Hot Electron-Driven Photocatalysis Using Sub-5 nm Gap Plasmonic Nanofinger Arrays
Abstract
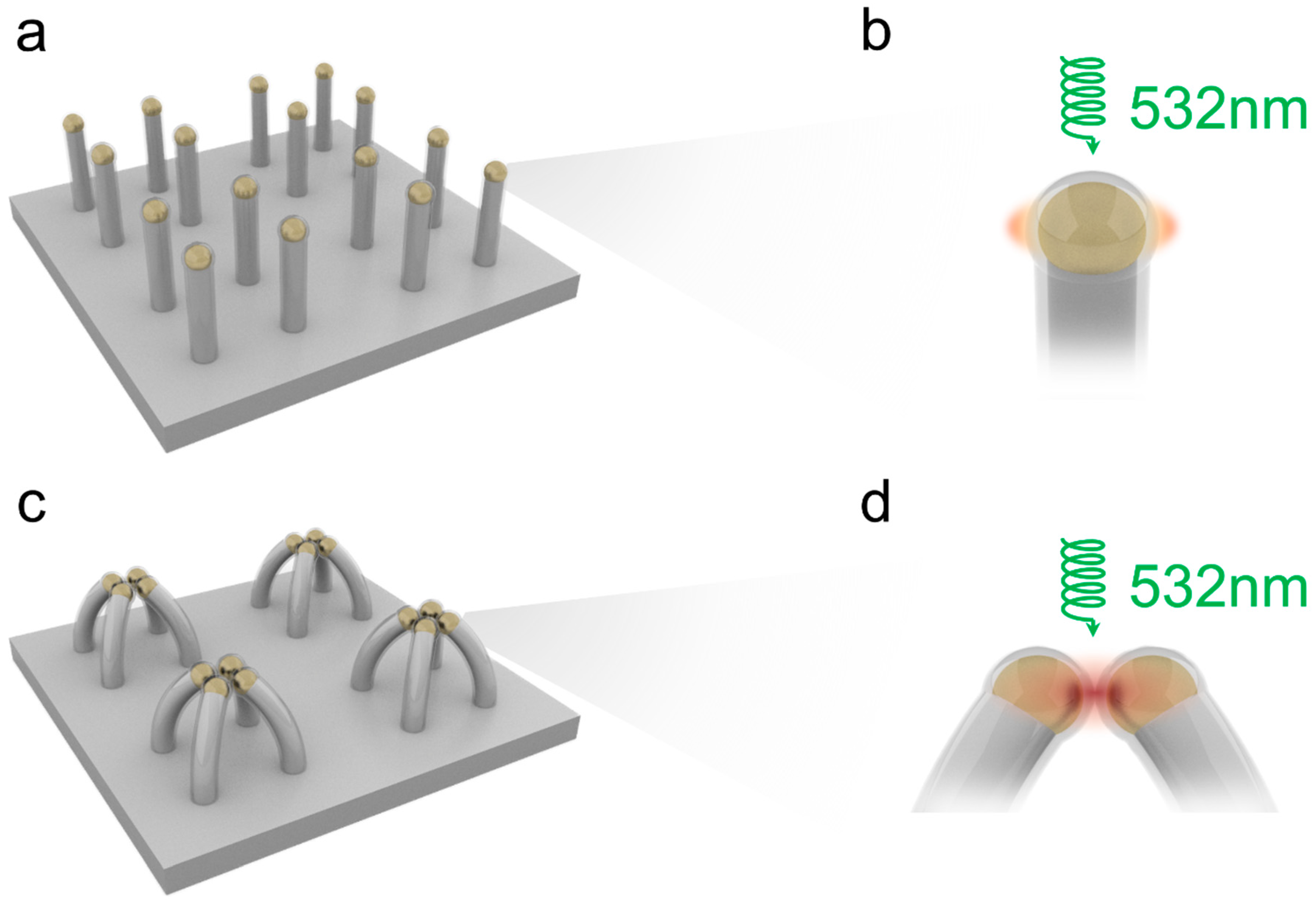
:1. Introduction
2. Materials and Methods
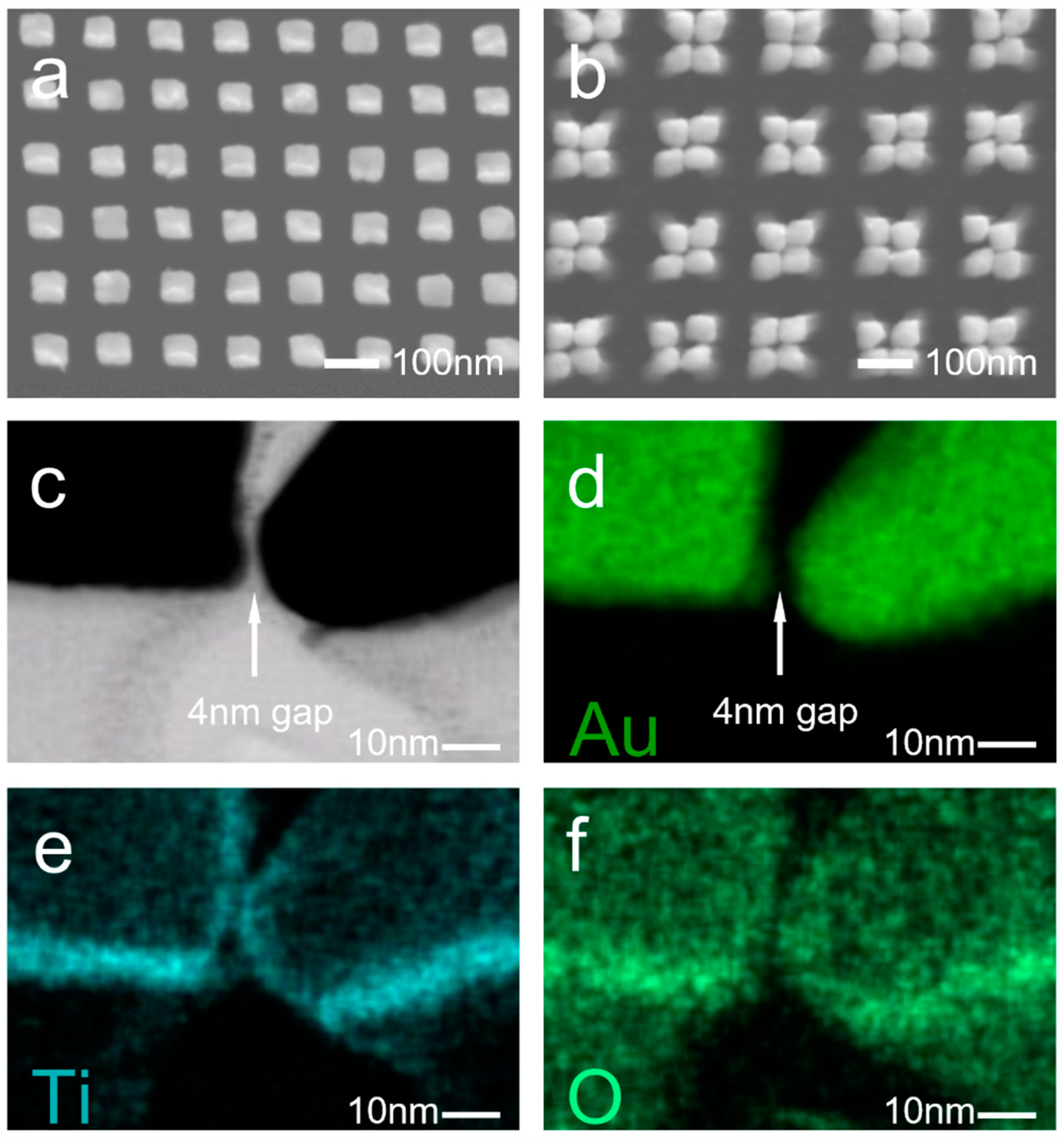
2.1. Fabrication of the Device
2.2. Characterization
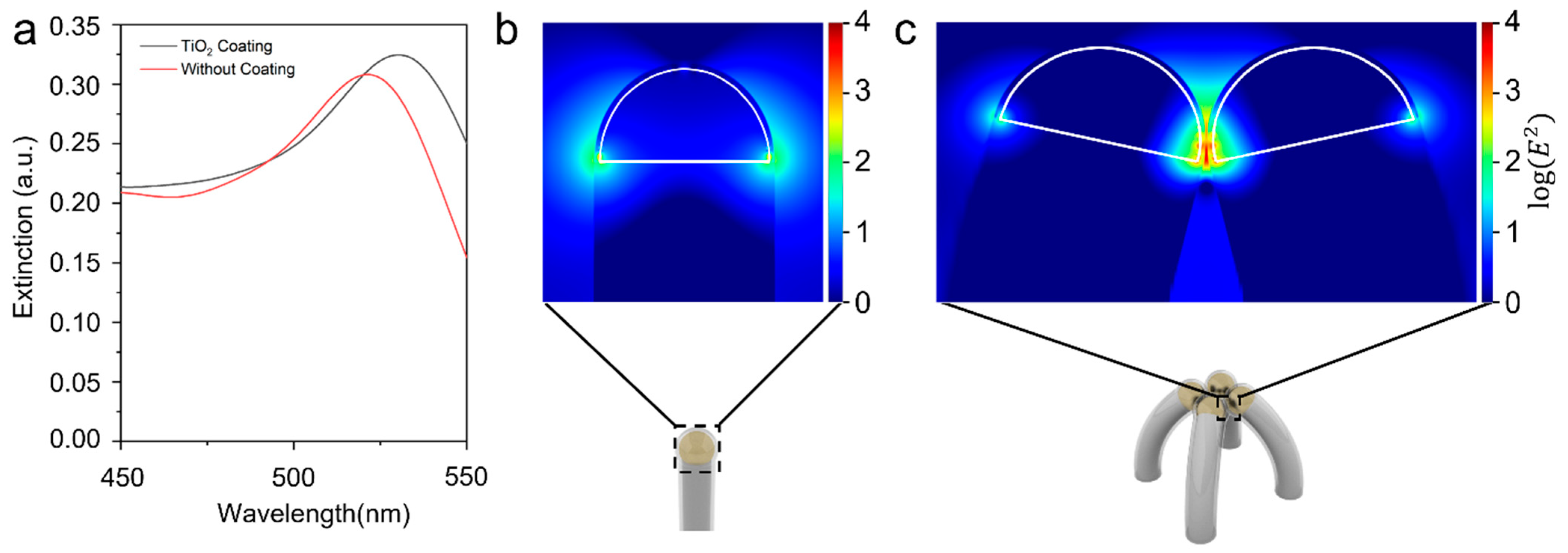
2.3. Electromagnetic Field Simulation
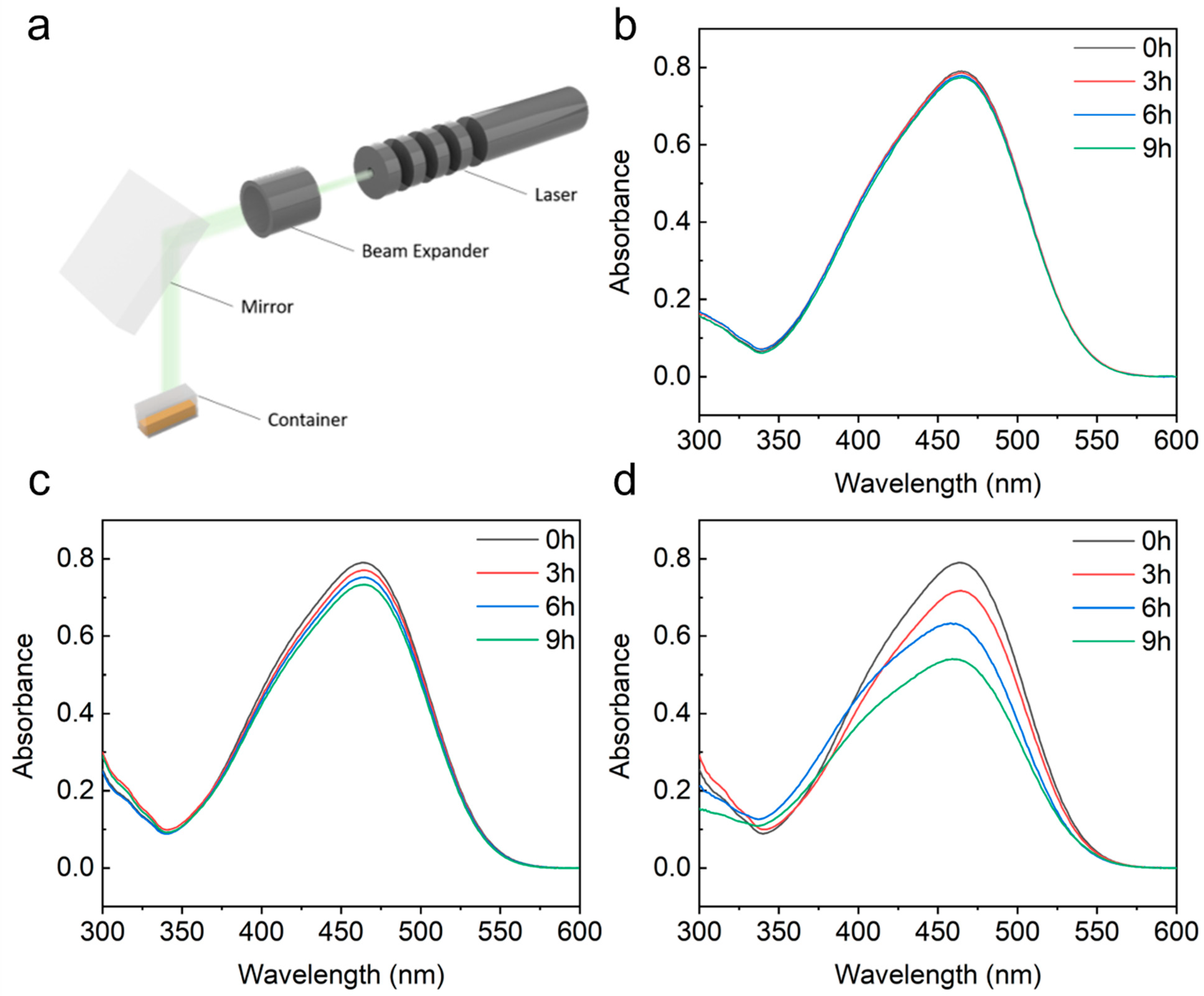
2.4. Photocatalysis Measurements
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Linic, S.; Aslam, U.; Boerigter, C.; Morabito, M. Photochemical transformations on plasmonic metal nanoparticles. Nat. Mater. 2015, 14, 567–576. [Google Scholar] [CrossRef] [PubMed]
- Linic, S.; Christopher, P.; Ingram, D.B. Plasmonic-metal nanostructures for efficient conversion of solar to chemical energy. Nat. Mater. 2011, 10, 911–921. [Google Scholar] [CrossRef] [PubMed]
- Hou, W.B.; Cronin, S.B. A Review of Surface Plasmon Resonance-Enhanced Photocatalysis. Adv. Funct. Mater. 2013, 23, 1612–1619. [Google Scholar] [CrossRef]
- Mubeen, S.; Lee, J.; Singh, N.; Kramer, S.; Stucky, G.D.; Moskovits, M. An autonomous photosynthetic device in which all charge carriers derive from surface plasmons. Nat. Nanotechnol. 2013, 8, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Sil, D.; Gilroy, K.D.; Niaux, A.; Boulesbaa, A.; Neretina, S.; Borguet, E. Seeing Is Believing: Hot Electron Based Gold Nanoplasmonic Optical Hydrogen Sensor. ACS Nano 2014, 8, 7755–7762. [Google Scholar] [CrossRef]
- Hoffmann, M.R.; Martin, S.T.; Choi, W.Y.; Bahnemann, D.W. Environmental Applications of Semiconductor Photocatalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar] [CrossRef]
- Halas, N.J.; Lal, S.; Chang, W.S.; Link, S.; Nordlander, P. Plasmons in strongly coupled metallic nanostructures. Chem. Rev. 2011, 111, 3913–3961. [Google Scholar] [CrossRef]
- Sundararaman, R.; Narang, P.; Jermyn, A.S.; Goddard, W.A., III; Atwater, H.A. Theoretical predictions for hot-carrier generation from surface plasmon decay. Nat. Commun. 2014, 5, 5788. [Google Scholar] [CrossRef] [Green Version]
- Manjavacas, A.; Liu, J.G.; Kulkarni, V.; Nordlander, P. Plasmon-induced hot carriers in metallic nanoparticles. ACS Nano 2014, 8, 7630–7638. [Google Scholar] [CrossRef]
- Cortés, E.; Xie, W.; Cambiasso, J.; Jermyn, A.S.; Sundararaman, R.; Narang, P.; Schlücker, S.; Maier, S.A. Plasmonic hot electron transport drives nano-localized chemistry. Nat. Commun. 2017, 8, 14880. [Google Scholar] [CrossRef]
- Govorov, A.O.; Zhang, H.; Gun’ko, Y.K. Theory of photoinjection of hot plasmonic carriers from metal nanostructures into semiconductors and surface molecules. J. Phys. Chem. C 2013, 117, 16616–16631. [Google Scholar] [CrossRef]
- Clavero, C. Plasmon-induced hot-electron generation at nanoparticle/metal-oxide interfaces for photovoltaic and photocatalytic devices. Nat. Photon. 2014, 8, 95–103. [Google Scholar] [CrossRef]
- Li, S.; Miao, P.; Zhang, Y.; Wu, J.; Zhang, B.; Du, Y.; Han, X.; Sun, J.; Xu, P. Recent Advances in Plasmonic Nanostructures for Enhanced Photocatalysis and Electrocatalysis. Adv. Mater. 2021, 33, e2000086. [Google Scholar] [CrossRef] [PubMed]
- Linic, S.; Chavez, S.; Elias, R. Flow and extraction of energy and charge carriers in hybrid plasmonic nanostructures. Nat. Mater. 2021, 20, 916–924. [Google Scholar] [CrossRef] [PubMed]
- Amidani, L.; Naldoni, A.; Malvestuto, M.; Marelli, M.; Glatzel, P.; Dal Santo, V.; Boscherini, F. Probing Long-Lived Plasmonic-Generated Charges in TiO2/Au by High-Resolution X-ray Absorption Spectroscopy. Angew. Chem. Int. Ed. 2015, 54, 5413–5416. [Google Scholar] [CrossRef]
- DuChene, J.S.; Sweeny, B.C.; Johnston-Peck, A.C.; Su, D.; Stach, E.A.; Wei, W.D. Prolonged hot electron dynamics in plasmonic-metal/semiconductor heterostructures with implications for solar photocatalysis. Angew. Chem. Int. Ed. 2014, 53, 7887–7891. [Google Scholar] [CrossRef]
- Liu, Z.; Hou, W.; Pavaskar, P.; Aykol, M.; Cronin, S.B. Plasmon resonant enhancement of photocatalytic water splitting under visible illumination. Nano Lett. 2011, 11, 1111–1116. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, T.; Gong, J. Mechanistic understanding of the plasmonic enhancement for solar water splitting. Adv. Mater. 2015, 27, 5328–5342. [Google Scholar] [CrossRef]
- Abed, J.; Rajput, N.S.; Moutaouakil, A.E.; Jouiad, M. Recent advances in the design of plasmonic Au/TiO2 nanostructures for enhanced photocatalytic water splitting. Nanomaterials 2020, 10, 2260. [Google Scholar] [CrossRef]
- Nahar, S.; Zain, M.; Kadhum, A.A.H.; Hasan, H.A.; Hasan, M.R. Advances in photocatalytic CO2 reduction with water: A review. Materials 2017, 10, 629. [Google Scholar] [CrossRef]
- Jiang, X.; Huang, J.; Bi, Z.; Ni, W.; Gurzadyan, G.; Zhu, Y.; Zhang, Z. Plasmonic Active “Hot Spots”-Confined Photocatalytic CO2 Reduction with High Selectivity for CH4 Production. Adv. Mater. 2022, 34, 2109330. [Google Scholar] [CrossRef] [PubMed]
- Pincella, F.; Isozaki, K.; Miki, K. A visible light-driven plasmonic photocatalyst. Light-Sci. Appl. 2014, 3, e133. [Google Scholar] [CrossRef] [Green Version]
- Bian, Z.; Tachikawa, T.; Zhang, P.; Fujitsuka, M.; Majima, T. Au/TiO2 superstructure-based plasmonic photocatalysts exhibiting efficient charge separation and unprecedented activity. J. Am. Chem. Soc. 2014, 136, 458–465. [Google Scholar] [CrossRef] [PubMed]
- Salmistraro, M.; Schwartzberg, A.; Bao, W.; Depero, L.E.; Weber-Bargioni, A.; Cabrini, S.; Alessandri, I. Triggering and Monitoring Plasmon-Enhanced Reactions by Optical Nanoantennas Coupled to Photocatalytic Beads. Small 2013, 9, 3301–3307. [Google Scholar] [CrossRef] [PubMed]
- Salmistraro, M.; Sassolini, S.; Weber-Bargioni, A.; Cabrini, S.; Alessandri, I. Fabrication of gold nanoantennas on SiO2/TiO2 core/shell beads to study photon-driven surface reactions. Microelectron. Eng. 2015, 143, 69–73. [Google Scholar] [CrossRef] [Green Version]
- Song, B.X.; Yao, Y.H.; Groenewald, R.E.; Wang, Y.X.; Liu, H.; Wang, Y.F.; Li, Y.R.; Liu, F.X.; Cronin, S.B.; Schwartzberg, A.M.; et al. Probing Gap Plasmons Down to Subnanometer Scales Using Collapsible Nanofingers. ACS Nano 2017, 11, 5836–5843. [Google Scholar] [CrossRef] [Green Version]
- Song, B.; Jiang, Z.; Liu, Z.; Wang, Y.; Liu, F.; Cronin, S.B.; Yang, H.; Meng, D.; Chen, B.; Hu, P.; et al. Probing the Mechanisms of Strong Fluorescence Enhancement in Plasmonic Nanogaps with Sub-Nanometer Precision. ACS Nano 2020, 14, 14769–14778. [Google Scholar] [CrossRef]
- Liu, F.; Song, B.; Su, G.; Liang, O.; Zhan, P.; Wang, H.; Wu, W.; Xie, Y.; Wang, Z. Sculpting Extreme Electromagnetic Field Enhancement in Free Space for Molecule Sensing. Small 2018, 14, e1801146. [Google Scholar] [CrossRef]
- Yang, L.; Wang, H.; Fang, Y.; Li, Z. Polarization State of Light Scattered from Quantum Plasmonic Dimer Antennas. ACS Nano 2016, 10, 1580–1588. [Google Scholar] [CrossRef]
- Duan, H.; Hu, H.; Kumar, K.; Shen, Z.; Yang, J.K. Direct and reliable patterning of plasmonic nanostructures with sub-10-nm gaps. ACS Nano 2011, 5, 7593–7600. [Google Scholar] [CrossRef]
- Kim, A.; Ou, F.S.; Ohlberg, D.A.; Hu, M.; Williams, R.S.; Li, Z. Study of molecular trapping inside gold nanofinger arrays on surface-enhanced Raman substrates. J. Am. Chem. Soc. 2011, 133, 8234–8239. [Google Scholar] [CrossRef] [PubMed]
- Todisco, F.; Esposito, M.; Panaro, S.; De Giorgi, M.; Dominici, L.; Ballarini, D.; Fernandez-Dominguez, A.I.; Tasco, V.; Cuscuna, M.; Passaseo, A.; et al. Toward Cavity Quantum Electrodynamics with Hybrid Photon Gap-Plasmon States. ACS Nano 2016, 10, 11360–11368. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Y.; van de Groep, J.; Talin, A.A.; Brongersma, M.L. Dynamic Tuning of Gap Plasmon Resonances Using a Solid-State Electrochromic Device. Nano Lett. 2019, 19, 7988–7995. [Google Scholar] [CrossRef] [PubMed]
- Li, G.C.; Zhang, Y.L.; Jiang, J.; Luo, Y.; Lei, D.Y. Metal-Substrate-Mediated Plasmon Hybridization in a Nanoparticle Dimer for Photoluminescence Line-Width Shrinking and Intensity Enhancement. ACS Nano 2017, 11, 3067–3080. [Google Scholar] [CrossRef] [PubMed]
- Lumdee, C.; Yun, B.F.; Kik, P.G. Gap-Plasmon Enhanced Gold Nanoparticle Photoluminescence. ACS Photonics 2014, 1, 1224–1230. [Google Scholar] [CrossRef]
- Marinica, D.C.; Kazansky, A.K.; Nordlander, P.; Aizpurua, J.; Borisov, A.G. Quantum plasmonics: Nonlinear effects in the field enhancement of a plasmonic nanoparticle dimer. Nano Lett. 2012, 12, 1333–1339. [Google Scholar] [CrossRef] [PubMed]
- Tame, M.S.; McEnery, K.R.; Ozdemir, S.K.; Lee, J.; Maier, S.A.; Kim, M.S. Quantum plasmonics. Nat. Phys. 2013, 9, 329–340. [Google Scholar] [CrossRef] [Green Version]
- Esteban, R.; Borisov, A.G.; Nordlander, P.; Aizpurua, J. Bridging quantum and classical plasmonics with a quantum-corrected model. Nat. Commun. 2012, 3, 825. [Google Scholar] [CrossRef] [Green Version]
- Lin, J.J.; Shen, J.X.; Wang, R.J.; Cui, J.J.; Zhou, W.J.; Hu, P.G.; Liu, D.O.; Liu, H.; Wang, J.Y.; Boughton, R.I.; et al. Nano-p-n junctions on surface-coarsened TiO2 nanobelts with enhanced photocatalytic activity. J. Mater. Chem. 2011, 21, 5106–5113. [Google Scholar] [CrossRef]
- George, S.M. Atomic layer deposition: An overview. Chem. Rev. 2010, 110, 111–131. [Google Scholar] [CrossRef]
- Garcia de Abajo, F.J. Nonlocal effects in the plasmons of strongly interacting nanoparticles, dimers, and waveguides. J. Phys. Chem. C 2008, 112, 17983–17987. [Google Scholar] [CrossRef]
- Dong, T.Y.; Ma, X.K.; Mittra, R. Optical response in subnanometer gaps due to nonlocal response and quantum tunneling. Appl. Phys. Lett. 2012, 101, 233111. [Google Scholar] [CrossRef]
- Teperik, T.V.; Nordlander, P.; Aizpurua, J.; Borisov, A.G. Robust subnanometric plasmon ruler by rescaling of the nonlocal optical response. Phys. Rev. Lett. 2013, 110, 263901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jain, P.K.; Huang, W.Y.; El-Sayed, M.A. On the universal scaling behavior of the distance decay of plasmon coupling in metal nanoparticle pairs: A plasmon ruler equation. Nano Lett. 2007, 7, 2080–2088. [Google Scholar] [CrossRef]
- Romero, I.; Aizpurua, J.; Bryant, G.W.; de Abajo, F.J.G. Plasmons in nearly touching metallic nanoparticles: Singular response in the limit of touching dimers. Opt. Express 2006, 14, 9988–9999. [Google Scholar] [CrossRef] [PubMed]
- McMahon, J.M.; Gray, S.K.; Schatz, G.C. Optical properties of nanowire dimers with a spatially nonlocal dielectric function. Nano Lett. 2010, 10, 3473–3481. [Google Scholar] [CrossRef] [PubMed]
- David, C.; de Abajo, F.J.G. Spatial Nonlocality in the Optical Response of Metal Nanoparticles. J. Phys. Chem. C 2011, 115, 19470–19475. [Google Scholar] [CrossRef]
- Teperik, T.V.; Nordlander, P.; Aizpurua, J.; Borisov, A.G. Quantum effects and nonlocality in strongly coupled plasmonic nanowire dimers. Opt. Express 2013, 21, 27306–27325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toscano, G.; Raza, S.; Xiao, S.; Wubs, M.; Jauho, A.P.; Bozhevolnyi, S.I.; Mortensen, N.A. Surface-enhanced Raman spectroscopy: Nonlocal limitations. Opt. Lett. 2012, 37, 2538–2540. [Google Scholar] [CrossRef] [Green Version]
- Tan, S.F. Quantum plasmon resonances controlled by molecular tunnel junction. In Molecular Electronic Control over Tunneling Charge Transfer Plasmons Modes; Springer: Cham, Switzerland, 2018; pp. 51–67. [Google Scholar]
- Nordlander, P.; Oubre, C.; Prodan, E.; Li, K.; Stockman, M.I. Plasmon hybridization in nanoparticle dimers. Nano Lett. 2004, 4, 899–903. [Google Scholar] [CrossRef]
- Tiwana, P.; Docampo, P.; Johnston, M.B.; Snaith, H.J.; Herz, L.M. Electron Mobility and Injection Dynamics in Mesoporous ZnO, SnO2, and TiO2 Films Used in Dye-Sensitized Solar Cells. ACS Nano 2011, 5, 5158–5166. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, M.; Matsuda, K.; Tanaka, M.; Kuboyama, S.; Uto, K.; Wada, N.; Kawazumi, H.; Tsuji, T.; Ago, H.; Hayashi, J.I. Enhanced photocatalytic degradation of methyl orange by Au/TiO2 nanoparticles under neutral and acidic solutions. ChemistrySelect 2018, 3, 1432–1438. [Google Scholar] [CrossRef]
- Malik, R.; Tomer, V.K.; Joshi, N.; Dankwort, T.; Lin, L.; Kienle, L. Au–TiO2-loaded cubic g-C3N4 nanohybrids for photocatalytic and volatile organic amine sensing applications. ACS Appl. Mater. Interfaces 2018, 10, 34087–34097. [Google Scholar] [CrossRef] [PubMed]
- Alessandri, I. 4-Aminothiophenol Photodimerization without Plasmons. Angew. Chem. 2022, 134, e202205013. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Chen, B.; Meng, D.; Song, B.; Liu, Z.; Hu, P.; Yang, H.; Ou, T.-H.; Liu, F.; Pi, H.; et al. Hot Electron-Driven Photocatalysis Using Sub-5 nm Gap Plasmonic Nanofinger Arrays. Nanomaterials 2022, 12, 3730. https://doi.org/10.3390/nano12213730
Wang Y, Chen B, Meng D, Song B, Liu Z, Hu P, Yang H, Ou T-H, Liu F, Pi H, et al. Hot Electron-Driven Photocatalysis Using Sub-5 nm Gap Plasmonic Nanofinger Arrays. Nanomaterials. 2022; 12(21):3730. https://doi.org/10.3390/nano12213730
Chicago/Turabian StyleWang, Yunxiang, Buyun Chen, Deming Meng, Boxiang Song, Zerui Liu, Pan Hu, Hao Yang, Tse-Hsien Ou, Fanxin Liu, Halton Pi, and et al. 2022. "Hot Electron-Driven Photocatalysis Using Sub-5 nm Gap Plasmonic Nanofinger Arrays" Nanomaterials 12, no. 21: 3730. https://doi.org/10.3390/nano12213730
APA StyleWang, Y., Chen, B., Meng, D., Song, B., Liu, Z., Hu, P., Yang, H., Ou, T.-H., Liu, F., Pi, H., Pi, I., Pi, I., & Wu, W. (2022). Hot Electron-Driven Photocatalysis Using Sub-5 nm Gap Plasmonic Nanofinger Arrays. Nanomaterials, 12(21), 3730. https://doi.org/10.3390/nano12213730






