Coating of Flexible PDMS Substrates through Matrix-Assisted Pulsed Laser Evaporation (MAPLE) with a New-Concept Biocompatible Graphenic Material
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Instrumental Methods
2.3. Preparation of the PDMS Substrates and GL Suspension for MAPLE Deposition
2.4. MAPLE Deposition
2.5. Biological Studies
2.5.1. In Vitro Toxicity Assessment
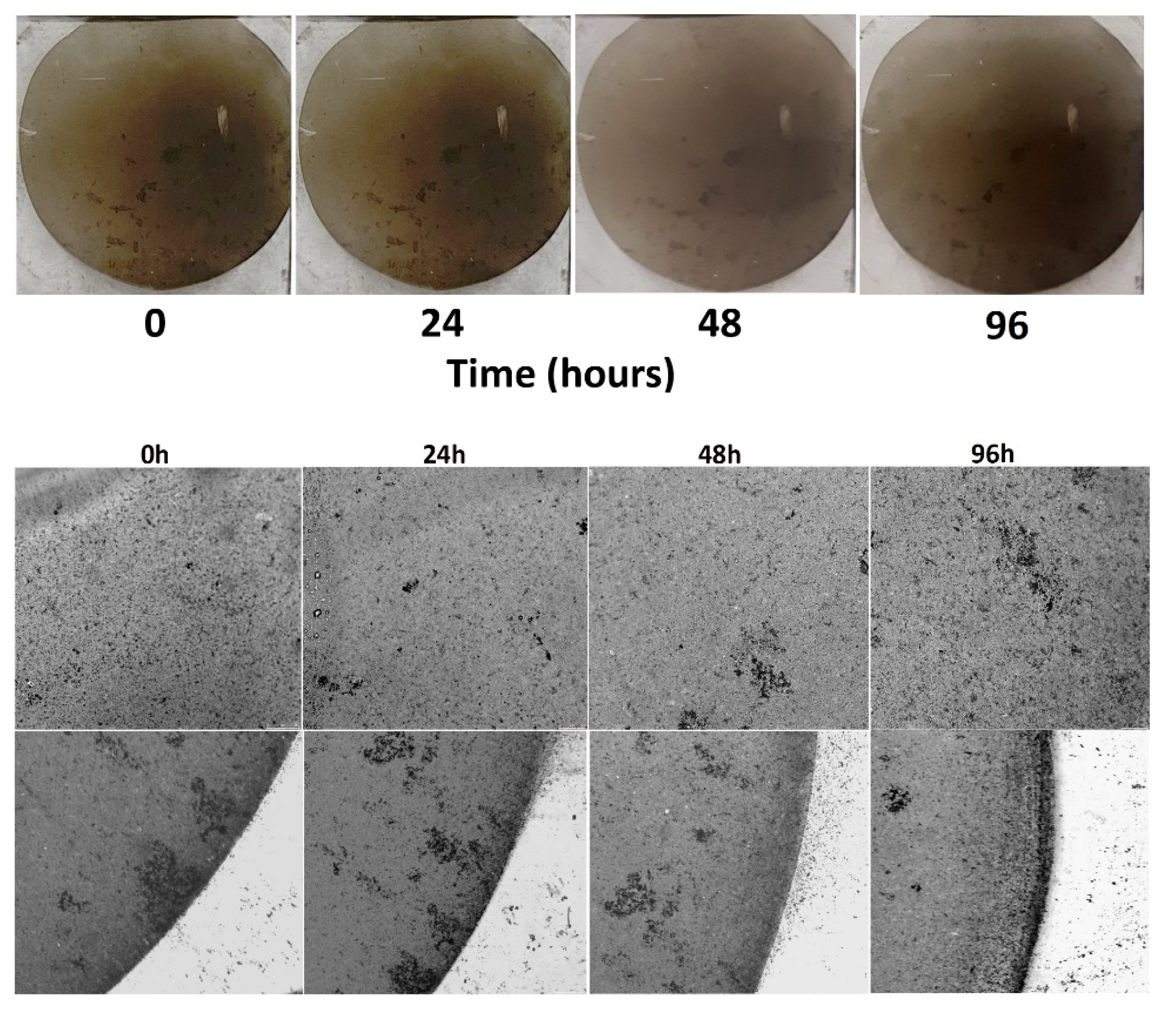
2.5.2. Light Microscopy
2.5.3. Statistical Analysis
3. Results and Discussion
3.1. Threshold Identification
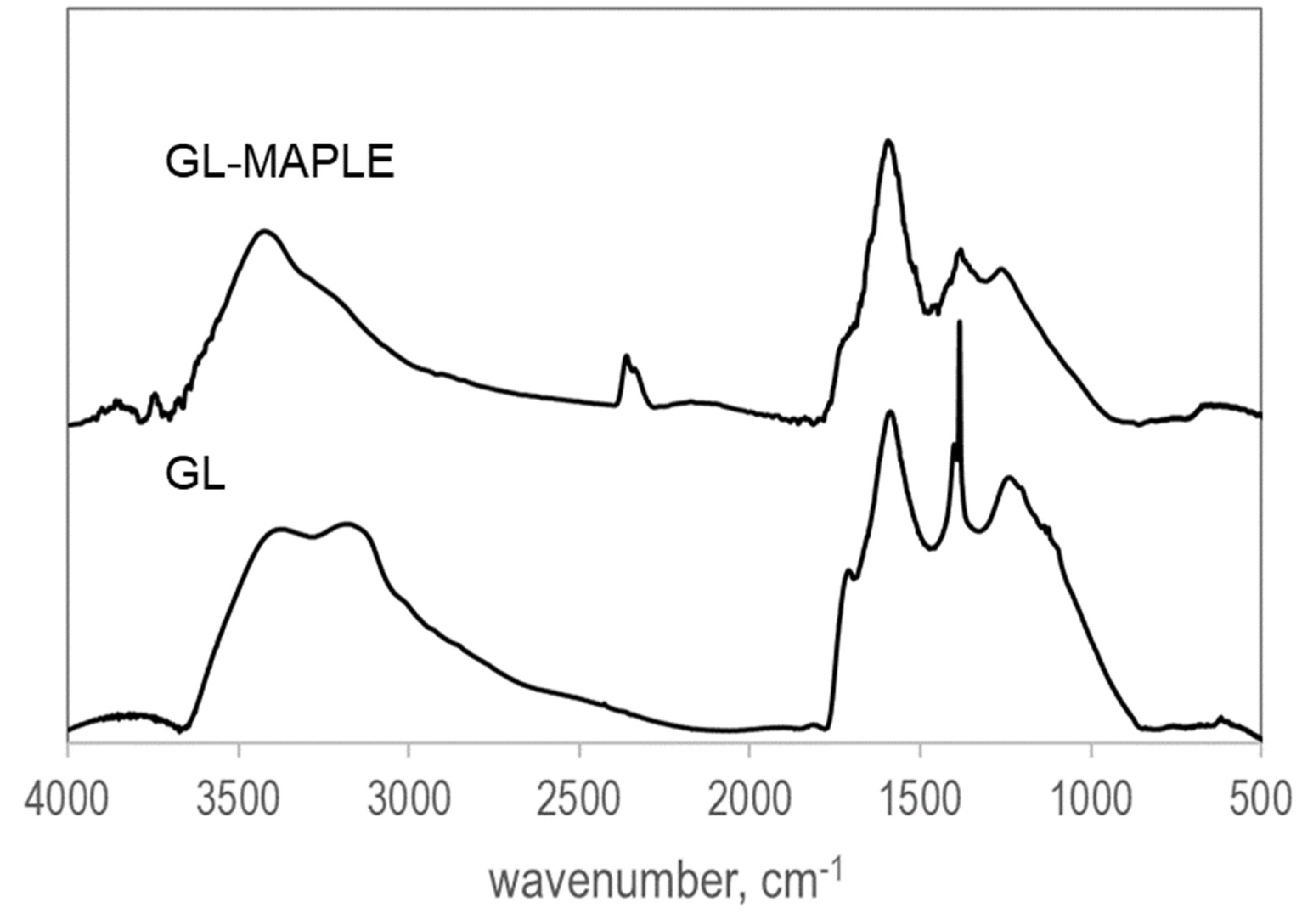
3.2. FTIR Analysis
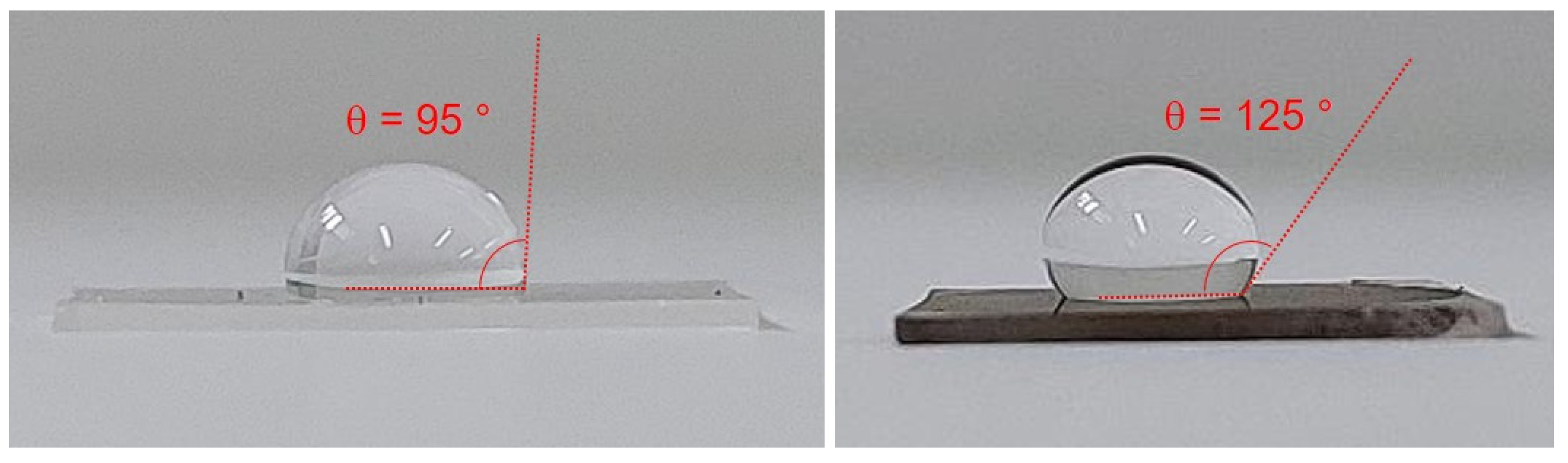
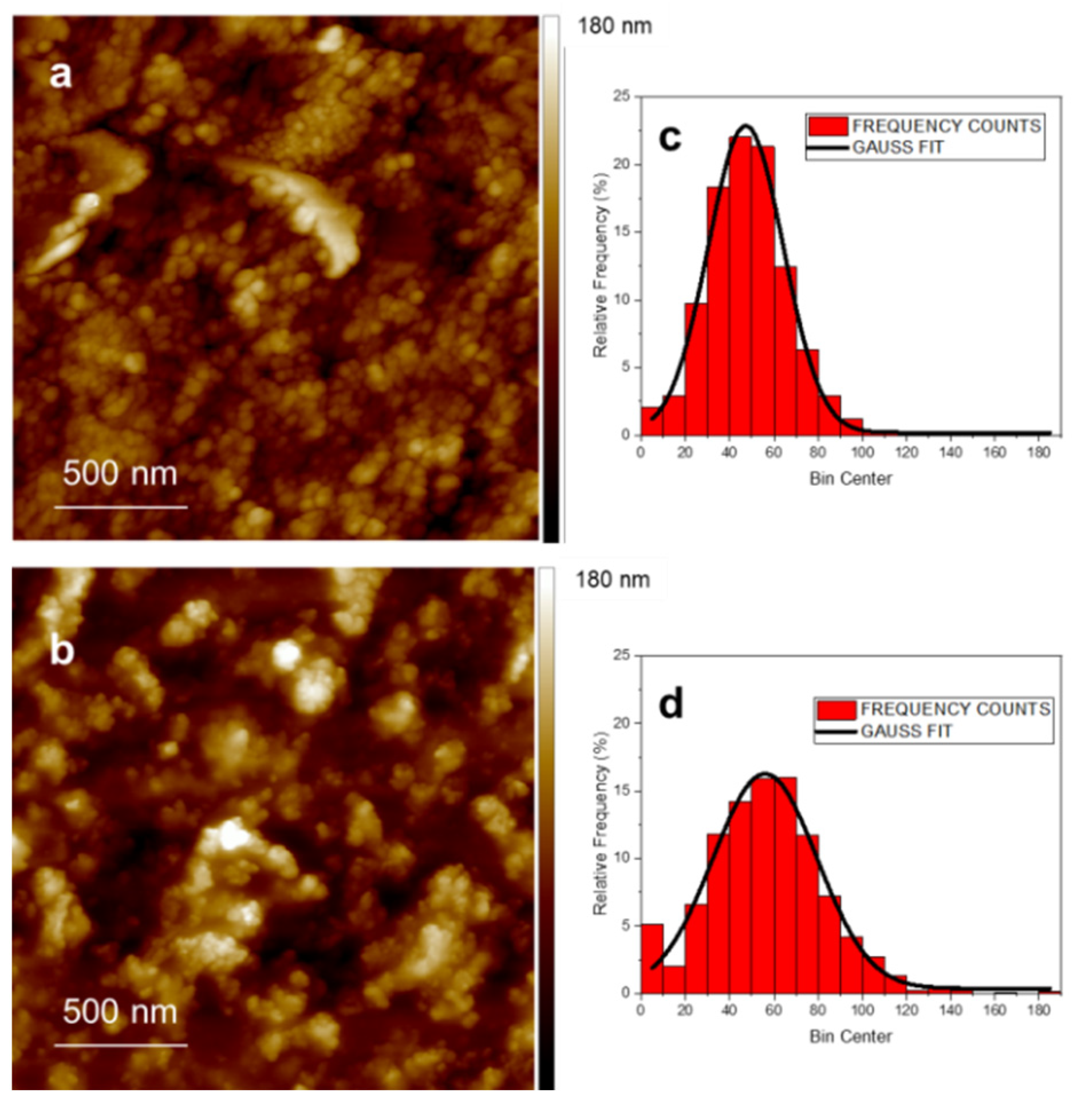
3.3. Surface Properties and AFM Imaging
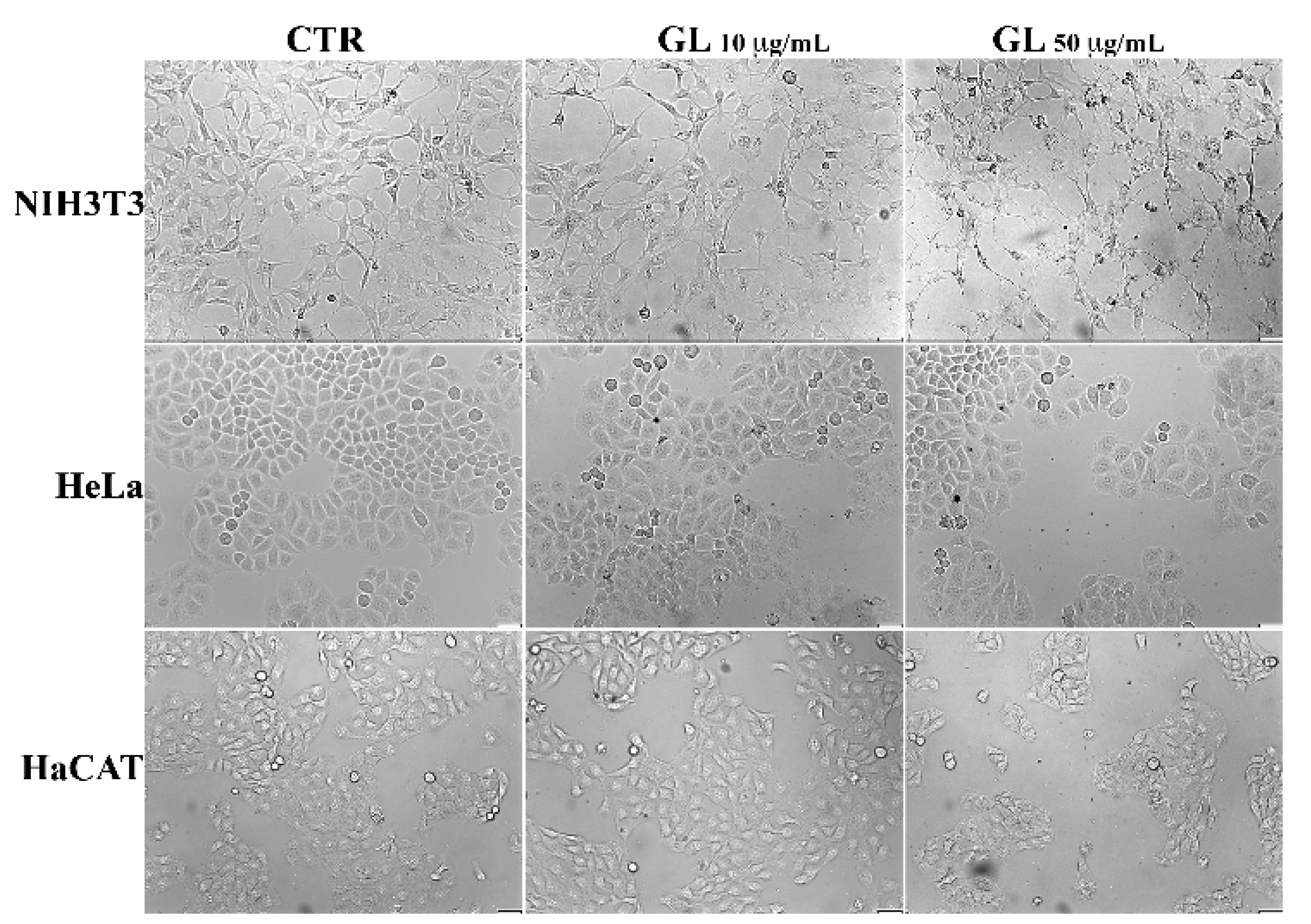
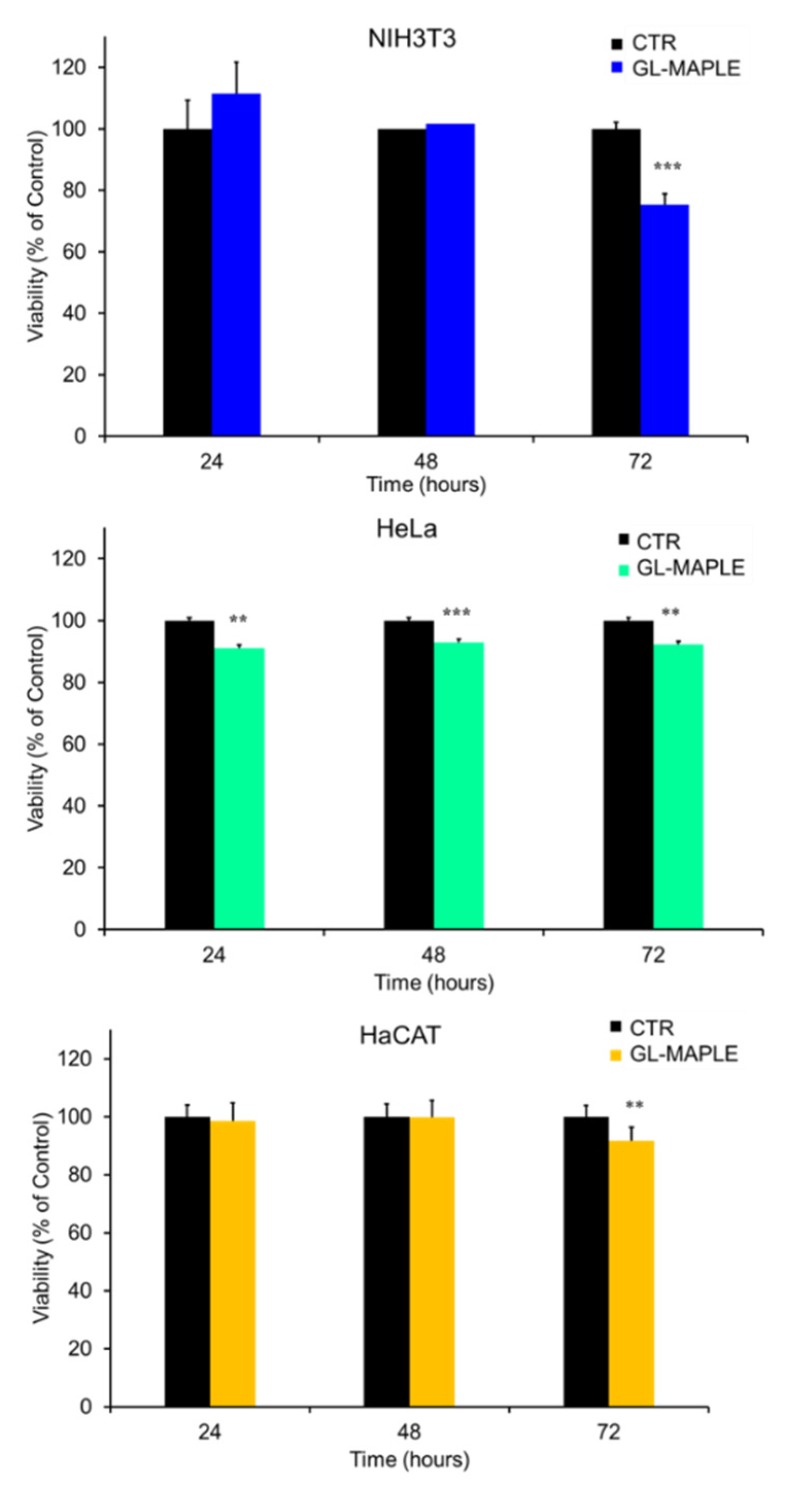
3.4. Biotoxicity Assessment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Leonidou, L.; Gogos, C.A. Catheter-related bloodstream infections: Catheter management according to pathogen. Int. J. Antimicrob. Agents 2010, 36, S26–S32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yousif, A.; Jamal, M.A.; Raad, I. Biofilm-based Healthcare-associated Infections. In Advances in Experimental Medicine and Biology; Donelli, G., Ed.; Springer: Cham, Germany, 2015; Volume 830, pp. 157–179. [Google Scholar]
- Ricardo, S.I.C.; Anjos, I.I.L.; Monge, N.; Faustino, C.M.C.; Ribeiro, I.A.C. A glance at antimicrobial strategies to prevent catheter-associated medical infections. ACS Infect. Dis. 2020, 6, 3109–3130. [Google Scholar] [CrossRef] [PubMed]
- Andersen, M.J.; Flores-Mireles, A.L. Urinary Catheter Coating Modifications: The Race against Catheter-Associated Infections. Coatings 2020, 10, 23. [Google Scholar] [CrossRef] [Green Version]
- Maan, A.M.C.; Hofman, A.H.; de Vos, W.M.; Kamperman, M. Recent Developments and Practical Feasibility of Polymer-Based Antifouling Coatings. Adv. Funct. Mater. 2020, 30, 2000936. [Google Scholar] [CrossRef]
- Singha, P.; Locklin, J.; Handa, H. A review of the recent advances in antimicrobial coatings for urinary catheters. Acta Biomater. 2017, 50, 20–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Z.-H.; Peng, S.-W.; Hsieh, S.-L.; Kirankumar, R.; Huang, P.-F.; Chang, T.-M.; Dwivedi, A.K.; Chen, N.-F.; Wu, H.-M.; Hsieh, S. Polydopamine Ultrathin Film Growth on Mica via In-Situ Polymerization of Dopamine with Applications for Silver-Based Antimicrobial Coatings. Materials 2021, 14, 671. [Google Scholar] [CrossRef]
- Hung, H.-S.; Yu, A.Y.-H.; Hsieh, S.-C.; Kung, M.-L.; Huang, H.-Y.; Fu, R.-H.; Yeh, C.-A.; Hsu, S.-h. Enhanced Biocompatibility and Differentiation Capacity of Mesenchymal Stem Cells on Poly(dimethylsiloxane) by Topographically Patterned Dopamine. ACS Appl. Mater. Interfaces 2020, 12, 44393–44406. [Google Scholar] [CrossRef]
- Pandit, S.; Gaska, K.; Kádár, R.; Mijakovic, I. Graphene-Based Antimicrobial Biomedical Surfaces. ChemPhysChem 2021, 22, 250–263. [Google Scholar] [CrossRef]
- Staneva, A.D.; Dimitrov, D.K.; Gospodinova, D.N.; Vladkova, T.G. Antibiofouling Activity of Graphene Materials and Graphene-Based Antimicrobial Coatings. Microorganisms 2021, 9, 1839. [Google Scholar] [CrossRef]
- Yager, T.; Lartsev, A.; Yakimova, R.; Lara-Avila, S.; Kubatkin, S. Wafer-scale homogeneity of transport properties in epitaxial graphene on SiC. Carbon 2015, 87, 409–414. [Google Scholar] [CrossRef]
- Rodriguez, J.A.; Liu, P.; Dvorak, J.; Jirsak, T. The interaction of oxygen with TiC (001): Photoemission and first-principles studies. J. Chem. Phys. 2004, 121, 465. [Google Scholar] [CrossRef] [PubMed]
- Grzybowski, B.A.; Bishop, K.J.M.; Campbell, C.J.; Fialkowski, M.; Smoukov, S.K. Micro- and nanotechnology via reaction–diffusion. Soft Matter 2005, 1, 114–128. [Google Scholar] [CrossRef]
- Hu, H.; Gopinadhan, M.; Osuji, C.O. Directed self-assembly of block copolymers: A tutorial review of strategies for enabling nanotechnology with soft matter. Soft Matter 2014, 10, 3867–3889. [Google Scholar] [CrossRef] [PubMed]
- Kunakova, G.; Surendran, A.P.; Montemurro, D.; Salvato, M.; Golubev, D.; Andzane, J.; Erts, D.; Bauch, T.; Lombardi, F.J. Topological insulator nanoribbon Josephson junctions: Evidence for size effects in transport properties. Appl. Phys. 2020, 128, 194304. [Google Scholar] [CrossRef]
- Mahashabde, S.; Otto, E.; Montemurro, D.; de Graaf, S.; Kubatkin, S.; Danilov, A. Fast Tunable High-Q-Factor Superconducting Microwave Resonators. Phys. Rev. Appl. 2020, 14, 044040. [Google Scholar] [CrossRef]
- Trabaldo, E.; Ruffieux, S.; Andersson, E.; Arpaia, R.; Montemurro, D.; Schneiderman, J.F.; Kalaboukhov, A.; Winkler, D.; Lombardi, F.; Bauch, T. Properties of grooved Dayem bridge based YBa2Cu3O7−δ superconducting quantum interference devices and magnetometers. Appl. Phys. Lett. 2020, 116, 132601. [Google Scholar] [CrossRef] [Green Version]
- Shen, K.M.; Davis, J.C.S. Cuprate high-Tc superconductors. Mater. Today 2008, 11, 14–21. [Google Scholar]
- Noimark, S.; Dunnill, C.W.; Wilson, M.; Parkin, I.P. The role of surfaces in catheter-associated infections. Chem. Soc. Rev. 2009, 38, 3435–3448. [Google Scholar] [CrossRef]
- Stiff-Roberts, A.D.; Ge, W. Organic/hybrid thin films deposited by matrix-assisted pulsed laser evaporation (MAPLE). Appl. Phys. Rev. 2017, 4, 041303. [Google Scholar] [CrossRef]
- Chrisey, D.B.; Piqué, A.; McGill, R.A.; Horwitz, J.S.; Ringeisen, B.R.; Bubb, D.M.; Wu, P.K. Laser deposition of polymer and biomaterial films. Chem. Rev. 2003, 103, 553–576. [Google Scholar] [CrossRef]
- Bloisi, F.; Pezzella, A.; Barra, M.; Alfè, M.; Chiarella, F.; Cassinese, A.; Vicari, L. Effect of substrate temperature on MAPLE deposition of synthetic eumelanin films. Appl. Phys. A 2011, 105, 619–627. [Google Scholar] [CrossRef]
- Bloisi, F.; Vicari, L.R.M.; Papa, R.; Califano, V.; Pedrazzani, R.; Bontempi, E.; Depero, L.E. Biomaterial thin film deposition and characterization by means of MAPLE technique. Mater. Sci. Eng. C 2007, 27, 1185–1190. [Google Scholar] [CrossRef]
- Califano, V.; Bloisi, F.; Perretta, G.; Aronne, A.; Ausanio, G.; Costantini, A.; Vicari, L. Frozen Microemulsions for MAPLE Immobilization of Lipase. Molecules 2017, 22, 2153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ausanio, G.; Califano, V.; Costantini, A.; Perretta, G.; Aronne, A.; Pepe, G.P.; Sannino, F.; Vicari, L. Matrix-assisted pulsed laser evaporation of β-glucosidase from a dopa/quinone target. Enzyme Microb. Technol. 2020, 132, 109414. [Google Scholar] [CrossRef] [PubMed]
- Ausanio, G.; Califano, V.; Costantini, A.; Pepe, G.P.; Vicari, C.; Vicari, L.R.M. Laser deposition from a lava stone cup. Opt. Laser Technol. 2020, 131, 106432. [Google Scholar] [CrossRef]
- Marturano, V.; Abate, F.; Ambrogi, V.; Califano, V.; Cerruti, P.; Pepe, G.P.; Vicari, L.R.M.; Ausanio, G. Smart coatings prepared via maple deposition of polymer nanocapsules for light-induced release. Molecules 2021, 26, 2736. [Google Scholar] [CrossRef]
- Socol, M.; Preda, N.; Costas, A.; Breazu, C.; Stanculescu, A.; Rasog, O.; Popescu-Pelin, G.; Mihailescu, A.; Socol, G. Hybrid organic-inorganic thin films based on zinc phthalocyanine and zinc oxide deposited by MAPLE. Appl. Surf. Sci. 2020, 503, 144317. [Google Scholar] [CrossRef]
- Dinca, V.; Viespe, C.; Brajnicov, S.; Constantinoiu, I.; Moldovan, A.; Bonciu, A.; Toader, C.N.; Ginghina, R.E.; Grigoriu, N.; Dinescu, M.; et al. MAPLE Assembled Acetylcholinesterase–Polyethylenimine Hybrid and Multilayered Interfaces for Toxic Gases Detection. Sensors 2018, 18, 4265. [Google Scholar] [CrossRef] [Green Version]
- Queralto, A.; Perez del Pino, A.; Logofatu, C.; Calota, A.; Amade, R.; Alshaikh, I.; Bertran, E.; Urzica, I.; Gyorgy, E. MAPLE synthesis of reduced graphene oxide/silver nanocomposite electrodes: Influence of target composition and gas ambience. J. Alloys Compd. 2017, 726, 1003. [Google Scholar] [CrossRef] [Green Version]
- Perez del Pino, A.; Gyorgy, E.; Logofatu, C.; Duta, A. Study of the deposition of graphene oxide by matrix-assisted pulsed laser evaporation. J. Phys. D Appl. Phys. 2013, 46, 505309. [Google Scholar] [CrossRef]
- Perez del Pino, A.; Gyogy, E.; Cabana, L.; Ballesteros, B.; Tobias, G. Deposition of functionalized single wall carbon nanotubes through matrix assisted pulsed laser evaporation. Carbon 2012, 50, 4450–4458. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Bonaccorso, F.; Fal’Ko, V.; Novoselov, K.S.; Roche, S.; Bøggild, P.; Borini, S.; Koppens, F.H.L.; Palermo, V.; Pugno, N.; et al. Science and technology roadmap for graphene, related two-dimensional crystals, and hybrid systems. Nanoscale 2015, 7, 4598–4810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tiwari, S.K.; Sahoo, S.; Wang, N.; Huczko, A. Graphene research and their outputs: Status and prospect. J. Sci. Adv. Mater Dev. 2020, 5, 10–29. [Google Scholar] [CrossRef]
- Bonavolontà, C.; Camerlingo, C.; Carotenuto, G.; De Nicola, S.; Longo, A.; Meola, C.; Boccardi, S.; Palomba, M.; Pepe, G.P.; Valentino, M. Characterization of piezoresistive properties of graphene-supported polymer coating for strain sensor applications. Sens. Actuators A Phys. 2016, 252, 26–32. [Google Scholar] [CrossRef]
- Gomes, R.N.; Borges, I.; Pereira, A.T.; Maia, A.F.; Pestana, M.; Magalhães, F.D.; Pinto, A.M.; Gonçalves, I.C. Antimicrobial graphene nanoplatelets coatings for silicone catheters. Carbon 2018, 139, 635–647. [Google Scholar] [CrossRef]
- Backes, C.; Abdelkader, A.M.; Alonso, C.; Andrieux-Ledier, A.; Arenal, R.; Azpeitia, J.; Balakrishnan, N.; Banszerus, L.; Barjon, J.; Bartali, R.; et al. Production and processing of graphene and related materials. 2D Mater. 2020, 7, 022001. [Google Scholar] [CrossRef]
- Jaworski, S.; Biniecka, P.; Bugajska, Ż.; Daniluk, K.; Dyjak, S.; Strojny, B.; Kutwin, M.; Wierzbicki, M.; Grodzik, M.; Chwalibog, A. Analysis of the cytotoxicity of hierarchical nanoporous graphenic carbon against human glioblastoma grade IV cells. Int. J. Nanomed. 2017, 12, 3839–3849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bugajska, Ż.; Jaworski, S.; Daniluk, K.; Biniecka, P.; Sosnowska, M. Interaction of hierarchical nanoporous carbons (HNCs) with chicken embryo red blood cells (RBC). Ann. Wars. Univ. Life Sci.-SGGW Anim. Sci. 2017, 56, 37–42. [Google Scholar] [CrossRef]
- d’Amora, M.; Alfe, M.; Gargiulo, V.; Giordani, S. Graphene-like layers from carbon black: In vivo toxicity assessment. Nanomaterials 2020, 10, 1472. [Google Scholar] [CrossRef]
- Hotowy, A.; Grodzik, M.; Zielińska-Górska, M.; Chojnacka, N.; Kurantowicz, N.; Dyjak, S.; Strojny, B.; Kutwin, M.; Chwalibog, A.; Sawosz, E. Silver and Graphenic Carbon Nanostructures Differentially Influence the Morphology and Viability of Cardiac Progenitor Cells. Materials 2020, 13, 2159. [Google Scholar] [CrossRef]
- Saha, S.; Lakhe, P.; Mason, M.J.; Coleman, B.J.; Arole, K.; Zhao, X.; Yakovlev, S.; Uppili, S.; Green, M.J.; Hule, R.A. Sustainable production of graphene from petroleum coke using electrochemical exfoliation. 2D Mater Appl. 2021, 5, 75. [Google Scholar] [CrossRef]
- Sierra, U.; Álvarez, P.; Blanco, C.; Granda, M.; Santamaria, R.; Menendez, R. New alternatives to graphite for producing graphene materials. Carbon 2015, 93, 812–818. [Google Scholar] [CrossRef]
- Sierra, U.; Álvarez, P.; Blanco, C.; Granda, M.; Santamaría, R.; Menéndez, R. Cokes of different origin as precursors of graphene oxide. Fuel 2016, 166, 400–403. [Google Scholar] [CrossRef]
- Sierra, U.; Mercado, A.; Cuara, E.; Barriga-Castro, E.D.; Cortés, A.; Gallardo-Vega, C.; Fernández, S. Coke-derived few layer graphene-like materials by mild planetary milling exfoliation. Fuel 2020, 262, 116455. [Google Scholar] [CrossRef]
- Vieira, O.; Ribeiro, R.S.; Diaz de Tuesta, J.L.; Gomes, H.T.; Silva, A.M.T. A systematic literature review on the conversion of plastic wastes into valuable 2D graphene-based materials. Chem. Eng. J. 2022, 428, 131399. [Google Scholar] [CrossRef]
- Kong, X.; Zhu, Y.; Lei, H.; Wang, C.; Zhao, Y.; Huo, E.; Lin, X.; Zhang, Q.; Qian, M.; Mateo, W.; et al. Synthesis of graphene-like carbon from biomass pyrolysis and its applications. Chem. Eng. J. 2020, 399, 125808. [Google Scholar] [CrossRef]
- Raghavan, N.; Thangavel, S.; Venugopal, G. A short review on preparation of graphene from waste and bioprecursors. Appl. Mater. Today 2017, 7, 246–254. [Google Scholar] [CrossRef]
- Poorna, A.R.; Saravanathamizhan, R.; Balasubramanian, N. Graphene and graphene-like structure frombiomass for Electrochemical Energy Storage application-A Review. Electrochem. Sci. Adv. 2021, 1, e2000028. [Google Scholar] [CrossRef]
- Safian, M.T.U.; Haron, U.S.; Ibrahim, M.M. A review on bio-based graphene derived from biomass wastes. BioResources 2020, 15, 9756. [Google Scholar] [CrossRef]
- Alfè, M.; Gargiulo, V.; Di Capua, R.; Chiarella, F.; Rouzaud, J.N.; Vergara, A.; Ciajolo, A. Wet Chemical Method for Making Graphene-like Films from Carbon Black. ACS Appl. Mater. Interfaces 2012, 4, 4491–4498. [Google Scholar] [CrossRef] [Green Version]
- Alfè, M.; Gargiulo, V.; Di Capua, R. Tuning the surface morphology of self-assembled graphene-like thin films through pH variation. Appl. Surf. Sci. 2015, 353, 628–635. [Google Scholar] [CrossRef]
- Gargiulo, V.; Alfè, M.; Di Capua, R.; Togna, A.R.; Cammisotto, V.; Fiorito, S.; Musto, A.; Navarra, A.; Parisi, A.S.; Pezzella, A. Supplementing π-systems: Eumelanin and graphene-like integration towards highly conductive materials for the mammalian cell culture bio-interface. J. Mater. Chem. B 2015, 3, 5070–5079. [Google Scholar] [CrossRef] [PubMed]
- Gargiulo, V.; Alfano, B.; Di Capua, R.; Alfé, M.; Vorokhta, M.; Polichetti, T.; Massera, E.; Miglietta, M.L.; Schiattarella, C.; Di Francia, G. Graphene-like layers as promising chemiresistive sensing material for detection of alcohols at low concentration. J. Appl. Phys. 2018, 123, 024503. [Google Scholar] [CrossRef]
- Olivi, M.; Alfè, M.; Gargiulo, V.; Valle, F.; Mura, F.; Di Giosia, M.; Rapino, S.; Palleschi, C.; Uccelletti, D.; Fiorito, S. Antimicrobial properties of graphene-like nanoparticles: Coating effect on Staphylococcus aureus. J. Nano. Res. 2016, 18, 358. [Google Scholar] [CrossRef]
- Di Capua, R.; Gargiulo, V.; Alfè, M.; De Luca, G.; Skála, T.; Mali, G.; Pezzella, A. Eumelanin Graphene-Like Integration: The Impact on Physical Properties and Electrical Conductivity. Front. Chem. 2019, 7, 121. [Google Scholar] [CrossRef] [Green Version]
- Sohaebuddin, S.K.; Thevenot, P.T.; Baker, D.; Eaton, J.W.; Tang, L. Nanomaterial cytotoxicity is composition, size, and cell type dependent. Part. Fibre Toxicol. 2010, 7, 22. [Google Scholar] [CrossRef] [Green Version]
- Steiner, M.A.; Fitz-Gerald, J.M. Dynamics of the inverse MAPLE nanoparticle deposition process. Appl. Phys. A 2015, 119, 629–638. [Google Scholar] [CrossRef]
- Arnal, C.; Alfè, M.; Gargiulo, V.; Ciajolo, A.; Alzueta, M.U.; Millera, A.; Bilbao, R. Cleaner Combustion: Green Energy and Technology; Battin-Leclerc, F., Simmie, J., Blurock, E., Eds.; Springer: London, UK, 2003; pp. 333–362. [Google Scholar]
- García Lebière, P.; György, E.; Logofatu, C.; Naumenko, D.; Amenitsch, H.; Rajak, P.; Ciancio, R.; Pérez del Pino, Á. Unravelling the origin of the capacitance in nanostructured nitrogen-doped carbon-NiO hybrid electrodes deposited with laser. Ceram. Int. 2022, 48, 15877–15888. [Google Scholar] [CrossRef]
- Gies, V.; Zou, S. Systematic toxicity investigation of graphene oxide: Evaluation of assay selection, cell type, exposure period and flake size. Toxicol. Res. 2018, 7, 93–101. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alfe, M.; Minopoli, G.; Tartaglia, M.; Gargiulo, V.; Caruso, U.; Pepe, G.P.; Ausanio, G. Coating of Flexible PDMS Substrates through Matrix-Assisted Pulsed Laser Evaporation (MAPLE) with a New-Concept Biocompatible Graphenic Material. Nanomaterials 2022, 12, 3663. https://doi.org/10.3390/nano12203663
Alfe M, Minopoli G, Tartaglia M, Gargiulo V, Caruso U, Pepe GP, Ausanio G. Coating of Flexible PDMS Substrates through Matrix-Assisted Pulsed Laser Evaporation (MAPLE) with a New-Concept Biocompatible Graphenic Material. Nanomaterials. 2022; 12(20):3663. https://doi.org/10.3390/nano12203663
Chicago/Turabian StyleAlfe, Michela, Giuseppina Minopoli, Massimiliano Tartaglia, Valentina Gargiulo, Ugo Caruso, Giovanni Piero Pepe, and Giovanni Ausanio. 2022. "Coating of Flexible PDMS Substrates through Matrix-Assisted Pulsed Laser Evaporation (MAPLE) with a New-Concept Biocompatible Graphenic Material" Nanomaterials 12, no. 20: 3663. https://doi.org/10.3390/nano12203663







