Storage of Lithium-Ion by Phase Engineered MoO3 Homojunctions
Abstract
1. Introduction
2. Materials and Methods
2.1. Material Preparation
2.2. Characterization
2.3. Electrochemical Measurements
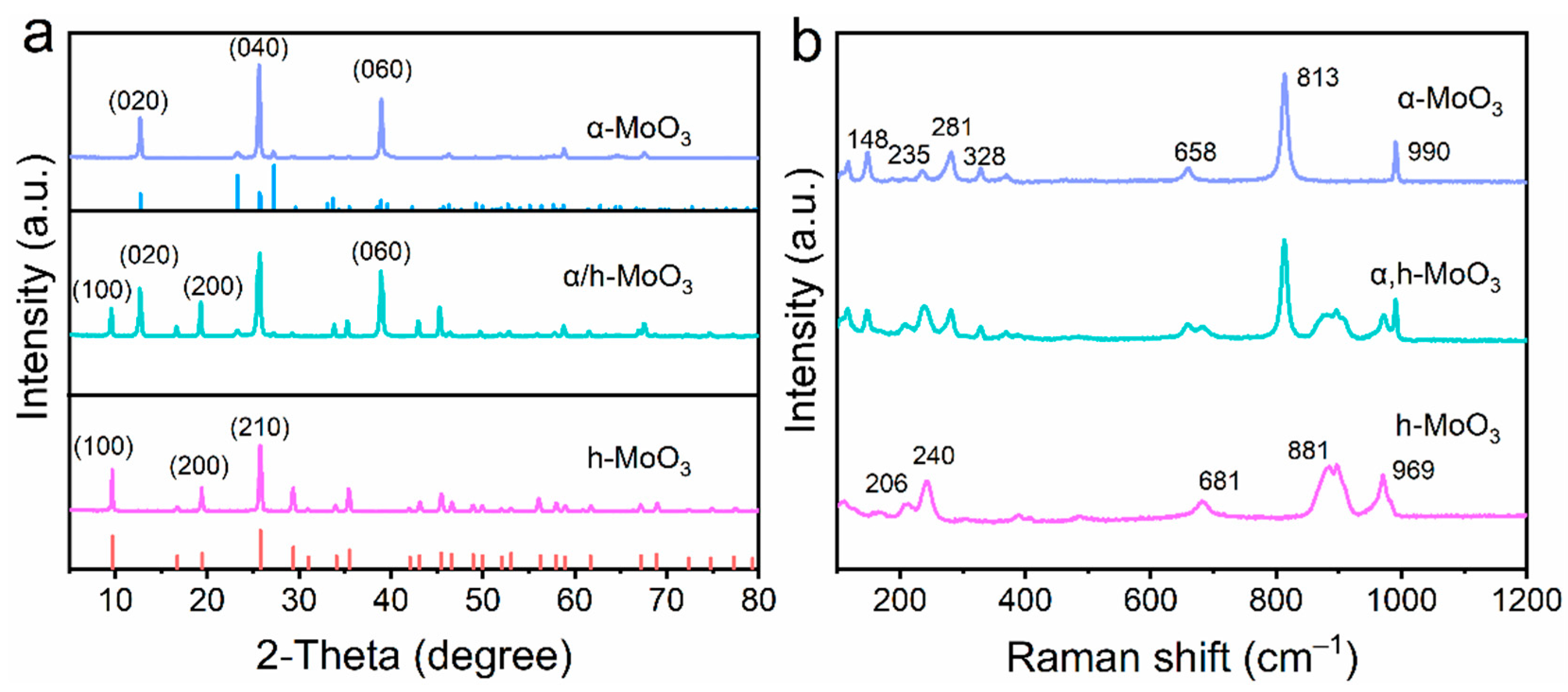
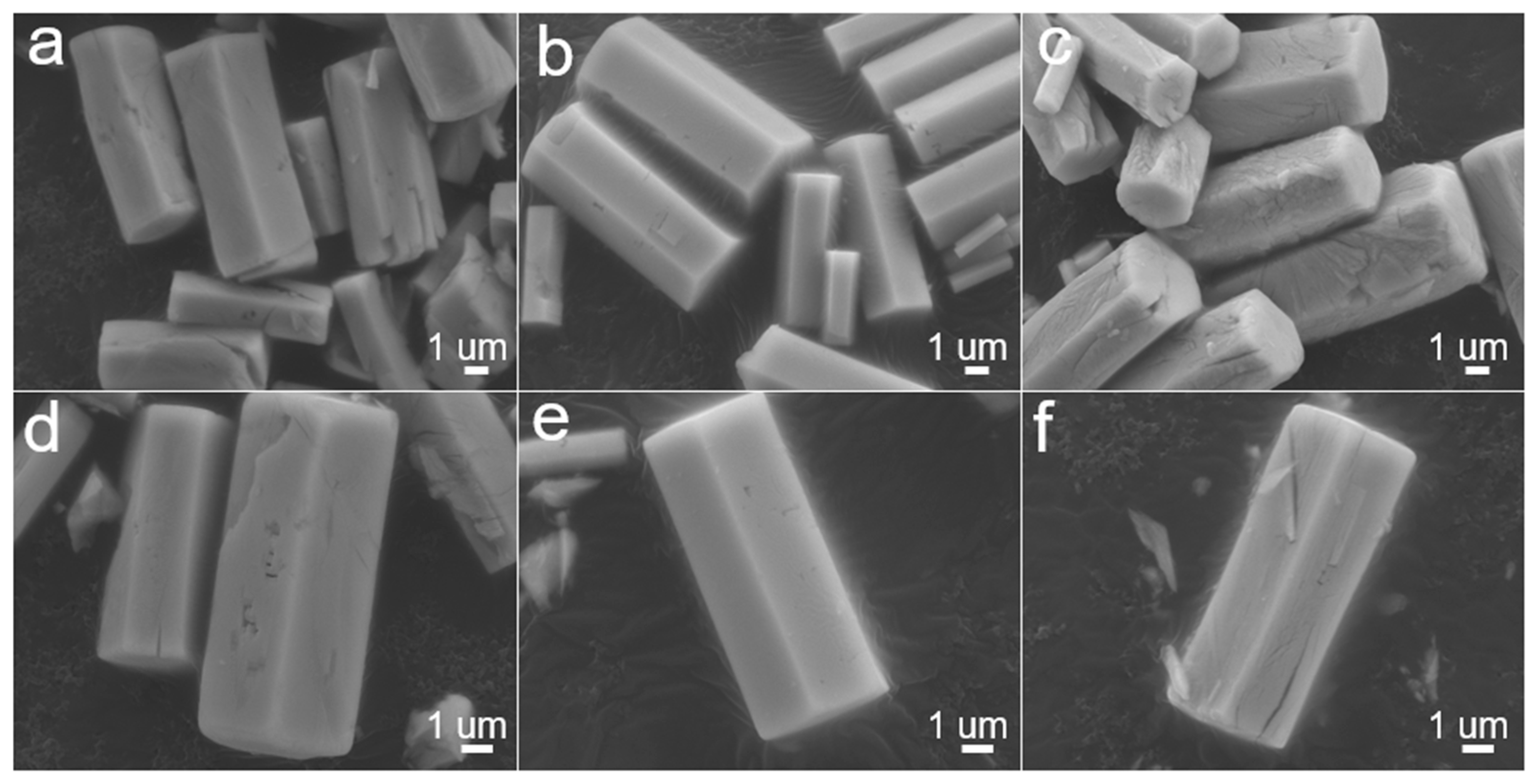
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, M.; Lu, J.; Chen, Z.; Amine, K. 30 Years of lithium-ion batteries. Adv. Mater. 2018, 3, 589–599. [Google Scholar] [CrossRef]
- Xie, J.; Lu, Y.C. A Retrospective on lithium-ion batteries. Nat. Commun. 2020, 11, 2499. [Google Scholar] [CrossRef]
- Tarascon, J.M.; Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 2001, 414, 359–367. [Google Scholar] [CrossRef]
- Lu, Y.; Shin, K.H.; Yu, Y.; Hu, Y.; Liang, J.; Chen, K.; Yuan, H.; Park, H.S.; Wang, D. Multiple active sites carbonaceous anodes for Na+ storage: Synthesis, electrochemical properties and reaction mechanism analysis. Adv. Funct. Mater. 2021, 31, 2007247. [Google Scholar] [CrossRef]
- Fang, S.; Bresser, D.; Passerini, S. Transition metal oxide anodes for electrochemical energy storage in lithium- and sodium-ion batteries. Adv. Energy Mater. 2020, 10, 1902485. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, Y.; Ni, J.; Li, L. Molybdenum-Based materials for sodium-ion batteries. InfoMat 2021, 3, 339–352. [Google Scholar] [CrossRef]
- Xie, J.; Zhang, H.; Liu, Q.; Liu, X.; Lu, X. Recent progress of molybdenum-based materials in aqueous rechargeable batteries. Mater. Today Adv. 2020, 8, 100100. [Google Scholar] [CrossRef]
- Chen, M.; Wang, Z.; Wang, Y.; Li, Y.; Chen, Q. Sodium-Ion storage mechanisms and design strategies of Molybdenum-Based materials: A Review. Appl. Mater. Today 2021, 23, 100985. [Google Scholar] [CrossRef]
- Hu, B.; Mai, L.; Chen, W.; Yang, F. From MoO3 Nanobelts to MoO2 nanorods: Structure transformation and electrical transport. ACS Nano 2009, 3, 478–482. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.S.; Lee, S.; Ahn, D.; Dillon, A.C.; Lee, S.-H. Electrochemical reactivity of ball-milled MoO3−y as anode materials for lithium-ion batteries. J. Power Sources 2009, 188, 286–291. [Google Scholar] [CrossRef]
- Yang, C.; Lua, H.; Li, C.; Wang, L.; Wang, H. Spatially-confined electrochemical reactions of MoO3 nanobelts for reversible high capacity: Critical roles of glucose. Chem. Eng. J. 2018, 337, 1–9. [Google Scholar] [CrossRef]
- Yang, C.; Liu, X.; Yang, Z.; Gu, L.; Yu, Y. Improvement of Lithium storage performance of Molybdenum Trioxide by a synergistic effect of surface coating and oxygen vacancies. Adv. Mater. Interfaces 2016, 3, 1600730. [Google Scholar]
- Chen, J.S.; Cheah, Y.L.; Madhavi, S.; Lou, X.W. Fast synthesis of r-MoO3 nanorods with controlled aspect ratios and their enhanced lithium storage capabilities. J. Phys. Chem. C 2010, 114, 8675–8678. [Google Scholar] [CrossRef]
- Yan, Y.; Li, S.; Yuan, B.; Hu, R.; Yang, L.; Liu, J.; Liu, J.; Wang, Y.; Luo, Z.; Ying, H.; et al. Flowerlike Ti-doped MoO3 conductive anode fabricated by a novel NiTi dealloying method: Greatly enhanced reversibility of the conversion and intercalation reaction. ACS Appl. Mater. Interfaces 2020, 12, 8240–8248. [Google Scholar] [CrossRef]
- Sahu, S.R.; Rikka, V.R.; Haridoss, P.; Chatterjee, A.; Gopalan, R.; Prakash, R. A NOVEL α-MoO3/single-walled carbon nanohorns composite as high-performance anode material for fast-charging lithium-ion battery. Adv. Energy Mater. 2020, 10, 2001627. [Google Scholar] [CrossRef]
- Zhou, X.; Gao, Y.; Lyu, L.-H.; Liang, Y.; Li, Z. General construction of molybdenum-based compounds embedded in flexible 3D interconnected porous carbon nanofibers with protective porous shell for high-performance lithium-ion battery. Carbon 2021, 179, 142–150. [Google Scholar] [CrossRef]
- Xia, Q.; Zhao, H.; Du, Z.; Zeng, Z.; Gao, C.; Zhang, Z.; Du, X.; Kulka, A.; Swierczek, K. Facile synthesis of MoO3/carbon nanobelts as high-performance anode material for lithium ion batteries. Electrochim. Acta 2015, 180, 947–956. [Google Scholar] [CrossRef]
- Jiang, Y.; Sun, M.; Ni, J.; Li, L. Ultrastable sodium storage in MoO3 nanotube arrays enabled by surface phosphorylation. ACS Appl. Mater. Interfaces 2019, 11, 37761–37767. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Xu, C.; Yang, H.; Qian, G.; Hua, S.; Liu, J.; Zheng, X.; Lu, X. Atomic modulation triggering improved performance of MoO3 nanobelts for fiber-shaped supercapacitors. Small 2020, 16, 1905778. [Google Scholar] [CrossRef]
- Chen, Y.; Lai, Z.; Zhang, X.; Fan, Z.; He, Q.; Tan, C.; Zhang, H. Phase engineering of nanomaterials. Nat. Rev. Chem. 2020, 4, 243–256. [Google Scholar] [CrossRef]
- Hao, J.N.; Zhang, J.; Xia, G.L.; Liu, Y.J.; Zheng, Y.; Zhang, W.C.; Tang, Y.B.; Pang, W.K.; Guo, Z.P. Heterostructure manipulation via in situ localized phase transformation for high-rate and highly durable lithium ion storage. ACS Nano 2018, 12, 10430–10438. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, J.; Chen, Q.; Xia, X.; Chen, M. Emerging of heterostructure materials in energy storage: A review. Adv. Mater. 2021, 33, 2100855. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhou, X.; Zhai, W.; Lu, S.; Liang, J.; He, Z.; Long, H.; Xiong, T.; Sun, H.; He, Q.; et al. Phase Engineering of Nanomaterials for Clean Energy and Catalytic Applications. Adv. Energy Mater. 2020, 10, 2002019. [Google Scholar] [CrossRef]
- Liang, G.; Wang, Y.; Huang, Z.; Mo, F.; Li, X.; Yang, Q.; Wang, D.; Li, H.; Chen, S.; Zhi, C. Initiating hexagonal MoO3 for superb-stable and fast NH4+ storage based on hydrogen bond chemistry. Adv. Mater. 2020, 32, 1907802. [Google Scholar] [CrossRef] [PubMed]
- Almodóva, P.; López, M.L.; Julio, R.-C.; Nappini, S.; Magnano, E.; José, M.G.-C.; Carlos, D.-G. Synthesis, characterization and electrochemical assessment of hexagonal molybdenum trioxide (h-MoO3) micro-composites with graphite, graphene and graphene oxide for lithium ion batteries. Electrochim. Acta 2021, 365, 137355. [Google Scholar] [CrossRef]
- Leng, K.; Chen, Z.; Zhao, X.; Tang, W.; Tian, B.; Nai, C.; Zhou, W.; Loh, K.P. Phase-junction electrocatalysts towards enhanced hydrogen evolution reaction in alkaline media. Angew. Chem. Int. Ed. 2021, 60, 259–267. [Google Scholar]
- Zheng, L.; Xu, Y.; Jin, D.; Xie, Y. Novel metastable hexagonal MoO3 nanobelts: Synthesis, photochromic, and electrochromic properties. Chem. Mater. 2009, 21, 5681–5690. [Google Scholar] [CrossRef]
- Shen, F.; Sun, Z.; Zhao, L.; Xia, Y.; Shao, Y.; Cai, J.; Li, S.; Lu, C.; Tong, X.; Zhao, Y.; et al. Triggering the phase transition and capacity enhancement of Nb2O5 for fast-charging lithiumion storage. J. Mater. Chem. A 2021, 9, 14534. [Google Scholar] [CrossRef]
- Li, X.; Pan, D.; Deng, J.; Wang, R.; Huang, J.; Lu, W.; Yao, T.; Wang, X.; Zhang, Y.; Xu, L.; et al. Phase-junction engineering boosts the performance of CoSe2 for efficient sodium/potassium storage. J. Mater. Chem. A 2021, 9, 25954–25963. [Google Scholar] [CrossRef]
- Lunk, H.-J.; Hartl, H.; Hartl, M.A.; Fait, M.J.G.; Shenderovich, I.G.; Feist, M.; Frisk, T.A.; Daemen, L.L.; Mauder, D.; Eckelt, R.; et al. “Hexagonal Molybdenum Trioxide”; Known for 100 years and still a fount of new discoveries. Inorg. Chem. 2010, 49, 9400–9408. [Google Scholar] [CrossRef]
- Ding, J.; Abbas, S.A.; Hanmandlu, C.; Lin, L.; Lai, C.-S.; Wang, P.-C.; Li, L.-J.; Chu, C.-W.; Chang, C.-C. Facile synthesis of carbon/MoO3 nanocomposites as stable battery anodes. J. Power Sources 2017, 348, 270–280. [Google Scholar] [CrossRef]
- Li, S.; Cui, Y.; Kang, R.; Zou, B.; Ng, D.H.L.; El-Khodary, S.A.; Liu, X.; Qiu, J.; Lian, J.; Li, H. Oxygen vacancies boosted the electrochemical kinetics of Nb2O5−x for superior lithium storage. Chem. Commun. 2021, 57, 8182–8185. [Google Scholar] [CrossRef] [PubMed]
- Zou, B.; Zhang, W.; Cui, Y.; Li, S.; Li, G.; Liu, X.; Ng, D.H.L.; Qiu, J.; Lian, J. Interfacial engineering for metal oxides/nitrides nano-heterojunctions towards high-rate lithium-ion storage. J. Mater. Chem. A 2022, 10, 7391–7398. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhou, T.; Zhang, C.; Mao, J.; Liu, H.; Guo, Z. Boosted charge transfer in SnS/SnO2 heterostructures: Toward high rate capability for sodium-ion batteries. Angew. Chem. Int. Ed. 2016, 55, 3408–3413. [Google Scholar] [CrossRef] [PubMed]
- John, W.; Polleux, J.; Lim, J.; Dunn, B. Pseudocapacitive contributions to electrochemical energy storage in TiO2 (Anatase) nanoparticles. J. Phys. Chem. C 2007, 111, 14925–14931. [Google Scholar]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ng, D.H.L.; Li, S.; Li, J.; Huang, J.; Cui, Y.; Lian, J.; Wang, C. Storage of Lithium-Ion by Phase Engineered MoO3 Homojunctions. Nanomaterials 2022, 12, 3762. https://doi.org/10.3390/nano12213762
Ng DHL, Li S, Li J, Huang J, Cui Y, Lian J, Wang C. Storage of Lithium-Ion by Phase Engineered MoO3 Homojunctions. Nanomaterials. 2022; 12(21):3762. https://doi.org/10.3390/nano12213762
Chicago/Turabian StyleNg, Dickon H. L., Sheng Li, Jun Li, Jinning Huang, Yingxue Cui, Jiabiao Lian, and Chuan Wang. 2022. "Storage of Lithium-Ion by Phase Engineered MoO3 Homojunctions" Nanomaterials 12, no. 21: 3762. https://doi.org/10.3390/nano12213762
APA StyleNg, D. H. L., Li, S., Li, J., Huang, J., Cui, Y., Lian, J., & Wang, C. (2022). Storage of Lithium-Ion by Phase Engineered MoO3 Homojunctions. Nanomaterials, 12(21), 3762. https://doi.org/10.3390/nano12213762






