applications. J. Mater. Chem. 2012, 22, 6526–6535. [Google Scholar] [CrossRef]
- Zhang, L.; Herrmann, L.O.; Baumberg, J.J. Size dependent plasmonic effect on BiVO4 photoanodes for solar water splitting. Sci. Rep. 2015, 5, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Pihosh, Y.; Turkevych, I.; Mawatari, K.; Uemura, J.; Kazoe, Y.; Kosar, S.; Makita, K.; Sugaya, T.; Matsui, T.; Fujita, D.; et al. Photocatalytic generation of hydrogen by core-shell WO3/BiVO4 nanorods with ultimate water splitting efficiency. Sci. Rep. 2015, 5, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Yin, X.; Qiu, W.; Li, W.; Li, C.; Wang, K.; Yang, X.; Du, L.; Liu, Y.; Li, J. High porosity Mo doped BiVO4 film by vanadium re-substitution for efficient photoelectrochemical water splitting. Chem. Eng. J. 2020, 389, 124365. [Google Scholar] [CrossRef]
- Liu, Y.; Wygant, B.R.; Kawashima, K.; Mabayoje, O.; Hong, T.E.; Lee, S.G.; Lin, J.; Kim, J.H.; Yubuta, K.; Li, W.; et al. Facet effect on the photoelectrochemical performance of a WO3/BiVO4 heterojunction photoanode. Appl. Catal. B Environ. 2019, 245, 227–239. [Google Scholar] [CrossRef]
- Zhou, S.; Chen, K.; Huang, J.; Wang, L.; Zhang, M.; Bai, B.; Liu, H.; Wang, Q. Preparation of heterometallic CoNi-MOFs-modified BiVO4: A steady photoanode for improved performance in photoelectrochemical water splitting. Appl. Catal. B Environ. 2020, 266, 118513. [Google Scholar] [CrossRef]
- Chen, H.M.; Chen, C.K.; Chen, C.J.; Chen, L.C.; Wu, P.C.; Cheng, B.H.; Ho, Y.Z.; Tseng, M.L.; Hsu, Y.Y.; Chan, T.S.; et al. Plasmon inducing effects for enhanced photoelectrochemical water splitting: X-ray absorption approach to electronic structures. ACS Nano 2012, 6, 7362–7372. [Google Scholar] [CrossRef]
- Abdi, F.F.; Dabirian, A.; Dam, B.; Krol, R.V.D. Plasmonic enhancement of the optical absorption and catalytic efficiency of BiVO4 photoanodes decorated with Ag@ SiO2 core–shell nanoparticles. Phys. Chem. Chem. Phys. 2014, 16, 15272–15277. [Google Scholar] [CrossRef]
- Wang, Z.; Xue, J.; Pan, H.; Wu, L.; Dong, J.; Cao, H.; Sun, S.; Gao, C.; Zhu, X.; Bao, J. Establishing a new hot electron transfer channel by ion doping in a plasmonic metal/semiconductor photocatalyst. Phys. Chem. Chem. Phys. 2020, 22, 15795–15798. [Google Scholar] [CrossRef] [PubMed]
- West, P.R.; Ishii, S.; Naik, G.V.; Emani, N.K.; Shalaev, V.M.; Boltasseva, A. Searching for better plasmonic materials. Laser Photonics Rev. 2010, 4, 795–808. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.G.; Moon, C.W.; Park, H.; Sohn, W.; Kang, S.B.; Lee, S.; Choi, K.J.; Jang, H.W. Dominance of Plasmonic Resonant Energy Transfer over Direct Electron Transfer in Substantially Enhanced Water Oxidation Activity of BiVO4 by Shape-Controlled Au Nanoparticles. Small 2017, 13, 1701644. [Google Scholar] [CrossRef]
- Bai, S.; Jiang, J.; Zhang, Q.; Xiong, Y. Steering charge kinetics in photocatalysis: Intersection of materials syntheses, characterization techniques and theoretical simulations. Chem. Soc. Rev. 2015, 44, 2893–2939. [Google Scholar] [CrossRef]
- Patil, S.S.; Mali, M.G.; Hassan, M.A.; Patil, D.R.; Kolekar, S.S.; Ryu, S.W. One-pot in situ hydrothermal growth of BiVO4/Ag/rGO hybrid architectures for solar water splitting and environmental remediation. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Tayebi, M.; Tayyebi, A.; Lee, B.K.; Lee, C.H.; Lim, D.H. The effect of silver doping on photoelectrochemical (PEC) properties of bismuth vanadate for hydrogen production. Sol. Energy Mater. Sol. Cells 2019, 200, 109943. [Google Scholar] [CrossRef]
- Jeong, S.Y.; Shin, H.M.; Jo, Y.R.; Kim, Y.J.; Kim, S.; Lee, W.J.; Lee, G.J.; Song, J.; Moon, B.J.; Seo, S.; et al. Plasmonic silver nanoparticle-impregnated nanocomposite BiVO4 photoanode for plasmon-enhanced photocatalytic water splitting. J. Phys. Chem C 2018, 122, 7088–7093. [Google Scholar] [CrossRef]
- Kim, T.W.; Choi, K.S. Nanoporous BiVO4 photoanodes with dual-layer oxygen evolution catalysts for solar water splitting. Science 2014, 343, 990–994. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Luo, W.; Yan, S.; Feng, J.; Zhao, Z.; Zhu, Y.; Li, Z.; Zou, Z. BiVO4 nano–leaves: Mild synthesis and improved photocatalytic activity for O2 production under visible light irradiation. CrystEngComm 2011, 13, 2500–2504. [Google Scholar] [CrossRef]
- Baruah, P.K.; Singh, A.; Rangan, L.; Sharma, A.K.; Khare, A. Elucidation of size, structure, surface plasmon resonance, and photoluminescence of Ag nanoparticles synthesized by pulsed laser ablation in distilled water and its viability as SERS substrate. Appl. Phys. A 2020, 126, 1–14. [Google Scholar] [CrossRef]
- Le, H.V.; Nguyen, M.D.; Pham, Y.T.H.; Nguyen, D.N.; Le, L.T.; Han, H.; Tran, P.D. Decoration of AgOx hole collector to boost photocatalytic water oxidation activity of BiVO4 photoanode. Mater. Today Energy 2021, 21, 100762. [Google Scholar] [CrossRef]
- Zhou, B.; Zhao, X.; Liu, H.; Qu, J.; Huang, C.P. Synthesis of visible-light sensitive M–BiVO4 (M = Ag, Co, and Ni) for the photocatalytic degradation of organic pollutants. Sep. Purif. Technol. 2011, 77, 275–282. [Google Scholar] [CrossRef]
- Hong, W.T.; Risch, M.; Stoerzinger, K.A.; Grimaud, A.; Suntivich, J.; Yang, S.H. Toward the rational design of non-precious transition metal oxides for oxygen electrocatalysis. Energy Environ. Sci. 2015, 8, 1404–1427. [Google Scholar] [CrossRef] [Green Version]
- Yin, J.; Li, Y.; Lv, F.; Lu, M.; Sun, K.; Wang, W.; Wang, L.; Cheng, F.; Li, Y.; Xi, P.; et al. Oxygen vacancies dominated NiS2/CoS2 interface porous nanowires for portable Zn-air batteries driven water splitting. Adv. Mater. 2017, 29, 1704681. [Google Scholar] [CrossRef]
- Tao, H.B.; Fang, L.; Chen, J.; Yang, H.B.; Gao, J.; Miao, J.; Chen, S.; Liu, B. Identification of surface reactivity descriptor for transition metal oxides in oxygen evolution reaction. J. Am. Chem. Soc. 2016, 138, 9978–9985. [Google Scholar] [CrossRef]
- Zhao, Y.; Chang, C.; Teng, F.; Zhao, Y.; Chen, G.; Shi, R.; Waterhouse, G.I.N.; Huang, W.; Zhang, T. Defect-engineered ultrathin d-MnO2 nanosheet arrays as bifunctional electrodes for efficient overall water splitting. Adv. Energy Mater. 2017, 7, 1700005. [Google Scholar] [CrossRef]
- Zhu, Y.; Liu, X.; Jin, S.; Chen, H.; Lee, W.; Liu, M.; Chen, Y. Anionic defect engineering of transition metal oxides for oxygen reduction and evolution reactions. J. Mater. Chem. 2019, 7, 5875. [Google Scholar] [CrossRef]
- Yan, D.; Li, Y.; Hou, J.; Chen, R.; Dai, L.; Wang, S. Defect chemistry of nonprecious-metal electrocatalysts for oxygen reactions. Adv. Mater. 2017, 29, 1606459. [Google Scholar] [CrossRef] [PubMed]
- Gan, J.; Lu, X.; Wu, J.; Xie, S.; Zhai, T.; Yu, M.; Zhang, Z.; Mao, Y.; Wang, S.C.; Shen, Y.; et al. Oxygen vacancies promoting photoelectrochemical performance of In2O3 nanocubes. Sci. Rep. 2013, 3, 2021. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kato, K.; Uemura, Y.; Asakura, K.; Yamakata, A. Role of oxygen vacancy in the photocarrier dynamics of WO3 photocatalysts: The case of recombination centers. J. Phys. Chem. C 2022, 126, 9257–9263. [Google Scholar] [CrossRef]
- Stathi, P.; Solakidou, M.; Deligiannakis, Y. Lattice Defects Engineering in W-, Zr-doped BiVO4 by Flame Spray Pyrolysis: Enhancing Photocatalytic O2 Evolution. Nanomaterials 2021, 11, 501. [Google Scholar] [CrossRef]
- Ullah, H.; Tahir, A.A.; Mallick, T.K. Structural and electronic properties of oxygen defective and Se-doped p-type BiVO4 (001) thin film for the applications of photocatalysis. Appl. Catal. B Environ. 2018, 224, 895–903. [Google Scholar] [CrossRef] [Green Version]
- Österbacka, N.; Wiktor, J. Influence of Oxygen Vacancies on the Structure of BiVO4. J. Phys. Chem C 2021, 125, 1200–1207. [Google Scholar] [CrossRef]
- Jovic, V.; Laverock, J.; Rettie, A.J.E.; Zhou, J.S.; Mullins, C.B.; Singh, V.R.; Lamoureux, B.; Wilson, D.; Su, T.Y.; Jovic, B.; et al. Soft X-ray spectroscopic studies of the electronic structure of M: BiVO4 (M = Mo, W) single crystals. J. Mater. Chem. A 2015, 3, 23743–23753. [Google Scholar] [CrossRef] [Green Version]
- Nie, K.; Kashtanov, S.; Wei, Y.; Liu, Y.S.; Zhang, H.; Kapilashrami, M.; Ye, Y.; Glans, P.A.; Zhong, J.; Vayssieres, L.; et al. Atomic-scale understanding of the electronic structure-crystal facets synergy of nanopyramidal CoPi/BiVO4 hybrid photocatalyst for efficient solar water oxidation. Nano Energy 2018, 53, 483–491. [Google Scholar] [CrossRef] [Green Version]
- Gu, X.; Luo, Y.; Li, Q.; Wang, R.; Fu, S.; Lv, X.; He, Q.; Zhang, Y.; Yan, Q.; Xu, X.; et al. First-principle insight into the effects of oxygen vacancies on the electronic, photocatalytic, and optical properties of monoclinic BiVO4 (001). Front. Chem. 2020, 8, 1110. [Google Scholar] [CrossRef]
- Cooper, J.K.; Gul, S.; Toma, F.M.; Chen, L.; Glans, P.A.; Guo, J.; Ager, J.W.; Yano, J.; Sharp, I.D. Electronic structure of monoclinic BiVO4. Chem. Mater. 2014, 26, 5365–5373. [Google Scholar] [CrossRef]
- Pattengale, B.; Ludwig, J.; Huang, J. Atomic insight into the W-doping effect on carrier dynamics and photoelectrochemical properties of BiVO4. J. Phys. Chem. C 2016, 120, 1421–1427. [Google Scholar] [CrossRef]
- Ding, K.; Chen, B.; Fang, Z.; Zhang, Y. Density functional theory study on the electronic and optical properties of three crystalline phases of BiVO4. Theor. Chem. Acc. 2013, 132, 1352. [Google Scholar] [CrossRef]
- Fu, Y.; Dong, C.L.; Zhou, W.; Lu, Y.R.; Huang, Y.C.; Liu, Y.; Guo, P.; Zhao, L.; Chou, W.C.; Shen, S.H. A ternary nanostructured α-Fe2O3/Au/TiO2 photoanode with reconstructed interfaces for efficient photoelectrocatalytic water splitting. Appl. Catal. B Environ. 2020, 260, 118206. [Google Scholar] [CrossRef]
- Liu, L.; Chan, J.; Sham, T.K. Calcination-induced phase transformation and accompanying optical luminescence of TiO2 nanotubes: An X-ray absorption near-edge structures and X-ray excited optical luminescence study. J. Phys. Chem. C 2010, 114, 21353–21359. [Google Scholar] [CrossRef]
- Liu, L.; Sun, X. X-ray Excited Optical Luminescence and Its Applications. In Synchrotron Radiation Applications; World Scientific: Singapore, 2018; pp. 493–534. [Google Scholar]
- Liu, L.; Sham, T.K. Luminescence from TiO2 Nanotubes and Related Nanostructures Investigated Using Synchrotron X-Ray Absorption Near-Edge Structure and X-Ray Excited Optical Luminescence. In Titanium Dioxide: Material for a Sustainable Environment; IntechOpen: London, UK, 2018; p. 191. [Google Scholar]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nga, T.T.T.; Huang, Y.-C.; Chen, J.-L.; Chen, C.-L.; Lin, B.-H.; Yeh, P.-H.; Du, C.-H.; Chiou, J.-W.; Pong, W.-F.; Arul, K.T.; et al. Effect of Ag-Decorated BiVO4 on Photoelectrochemical Water Splitting: An X-ray Absorption Spectroscopic Investigation. Nanomaterials 2022, 12, 3659. https://doi.org/10.3390/nano12203659
Nga TTT, Huang Y-C, Chen J-L, Chen C-L, Lin B-H, Yeh P-H, Du C-H, Chiou J-W, Pong W-F, Arul KT, et al. Effect of Ag-Decorated BiVO4 on Photoelectrochemical Water Splitting: An X-ray Absorption Spectroscopic Investigation. Nanomaterials. 2022; 12(20):3659. https://doi.org/10.3390/nano12203659
Chicago/Turabian StyleNga, Ta Thi Thuy, Yu-Cheng Huang, Jeng-Lung Chen, Chi-Liang Chen, Bi-Hsuan Lin, Ping-Hung Yeh, Chao-Hung Du, Jau-Wern Chiou, Way-Faung Pong, K. Thanigai Arul, and et al. 2022. "Effect of Ag-Decorated BiVO4 on Photoelectrochemical Water Splitting: An X-ray Absorption Spectroscopic Investigation" Nanomaterials 12, no. 20: 3659. https://doi.org/10.3390/nano12203659





