High-Performance Ternary NiCoMo Electrocatalyst with Three-Dimensional Nanosheets Array Structure
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Nickel-Cobalt-Molybdenum Layered Double Hydroxide Materials
2.3. Characterization
2.4. Electrochemical Measurements
3. Results and Discussion
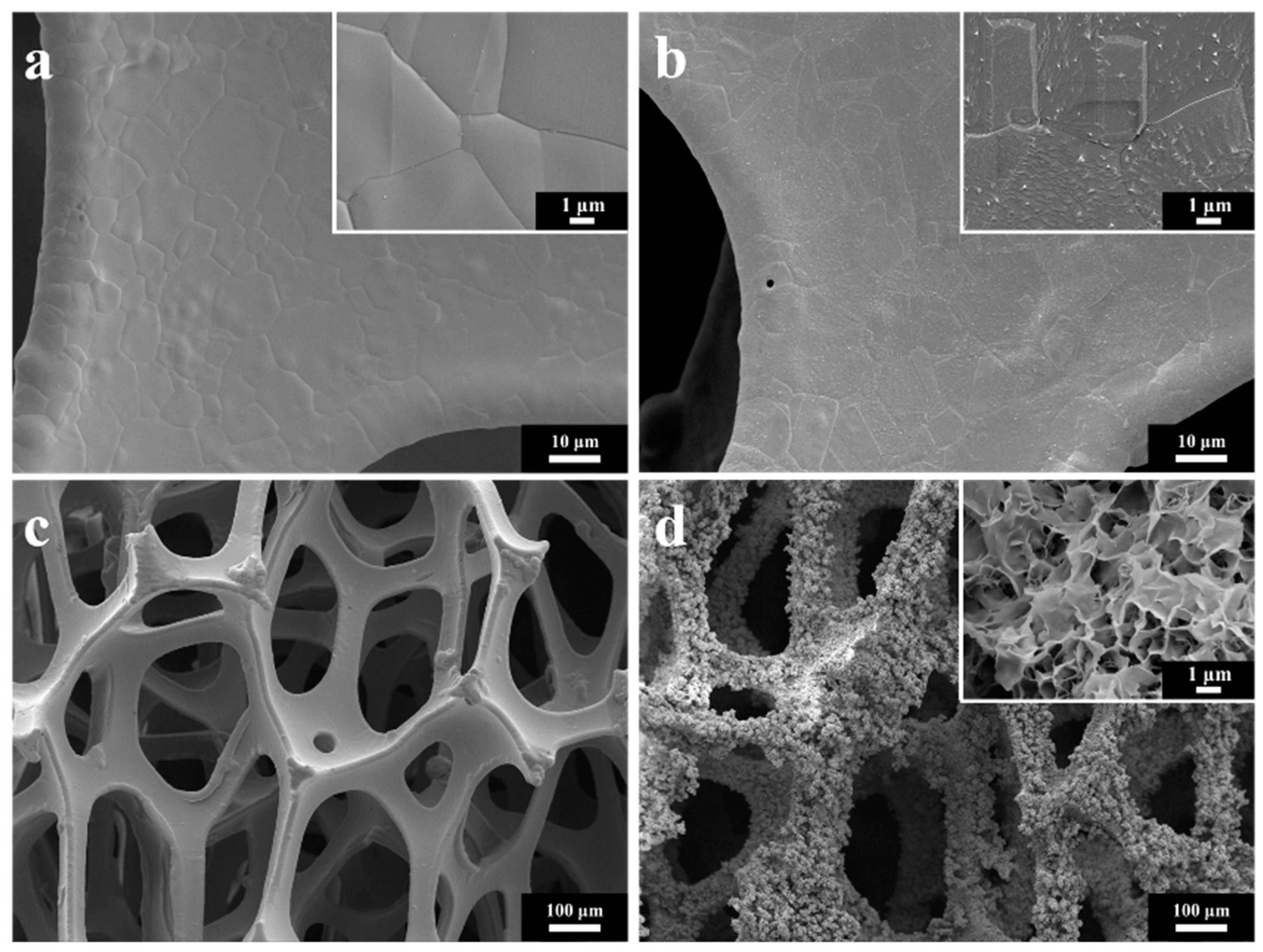
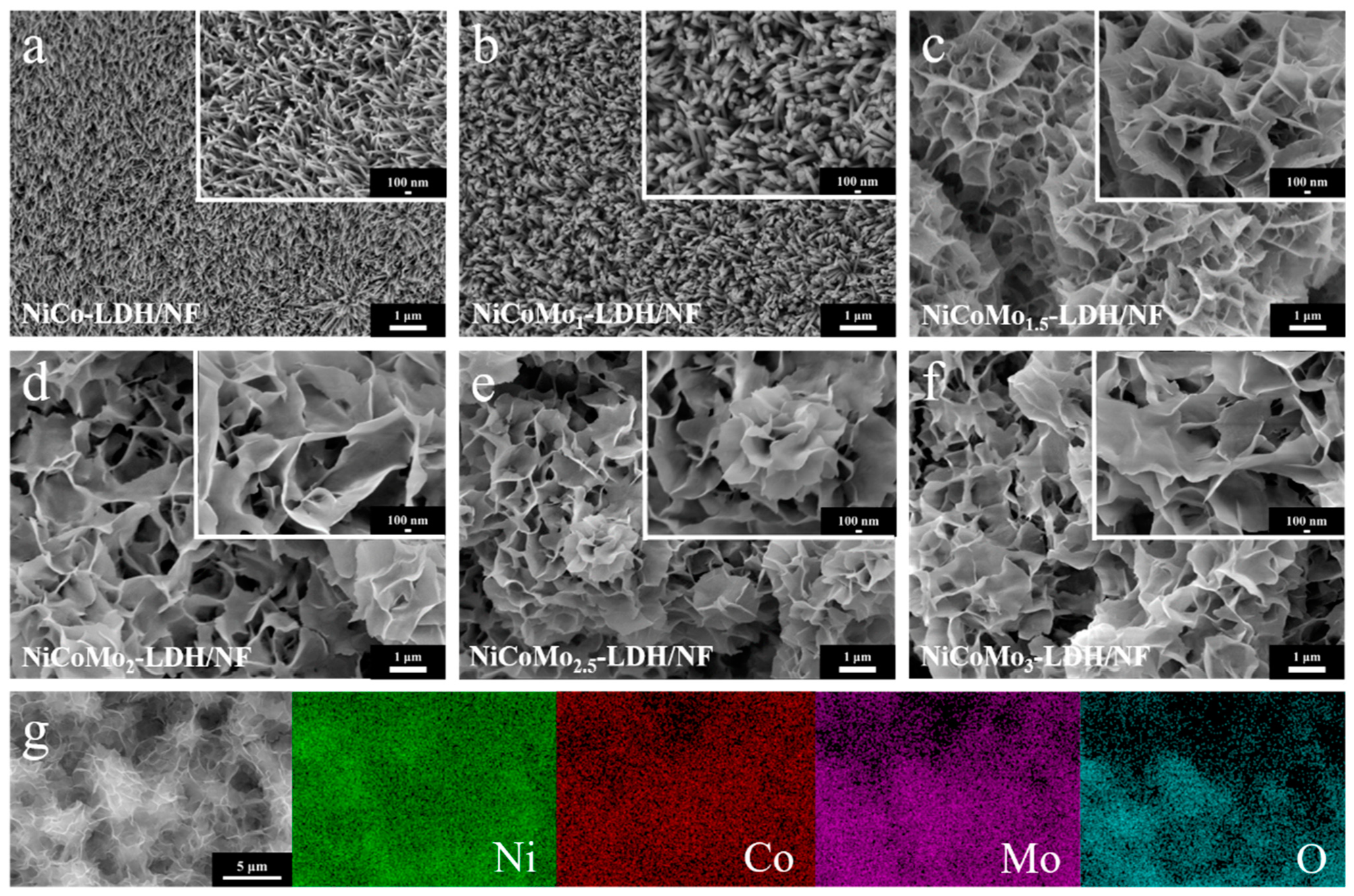
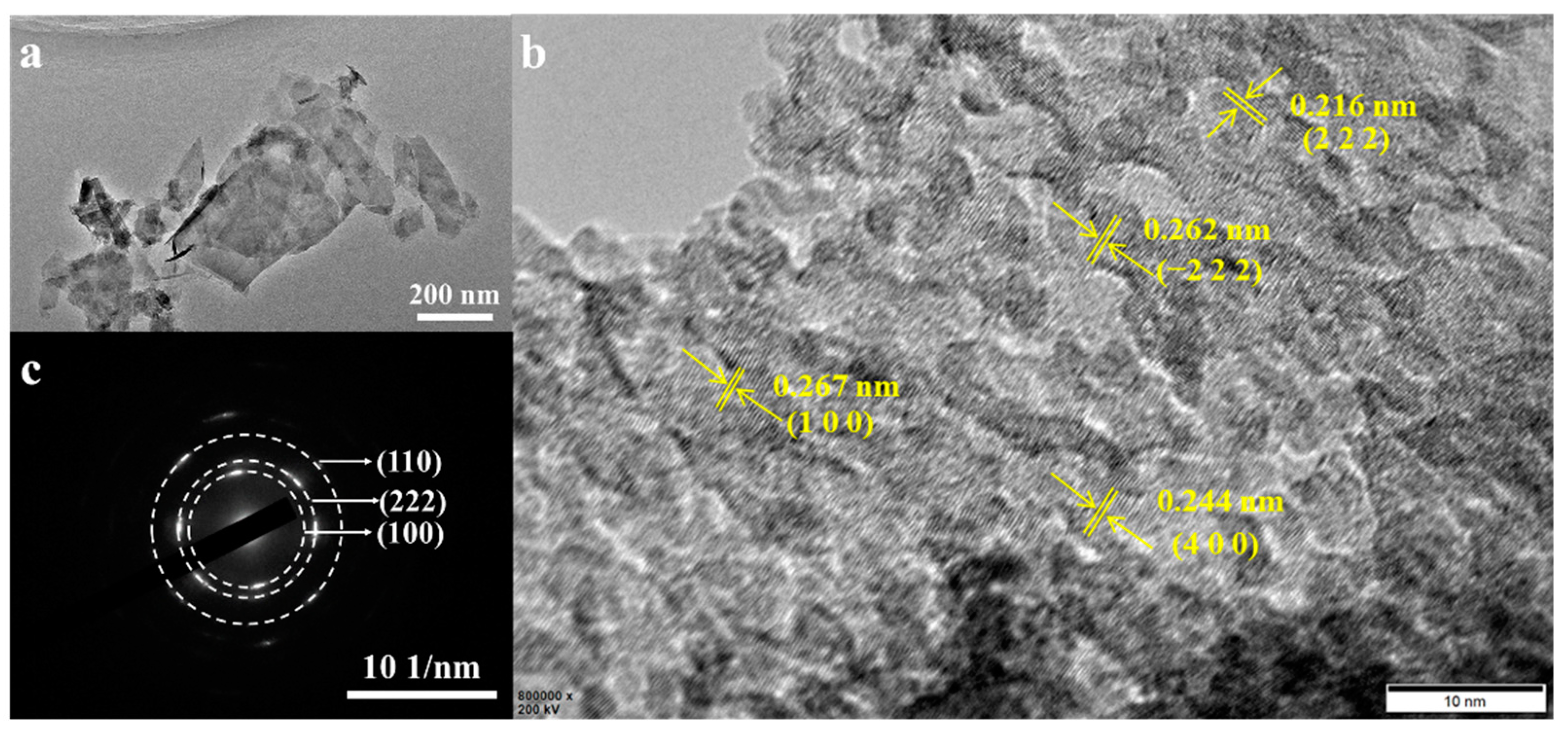
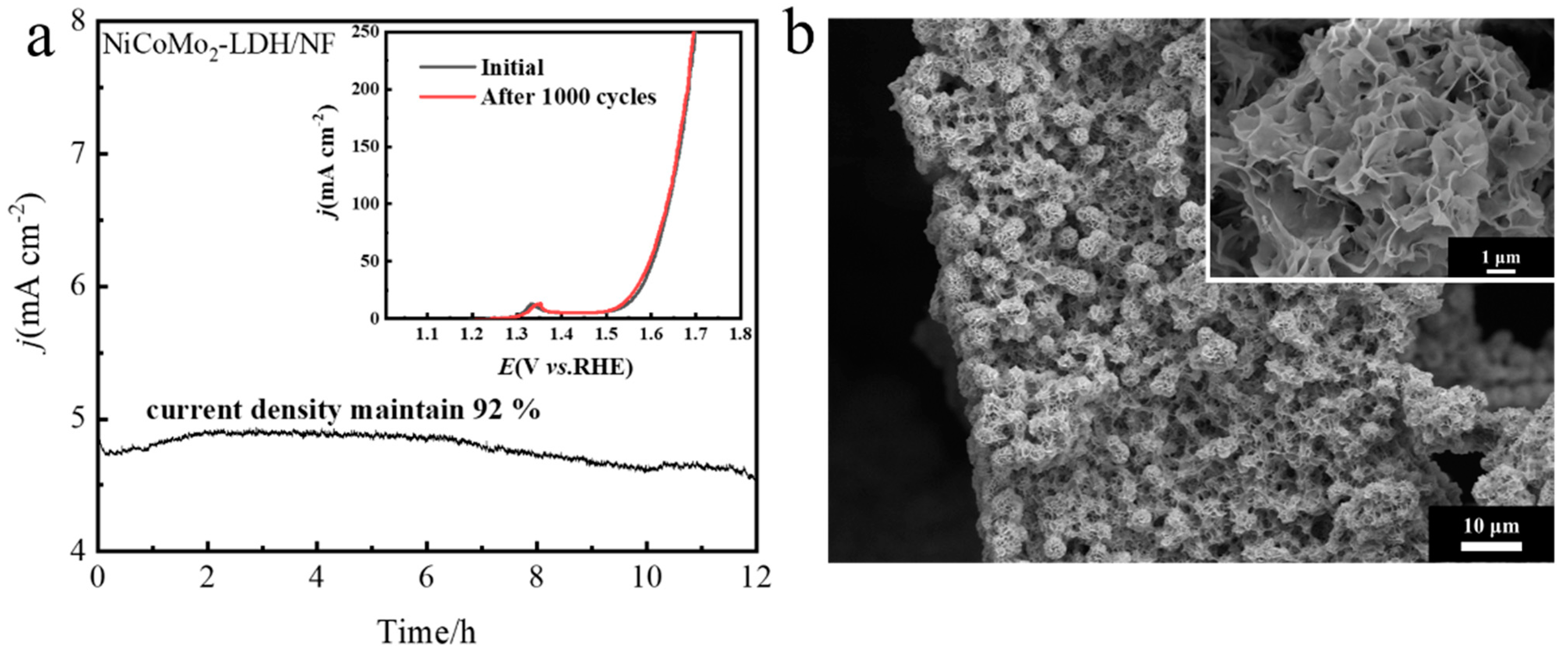
3.1. Structure and Morphology Characterization of NiCoMo-LDH/NF Catalyst
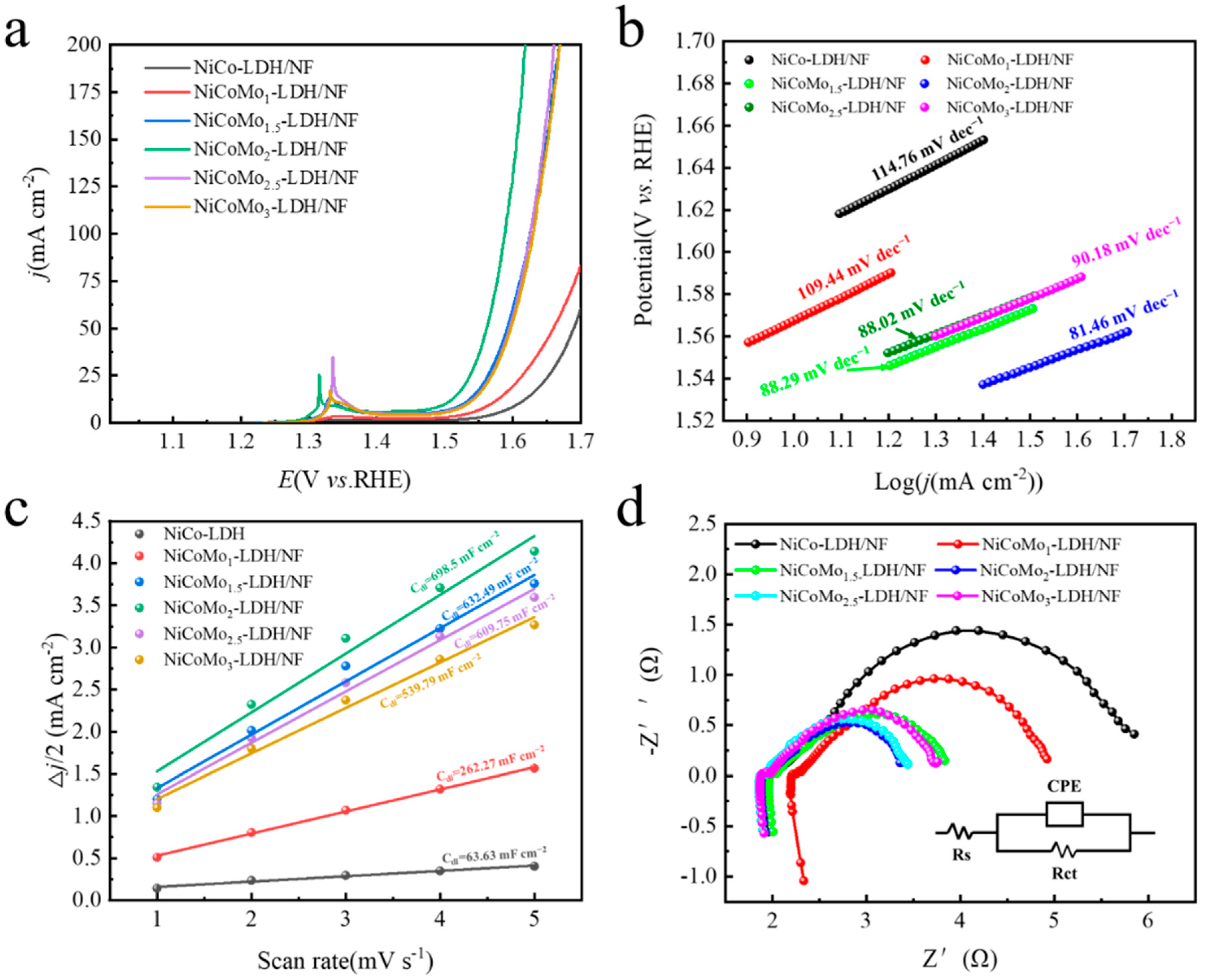
3.2. Oxygen Evolution Reaction Performance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zou, X.; Zhang, Y. Noble metal-free hydrogen evolution catalysts for water splitting. Chem. Soc. Rev. 2015, 44, 5148–5180. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Ge, R.; Zhang, Y.; Liu, D.; Wu, D.; Sun, X.; Du, B.; Wei, Q. Cobalt–borate nanowire array as a high-performance catalyst for oxygen evolution reaction in near-neutral media. J. Mater. Chem. A 2017, 5, 7291–7294. [Google Scholar] [CrossRef]
- Thenuwara, A.C.; Shumlas, S.L.; Attanayake, N.H.; Aulin, Y.V.; McKendry, I.G.; Qiao, Q.; Zhu, Y.; Borguet, E.; Zdilla, M.J.; Strongin, D.R. Intercalation of Cobalt into the Interlayer of Birnessite Improves Oxygen Evolution Catalysis. ACS Catal. 2016, 6, 7739–7743. [Google Scholar] [CrossRef]
- Esswein, A.J.; Nocera, D.G. Hydrogen Production by Molecular Photocatalysis. Chem. Rev. 2007, 107, 4022–4047. [Google Scholar] [CrossRef]
- Veziroğlu, T.N.; Şahi, S. 21st Century’s energy: Hydrogen energy system. Energy Convers. Manag. 2008, 49, 1820–1831. [Google Scholar] [CrossRef]
- Masa, J.; Piontek, S.; Wilde, P.; Antoni, H.; Eckhard, T.; Chen, Y.-T.; Muhler, M.; Apfel, U.; Schuhmann, W. Ni-Metalloid (B, Si, P, As, and Te) Alloys as Water Oxidation Electrocatalysts. Adv. Energy Mater. 2019, 9, 1900796. [Google Scholar] [CrossRef]
- He, Q.; Xie, H.; Rehman, Z.U.; Wang, C.; Wan, P.; Jiang, H.; Chu, W.; Song, L. Highly defective Fe-based oxyhydroxides from electrochemical reconstruction for efficient oxygen evolution catalysis. ACS Energy Lett. 2018, 3, 861–868. [Google Scholar] [CrossRef]
- Song, F.; Hu, X. Ultrathin cobalt-manganese layered double hydroxide is an efficient oxygen evolution catalyst. J. Am. Chem. Soc. 2014, 136, 16481–16484. [Google Scholar] [CrossRef]
- Fan, K.; Chen, H.; Ji, Y.; Huang, H.; Claesson, H.H.P.M.; Daniel, Q.; Philippe, B.; Rensmo, B.P.H.; Li, F.; Luo, Y.; et al. Nickel-vanadium monolayer double hydroxide for efficient electrochemical water oxidation. Nat. Commun. 2016, 7, 11981. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Dang, L.; Shearer, M.J.; Sheng, H.; Li, W.; Chen, J.; Xiao, P.; Zhang, Y.; Hamers, R.J.; Jin, S. Highly Active Trimetallic NiFeCr Layered Double Hydroxide Electrocatalysts for Oxygen Evolution Reaction. Adv. Energy Mater. 2018, 8, 1703189. [Google Scholar] [CrossRef]
- Liu, H.; Wang, Y.; Lu, X.; Hu, Y.; Zhu, G.; Chen, R.; Ma, L.; Zhu, H.; Tie, Z.; Liu, J.; et al. The effects of Al substitution and partial dissolution on ultrathin NiFeAl trinary layered double hydroxide nanosheets for oxygen evolution reaction in alkaline solution. Nano Energy 2017, 35, 350–357. [Google Scholar] [CrossRef]
- Ma, F.; Wu, Q.; Liu, M.; Zheng, L.; Tong, F.; Wang, Z.; Wang, P.; Liu, Y.; Cheng, H.; Dai, Y.; et al. Surface Fluorination Engineering of NiFe Prussian Blue Analogue Derivatives for Highly Efficient Oxygen Evolution Reaction. ACS Appl. Mater. Interfaces 2021, 13, 5142–5152. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Xu, W.; Zhu, W.; Yang, Q.; Lei, X.; Liu, J.; Li, Y.; Sun, X.; Duan, X. Three-dimensional NiFe layered double hydroxide film for high-efficiency oxygen evolution reaction. Chem. Commun. 2014, 50, 6479–6482. [Google Scholar] [CrossRef]
- Li, Z.; Shao, M.; An, H.; Wang, Z.; Xu, S.; Wei, M.; Evans, D.G.; Duan, X. Fast electrosynthesis of Fe-containing layered double hydroxide arrays toward highly efficient electrocatalytic oxidation reactions. Chem. Sci. 2015, 6, 6624–6631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, J.; Im, J.H.; Mayer, M.T.; Schreier, M.; Nazeeruddin, M.K.; Park, N.G.; Tilley, S.D.; Fan, H.J.; Grätzel, M. Water photolysis at 12.3% efficiency via perovskite photovoltaics and Earth-abundant catalysts. Science 2014, 345, 1593–1596. [Google Scholar] [CrossRef]
- Ye, C.; Zhang, L.; Yue, L.; Deng, B.; Cao, Y.; Liu, Q.; Luo, Y.; Lu, S.; Zheng, B.; Sun, X. A NiCo LDH nanosheet array on graphite felt: An efficient 3D electrocatalyst for the oxygen evolution reaction in alkaline media. Inorg. Chem. Front. 2021, 8, 3162–3166. [Google Scholar] [CrossRef]
- Feng, L.; Du, Y.; Huang, J.; Cao, L.; Feng, L.; Feng, Y.; Liu, Q.; Yang, D.; Kajiyoshi, K. Nanoporous NiAl-LDH nanosheet arrays with optimized Ni active sites for efficient electrocatalytic alkaline water splitting. Sustain. Energy Fuels 2020, 4, 2850–2858. [Google Scholar] [CrossRef]
- Li P, Duan X, Kuang Y, Li Y, Zhang G, Liu W, et al Tuning Electronic Structure of NiFe Layered Double Hydroxides with Vanadium Doping toward High Efficient Electrocatalytic Water Oxidation. Adv. Energy Mater. 2018, 8, 1703341. [Google Scholar] [CrossRef]
- Long, X.; Xiao, S.; Wang, Z.; Zheng, X.; Yang, S. Co intake mediated formation of ultrathin nanosheets of transition metal LDH-an advanced electrocatalyst for oxygen evolution reaction. Chem. Commun. 2015, 51, 1120–1123. [Google Scholar] [CrossRef]
- Yan, X.; Hu, Q.-T.; Liu, J.; Zhang, W.-D.; Gu, Z.-G. Ultrafine-grained NiCo layered double hydroxide nanosheets with abundant active edge sites for highly enhanced electro-oxidation of urea. Electrochim. Acta 2021, 368, 137648. [Google Scholar] [CrossRef]
- Lin, C.-H.; Chen, C.-L.; Wang, J.-H. Mechanistic Studies of Water–Gas-Shift Reaction on Transition Metals. J. Phys. Chem. C 2011, 115, 18582–18588. [Google Scholar] [CrossRef]
- Jiang, Y.-F.; Yuan, C.-Z.; Zhou, X.; Liu, Y.-N.; Zhao, Z.-W.; Zhao, S.-J.; Xu, A.-W. Selenium phosphorus co-doped cobalt oxide nanosheets anchored on Co foil: A self-supported and stable bifunctional electrode for efficient electrochemical water splitting. Electrochim. Acta 2018, 292, 247–255. [Google Scholar] [CrossRef]
- Wang, J.-W.; Wang, Y.-F.; Zhang, J.-G.; Yu, Y.-L.; Zhou, G.-G.; Cheng, L.; Wang, L.-S.; Fang, Z.Z. Optimization of electrocatalytic properties of NiMoCo foam electrode for water electrolysis by post-treatment processing. Rare Met. 2015, 34, 802–807. [Google Scholar] [CrossRef]
- Hu, K.; Wu, M.; Hinokuma, S.; Ohto, T.; Wakisaka, M.; Fujita, J.-I.; Ito, Y. Boosting electrochemical water splitting via ternary NiMoCo hybrid nanowire arrays. J. Mater. Chem. A 2019, 7, 2156–2164. [Google Scholar] [CrossRef]
- He, W.; Wei, W.; Wen, B.; Chen, D.; Zhang, J.; Jiang, Y.; Dong, G.; Meng, Y.; Zhou, G.; Liu, J.M.; et al. Lamellar NiMoCo@CuS enabling electrocatalytic activity and stability for hydrogen evolution. Chem. Commun. 2019, 55, 10555–10558. [Google Scholar] [CrossRef]
- Tiwari, A.P.; Lee, K.; Kim, K.; Kim, J.; Novak, T.G.; Jeon, S. Conformally Coated Nickel Phosphide on 3D, Ordered Nanoporous Nickel for Highly Active and Durable Hydrogen Evolution. ACS Sustain. Chem. Eng. 2019, 7, 11500–11510. [Google Scholar] [CrossRef]
- Bae, S.H.; Kim, J.E.; Randriamahazaka, H.; Moon, S.Y.; Park, J.Y.; Oh, I.K. Seamlessly conductive 3D nanoarchitecture of core–shell Ni-Co nanowire network for highly efficient oxygen evolution. Adv. Energy Mater. 2017, 7, 1601492. [Google Scholar] [CrossRef]
- Li, X.; Lin, S.-Y.; Zhang, M.; Jiang, G.; Gao, H. Construction of Hierarchical Ni(OH)2@CoMoO4 Nanoflake Composite for High-Performance Supercapacitors. Nano 2016, 11, 1650050. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Zhao, D.; Liu, Y.; Tong, Y.; Wu, X.; Shen, G. NiMoCo layered double hydroxides for electrocatalyst and supercapacitor electrode. Sci. China Mater. 2020, 64, 581–591. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, W.; Xiao, Y.; Shi, Z.; Cao, X.; Tang, Y.; Gao, Q. CoNiSe2 heteronanorods decorated with layered-double-hydroxides for efficient hydrogen evolution. Appl. Catal. B Environ. 2018, 242, 132–139. [Google Scholar] [CrossRef]
- Liang, H.; Gandi, A.N.; Anjum, D.H.; Wang, X.; Schwingenschlogl, U.; Alshareef, H.N. Plasma-Assisted Synthesis of NiCoP for Efficient Overall Water Splitting. Nano Lett. 2016, 16, 7718–7725. [Google Scholar] [CrossRef] [PubMed]
- Pintado, S.; Goberna-Ferron, S.; Escudero-Adan, E.C.; Galan-Mascaros, J.R. Fast and persistent electrocatalytic water oxidation by Co-Fe Prussian blue coordination polymers. J. Am. Chem. Soc. 2013, 135, 13270–13273. [Google Scholar] [CrossRef] [PubMed]
- Ge, R.; Ren, X.; Ji, X.; Liu, Z.; Du, G.; Asiri, A.M.; Sun, X.; Chen, L. Benzoate Anion-Intercalated Layered Cobalt Hydroxide Nanoarray: An Efficient Electrocatalyst for the Oxygen Evolution Reaction. ChemSusChem 2017, 10, 4004–4008. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Xia, Z.; Zhang, Z.; Ma, Y.; Qu, Y. One-step synthesis of multi-walled carbon nanotubes/ultra-thin Ni(OH)2 nanoplate composite as efficient catalysts for water oxidation. J. Mater. Chem. A 2014, 2, 11799–11806. [Google Scholar] [CrossRef]
- Rovetta, A.A.; Browne, M.P.; Harvey, A.; Godwin, I.J.; Coleman, J.N.; Lyons, M.E. Cobalt hydroxide nanoflakes and their application as supercapacitors and oxygen evolution catalysts. Nanotechnology 2017, 28, 375401. [Google Scholar] [CrossRef]
- Ai, L.; Niu, Z.; Jiang, J. Mechanistic insight into oxygen evolution electrocatalysis of surface phosphate modified cobalt phosphide nanorod bundles and their superior performance for overall water splitting. Electrochim. Acta 2017, 242, 355–363. [Google Scholar] [CrossRef]
- Zhao, Z.; Wu, H.; He, H.; Xu, X.; Jin, Y. A High-Performance Binary Ni-Co Hydroxide-based Water Oxidation Electrode with Three-Dimensional Coaxial Nanotube Array Structure. Adv. Funct. Mater. 2014, 24, 4698–4705. [Google Scholar] [CrossRef]
- Liang, H.; Meng, F.; Cabán-Acevedo, M.; Li, L.; Forticaux, A.; Xiu, L.; Wang, Z.; Jin, S. Hydrothermal Continuous Flow Synthesis and Exfoliation of NiCo Layered Double Hydroxide Nanosheets for Enhanced Oxygen Evolution Catalysis. Nano Lett. 2015, 15, 1421–1427. [Google Scholar] [CrossRef]
- Song, F.; Hu, X. Exfoliation of layered double hydroxides for enhanced oxygen evolution catalysis. Nat. Commun. 2014, 5, 4477. [Google Scholar] [CrossRef] [Green Version]
- Gao, X.; Zhang, H.; Li, Q.; Yu, X.; Hong, Z.; Zhang, X.; Liang, C.; Lin, Z. Hierarchical NiCo2O4 Hollow Microcuboids as Bifunctional Electrocatalysts for Overall Water-Splitting. Angew. Chem. Int. Ed. Engl. 2016, 55, 6290–6294. [Google Scholar] [CrossRef]
- Liu, Q.; Jin, J.; Zhang, J. NiCo2S4@graphene as a bifunctional electrocatalyst for oxygen reduction and evolution reactions. ACS Appl. Mater. Interfaces 2013, 5, 5002–5008. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Du, N.; Zheng, W.; Li, X.; Dai, Y.; He, G. Ultrathin Ni-Co double hydroxide nanosheets with conformal graphene coating for highly active oxygen evolution reaction and lithium ion battery anode materials. Chem. Eng. J. 2017, 327, 9–17. [Google Scholar] [CrossRef]
- Xu, J.; Zhao, Y.; Li, M.; Fan, G.; Yang, L.; Li, F. A strong coupled 2D metal-organic framework and ternary layered double hydroxide hierarchical nanocomposite as an excellent electrocatalyst for the oxygen evolution reaction. Electrochim. Acta 2019, 307, 275–284. [Google Scholar] [CrossRef]
- Shinde, D.V.; Trizio, L.D.; Dang, Z.; Prato, M.; Gaspari, R.; Manna, L. Hollow and Porous Nickel Cobalt Perselenide Nanostructured Microparticles for Enhanced Electrocatalytic Oxygen Evolution. Chem. Mater. 2017, 29, 7032–7041. [Google Scholar] [CrossRef]
- Jiang, J.; Zhang, A.; Li, L.; Ai, L. Nickel–cobalt layered double hydroxide nanosheets as high-performance electrocatalyst for oxygen evolution reaction. J. Power Source 2015, 278, 445–451. [Google Scholar] [CrossRef]
- Sayeed, M.A.; Herd, T.; O’Mullane, A.P. Direct electrochemical formation of nanostructured amorphous Co(OH)2 on gold electrodes with enhanced activity for the oxygen evolution reaction. J. Mater. Chem. A 2016, 4, 991–999. [Google Scholar] [CrossRef]



| Catalyst | Current Density (mA cm−2) | Overpotential (mV) | Reference |
|---|---|---|---|
| Ni(OH)2 | 10 | 595 | [34] |
| Co(OH)2 nanoflake/Ni foam | 10 | 280 | [35] |
| Co-Ni-B/NF | 10 | 313 | [36] |
| NiCo hydroxide | 10 | 460 | [37] |
| NiCo-LDH | 10 | 367 | [38] |
| NiCo-NS | 10 | 334 | [39] |
| NiCo2O4 hollow microcuboids | 10 | 277 | [40] |
| NiCo2S4@graphene | 10 | 470 | [41] |
| NixCo2x(OH)6x@eRG/NF | 10 | 280 | [42] |
| ZIF-67/CoNiAl-LDH/NF | 10 | 303 | [43] |
| (Ni1−xCo1+xSe4)MPs | 10 | 320 | [44] |
| NiCo-LDH nanosheets | 10 | 290 | [45] |
| Co(OH)2 | 10 | 360 | [46] |
| NiCoMo2-LDH/NF | 10 | 270 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Z.; Lu, Z.; Li, S.; Li, Y.; Tan, G.; Hao, Y.; Wang, Y.; Huang, Y.; Zhang, X.; Li, S.; et al. High-Performance Ternary NiCoMo Electrocatalyst with Three-Dimensional Nanosheets Array Structure. Nanomaterials 2022, 12, 3716. https://doi.org/10.3390/nano12213716
Zhou Z, Lu Z, Li S, Li Y, Tan G, Hao Y, Wang Y, Huang Y, Zhang X, Li S, et al. High-Performance Ternary NiCoMo Electrocatalyst with Three-Dimensional Nanosheets Array Structure. Nanomaterials. 2022; 12(21):3716. https://doi.org/10.3390/nano12213716
Chicago/Turabian StyleZhou, Zhihao, Zhi Lu, Shilin Li, Yiting Li, Gongliang Tan, Yang Hao, Yu Wang, Yuzhao Huang, Xuefeng Zhang, Shuaifang Li, and et al. 2022. "High-Performance Ternary NiCoMo Electrocatalyst with Three-Dimensional Nanosheets Array Structure" Nanomaterials 12, no. 21: 3716. https://doi.org/10.3390/nano12213716




