Gold-Nanoparticle Hybrid Nanostructures for Multimodal Cancer Therapy
Abstract
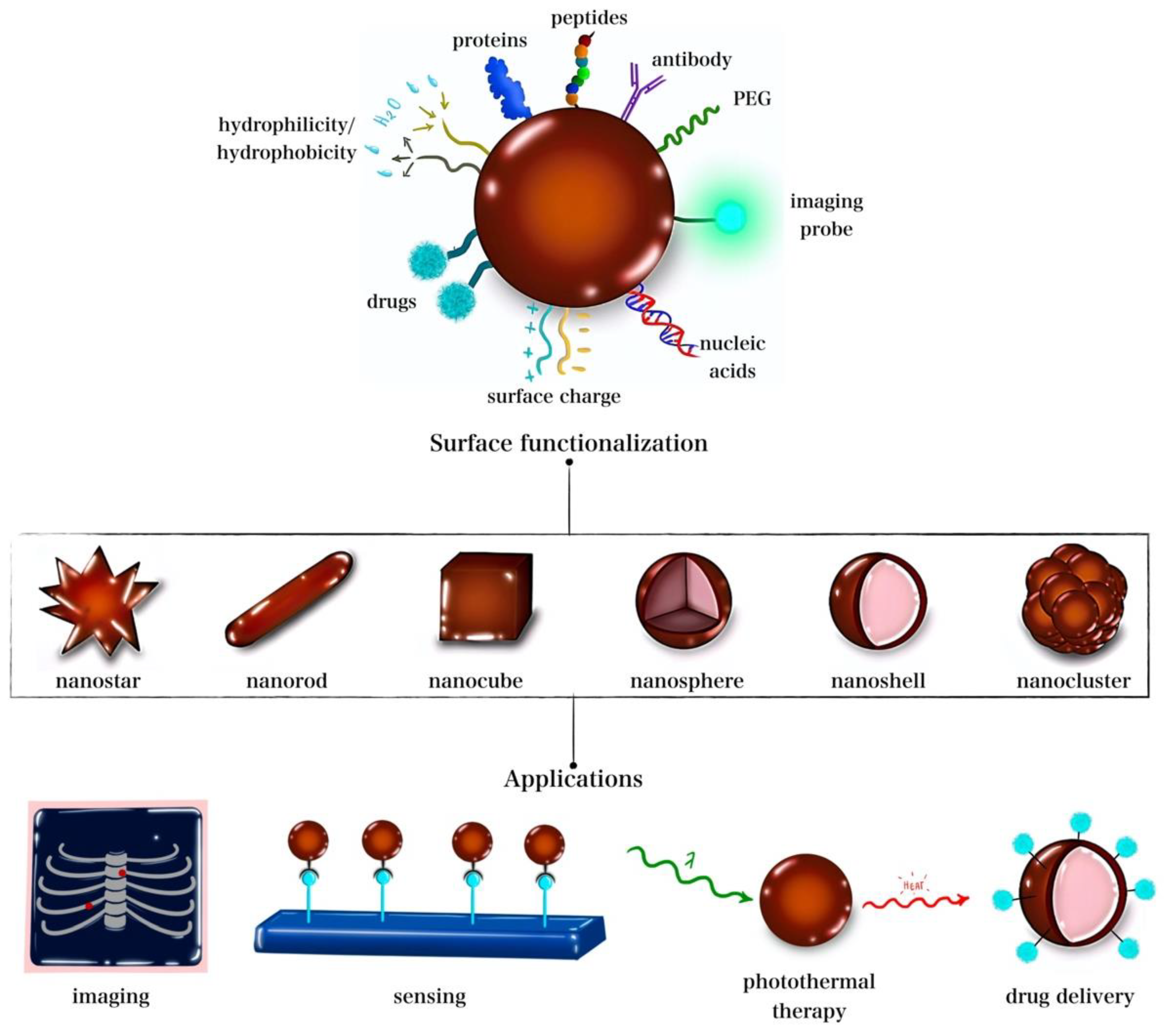
:1. Introduction
2. Smart Drug Delivery Nanocarriers
- (1)
- Metal-based nanocarriers are among the emerging materials for biomedicine and drug delivery applications [54]. GNPs and iron oxide nanoparticles (IONPs) have been increasingly studied for drug delivery purposes, as reviewed by Hossen et al. [53]. GNPs and IONPs share the common attractive feature of heat generation that can trigger drug release and/or kill cells via thermal ablation. Both nanoparticles have the benefits of easy synthesis and surface functionalization, [53] and serve as contrast agents to enhance imaging and achieve image-guided therapy [55,56,57]. Additionally, SPIONs exhibit the advantageous property of magnetic targeting via an external magnetic field for spatial targeting [58]. Venditti et al. reported that GNPs are used to improve the bioavailability of drugs [59]. Yet, the practical application of such metal-based nanocarriers can be limited by their potential toxicity [60];
- (2)
- Polymer-based smart nanocarriers include hydrogels and dendrimers. Dendrimers are large, highly branched polymers capable of loading drugs via entrapment in spaces within the network or by attaching to branching points (via hydrogen bonding or to surface groups via electrostatic interactions) [61,62]. Hydrogels, on the other hand, are composed of hydrophilic crosslinked polymer chains capable of cargo entrapment and delivery [63,64,65]. Dendrimers and hydrogels have been reported for the efficient delivery of genes, drugs, and proteins [66,67,68,69,70,71] and for stimulus-responsive release under various triggers, including temperature, pH, and redox conditions [72,73]. However, dendrimers suffer from their complicated and costly synthesis procedures, and both dendrimers and hydrogels are restricted by their ability to host solely hydrophilic drugs [60];
- (3)
- Lipid-based nanocarriers include liposomes and micelles. Liposomes, membrane-like self-assembled lipid bilayers, are utilized for the delivery of hydrophobic/hydrophilic drugs, genes, and proteins while possessing high biocompatibility and stimulus responsiveness (e.g., ultrasound and temperature responsiveness) [74]. Micelles are organic nanocarriers similar in structure to liposomes but made up of a single layer. Unlike liposomes, micelles can also be composed of amphiphilic polymers [75,76]. Micelles are used to transport hydrophobic drugs, genes, and proteins and exhibit stimulus responsiveness making them “smart” nanocarriers [77,78]. Liposomes are limited by their poor stability and possibility of triggering an immune response, while micelles are limited by their occasional cytotoxicity and degradability [60]. Several triggering mechanisms can be used to stimulate the release of encapsulated cargo from the nanocarriers [79]. The different types of nanocarriers and possible release trigger mechanisms are presented in Figure 2.
3. Organic GNP Nanohybrid Chemotherapeutic Platforms
3.1. Multimodal Liposome–GNP Nanohybrids
| Triggering Stimuli | Loaded Agents and Surface Modifications | Targeted Cancer Type | Release Mechanisms | Toxicity | References |
|---|---|---|---|---|---|
| NIR-generated heat | DOX PEG | Lung cancer | DOX release via heat-induced liposomal phase transition Hyperthermia and DOX synergy | PTT/chemotherapy toxicity in vitro | [34] |
| NIR-generated heat Low pH | DOX | Cervical cancer | DOX release via heat-induced liposomal phase transition and low-pH-induced membrane instability Hyperthermia and DOX synergy | PTT/chemotherapy toxicity in vitro and in vivo | [38] |
| NIR-generated heat | ZnPc | Breast cancer | Heat-triggered ZnPc release PDT and PTT synergy | PTT/PDT toxicity in vitro and in vivo | [92] |
| NIR-generated heat | DOX | Breast cancer | DOX release via heat-induced liposomal phase transition Hyperthermia and DOX synergy | PTT/chemotherapy toxicity in vitro and in vivo | [83] |
| NIR-generated heat | DOX | Breast cancer | DOX release via heat-induced liposomal phase transition Hyperthermia and DOX synergy | PTT/chemotherapy toxicity in vitro | [80] |
| NIR-generated heat | DOX Chitosan | Melanoma | DOX release via heat-induced liposomal phase transition | Chemotherapy-induced toxicity in vitro and in vivo | [88] |
| NIR-generated heat Low pH | Resveratrol Chitosan | Cervical cancer | Resveratrol release via pH-induced chitosan amine group protonation and heat-induced liposomal phase transition Hyperthermia and resveratrol synergy | PTT/resveratrol toxicity in vitro | [39] |
| NIR-generated heat Low pH | Oleanolic acid Chitosan | Osteosarcoma | Dual pH- and temperature-stimulated oleanolic acid release Hyperthermia and oleanolic acid synergy | PTT/oleanolic acid toxicity in vitro and in vivo | [81] |
| NIR-generated heat | DOX | Breast cancer | DOX release via heat-induced liposomal phase transition Hyperthermia and DOX synergy | PTT/chemotherapy toxicity in vitro and in vivo | [82] |
| NIR-generated heat | DOX | Liver cancer Breast cancer | DOX release via heat-induced liposomal phase transition Hyperthermia and DOX synergy | PTT/chemotherapy toxicity in vitro | [99,103] |
| NIR-generated heat | Betulinic acid | Cervical cancer Osteosarcoma | Betulinic acid release via heat-induced liposomal phase transition Hyperthermia and betulinic acid synergy | PTT/betulinic acid toxicity in vitro and in vivo | [98,100] |
| NIR-generated heat | Curcumin | Melanoma | Curcumin release via heat-induced liposomal phase transition Hyperthermia and curcumin synergy | PTT/chemotherapy toxicity in vitro | [97] |
| NIR-generated heat | DOX PEG Low-density lipoprotein receptor (LDLR)-binding peptide | Prostate cancer | LDLR-binding-peptide-mediated cellular uptake and tumor accumulation. DOX release via heat-induced liposomal phase transition Hyperthermia and DOX synergy | PTT/chemotherapy toxicity in vitro and in vivo | [101] |
| NIR-generated heat | DOX Folic acid | Breast cancer | Folic acid-mediated cellular uptake and tumor accumulation DOX release via heat-induced liposomal phase transition Hyperthermia and DOX synergy | PTT/chemotherapy toxicity in vitro and in vivo | [102] |
| NIR-generated heat | HER2 Cyclopamine | Breast cancer | Deeper tissue penetration via cyclopamine stroma destruction HER2-mediated tumor targeting Cyclopamine release via heat-induced liposomal phase transition Hyperthermia and cyclopamine synergy | PTT/chemotherapy toxicity in vitro and in vivo | [104] |
| NIR-generated heat | DOX ABC Folic acid | Breast cancer Sarcoma (S180) ascite cells were used for in vivo studies | DOX release via transient cavitation caused by carbon dioxide generated upon hyperthermia-induced ABC decomposition Improved tumor cell targeting via folic acid-mediated endocytosis Computed tomography contrast agent | Chemotherapy-induced tumor inhibition in vitro and in vivo | [105] |
| NIR-generated heat | PEG | Breast cancer | Tumor eradication via NIR-generated PTT | PTT-induced tumor growth inhibition in vitro and in vivo | [117] |
| NIR-generated heat | DOX | Breast cancer | DOX release via heat-induced liposomal phase transition | Chemotherapy-induced tumor inhibition in vitro and in vivo | [118] |
| NIR-generated heat | Calcein | Breast cancer | Hyperthermia-triggered calcein release Tumor eradication via NIR-generated PTT | PTT-induced cell death in vitro | [106] |
| NIR-generated heat | DOX Rose Bengal | Colon cancer Breast cancer | GNP-induced generation of singlet oxygen species (PDT) and DOX release PDT and DOX synergy | PDT and DOX-induced toxicity in vitro | [96] |
| NIR-generated heat | siRNA PBA RGD | Pancreatic cancer | PBA/GNP NIR-triggered siRNA release and PTT Gene therapy–PTT synergy PAI, PTI, and CT imaging contrast agents | K-Ras knockdown and PTT-induced toxicity in vitro and in vivo | [122] |
| NIR | DOX | Breast cancer | NIR-triggered DOX release | DOX-induced toxicity in vitro | [130] |
| Low pH Heat | DOX | Ovarian cancer Breast cancer | Low-pH- and hyperthermia-triggered DOX release | DOX-induced toxicity in vitro (to a lower extent than free DOX) | [131] |
3.2. Multimodal Polymer–GNP Nanohybrids
3.2.1. Multimodal Hydrogel–GNP Nanohybrids
3.2.2. Multimodal Micelle–GNP Nanohybrids
| Hydrogel–GNP Hybrids | ||||||
|---|---|---|---|---|---|---|
| Polymer | Triggering Stimuli | Targeted Cancer | Loaded Agents and Surface Modifications | Release Mechanisms | In Vitro/In Vivo Toxicity | Reference |
| Alginate | NIR-generated heat | Colon cancer | Cisplatin | Hyperthermia-triggered cisplatin release Chemotherapy, radiotherapy, and PTT synergy | Chemotherapy/radiotherapy/PTT synergy in vivo | [157] |
| Alginate | Fe3O4 | Colon cancer | DOX Iron oxide nanoparticles | Magnetically guided chemotherapy/PTT Iron oxide-enhanced MRI | Chemotherapy/PTT synergy in vivo | [159] |
| Chitosan | NIR-generated heat | Melanoma | Liposomal DOX | Hyperthermia-triggered DOX release | Chemotherapy toxicity in vivo | [88] |
| Chitosan PNIPAM | Low pH NIR-generated heat | Breast cancer | Curcumin | Hyperthermia- and low-pH-triggered curcumin release Chemotherapy/PTT synergy | Chemotherapy/PTT synergy in vitro | [35] |
| Chitosan | Low pH NIR-generated heat | Breast cancer | DOX Porous silica nanoparticles | Hyperthermia- and low-pH-triggered curcumin release Chemotherapy/PTT synergy | Chemotherapy/PTT synergy in vivo | [160] |
| PNIPAAm Carboxymethyl chitosan | NIR-generated heat | DOX Iron oxide nanoparticles | Heat-triggered DOX release | Chemotherapy toxicity in vitro | [36] | |
| PNIPAAm, HEMA, maleic acid, N,N’-bis(acryloyl)cystamine | Non-NIR-generated heat Redox Low pH | DOX 6-marcaptopurine PEG | pH-, redox-, and temperature-triggered drug release | Chemotherapy toxicity in vitro | [147] | |
| DNA | NIR-generated heat | Melanoma | DOX | Hyperthermia-triggered DOX release Chemotherapy/PTT synergy | Chemotherapy/PTT synergy in vitro and in vivo | [165] |
| Hyaluronic acid | Hyaluronidase (enzyme) NIR-generated heat | Stomach cancer | DOX Triphenylphosphine | HA- and triphenylphosphine-mediated targeting Hyaluronidase and hyperthermia-triggered DOX release Chemotherapy/PTT synergy | Chemotherapy/PTT synergy in vitro and in vivo | [166] |
| Hyaluronic acid | NIR-generated heat GSH | Breast cancer | DOX | GSH- and hyperthermia-triggered DOX release HA-mediated targeting Drug resistance reversal in vitro, possibly due to enhanced hyperthermia-induced membrane fluidity | Chemotherapy/PTT synergy in vitro | [173] |
| Micellar GNP hybrids | ||||||
| PLL | NIR-generated heat | Breast cancer | Paclitaxel | Hyperthermia-triggered paclitaxel release Chemotherapy/PTT synergy | Chemotherapy/PTT synergy in vitro and in vivo | [175] |
| PEG-b-PHEA | GSH | Cervical cancer | GW627368X Folic acid Lipoic acid | Folic and lipoic acid-mediated targeting Redox (GSH)-triggered GW627368X release Chemotherapy/PTT synergy | Chemotherapy/PTT synergy in vitro and in vivo | [180] |
| b-cyclodextrin-{poly(lactide)-poly(2-(d imethylamino) ethyl methacrylate)-poly[oligo(2-ethyl-2-oxazoline)methacrylate]}21 [b-CD-(PLAPDMAEMA-PEtOxMA)21] | Low pH | Liver cancer | DOX | Low-pH-triggered DOX release | Chemotherapy toxicity in vitro | [177] |
| poly(ethylene glycol)-b-poly(ε-caprolactone) (PEG- PCL-LA) | NIR-generated heat | Breast cancer | DOX | Hyperthermia-triggered DOX release Resistance reversal | Chemotherapy toxicity in vitro | [176] |
| poly(L-aspartate)-b-poly(ethylene glycol) copolymer | Low pH | Breast cancer | DOX Folic acid | FA-mediated targeting Low-pH-triggered DOX release | Chemotherapy toxicity in vitro | [178] |
| PEG-PAsp(DIP)-b-PAsp(MEA) | Low pH GSH NIR | Ovarian cancer | DOX | NIR-, low-pH-, and GSH-triggered DOX release PTT/chemotherapy synergy | Chemotherapy/PTT synergy in vitro and in vivo | [179] |
| poly(ethylene glycol)-block-poly(propylene glycol)-block-poly(ethylene glycol) | Low pH | Breast cancer | ZD6474 (dual tyrosine kinase inhibitor) | Low-pH-triggered ZD6474 release | Chemotherapy toxicity in vitro | [184] |
| Other polymeric GNP hybrids | ||||||
| DNA | Low pH DNase II (nuclease) | Breast cancer | HER2 affibody 5-fluorouracil DOX | Low-pH- and DNase II-triggered drug release HER2-affibody-mediated targeted and internalization | DOX/5-fluorouracil synergy in vitro | [136] |
| PLGA | NIR-generated heat | Cervical cancer | DOX | Hyperthermia-improved DOX release Chemotherapy/PTT synergy | Chemotherapy/PTT synergy in vitro | [138] |
| Polyrotaxanes | NIR-generated heat | _______ | DOX Cisplatin | Hyperthermia-triggered drug release | Chemotherapy toxicity in vitro | [33] |
4. Challenges and Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sibuyi, N.R.S.; Moabelo, K.L.; Fadaka, A.O.; Meyer, S.; Onani, M.O.; Madiehe, A.M.; Meyer, M. Multifunctional Gold Nanoparticles for Improved Diagnostic and Therapeutic Applications: A Review. Nanoscale Res. Lett. 2021, 16, 174. [Google Scholar] [CrossRef] [PubMed]
- Kong, F.-Y.; Zhang, J.-W.; Li, R.-F.; Wang, Z.-X.; Wang, W.-J.; Wang, W. Unique Roles of Gold Nanoparticles in Drug Delivery, Targeting and Imaging Applications. Molecules 2017, 22, 1445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tiwari, P.; Vig, K.; Dennis, V.; Singh, S. Functionalized Gold Nanoparticles and Their Biomedical Applications. Nanomaterials 2011, 1, 31–63. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, Q.W.; Wang, X.M. Gold Nanostructures for Bioimaging, Drug Delivery and Therapeutics. In Precious Metals for Biomedical Applications; Elsevier: Amsterdam, The Netherlands, 2014; pp. 163–176. ISBN 978-0-85709-434-6. [Google Scholar]
- Chandran, P.R.; Thomas, R.T. Gold Nanoparticles in Cancer Drug Delivery. In Nanotechnology Applications for Tissue Engineering; Elsevier: Amsterdam, The Netherlands, 2015; pp. 221–237. ISBN 978-0-323-32889-0. [Google Scholar]
- Maccora, D.; Dini, V.; Battocchio, C.; Fratoddi, I.; Cartoni, A.; Rotili, D.; Castagnola, M.; Faccini, R.; Bruno, I.; Scotognella, T.; et al. Gold Nanoparticles and Nanorods in Nuclear Medicine: A Mini Review. Appl. Sci. 2019, 9, 3232. [Google Scholar] [CrossRef] [Green Version]
- Rai, M.; Yadav, A. (Eds.) Nanobiotechnology in Neurodegenerative Diseases; Springer International Publishing: Cham, Switzerland, 2019; ISBN 978-3-030-30929-9. [Google Scholar]
- Rodzik-Czałka, Ł.; Lewandowska-Łańcucka, J.; Gatta, V.; Venditti, I.; Fratoddi, I.; Szuwarzyński, M.; Romek, M.; Nowakowska, M. Nucleobases Functionalized Quantum Dots and Gold Nanoparticles Bioconjugates as a Fluorescence Resonance Energy Transfer (FRET) System—Synthesis, Characterization and Potential Applications. J. Colloid Interface Sci. 2018, 514, 479–490. [Google Scholar] [CrossRef]
- Vines, J.B.; Yoon, J.-H.; Ryu, N.-E.; Lim, D.-J.; Park, H. Gold Nanoparticles for Photothermal Cancer Therapy. Front. Chem. 2019, 7, 167. [Google Scholar] [CrossRef] [Green Version]
- Sztandera, K.; Gorzkiewicz, M.; Klajnert-Maculewicz, B. Gold Nanoparticles in Cancer Treatment. Mol. Pharm. 2019, 16, 1–23. [Google Scholar] [CrossRef]
- Huang, X.; El-Sayed, M.A. Gold Nanoparticles: Optical Properties and Implementations in Cancer Diagnosis and Photothermal Therapy. J. Adv. Res. 2010, 1, 13–28. [Google Scholar] [CrossRef] [Green Version]
- Hu, X.; Zhang, Y.; Ding, T.; Liu, J.; Zhao, H. Multifunctional Gold Nanoparticles: A Novel Nanomaterial for Various Medical Applications and Biological Activities. Front. Bioeng. Biotechnol. 2020, 8, 990. [Google Scholar] [CrossRef]
- Yu, C.; Xu, L.; Zhang, Y.; Timashev, P.S.; Huang, Y.; Liang, X.-J. Polymer-Based Nanomaterials for Noninvasive Cancer Photothermal Therapy. ACS Appl. Polym. Mater. 2020, 2, 4289–4305. [Google Scholar] [CrossRef]
- Behrouzkia, Z.; Joveini, Z.; Keshavarzi, B.; Eyvazzadeh, N.; Aghdam, R.Z. Hyperthermia: How Can It Be Used? Oman Med. J. 2016, 31, 89–97. [Google Scholar] [CrossRef]
- Dunne, M.; Regenold, M.; Allen, C. Hyperthermia Can Alter Tumor Physiology and Improve Chemo- and Radio-Therapy Efficacy. Adv. Drug Deliv. Rev. 2020, 163–164, 98–124. [Google Scholar] [CrossRef] [PubMed]
- Beik, J.; Abed, Z.; Ghoreishi, F.S.; Hosseini-Nami, S.; Mehrzadi, S.; Shakeri-Zadeh, A.; Kamrava, S.K. Nanotechnology in Hyperthermia Cancer Therapy: From Fundamental Principles to Advanced Applications. J. Control. Release 2016, 235, 205–221. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, Y.; Wang, Y.; Zhu, W.; Li, G.; Ma, X.; Zhang, Y.; Chen, S.; Tiwari, S.; Shi, K.; et al. Comprehensive Understanding of Magnetic Hyperthermia for Improving Antitumor Therapeutic Efficacy. Theranostics 2020, 10, 3793–3815. [Google Scholar] [CrossRef] [PubMed]
- Petryk, A.A.; Giustini, A.J.; Gottesman, R.E.; Kaufman, P.A.; Hoopes, P.J. Magnetic Nanoparticle Hyperthermia Enhancement of Cisplatin Chemotherapy Cancer Treatment. Int. J. Hyperth. 2013, 29, 845–851. [Google Scholar] [CrossRef] [Green Version]
- Najahi-Missaoui, W.; Arnold, R.D.; Cummings, B.S. Safe Nanoparticles: Are We There Yet? IJMS 2020, 22, 385. [Google Scholar] [CrossRef]
- Jia, Y.-P.; Ma, B.-Y.; Wei, X.-W.; Qian, Z.-Y. The in Vitro and in Vivo Toxicity of Gold Nanoparticles. Chin. Chem. Lett. 2017, 28, 691–702. [Google Scholar] [CrossRef]
- Singh, A.K.; Srivastava, O.N. One-Step Green Synthesis of Gold Nanoparticles Using Black Cardamom and Effect of PH on Its Synthesis. Nanoscale Res. Lett. 2015, 10, 353. [Google Scholar] [CrossRef] [Green Version]
- Yang, W.; Xia, B.; Wang, L.; Ma, S.; Liang, H.; Wang, D.; Huang, J. Shape Effects of Gold Nanoparticles in Photothermal Cancer Therapy. Mater. Today Sustain. 2021, 13, 100078. [Google Scholar] [CrossRef]
- Chithrani, B.D.; Ghazani, A.A.; Chan, W.C.W. Determining the Size and Shape Dependence of Gold Nanoparticle Uptake into Mammalian Cells. Nano Lett. 2006, 6, 662–668. [Google Scholar] [CrossRef]
- Alamzadeh, Z.; Beik, J.; Mirrahimi, M.; Shakeri-Zadeh, A.; Ebrahimi, F.; Komeili, A.; Ghalandari, B.; Ghaznavi, H.; Kamrava, S.K.; Moustakis, C. Gold Nanoparticles Promote a Multimodal Synergistic Cancer Therapy Strategy by Co-Delivery of Thermo-Chemo-Radio Therapy. Eur. J. Pharm. Sci. 2020, 145, 105235. [Google Scholar] [CrossRef] [PubMed]
- Keshavarz, M.; Moloudi, K.; Paydar, R.; Abed, Z.; Beik, J.; Ghaznavi, H.; Shakeri-Zadeh, A. Alginate Hydrogel Co-Loaded with Cisplatin and Gold Nanoparticles for Computed Tomography Image-Guided Chemotherapy. J. Biomater. Appl. 2018, 33, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Hainfeld, J.F.; Dilmanian, F.A.; Zhong, Z.; Slatkin, D.N.; Kalef-Ezra, J.A.; Smilowitz, H.M. Gold Nanoparticles Enhance the Radiation Therapy of a Murine Squamous Cell Carcinoma. Phys. Med. Biol. 2010, 55, 3045–3059. [Google Scholar] [CrossRef] [PubMed]
- Piccolo, O.; Lincoln, J.D.; Melong, N.; Orr, B.C.; Fernandez, N.R.; Borsavage, J.; Berman, J.N.; Robar, J.; Ha, M.N. Radiation Dose Enhancement Using Gold Nanoparticles with a Diamond Linear Accelerator Target: A Multiple Cell Type Analysis. Sci. Rep. 2022, 12, 1559. [Google Scholar] [CrossRef] [PubMed]
- Popovtzer, R.; Agrawal, A.; Kotov, N.A.; Popovtzer, A.; Balter, J.; Carey, T.E.; Kopelman, R. Targeted Gold Nanoparticles Enable Molecular CT Imaging of Cancer. Nano Lett. 2008, 8, 4593–4596. [Google Scholar] [CrossRef] [Green Version]
- Chemla, Y.; Betzer, O.; Markus, A.; Farah, N.; Motiei, M.; Popovtzer, R.; Mandel, Y. Gold Nanoparticles for Multimodal High-Resolution Imaging of Transplanted Cells for Retinal Replacement Therapy. Nanomedicine 2019, 14, 1857–1871. [Google Scholar] [CrossRef]
- Mirrahimi, M.; Khateri, M.; Beik, J.; Ghoreishi, F.S.; Dezfuli, A.S.; Ghaznavi, H.; Shakeri-Zadeh, A. Enhancement of Chemoradiation by Co-incorporation of Gold Nanoparticles and Cisplatin into Alginate Hydrogel. J. Biomed. Mater. Res. 2019, 107, 2658–2663. [Google Scholar] [CrossRef]
- Hsiao, C.-W.; Chuang, E.-Y.; Chen, H.-L.; Wan, D.; Korupalli, C.; Liao, Z.-X.; Chiu, Y.-L.; Chia, W.-T.; Lin, K.-J.; Sung, H.-W. Photothermal Tumor Ablation in Mice with Repeated Therapy Sessions Using NIR-Absorbing Micellar Hydrogels Formed in Situ. Biomaterials 2015, 56, 26–35. [Google Scholar] [CrossRef]
- Tan, C.; Chen, J.; Wu, X.-J.; Zhang, H. Epitaxial Growth of Hybrid Nanostructures. Nat. Rev. Mater. 2018, 3, 17089. [Google Scholar] [CrossRef]
- Adeli, M.; Sarabi, R.S.; Yadollahi Farsi, R.; Mahmoudi, M.; Kalantari, M. Polyrotaxane/Gold Nanoparticle Hybrid Nanomaterials as Anticancer Drug Delivery Systems. J. Mater. Chem. 2011, 21, 18686. [Google Scholar] [CrossRef]
- Koga, K.; Tagami, T.; Ozeki, T. Gold Nanoparticle-Coated Thermosensitive Liposomes for the Triggered Release of Doxorubicin, and Photothermal Therapy Using a near-Infrared Laser. Colloids Surf. A Physicochem. Eng. Asp. 2021, 626, 127038. [Google Scholar] [CrossRef]
- Howaili, F.; Özliseli, E.; Küçüktürkmen, B.; Razavi, S.M.; Sadeghizadeh, M.; Rosenholm, J.M. Stimuli-Responsive, Plasmonic Nanogel for Dual Delivery of Curcumin and Photothermal Therapy for Cancer Treatment. Front. Chem. 2021, 8, 602941. [Google Scholar] [CrossRef] [PubMed]
- Pourjavadi, A.; Doroudian, M.; Bagherifard, M.; Bahmanpour, M. Magnetic and Light-Responsive Nanogels Based on Chitosan Functionalized with Au Nanoparticles and Poly(N-Isopropylacrylamide) as a Remotely Triggered Drug Carrier. New J. Chem. 2020, 44, 17302–17312. [Google Scholar] [CrossRef]
- Yang, L. Tumor Microenvironment and Metabolism. IJMS 2017, 18, 2729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xing, S.; Zhang, X.; Luo, L.; Cao, W.; Li, L.; He, Y.; An, J.; Gao, D. Doxorubicin/Gold Nanoparticles Coated with Liposomes for Chemo-Photothermal Synergetic Antitumor Therapy. Nanotechnology 2018, 29, 405101. [Google Scholar] [CrossRef]
- Wang, M.; Liu, Y.; Zhang, X.; Luo, L.; Li, L.; Xing, S.; He, Y.; Cao, W.; Zhu, R.; Gao, D. Gold Nanoshell Coated Thermo-PH Dual Responsive Liposomes for Resveratrol Delivery and Chemo-Photothermal Synergistic Cancer Therapy. J. Mater. Chem. B 2017, 4, 1–12. [Google Scholar] [CrossRef]
- Alle, M.; Sharma, G.; Lee, S.-H.; Kim, J.-C. Next-Generation Engineered Nanogold for Multimodal Cancer Therapy and Imaging: A Clinical Perspectives. J. Nanobiotechnol. 2022, 20, 222. [Google Scholar] [CrossRef]
- Mauro, N.; Utzeri, M.A.; Varvarà, P.; Cavallaro, G. Functionalization of Metal and Carbon Nanoparticles with Potential in Cancer Theranostics. Molecules 2021, 26, 3085. [Google Scholar] [CrossRef]
- Debela, D.T.; Muzazu, S.G.; Heraro, K.D.; Ndalama, M.T.; Mesele, B.W.; Haile, D.C.; Kitui, S.K.; Manyazewal, T. New Approaches and Procedures for Cancer Treatment: Current Perspectives. SAGE Open Med. 2021, 9, 20503121211034366. [Google Scholar] [CrossRef]
- Bao, Q.-Y.; Zhang, N.; Geng, D.-D.; Xue, J.-W.; Merritt, M.; Zhang, C.; Ding, Y. The Enhanced Longevity and Liver Targetability of Paclitaxel by Hybrid Liposomes Encapsulating Paclitaxel-Conjugated Gold Nanoparticles. Int. J. Pharm. 2014, 477, 408–415. [Google Scholar] [CrossRef]
- Wang, W.; Shao, A.; Zhang, N.; Fang, J.; Ruan, J.J.; Ruan, B.H. Cationic Polymethacrylate-Modified Liposomes Significantly Enhanced Doxorubicin Delivery and Antitumor Activity. Sci. Rep. 2017, 7, 43036. [Google Scholar] [CrossRef] [Green Version]
- Chowdhury, N.; Chaudhry, S.; Hall, N.; Olverson, G.; Zhang, Q.-J.; Mandal, T.; Dash, S.; Kundu, A. Targeted Delivery of Doxorubicin Liposomes for Her-2+ Breast Cancer Treatment. AAPS Pharm. Sci. Tech. 2020, 21, 202. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, T.; Miao, Y.; Zhou, L.; Zhang, W. Dual-Responsive Doxorubicin-Loaded Nanomicelles for Enhanced Cancer Therapy. J. Nanobiotechnol. 2020, 18, 136. [Google Scholar] [CrossRef]
- Norouzi, M.; Yathindranath, V.; Thliveris, J.A.; Kopec, B.M.; Siahaan, T.J.; Miller, D.W. Doxorubicin-Loaded Iron Oxide Nanoparticles for Glioblastoma Therapy: A Combinational Approach for Enhanced Delivery of Nanoparticles. Sci. Rep. 2020, 10, 11292. [Google Scholar] [CrossRef]
- An, X. Stimuli-Responsive Liposome and Control Release Drug; Elsevier: Amsterdam, The Netherlands, 2017; Volume 31. [Google Scholar]
- Wang, D.; Green, M.D.; Chen, K.; Daengngam, C.; Kotsuchibashi, Y. Stimuli-Responsive Polymers: Design, Synthesis, Characterization, and Applications. Int. J. Polym. Sci. 2016, 2016, 6480259. [Google Scholar] [CrossRef]
- Ortega-García, A.; Martínez-Bernal, B.G.; Ceja, I.; Mendizábal, E.; Puig-Arévalo, J.E.; Pérez-Carrillo, L.A. Drug Delivery from Stimuli-Responsive Poly(N-Isopropylacrylamide-Co-N-Isopropylmethacrylamide)/Chitosan Core/Shell Nanohydrogels. Polymers 2022, 14, 522. [Google Scholar] [CrossRef] [PubMed]
- Harris, M.; Ahmed, H.; Barr, B.; LeVine, D.; Pace, L.; Mohapatra, A.; Morshed, B.; Bumgardner, J.D.; Jennings, J.A. Magnetic Stimuli-Responsive Chitosan-Based Drug Delivery Biocomposite for Multiple Triggered Release. Int. J. Biol. Macromol. 2017, 104, 1407–1414. [Google Scholar] [CrossRef] [PubMed]
- Hajebi, S.; Rabiee, N.; Bagherzadeh, M.; Ahmadi, S.; Rabiee, M.; Roghani-Mamaqani, H.; Tahriri, M.; Tayebi, L.; Hamblin, M.R. Stimulus-Responsive Polymeric Nanogels as Smart Drug Delivery Systems. Acta Biomater. 2019, 92, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Hossen, S.; Hossain, M.K.; Basher, M.K.; Mia, M.N.H.; Rahman, M.T.; Uddin, M.J. Smart Nanocarrier-Based Drug Delivery Systems for Cancer Therapy and Toxicity Studies: A Review. J. Adv. Res. 2019, 15, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Venditti, I.; Testa, G.; Sciubba, F.; Carlini, L.; Porcaro, F.; Meneghini, C.; Mobilio, S.; Battocchio, C.; Fratoddi, I. Hydrophilic Metal Nanoparticles Functionalized by 2-Diethylaminoethanethiol: A Close Look at the Metal−Ligand Interaction and Interface Chemical Structure. J. Phys. Chem. C 2017, 121, 8002–8013. [Google Scholar] [CrossRef]
- Yin, X.; Russek, S.E.; Zabow, G.; Sun, F.; Mohapatra, J.; Keenan, K.E.; Boss, M.A.; Zeng, H.; Liu, J.P.; Viert, A.; et al. Large T1 Contrast Enhancement Using Superparamagnetic Nanoparticles in Ultra-Low Field MRI. Sci. Rep. 2018, 8, 11863. [Google Scholar] [CrossRef] [Green Version]
- Luo, D.; Wang, X.; Burda, C.; Basilion, J.P. Recent Development of Gold Nanoparticles as Contrast Agents for Cancer Diagnosis. Cancers 2021, 13, 1825. [Google Scholar] [CrossRef]
- Fan, M.; Han, Y.; Gao, S.; Yan, H.; Cao, L.; Li, Z.; Liang, X.-J.; Zhang, J. Ultrasmall Gold Nanoparticles in Cancer Diagnosis and Therapy. Theranostics 2020, 10, 4944–4957. [Google Scholar] [CrossRef]
- Fontes de Paula Aguiar, M.; Bustamante Mamani, J.; Klei Felix, T.; Ferreira dos Reis, R.; Rodrigues da Silva, H.; Nucci, L.P.; Nucci-da-Silva, M.P.; Gamarra, L.F. Magnetic Targeting with Superparamagnetic Iron Oxide Nanoparticles for In Vivo Glioma. Nanotechnol. Rev. 2017, 6, 449–472. [Google Scholar] [CrossRef]
- Venditti, I.; Iucci, G.; Fratoddi, I.; Cipolletti, M.; Montalesi, E.; Marino, M.; Secchi, V.; Battocchio, C. Direct Conjugation of Resveratrol on Hydrophilic Gold Nanoparticles: Structural and Cytotoxic Studies for Biomedical Applications. Nanomaterials 2020, 10, 1898. [Google Scholar] [CrossRef] [PubMed]
- AlSawaftah, N.M.; Awad, N.S.; Pitt, W.G.; Husseini, G.A. PH-Responsive Nanocarriers in Cancer Therapy. Polymers 2022, 14, 936. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, A. Dendrimers for Drug Delivery. Molecules 2018, 23, 938. [Google Scholar] [CrossRef] [Green Version]
- Choudhary, S.; Gupta, L.; Rani, S.; Dave, K.; Gupta, U. Impact of Dendrimers on Solubility of Hydrophobic Drug Molecules. Front. Pharmacol. 2017, 8, 261. [Google Scholar] [CrossRef] [Green Version]
- de Lima, C.S.A.; Balogh, T.S.; Varca, J.P.R.O.; Varca, G.H.C.; Lugão, A.B.; Camacho-Cruz, L.A.; Bucio, E.; Kadlubowski, S.S. An Updated Review of Macro, Micro, and Nanostructured Hydrogels for Biomedical and Pharmaceutical Applications. Pharmaceutics 2020, 12, 970. [Google Scholar] [CrossRef] [PubMed]
- Chai, Q.; Jiao, Y.; Yu, X. Hydrogels for Biomedical Applications: Their Characteristics and the Mechanisms behind Them. Gels 2017, 3, 6. [Google Scholar] [CrossRef]
- Kopeček, J.; Yang, J. Hydrogels as Smart Biomaterials. Polym. Int. 2007, 56, 1078–1098. [Google Scholar] [CrossRef]
- Santander-Ortega, M.J.; Lozano, M.V.; Uchegbu, I.F.; Schätzlein, A.G. Dendrimers for Gene Therapy. In Polymers and Nanomaterials for Gene Therapy; Elsevier: Amsterdam, The Netherlands, 2016; pp. 113–146. ISBN 978-0-08-100520-0. [Google Scholar]
- Lv, J.; Wang, C.; Li, H.; Li, Z.; Fan, Q.; Zhang, Y.; Li, Y.; Wang, H.; Cheng, Y. Bifunctional and Bioreducible Dendrimer Bearing a Fluoroalkyl Tail for Efficient Protein Delivery Both In Vitro and In Vivo. Nano Lett. 2020, 20, 8600–8607. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wan, T.; Wang, H.; Zhang, S.; Ping, Y.; Cheng, Y. A Boronic Acid–Rich Dendrimer with Robust and Unprecedented Efficiency for Cytosolic Protein Delivery and CRISPR-Cas9 Gene Editing. Sci. Adv. 2019, 5, eaaw8922. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kandil, R.; Merkel, O.M. Recent Progress of Polymeric Nanogels for Gene Delivery. Curr. Opin. Colloid Interface Sci. 2019, 39, 11–23. [Google Scholar] [CrossRef]
- Kousalová, J.; Etrych, T. Polymeric Nanogels as Drug Delivery Systems. Physiol. Res. 2018, 67, S305–S317. [Google Scholar] [CrossRef]
- Xu, X.; Shen, S.; Mo, R. Bioresponsive Nanogels for Protein Delivery. View 2022, 3, 20200136. [Google Scholar] [CrossRef]
- Wang, H.; Huang, Q.; Chang, H.; Xiao, J.; Cheng, Y. Stimuli-Responsive Dendrimers in Drug Delivery. Biomater. Sci. 2016, 4, 375–390. [Google Scholar] [CrossRef]
- Chacko, R.T.; Ventura, J.; Zhuang, J.; Thayumanavan, S. Polymer Nanogels: A Versatile Nanoscopic Drug Delivery Platform. Adv. Drug Deliv. Rev. 2012, 64, 836–851. [Google Scholar] [CrossRef] [Green Version]
- Bahutair, W.N.; Abuwatfa, W.H.; Husseini, G.A. Ultrasound Triggering of Liposomal Nanodrugs for Cancer Therapy: A Review. Nanomaterials 2022, 12, 3051. [Google Scholar] [CrossRef]
- Hanafy, N.; El-Kemary, M.; Leporatti, S. Micelles Structure Development as a Strategy to Improve Smart Cancer Therapy. Cancers 2018, 10, 238. [Google Scholar] [CrossRef]
- Ghezzi, M.; Pescina, S.; Padula, C.; Santi, P.; Del Favero, E.; Cantù, L.; Nicoli, S. Polymeric Micelles in Drug Delivery: An Insight of the Techniques for Their Characterization and Assessment in Biorelevant Conditions. J. Control. Release 2021, 332, 312–336. [Google Scholar] [CrossRef] [PubMed]
- Jhaveri, A.M.; Torchilin, V.P. Multifunctional Polymeric Micelles for Delivery of Drugs and SiRNA. Front. Pharmacol. 2014, 5, 77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Q.; Zhang, L.; Yang, T.; Wu, H. Stimuli-Responsive Polymeric Micelles for Drug Delivery and Cancer Therapy. IJN 2018, 13, 2921–2942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mi, P. Stimuli-Responsive Nanocarriers for Drug Delivery, Tumor Imaging, Therapy and Theranostics. Theranostics 2020, 10, 4557–4588. [Google Scholar] [CrossRef]
- Ou, Y.-C.; Webb, J.A.; Faley, S.; Shae, D.; Talbert, E.M.; Lin, S.; Cutright, C.C.; Wilson, J.T.; Bellan, L.M.; Bardhan, R. Gold Nanoantenna-Mediated Photothermal Drug Delivery from Thermosensitive Liposomes in Breast Cancer. ACS Omega 2016, 1, 234–243. [Google Scholar] [CrossRef]
- Luo, L.; Bian, Y.; Liu, Y.; Zhang, X.; Wang, M.; Xing, S.; Li, L.; Gao, D. Combined Near Infrared Photothermal Therapy and Chemotherapy Using Gold Nanoshells Coated Liposomes to Enhance Antitumor Effect. Small 2016, 12, 4103–4112. [Google Scholar] [CrossRef]
- He, H.; Liu, L.; Zhang, S.; Zheng, M.; Ma, A.; Chen, Z.; Pan, H.; Zhou, H.; Liang, R.; Cai, L. Smart Gold Nanocages for Mild Heat-Triggered Drug Release and Breaking Chemoresistance. J. Control. Release 2020, 323, 387–397. [Google Scholar] [CrossRef]
- Li, Y.; He, D.; Tu, J.; Wang, R.; Zu, C.; Chen, Y.; Yang, W.; Shi, D.; Webster, T.J.; Shen, Y. Comparative Effect of Wrapping Solid Gold Nanoparticles and Hollow Gold Nanoparticles with Doxorubicin-Loaded Thermosensitive Liposomes for Cancer Thermo-Chemotherapy. Nanoscale 2018, 10, 8628–8641. [Google Scholar] [CrossRef]
- Hossann, M.; Kneidl, B.; Peller, M.; Lindner, L.; Winter, G. Thermosensitive Liposomal Drug Delivery Systems: State of the Art Review. IJN 2014, 9, 4387–4398. [Google Scholar] [CrossRef] [Green Version]
- Sercombe, L.; Veerati, T.; Moheimani, F.; Wu, S.Y.; Sood, A.K.; Hua, S. Advances and Challenges of Liposome Assisted Drug Delivery. Front. Pharmacol. 2015, 6, 286. [Google Scholar] [CrossRef]
- Olusanya, T.; Haj Ahmad, R.; Ibegbu, D.; Smith, J.; Elkordy, A. Liposomal Drug Delivery Systems and Anticancer Drugs. Molecules 2018, 23, 907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhardwaj, V.; Kaushik, A.; Khatib, Z.M.; Nair, M.; McGoron, A.J. Recalcitrant Issues and New Frontiers in Nano-Pharmacology. Front. Pharmacol. 2019, 10, 1369. [Google Scholar] [CrossRef] [PubMed]
- Won, J.E.; Wi, T.I.; Lee, C.M.; Lee, J.H.; Kang, T.H.; Lee, J.-W.; Shin, B.C.; Lee, Y.; Park, Y.-M.; Han, H.D. NIR Irradiation-Controlled Drug Release Utilizing Injectable Hydrogels Containing Gold-Labeled Liposomes for the Treatment of Melanoma Cancer. Acta Biomater. 2021, 136, 508–518. [Google Scholar] [CrossRef]
- Lorusso, D.; Di Stefano, A.; Carone, V.; Fagotti, A.; Pisconti, S.; Scambia, G. Pegylated Liposomal Doxorubicin-Related Palmar-Plantar Erythrodysesthesia (‘Hand-Foot’ Syndrome). Ann. Oncol. 2007, 18, 1159–1164. [Google Scholar] [CrossRef]
- Seynhaeve, A.L.B. Intact Doxil Is Taken up Intracellularly and Released Doxorubicin Sequesters in the Lysosome: Evaluated by in Vitro/in Vivo Live Cell Imaging. J. Control. Release 2013, 172, 330–340. [Google Scholar] [CrossRef]
- Chaikomon, K.; Chattong, S.; Chaiya, T.; Tiwawech, D.; Sritana-anant, Y.; Sereemaspun, A.; Manotham, K. Doxorubicin-Conjugated Dexamethasone Induced MCF-7 Apoptosis without Entering the Nucleus and Able to Overcome MDR-1-Induced Resistance. DDDT 2018, 12, 2361–2369. [Google Scholar] [CrossRef] [Green Version]
- Thakur, N.S.; Patel, G.; Kushwah, V.; Jain, S.; Banerjee, U.C. Self-Assembled Gold Nanoparticle−Lipid Nanocomposites for On- Demand Delivery, Tumor Accumulation, and Combined Photothermal−Photodynamic Therapy. ACS Appl. Bio Mater. 2019, 2, 349–361. [Google Scholar] [CrossRef]
- Kang, Z.; Yan, X.; Zhao, L.; Liao, Q.; Zhao, K.; Du, H.; Zhang, X.; Zhang, X.; Zhang, Y. Gold Nanoparticle/ZnO Nanorod Hybrids for Enhanced Reactive Oxygen Species Generation and Photodynamic Therapy. Nano Res. 2015, 8, 2004–2014. [Google Scholar] [CrossRef]
- Zhao, T.; Yu, K.; Li, L.; Zhang, T.; Guan, Z.; Gao, N.; Yuan, P.; Li, S.; Yao, S.Q.; Xu, Q.-H.; et al. Gold Nanorod Enhanced Two-Photon Excitation Fluorescence of Photosensitizers for Two-Photon Imaging and Photodynamic Therapy. ACS Appl. Mater. Interfaces 2014, 6, 2700–2708. [Google Scholar] [CrossRef]
- Srivatsan, A.; Jenkins, S.V.; Jeon, M.; Wu, Z.; Kim, C.; Chen, J.; Pandey, R. Gold Nanocage-Photosensitizer Conjugates for Dual-Modal Image-Guided Enhanced Photodynamic Therapy. Theranostics 2014, 4, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Kautzka, Z.; Clement, S.; Goldys, E.M.; Deng, W. Light-Triggered Liposomal Cargo Delivery Platform Incorporating Photosensitizers and Gold Nanoparticles for Enhanced Singlet Oxygen Generation and Increased Cytotoxicity. IJN 2017, 12, 969–977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, S.P.; Alvi, S.B.; Pemmaraju, D.B.; Singh, A.D.; Manda, S.V.; Srivastava, R.; Rengan, A.K. NIR Triggered Liposome Gold Nanoparticles Entrapping Curcumin as in Situ Adjuvant for Photothermal Treatment of Skin Cancer. Int. J. Biol. Macromol. 2018, 110, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, X.; Luo, L.; Li, L.; Zhu, R.Y.; Li, A.; He, Y.; Cao, W.; Niu, K.; Liu, H.; et al. Gold-Nanobranched-Shell Based Drug Vehicles with Ultrahigh Photothermal Efficiency for Chemo-Photothermal Therapy. Nanomed. Nanotechnol. Biol. Med. 2019, 18, 303–314. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, D.S.; Prasad, R.; Devrukhkar, J.; Selvaraj, K.; Srivastava, R. Disintegrable NIR Light Triggered Gold Nanorods Supported Liposomal Nanohybrids for Cancer Theranostics. Bioconjugate Chem. 2018, 29, 1510–1518. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, X.; Liu, Z.; Wang, L.; Luo, L.; Wang, M.; Wang, Q.; Gao, D. Gold Nanoshell-Based Betulinic Acid Liposomes for Synergistic Chemo-Photothermal Therapy. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 1891–1900. [Google Scholar] [CrossRef]
- Hua, H.; Zhang, N.; Liu, D.; Song, L.; Liu, T.; Li, S.; Zhao, Y. Multifunctional Gold Nanorods and Docetaxel-Encapsulated Liposomes for Combined Thermo- and Chemotherapy. IJN 2017, 12, 7869–7884. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, V.D.; Min, H.-K.; Kim, C.-S.; Han, J.; Park, J.-O.; Choi, E. Folate Receptor-Targeted Liposomal Nanocomplex for Effective Synergistic Photothermal-Chemotherapy of Breast Cancer in Vivo. Colloids Surf. B Biointerfaces 2019, 173, 539–548. [Google Scholar] [CrossRef]
- You, J.; Zhang, P.; Hu, F.; Du, Y.; Yuan, H.; Zhu, J.; Wang, Z.; Zhou, J.; Li, C. Near-Infrared Light-Sensitive Liposomes for the Enhanced Photothermal Tumor Treatment by the Combination with Chemotherapy. Pharm. Res. 2014, 31, 554–565. [Google Scholar] [CrossRef]
- Li, Y.; Song, W.; Hu, Y.; Xia, Y.; Li, Z.; Lu, Y.; Shen, Y. “Petal-like” Size-Tunable Gold Wrapped Immunoliposome to Enhance Tumor Deep Penetration for Multimodal Guided Two-Step Strategy. J. Nanobiotechnol. 2021, 19, 293. [Google Scholar] [CrossRef]
- Zhang, N.; Li, J.; Hou, R.; Zhang, J.; Wang, P.; Liu, X.; Zhang, Z. Bubble-Generating Nano-Lipid Carriers for Ultrasound/CT Imaging-Guided Efficient Tumor Therapy. Int. J. Pharm. 2017, 534, 251–262. [Google Scholar] [CrossRef]
- Rengan, A.K.; Jagtap, M.; De, A.; Banerjee, R.; Srivastava, R. Multifunctional Gold Coated Thermo-Sensitive Liposomes for Multimodal Imaging and Photo-Thermal Therapy of Breast Cancer Cells. Nanoscale 2014, 6, 916–923. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-M.; Choi, H.E.; Kudaibergen, D.; Kim, J.-H.; Kim, K.S. Recent Advances in Hollow Gold Nanostructures for Biomedical Applications. Front. Chem. 2021, 9, 699284. [Google Scholar] [CrossRef]
- You, J.; Zhang, G.; Li, C. Exceptionally High Payload of Doxorubicin in Hollow Gold Nanospheres for Near-Infrared Light-Triggered Drug Release. ACS Nano 2010, 4, 1033–1041. [Google Scholar] [CrossRef] [Green Version]
- Xiong, C.; Lu, W.; Zhou, M.; Wen, X.; Li, C. Cisplatin-Loaded Hollow Gold Nanoparticles for Laser-Triggered Release. Cancer Nano 2018, 9, 6. [Google Scholar] [CrossRef] [Green Version]
- Sonkar, R.; Sonali; Jha, A.; Viswanadh, M.K.; Burande, A.S.; Narendra; Pawde, D.M.; Patel, K.K.; Singh, M.; Koch, B.; et al. Gold Liposomes for Brain-Targeted Drug Delivery: Formulation and Brain Distribution Kinetics. Mater. Sci. Eng. C 2021, 120, 111652. [Google Scholar] [CrossRef] [PubMed]
- Kirui, D.K.; Celia, C.; Molinaro, R.; Bansal, S.S.; Cosco, D.; Fresta, M.; Shen, H.; Ferrari, M. Mild Hyperthermia Enhances Transport of Liposomal Gemcitabine and Improves In Vivo Therapeutic Response. Adv. Healthc. Mater. 2015, 4, 1092–1103. [Google Scholar] [CrossRef] [Green Version]
- Zhang, N.; Chen, H.; Liu, A.-Y.; Shen, J.-J.; Shah, V.; Zhang, C.; Hong, J.; Ding, Y. Gold Conjugate-Based Liposomes with Hybrid Cluster Bomb Structure for Liver Cancer Therapy. Biomaterials 2016, 74, 280–291. [Google Scholar] [CrossRef]
- Hamzawy, M.A.; Abo-youssef, A.M.; Salem, H.F.; Mohammed, S.A. Antitumor Activity of Intratracheal Inhalation of Temozolomide (TMZ) Loaded into Gold Nanoparticles and/or Liposomes against Urethane-Induced Lung Cancer in BALB/c Mice. Drug Deliv. 2017, 24, 599–607. [Google Scholar] [CrossRef] [Green Version]
- Deng, W.; Chen, W.; Clement, S.; Guller, A.; Zhao, Z.; Engel, A.; Goldys, E.M. Controlled Gene and Drug Release from a Liposomal Delivery Platform Triggered by X-Ray Radiation. Nat. Commun. 2018, 9, 2713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noh, J.; Kwon, B.; Han, E.; Park, M.; Yang, W.; Cho, W.; Yoo, W.; Khang, G.; Lee, D. Amplification of Oxidative Stress by a Dual Stimuli-Responsive Hybrid Drug Enhances Cancer Cell Death. Nat. Commun. 2015, 6, 6907. [Google Scholar] [CrossRef]
- Wei, X.; Liao, J.; Davoudi, Z.; Zheng, H.; Chen, J.; Li, D.; Xiong, X.; Yin, Y.; Yu, X.; Xiong, J.; et al. Folate Receptor-Targeted and GSH-Responsive Carboxymethyl Chitosan Nanoparticles Containing Covalently Entrapped 6-Mercaptopurine for Enhanced Intracellular Drug Delivery in Leukemia. Mar. Drugs 2018, 16, 439. [Google Scholar] [CrossRef] [Green Version]
- Jeon, M.; Kim, G.; Lee, W.; Baek, S.; Jung, H.N.; Im, H.-J. Development of Theranostic Dual-Layered Au-Liposome for Effective Tumor Targeting and Photothermal Therapy. J. Nanobiotechnol. 2021, 19, 262. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.J.; Byeon, Y.; Jeon, H.N.; Cho, S.H.; Han, H.D.; Shin, B.C. Gold Cluster-Labeled Thermosensitive Liposmes Enhance Triggered Drug Release in the Tumor Microenvironment by a Photothermal Effect. J. Control. Release 2015, 216, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Du, B.; Gu, X.; Han, X.; Ding, G.; Wang, Y.; Li, D.; Wang, E.; Wang, J. Lipid-Coated Gold Nanoparticles Functionalized by Folic Acid as Gene Vectors for Targeted Gene Delivery in Vitro and in Vivo. ChemMedChem 2017, 12, 1768–1775. [Google Scholar] [CrossRef]
- Refaat, A.; del Rosal, B.; Palasubramaniam, J.; Pietersz, G.; Wang, X.; Moulton, S.E.; Peter, K. Near-Infrared Light-Responsive Liposomes for Protein Delivery: Towards Bleeding-Free Photothermally-Assisted Thrombolysis. J. Control. Release 2021, 337, 212–223. [Google Scholar] [CrossRef]
- Grafals-Ruiz, N.; Rios-Vicil, C.I.; Lozada-Delgado, E.L.; Quiñones-Díaz, B.I.; Noriega-Rivera, R.A.; Martínez-Zayas, G.; Santana-Rivera, Y.; Santiago-Sánchez, G.S.; Valiyeva, F.; Vivas-Mejía, P.E. Brain Targeted Gold Liposomes Improve RNAi Delivery for Glioblastoma. IJN 2020, 15, 2809–2828. [Google Scholar] [CrossRef] [Green Version]
- Jia, X.; Lv, M.; Fei, Y.; Dong, Q.; Wang, H.; Liu, Q.; Li, D.; Wang, J.; Wang, E. Facile One-Step Synthesis of NIR-Responsive SiRNA-Inorganic Hybrid Nanoplatform for Imaging-Guided Photothermal and Gene Synergistic Therapy. Biomaterials 2022, 282, 121404. [Google Scholar] [CrossRef]
- Skalickova, S.; Nejdl, L.; Kudr, J.; Ruttkay-Nedecky, B.; Jimenez Jimenez, A.; Kopel, P.; Kremplova, M.; Masarik, M.; Stiborova, M.; Eckschlager, T.; et al. Fluorescence Characterization of Gold Modified Liposomes with Antisense N-Myc DNA Bound to the Magnetisable Particles with Encapsulated Anticancer Drugs (Doxorubicin, Ellipticine and Etoposide). Sensors 2016, 16, 290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- An, S.S.; Kang, J.H.; Ko, Y.T. Lipid-Coated Gold Nanocomposites for Enhanced Cancer Therapy. IJN 2015, 10, 33–45. [Google Scholar] [CrossRef] [Green Version]
- Kunjiappan, S.; Panneerselvam, T.; Somasundaram, B.; Arunachalam, S.; Sankaranarayanan, M.; Parasuraman, P. Preparation of Liposomes Encapsulated Epirubicin-Gold Nanoparticles for Tumor Specific Delivery and Release. Biomed. Phys. Eng. Express 2018, 4, 045027. [Google Scholar] [CrossRef]
- Guité-Verret, A.; Vachon, M. The Incurable Metastatic Breast Cancer Experience through Metaphors: The Fight and the Unveiling. Int. J. Qual. Stud. Health Well-Being 2021, 16, 1971597. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Cancer Statistics for the Year 2020: An Overview. Int. J. Cancer 2021, 149, 778–789. [Google Scholar] [CrossRef] [PubMed]
- Westphal, T.; Gampenrieder, S.P.; Rinnerthaler, G.; Greil, R. Cure in Metastatic Breast Cancer. Memo 2018, 11, 172–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prasad, R.; Jain, N.K.; Yadav, A.S.; Chauhan, D.S.; Devrukhkar, J.; Kumawat, M.K.; Shinde, S.; Gorain, M.; Thakor, A.S.; Kundu, G.C.; et al. Liposomal Nanotheranostics for Multimode Targeted in Vivo Bioimaging and Near-infrared Light Mediated Cancer Therapy. Commun. Biol. 2020, 3, 284. [Google Scholar] [CrossRef] [PubMed]
- Qin, G.; Li, Z.; Xia, R.; Li, F.; O’Neill, B.E.; Goodwin, J.T.; Khant, H.A.; Chiu, W.; Li, K.C. Partially Polymerized Liposomes: Stable against Leakage yet Capable of Instantaneous Release for Remote Controlled Drug Delivery. Nanotechnology 2011, 22, 155605. [Google Scholar] [CrossRef] [Green Version]
- García, M.C.; Calderón-Montaño, J.M.; Rueda, M.; Longhi, M.; Rabasco, A.M.; López-Lázaro, M.; Prieto-Dapena, F.; González-Rodríguez, M.L. PH-Temperature Dual-Sensitive Nucleolipid-Containing Stealth Liposomes Anchored with PEGylated AuNPs for Triggering Delivery of Doxorubicin. Int. J. Pharm. 2022, 619, 121691. [Google Scholar] [CrossRef] [PubMed]
- Vigata, M.; Meinert, C.; Hutmacher, D.W.; Bock, N. Hydrogels as Drug Delivery Systems: A Review of Current Characterization and Evaluation Techniques. Pharmaceutics 2020, 12, 1188. [Google Scholar] [CrossRef]
- Das, S.S.; Bharadwaj, P.; Bilal, M.; Barani, M.; Rahdar, A.; Taboada, P.; Bungau, S.; Kyzas, G.Z. Stimuli-Responsive Polymeric Nanocarriers for Drug Delivery, Imaging, and Theragnosis. Polymers 2020, 12, 1397. [Google Scholar] [CrossRef]
- Thelu, H.V.P.; Albert, S.K.; Golla, M.; Krishnan, N.; Ram, D.; Srinivasula, S.M.; Varghese, R. Size Controllable DNA Nanogels from the Self-Assembly of DNA Nanostructures through Multivalent Host–Guest Interactions. Nanoscale 2018, 10, 222–230. [Google Scholar] [CrossRef]
- Yao, C.; Yuan, Y.; Yang, D. Magnetic DNA Nanogels for Targeting Delivery and Multistimuli-Triggered Release of Anticancer Drugs. ACS Appl. Bio Mater. 2018, 1, 2012–2020. [Google Scholar] [CrossRef]
- Zhang, C. Co-Delivery of 5-Fluorodeoxyuridine and Doxorubicin via Gold Nanoparticle Equipped with Affibody-DNA Hybrid Strands for Targeted Synergistic Chemotherapy of HER2 Overexpressing Breast Cancer. Sci. Rep. 2020, 10, 22015. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Peng, X.; Daley, J.; Yang, L.; Shen, J.; Nguyen, N.; Bae, G.; Niu, H.; Peng, Y.; Hsieh, H.-J.; et al. Inhibition of DNA2 Nuclease as a Therapeutic Strategy Targeting Replication Stress in Cancer Cells. Oncogenesis 2017, 6, e319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, H.; Yang, J.; Lee, J.; Haam, S.; Choi, I.-H.; Yoo, K.-H. Multifunctional Nanoparticles for Combined Doxorubicin and Photothermal Treatments. ACS Nano 2009, 3, 2919–2926. [Google Scholar] [CrossRef] [PubMed]
- Dai, M.; Ja, F. Engineered Protein Polymer-Gold Nanoparticle Hybrid Materials for Small Molecule Delivery. J. Nanomed. Nanotechnol. 2016, 7, 356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Narayanaswamy, R.; Torchilin, V.P. Hydrogels and Their Applications in Targeted Drug Delivery. Molecules 2019, 24, 603. [Google Scholar] [CrossRef] [Green Version]
- Hoare, T.R.; Kohane, D.S. Hydrogels in Drug Delivery: Progress and Challenges. Polymer 2008, 49, 1993–2007. [Google Scholar] [CrossRef] [Green Version]
- Cuggino, J.C.; Blanco, E.R.O.; Gugliotta, L.M.; Alvarez Igarzabal, C.I.; Calderón, M. Crossing Biological Barriers with Nanogels to Improve Drug Delivery Performance. J. Control. Release 2019, 307, 221–246. [Google Scholar] [CrossRef]
- Soleimani, K.; Arkan, E.; Derakhshankhah, H.; Haghshenas, B.; Jahanban-Esfahlan, R.; Jaymand, M. A Novel Bioreducible and PH-Responsive Magnetic Nanohydrogel Based on β-Cyclodextrin for Chemo/Hyperthermia Therapy of Cancer. Carbohydr. Polym. 2021, 252, 117229. [Google Scholar] [CrossRef]
- Häring, M.; Schiller, J.; Mayr, J.; Grijalvo, S.; Eritja, R.; Díaz, D. Magnetic Gel Composites for Hyperthermia Cancer Therapy. Gels 2015, 1, 135–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiang, W.-H.; Ho, V.T.; Chen, H.-H.; Huang, W.-C.; Huang, Y.-F.; Lin, S.-C.; Chern, C.-S.; Chiu, H.-C. Superparamagnetic Hollow Hybrid Nanogels as a Potential Guidable Vehicle System of Stimuli-Mediated MR Imaging and Multiple Cancer Therapeutics. Langmuir 2013, 29, 6434–6443. [Google Scholar] [CrossRef]
- Jin, R.-M.; Yao, M.-H.; Yang, J.; Zhao, D.-H.; Zhao, Y.-D.; Liu, B. One-Step in Situ Synthesis of Polypeptide–Gold Nanoparticles Hybrid Nanogels and Their Application in Targeted Photoacoustic Imaging. ACS Sustain. Chem. Eng. 2017, 5, 9841–9847. [Google Scholar] [CrossRef]
- Ghorbani, M.; Hamishehkar, H. A Novel Multi Stimuli-Responsive PEGylated Hybrid Gold/Nanogels for Co-Delivery of Doxorubicin and 6-mercaptopurine. Mater. Sci. Eng. C 2018, 92, 599–611. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhu, Y.; Qu, L.; Wu, H.; Kong, H.; Yang, Z.; Chen, D.; Mäkilä, E.; Salonen, J.; Santos, H.A.; et al. Gold Nanorods Conjugated Porous Silicon Nanoparticles Encapsulated in Calcium Alginate Nano Hydrogels Using Microemulsion Templates. Nano Lett. 2018, 18, 1448–1453. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wu, H.; Zhou, J.; Xu, P.; Wang, C.; Shi, R.; Wang, H.; Wang, H.; Guo, Z.; Chen, Q. In Situ One-Pot Synthesis of MOF-Polydopamine Hybrid Nanogels with Enhanced Photothermal Effect for Targeted Cancer Therapy. Adv. Sci. 2018, 5, 1800287. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wu, J.; Wang, J.; Wang, X.; Wu, C.; Chen, M.; Wu, Q.; Lesniak, M.S.; Mi, Y.; Cheng, Y.; et al. A Neutrophil-Inspired Supramolecular Nanogel for Magnetocaloric–Enzymatic Tandem Therapy. Angew. Chem. Int. Ed. 2020, 59, 3732–3738. [Google Scholar] [CrossRef]
- Qu, Y.; Chu, B.; Wei, X.; Lei, M.; Hu, D.; Zha, R.; Zhong, L.; Wang, M.; Wang, F.; Qian, Z. Redox/PH Dual-Stimuli Responsive Camptothecin Prodrug Nanogels for “on-Demand” Drug Delivery. J. Control. Release 2019, 296, 93–106. [Google Scholar] [CrossRef]
- Gao, W.; Zhang, Y.; Zhang, Q.; Zhang, L. Nanoparticle-Hydrogel: A Hybrid Biomaterial System for Localized Drug Delivery. Ann. Biomed. Eng. 2016, 44, 2049–2061. [Google Scholar] [CrossRef] [Green Version]
- Kiseleva, M.; Omar, M.M.; Boisselier, É.; Selivanova, S.V.; Fortin, M.-A. A Three-Dimensional Printable Hydrogel Formulation for the Local Delivery of Therapeutic Nanoparticles to Cervical Cancer. ACS Biomater. Sci. Eng. 2022, 8, 1200–1214. [Google Scholar] [CrossRef]
- Jeong, B.; Kim, S.W.; Bae, Y.H. Thermosensitive Sol–Gel Reversible Hydrogels. Adv. Drug Deliv. Rev. 2012, 64, 154–162. [Google Scholar] [CrossRef]
- Liu, M.; Huang, P.; Wang, W.; Feng, Z.; Zhang, J.; Deng, L.; Dong, A. Injectable Nanocomposite Hydrogel Co-Constructed by Gold Nanorods and Paclitaxel-Loaded Nanoparticles for Local Chemo- Photothermal Synergetic Cancer Therapy. J. Mater. Chem. B 2016, 4, 37–45. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and Biomedical Applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [Green Version]
- Mirrahimi, M.; Beik, J.; Mirrahimi, M.; Alamzadeh, Z.; Teymouri, S.; Mahabadi, V.P.; Eslahi, N.; Ebrahimi Tazehmahalleh, F.; Ghaznavi, H.; Shakeri-Zadeh, A.; et al. Triple Combination of Heat, Drug and Radiation Using Alginate Hydrogel Co-Loaded with Gold Nanoparticles and Cisplatin for Locally Synergistic Cancer Therapy. Int. J. Biol. Macromol. 2020, 158, 617–626. [Google Scholar] [CrossRef] [PubMed]
- Alamzadeh, Z.; Beik, J.; Pirhajati Mahabadi, V.; Abbasian Ardekani, A.; Ghader, A.; Kamrava, S.K.; Shiralizadeh Dezfuli, A.; Ghaznavi, H.; Shakeri-Zadeh, A. Ultrastructural and Optical Characteristics of Cancer Cells Treated by a Nanotechnology Based Chemo-Photothermal Therapy Method. J. Photochem. Photobiol. B Biol. 2019, 192, 19–25. [Google Scholar] [CrossRef]
- Khani, T.; Alamzadeh, Z.; Sarikhani, A.; Mousavi, M.; Mirrahimi, M.; Tabei, M.; Irajirad, R.; Abed, Z.; Beik, J. Fe3O4@Au Core–Shell Hybrid Nanocomposite for MRI-Guided Magnetic Targeted Photo-Chemotherapy. Lasers Med. Sci. 2022, 37, 2387–2395. [Google Scholar] [CrossRef] [PubMed]
- Xia, B.; Zhang, W.; Tong, H.; Li, J.; Chen, Z.; Shi, J. Multifunctional Chitosan/Porous Silicon@Au Nanocomposite Hydrogels for Long-Term and Repeatedly Localized Combinatorial Therapy of Cancer via a Single Injection. ACS Biomater. Sci. Eng. 2019, 5, 1857–1867. [Google Scholar] [CrossRef] [PubMed]
- Namgung, H.; Jo, S.; Lee, T.S. Fluorescence Modulation of Conjugated Polymer Nanoparticles Embedded in Poly(N-Isopropylacrylamide) Hydrogel. Polymers 2021, 13, 4315. [Google Scholar] [CrossRef]
- Bordat, A.; Boissenot, T.; Nicolas, J.; Tsapis, N. Thermoresponsive Polymer Nanocarriers for Biomedical Applications. Adv. Drug Deliv. Rev. 2019, 138, 167–192. [Google Scholar] [CrossRef]
- de Solorzano, I.O.; Prieto, M.; Mendoza, G.; Sebastian, V.; Arruebo, M. Triggered Drug Release from Hybrid Thermoresponsive Nanoparticles Using near Infrared Light. Nanomedicine 2020, 15, 219–234. [Google Scholar] [CrossRef]
- Yavuz, M.S.; Cheng, Y.; Chen, J.; Cobley, C.M.; Zhang, Q.; Rycenga, M.; Xie, J.; Kim, C.; Song, K.H.; Schwartz, A.G.; et al. Gold Nanocages Covered by Smart Polymers for Controlled Release with Near-Infrared Light. Nat. Mater. 2009, 8, 935–939. [Google Scholar] [CrossRef]
- Song, J.; Hwang, S.; Im, K.; Hur, J.; Nam, J.; Hwang, S.; Ahn, G.-O.; Kim, S.; Park, N. Light-Responsible DNA Hydrogel–Gold Nanoparticle Assembly for Synergistic Cancer Therapy. J. Mater. Chem. B 2015, 3, 1537–1543. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, M.; Han, Y.; Lai, J.; Chen, J. Multistage-Targeted Gold/Mesoporous Silica Nanocomposite Hydrogel as In Situ Injectable Drug Release System for Chemophotothermal Synergistic Cancer Therapy. ACS Appl. Bio Mater. 2020, 3, 421–431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, R.; Yang, J.; Zhao, D.; Hou, X.; Li, C.; Chen, W.; Zhao, Y.; Yin, Z.; Liu, B. Hollow Gold Nanoshells-Incorporated Injectable Genetically Engineered Hydrogel for Sustained Chemo-Photothermal Therapy of Tumor. J. Nanobiotechnol. 2019, 17, 99. [Google Scholar] [CrossRef] [Green Version]
- Baseeruddin Alvi, S.; Rajalakshmi, S.R.; Begum, N.; Jogdand, A.B.; Veeresh, B.; Rengan, A.K. In Situ Nanotransformable Hydrogel for Chemo-Photothermal Therapy of Localized Tumors and Targeted Therapy of Highly Metastatic Tumors. ACS Appl. Mater. Interfaces 2021, 13, 55862–55878. [Google Scholar] [CrossRef]
- Jin, H.; Liu, X.; Gui, R.; Wang, Z. Facile Synthesis of Gold Nanorods/Hydrogels Core/Shell Nanospheres for PH and near-Infrared-Light Induced Release of 5-Fluorouracil and Chemo-Photothermal Therapy. Colloids Surf. B Biointerfaces 2015, 128, 498–505. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Zhang, M.; Wang, J.; Wang, T.; Yao, Y.; Zhang, X.; Zhang, C.; Zhang, N. Thermosensitive Hydrogel Co-Loaded with Gold Nanoparticles and Doxorubicin for Effective Chemoradiotherapy. AAPS J. 2016, 18, 146–155. [Google Scholar] [CrossRef]
- Gonçalves, D.P.N.; Rodriguez, R.D.; Kurth, T.; Bray, L.J.; Binner, M.; Jungnickel, C.; Gür, F.N.; Poser, S.W.; Schmidt, T.L.; Zahn, D.R.T.; et al. Enhanced Targeting of Invasive Glioblastoma Cells by Peptide-Functionalized Gold Nanorods in Hydrogel-Based 3D Cultures. Acta Biomater. 2017, 58, 12–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yata, T.; Takahashi, Y.; Tan, M.; Nakatsuji, H.; Ohtsuki, S.; Murakami, T.; Imahori, H.; Umeki, Y.; Shiomi, T.; Takakura, Y.; et al. DNA Nanotechnology-Based Composite-Type Gold Nanoparticle-Immunostimulatory DNA Hydrogel for Tumor Photothermal Immunotherapy. Biomaterials 2017, 146, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Xu, Q.; Li, X.; Zhang, P.; Zhao, X.; Wang, Y. Redox-Responsive Hyaluronic Acid Nanogels for Hyperthermia- Assisted Chemotherapy to Overcome Multidrug Resistance. Carbohydr. Polym. 2019, 203, 378–385. [Google Scholar] [CrossRef]
- Bukowski, K.; Kciuk, M.; Kontek, R. Mechanisms of Multidrug Resistance in Cancer Chemotherapy. IJMS 2020, 21, 3233. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, Q.; Chen, J.; Liu, L.; Ding, L.; Shen, M.; Li, J.; Han, B.; Duan, Y. Temperature-Sensitive Gold Nanoparticle-Coated Pluronic-PLL Nanoparticles for Drug Delivery and Chemo-Photothermal Therapy. Theranostics 2017, 7, 4424–4444. [Google Scholar] [CrossRef]
- Zhong, Y.; Wang, C.; Cheng, L.; Meng, F.; Zhong, Z.; Liu, Z. Gold Nanorod-Cored Biodegradable Micelles as a Robust and Remotely Controllable Doxorubicin Release System for Potent Inhibition of Drug-Sensitive and -Resistant Cancer Cells. Biomacromolecules 2013, 14, 2411–2419. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Yao, N.; Qian, L.; Zhang, X.; Chen, Q.; Wang, J.; Zhang, L. PH-Responsive Unimolecular Micelle-Gold Nanoparticles-Drug Nanohybrid System for Cancer Theranostics. Acta Biomater. 2017, 58, 455–465. [Google Scholar] [CrossRef] [PubMed]
- Prabaharan, M.; Grailer, J.J.; Pilla, S.; Steeber, D.A.; Gong, S. Gold Nanoparticles with a Monolayer of Doxorubicin-Conjugated Amphiphilic Block Copolymer for Tumor-Targeted Drug Delivery. Biomaterials 2009, 30, 6065–6075. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Xiao, H.; Li, X.; Huang, Y.; Song, W.; Song, L.; Chen, M.; Cheng, D.; Shuai, X. Gold Nanocage Decorated PH-Sensitive Micelle for Highly Effective Photothermo-Chemotherapy and Photoacoustic Imaging. Acta Biomater. 2017, 64, 223–236. [Google Scholar] [CrossRef] [PubMed]
- Parida, S.; Maiti, C.; Rajesh, Y.; Dey, K.K.; Pal, I.; Parekh, A.; Patra, R.; Dhara, D.; Dutta, P.K.; Mandal, M. Gold Nanorod Embedded Reduction Responsive Block Copolymer Micelle-Triggered Drug Delivery Combined with Photothermal Ablation for Targeted Cancer Therapy. Biochim. Biophys. Acta (BBA) Gen. Subj. 2017, 1861, 3039–3052. [Google Scholar] [CrossRef] [PubMed]
- Aryal, S.; Pilla, S.; Gong, S. Multifunctional Nano-Micelles Formed by Amphiphilic Gold-Polycaprolactone-Methoxy Poly(Ethylene Glycol) (Au-PCL-MPEG) Nanoparticles for Potential Drug Delivery Applications. J. Nanosci. Nanotech. 2009, 9, 5701–5708. [Google Scholar] [CrossRef]
- Lin, Y.-C.; Perevedentseva, E.; Lin, Z.-R.; Chang, C.-C.; Chen, H.-H.; Yang, S.-M.; Lin, M.-D.; Karmenyan, A.; Speranza, G.; Minati, L.; et al. Multimodal Bioimaging Using Nanodiamond and Gold Hybrid Nanoparticles. Sci. Rep. 2022, 12, 5331. [Google Scholar] [CrossRef]
- Lin, W.; Zhang, X.; Qian, L.; Yao, N.; Pan, Y.; Zhang, L. Doxorubicin-Loaded Unimolecular Micelle-Stabilized Gold Nanoparticles as a Theranostic Nanoplatform for Tumor-Targeted Chemotherapy and Computed Tomography Imaging. Biomacromolecules 2017, 18, 3869–3880. [Google Scholar] [CrossRef]
- Sarkar, S.; Konar, S.; Prasad, P.N.; Rajput, S.; Kumar, B.N.P.; Rao, R.R.; Pathak, A.; Fisher, P.B.; Mandal, M. Micellear Gold Nanoparticles as Delivery Vehicles for Dual Tyrosine Kinase Inhibitor ZD6474 for Metastatic Breast Cancer Treatment. Langmuir 2017, 33, 7649–7659. [Google Scholar] [CrossRef]
- Sanzhakov, M.A.; Kudinov, V.A.; Baskaev, K.K.; Morozevich, G.E.; Stepanova, D.S.; Torkhovskaya, T.I.; Tereshkina, Y.A.; Korotkevich, E.I.; Tikhonova, E.G. Composite Phospholipid-Gold Nanoparticles with Targeted Fragment for Tumor Imaging. Biomed. Pharmacother. 2021, 142, 111985. [Google Scholar] [CrossRef] [PubMed]
- Fogel, D.B. Factors Associated with Clinical Trials That Fail and Opportunities for Improving the Likelihood of Success: A Review. Contemp. Clin. Trials Commun. 2018, 11, 156–164. [Google Scholar] [CrossRef]
- Foulkes, R.; Man, E.; Thind, J.; Yeung, S.; Joy, A.; Hoskins, C. The Regulation of Nanomaterials and Nanomedicines for Clinical Application: Current and Future Perspectives. Biomater. Sci. 2020, 8, 4653–4664. [Google Scholar] [CrossRef] [PubMed]
- Metselaar, J.M.; Lammers, T. Challenges in Nanomedicine Clinical Translation. Drug Deliv. Transl. Res. 2020, 10, 721–725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Begley, C.G.; Ellis, L.M. Raise Standards for Preclinical Cancer Research. Nature 2012, 483, 531–533. [Google Scholar] [CrossRef] [PubMed]
- Pompili, L.; Porru, M.; Caruso, C.; Biroccio, A.; Leonetti, C. Patient-Derived Xenografts: A Relevant Preclinical Model for Drug Development. J. Exp. Clin. Cancer Res. 2016, 35, 189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koga, Y.; Ochiai, A. Systematic Review of Patient-Derived Xenograft Models for Preclinical Studies of Anti-Cancer Drugs in Solid Tumors. Cells 2019, 8, 418. [Google Scholar] [CrossRef] [Green Version]
- Bromma, K.; Bannister, A.; Kowalewski, A.; Cicon, L.; Chithrani, D.B. Elucidating the Fate of Nanoparticles among Key Cell Components of the Tumor Microenvironment for Promoting Cancer Nanotechnology. Cancer Nano 2020, 11, 8. [Google Scholar] [CrossRef]
- Islam, R.; Maeda, H.; Fang, J. Factors Affecting the Dynamics and Heterogeneity of the EPR Effect: Pathophysiological and Pathoanatomic Features, Drug Formulations and Physicochemical Factors. Expert Opin. Drug Deliv. 2022, 19, 199–212. [Google Scholar] [CrossRef]
- Maeda, H. Toward a Full Understanding of the EPR Effect in Primary and Metastatic Tumors as Well as Issues Related to Its Heterogeneity. Adv. Drug Deliv. Rev. 2015, 91, 3–6. [Google Scholar] [CrossRef]
- Park, J.; Choi, Y.; Chang, H.; Um, W.; Ryu, J.H.; Kwon, I.C. Alliance with EPR Effect: Combined Strategies to Improve the EPR Effect in the Tumor Microenvironment. Theranostics 2019, 9, 8073–8090. [Google Scholar] [CrossRef]
- de la Harpe, K.; Kondiah, P.; Choonara, Y.; Marimuthu, T.; du Toit, L.; Pillay, V. The Hemocompatibility of Nanoparticles: A Review of Cell–Nanoparticle Interactions and Hemostasis. Cells 2019, 8, 1209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Bai, Y.; Jia, J.; Gao, N.; Li, Y.; Zhang, R.; Jiang, G.; Yan, B. Perturbation of Physiological Systems by Nanoparticles. Chem. Soc. Rev. 2014, 43, 3762–3809. [Google Scholar] [CrossRef] [PubMed]
- Hante, N.K.; Medina, C.; Santos-Martinez, M.J. Effect on Platelet Function of Metal-Based Nanoparticles Developed for Medical Applications. Front. Cardiovasc. Med. 2019, 6, 139. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.M.; Schoen, F.J. In Vivo Assessment of Tissue Compatibility. Biomater. Sci. 2020, 14, 102436512. [Google Scholar]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, A.A.; Abuwatfa, W.H.; Al-Sayah, M.H.; Husseini, G.A. Gold-Nanoparticle Hybrid Nanostructures for Multimodal Cancer Therapy. Nanomaterials 2022, 12, 3706. https://doi.org/10.3390/nano12203706
Ali AA, Abuwatfa WH, Al-Sayah MH, Husseini GA. Gold-Nanoparticle Hybrid Nanostructures for Multimodal Cancer Therapy. Nanomaterials. 2022; 12(20):3706. https://doi.org/10.3390/nano12203706
Chicago/Turabian StyleAli, Amaal Abdulraqeb, Waad H. Abuwatfa, Mohammad H. Al-Sayah, and Ghaleb A. Husseini. 2022. "Gold-Nanoparticle Hybrid Nanostructures for Multimodal Cancer Therapy" Nanomaterials 12, no. 20: 3706. https://doi.org/10.3390/nano12203706





