Citrullus colocynthis-Mediated Green Synthesis of Silver Nanoparticles and Their Antiproliferative Action against Breast Cancer Cells and Bactericidal Roles against Human Pathogens
Abstract
1. Introduction
2. Materials and Methods
2.1. Fruit Extraction and Preparation of AgNPs
2.2. Synthesis of AgNPs by Wet Chemical Methods
2.3. Characterization of Biosynthesized Cc-AgNPs
2.4. Antibacterial Activity
2.5. Cell Culture
2.6. MTT Cell Viability Assay
2.7. Colony Formation Assay
2.8. Scratch Assay
2.9. Spheroid Formation Assay
2.10. Determination of Cholesterol and Triglycerides
2.11. Gene Expression Analysis
2.12. Statistical Analysis
3. Results
3.1. UV-Vis Spectroscopy Analysis
3.2. XRD Analysis
3.3. FTIR Analysis
3.4. Morphological and Compositional Analysis
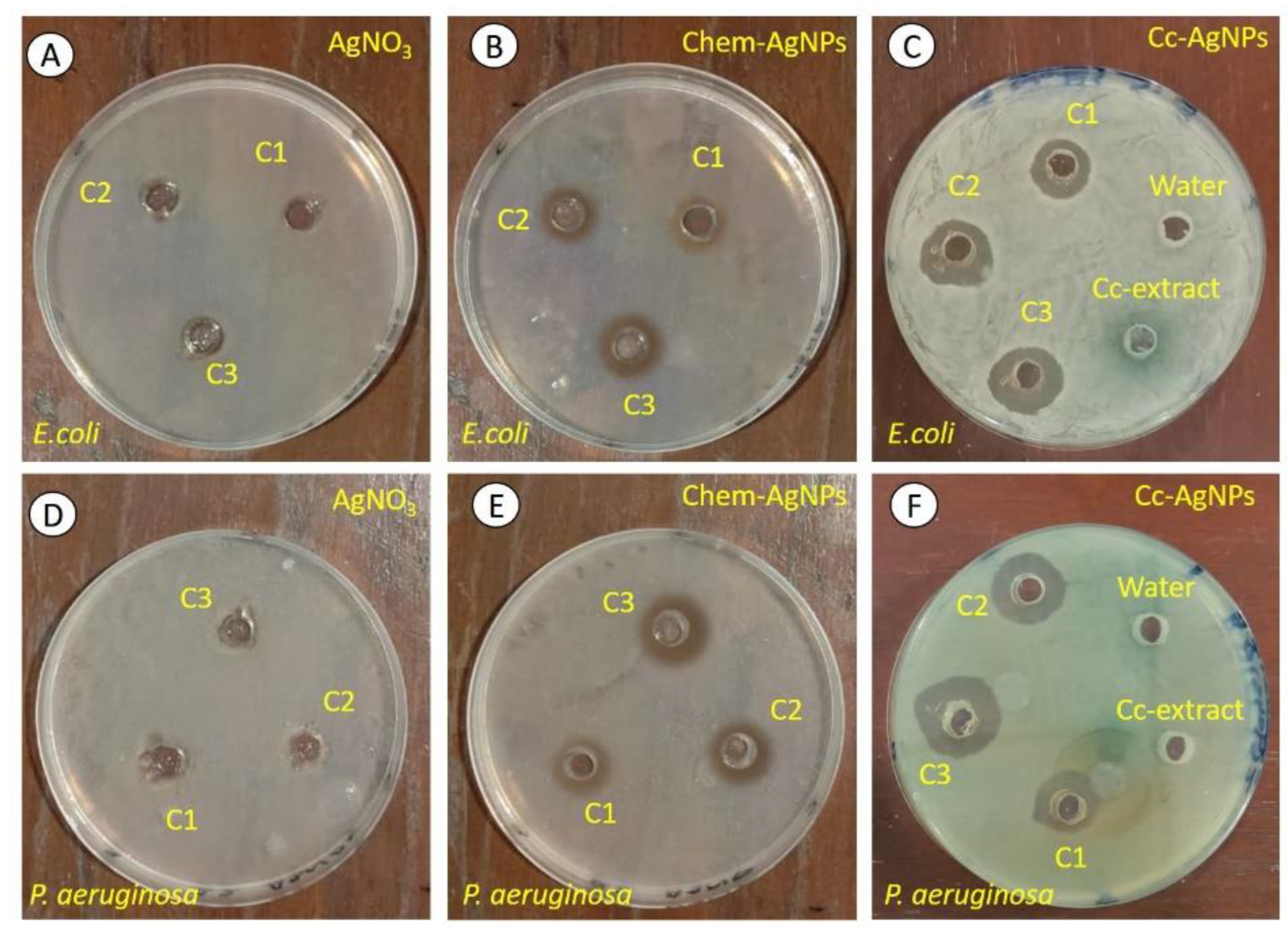
3.5. Cc-AgNP Treatment Significantly Inhibited the Growth of Pathogenic Bacteria
3.6. Reduced Cell Viability, Proliferation, and Growth of MCF7 Cells Treated with Cc-AgNPs
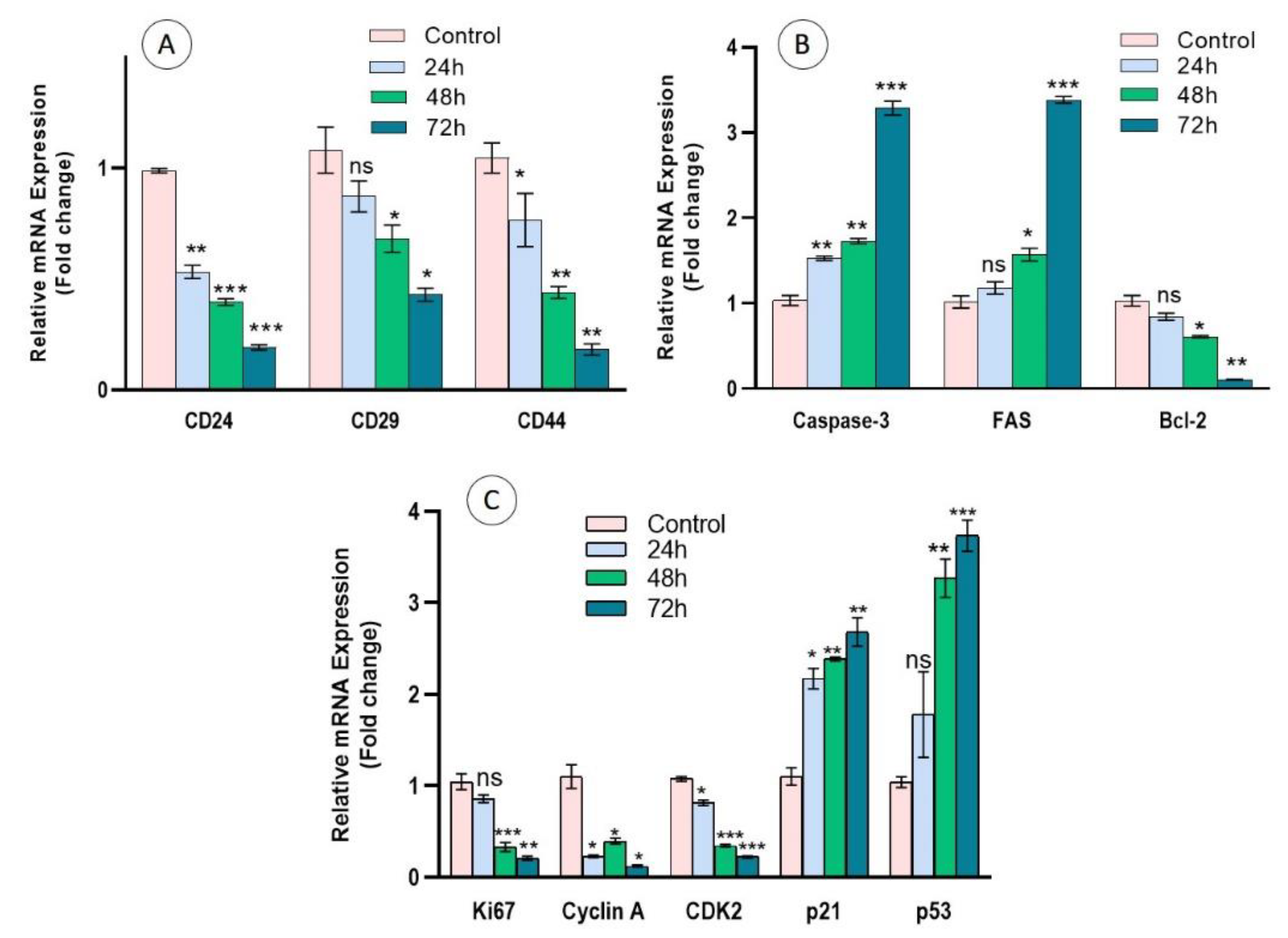
3.7. Regulation of Cell Surface Markers, Apoptosis, and Cell Proliferation Genes in Cc-AgNP-Treated MCF7 Cells
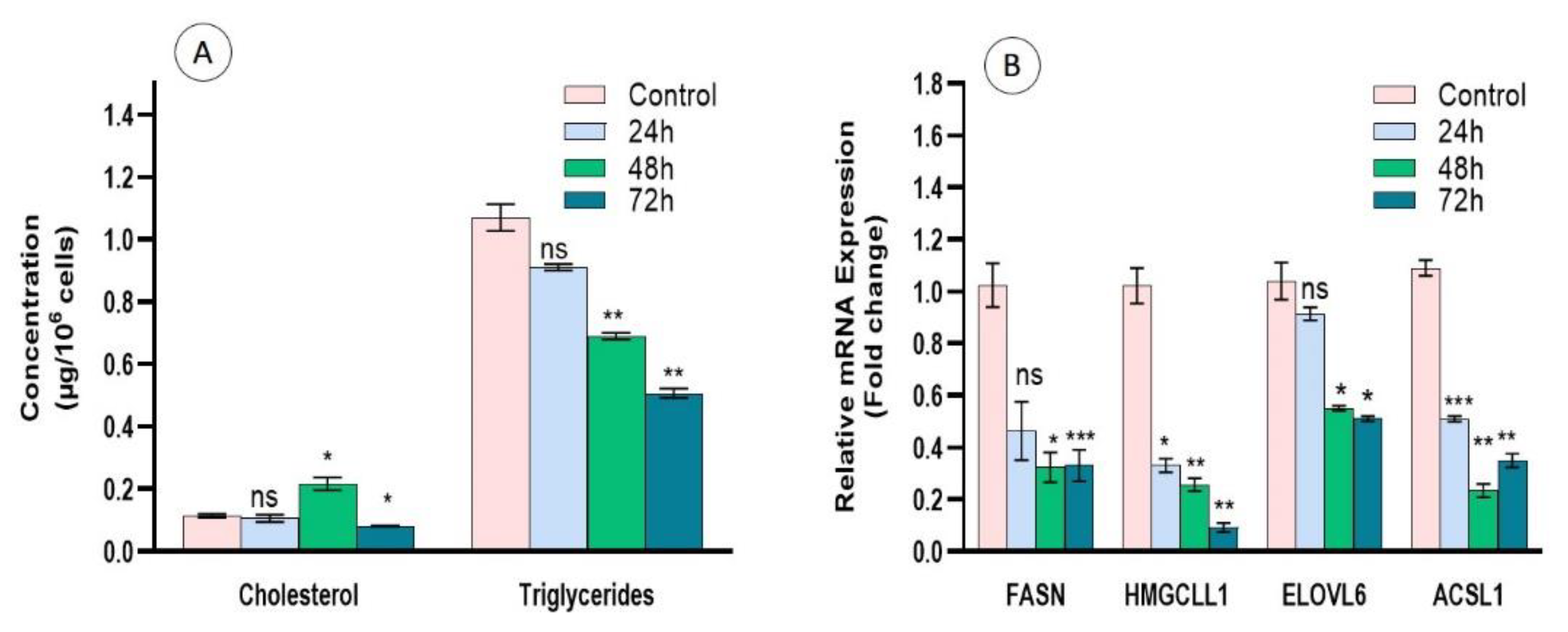
3.8. Regulation of Lipid Metabolism by Cc-AgNPs
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ahmad, S.; Munir, S.; Zeb, N.; Ullah, A.; Khan, B.; Ali, J.; Bilal, M.; Omer, M.; Alamzeb, M.; Salman, S.M.; et al. Green nanotechnology: A review on green synthesis of silver nanoparticles—An ecofriendly approach. Int. J. Nanomed. 2019, 14, 5087–5107. [Google Scholar] [CrossRef] [PubMed]
- Zahin, N.; Anwar, R.; Tewari, D.; Kabir, M.T.; Sajid, A.; Mathew, B.; Sahab-Uddin, M.; Aleya, L.; Abdel-Daim, M.M. Nanoparticles and its biomedical applications in health and diseases: Special focus on drug delivery. Environ. Sci. Pollut. Res. Int. 2020, 27, 19151–19168. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Hussain, Z.; Khan, J.A.; Murtaza, S. Nanotechnology: An Emerging Therapeutic Option for Breast Cancer. Crit. Rev. Eukaryot. Gene Expr. 2018, 28, 163–175. [Google Scholar] [CrossRef]
- Satyavani, K.; Gurudeeban, S.; Ramanathan, T.; Balasubramanian, T. Biomedical potential of silver nanoparticles synthesized from calli cells of Citrullus colocynthis (L.) Schrad. J. Nanobiotechnol. 2011, 9, 43. [Google Scholar] [CrossRef]
- Ahmed, S.; Munir, S.; Zeb, N.; Ullah, A.; Khan, B.; Ali, J.; Bilal, M.; Omer, M.; Alamzeb, M.; Ahmed, M.; et al. Phytochemical screening, total phenolic and flavonoids contents and antioxidant activities of Citrullus colocynthis L. and Cannabis sativa L. Appl. Ecol. Environ. Res. 2019, 17, 6961–6979. [Google Scholar] [CrossRef]
- Chowdhury, K.; Sharma, A.; Kumar, S.; Gunjan, G.K.; Nag, A.; Mandal, C.C. Colocynth extracts prevent epithelial to mesenchymal transition and stemness of breast cancer cells. Front. Pharmacol. 2017, 8, 593. [Google Scholar] [CrossRef]
- Gupta, S.C.; Tripathi, T.; Paswan, S.K.; Agarwal, A.G.; Rao, C.V.; Sidhu, O.P. Phytochemical investigation, antioxidant and wound healing activities of Citrullus colocynthis (bitter apple). Asian. Pac. J. Trop. Biomed. 2018, 8, 418–424. [Google Scholar] [CrossRef]
- Abu-Darwish, M.S.; Efferth, T. Medicinal plants from near east for cancer therapy. Front. Pharmacol. 2018, 9, 56. [Google Scholar] [CrossRef]
- Botcha, S.; Prattipati, S.D. Callus Extract Mediated Green Synthesis of Silver Nanoparticles, Their Characterization and Cytotoxicity Evaluation Against MDA-MB-231 and PC-3 Cells. BioNanoScience 2019, 10, 11–22. [Google Scholar] [CrossRef]
- Shawkey, A.M.; Rabeh, M.A.; Abdulall, A.K.; Abdellatif, A.O. Green nanotechnology: Anticancer Activity of Silver Nanoparticles using Citrullus colocynthis aqueous extracts. Int. J. Adv. Life Sci. 2013, 13, 60–70. [Google Scholar]
- Raza, M.A.; Kanwal, K.; Rauf, A.; Sabri, A.N.; Riaz, S.; Naseem, S. Size- and shape-dependent antibacterial studies of silver nanoparticles synthesized by wet chemical routes. Nanomaterials 2016, 6, 74. [Google Scholar] [CrossRef] [PubMed]
- Nasrollahzadeh, M.; Sajadi, S.M.; Maham, M. Green synthesis of palladium nanoparticles using Hippophae rhamnoides Linn leaf extract and their catalytic activity for the Suzuki–Miyaura coupling in water. J. Mol. Catal. A Chem. 2015, 396, 297–303. [Google Scholar] [CrossRef]
- Rajeshkumar, S.; Bharath, L.V. Mechanism of plant-mediated synthesis of silver nanoparticles—A review on biomolecules involved, characterization and antibacterial activity. Chem. Biol. Interact. 2017, 273, 219–227. [Google Scholar] [CrossRef]
- Suh, I.K.; Ohta, H.; Waseda, Y. High-temperature thermal expansion of six metallic elements measured by dilatation method and X-ray Diffraction Locality: Synthetic Sample: At T = 576 K. J. Mater. Sci. 1988, 23, 757–760. [Google Scholar] [CrossRef]
- Rasool, S.; Raza, M.A.; Manzoor, F.; Kanwal, Z.; Riaz, S.; Iqbal, M.J.; Naseem, S. Biosynthesis, characterization and anti-dengue vector activity of silver nanoparticles prepared from Azadirachta indica and Citrullus colocynthis. R. Soc.Open. Sci. 2020, 7, 200540. [Google Scholar] [CrossRef]
- Sujitha, V.; Murugan, K.; Paulpandi, M.; Panneerselvam, C.; Suresh, U.; Roni, M.; Nicoletti, M.; Higuchi, A.; Madhiyazhagan, P.; Subramaniam, J.; et al. Green-synthesized silver nanoparticles as a novel control tool against dengue virus (DEN-2) and its primary vector Aedes aegypti. Parasitol. Res. 2015, 114, 3315–3325. [Google Scholar] [CrossRef]
- Ravindran, A.; Chandran, P.; Khan, S.S. Biofunctionalized silver nanoparticles: Advances and prospects. Colloids. Surf. B Biointerfaces 2013, 105, 342–352. [Google Scholar] [CrossRef]
- Kanwal, Z.; Raza, M.A.; Manzoor, F.; Riaz, S.; Jabeen, G.; Fatima, S.; Naseem, S. A comparative assessment of nanotoxicity induced by metal (silver, nickel) and metal oxide (cobalt, chromium) nanoparticles in Labeo rohita. Nanomaterials 2019, 9, 309. [Google Scholar] [CrossRef]
- Mohamed, H.E.A.; Afridi, S.; Khalil, A.T.; Zohra, T.; Ali, M.; Alam, M.M.; Ikram, A.; Shinwari, Z.K.; Maaza, M. Phyto-fabricated Cr2O3 nanoparticle for multifunctional biomedical applications. Nanomedicine 2020, 15, 1653–1669. [Google Scholar] [CrossRef]
- Sangeetha, A.; Senthil Kumar, P.; Balakrishnan, S.; Manimaran, K.; Dhanalakshmi, M.; Sivakumar, T. In-Vitro antimicrobial activity of Madhuca indica and Cassia fistula leaves against food-borne pathogens. J. Pharmacogn. Phytochem. 2020, 9, 587–591. [Google Scholar] [CrossRef]
- Mostafa, A.A.; Al-Askar, A.A.; Almaary, K.S.; Dawoud, T.M.; Sholkamy, E.N.; Bakri, M.M. Antimicrobial activity of some plant extracts against bacterial strains causing food poisoning diseases. Saudi. J. Biol. Sci. 2018, 25, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Kumar Pandian, S.R.; Deepak, V.; Kalishwaralal, K.; Viswanathan, P.; Gurunathan, S. Mechanism of bactericidal activity of silver nitrate—A concentration dependent bi-functional molecule. Braz. J. Microbiol. 2010, 41, 805–809. [Google Scholar] [CrossRef] [PubMed]
- Pratama, M.A.; Ramahdita, G.; Yuwono, A.H. The Effect of Silver Nitrate Addition on Antibacterial Properties of Bone Scaffold Chitosan-Hydroxyapatite. AIP Conf. Proc. 2019, 2193, 020014. [Google Scholar] [CrossRef]
- Sankaranarayanan, A.; Munivel, G.; Karunakaran, G.; Kadaikunnan, S.; Alharbi, N.S.; Khaled, J.M.; Kuzentsov, D. Green synthesis of silver nanoparticles using Arachis hypogaea (ground nut) root extract for antibacterial and clinical applications. J. Clust. Sci. 2017, 28, 995–1008. [Google Scholar] [CrossRef]
- Abdulridha, M.K.; Al-Marzoqi, A.H.; Ghasemian, A. The anticancer efficiency of Citrullus colocynthis toward the colorectal cancer therapy. J. Gastrointest. Cancer. 2020, 51, 439–444. [Google Scholar] [CrossRef]
- Rezai, M.; Davoodi, A.; Asori, M.; Azadbakht, M. Cytotoxic activity of Citrullus colocynthis (L.) schrad fruit extract on gastric adenocarcinoma and breast cancer cell lines. Int. J. Pharm. Sci. Rev. Res. 2017, 45, 175–178. [Google Scholar]
- Kim, H.J.; Kim, J.B.; Lee, K.-M.; Shin, I.; Han, W.; Ko, E.; Bae, J.Y.; Noh, D.Y. Isolation of CD24high and CD24low/− cells from MCF-7: CD24 expression is positively related with proliferation, adhesion and invasion in MCF-7. Cancer Lett. 2007, 258, 98–108. [Google Scholar] [CrossRef]
- Zhang, G.; Zhao, H.; Wu, J.; Li, J.; Xiang, Y.; Wang, G.; Wu, L.; Jio, S. Adoptive immunotherapy for non-small cell lung cancer by NK and cytotoxic T lymphocytes mixed effector cells: Retrospective clinical observation. Int. Immunopharmacol. 2014, 21, 396–405. [Google Scholar] [CrossRef]
- Gianni, L.; Colleoni, M.; Bisagni, G.; Mansutti, M.; Zamagni, C.; Del Mastro, L.; Zambelli, S.; Frassoldati, A.; Barlera, S.; Valagussa, P.; et al. Ki67 during and after neoadjuvant trastuzumab, pertuzumab and palbociclib plus or minus fulvestrant in HER2 and ER-positive breast cancer: The NA-PHER2 Michelangelo study. J. Clin. Oncol. 2019, 37, 527. [Google Scholar] [CrossRef]
- Pathmanathan, N.; Balleine, R.L. Ki-67 is a prognostic parameter in breast cancer patients: Results of a large population-based cohort of a cancer registry. J. Clin. Pathol. 2013, 66, 512–516. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, J.; Ding, M.; Xu, K.; Li, L.; Mao, L.; Zheng, J. Ki67 targeted strategies for cancer therapy. Clin. Transl. Oncol. 2018, 20, 570–575. [Google Scholar] [CrossRef] [PubMed]
- De Boer, L.; Oakes, V.; Beamish, H.; Giles, N.; Stevens, F.; Somodevilla-Torres, M.; De Souza, C.; Gabrielli, B. Cyclin A/cdk2 coordinates centrosomal and nuclear mitotic events. Oncogene 2008, 27, 4261–4268. [Google Scholar] [CrossRef] [PubMed]
- Ming, P.; Cai, T.; Li, J.; Ning, Y.; Xie, S.; Tao, T.; Tang, F. A novel arylbenzofuran induces cervical cancer cell apoptosis and G1/S arrest through ERK-mediated Cdk2/cyclin-A signaling pathway. Oncotarget 2016, 7, 41843–41856. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yang, T.Y.; Chang, G.C.; Chen, K.C.; Hung, H.W.; Hsu, K.H.; Sheu, G.T.; Hsu, S.L. Sustained activation of ERK and Cdk2/cyclin-A signaling pathway by pemetrexed leading to S-phase arrest and apoptosis in human non-small cell lung cancer A549 cells. Eur. J. Pharmacol. 2011, 663, 17–26. [Google Scholar] [CrossRef]
- Du, J.; Sun, Y.; Lu, Y.Y.; Lau, E.; Zhao, M.; Zhou, Q.M.; Su, S.B. Berberine and evodiamine act synergistically against human breast cancer MCF-7 cells by inducing cell cycle arrest and apoptosis. Anticancer. Res. 2017, 37, 6141–6151. [Google Scholar] [CrossRef]
- Genov, M.; Kreiseder, B.; Nagl, M.; Drucker, E.; Wiederstein, M.; Muellauer, B.; Krebs, J.; Grohmann, T.; Pretsch, D.; Baumann, K.; et al. Tetrahydroanthraquinone derivative (±)-4-deoxyaustrocortilutein induces cell cycle arrest and apoptosis in melanoma cells via upregulation of p21 and p53 and downregulation of NF-kappaB. J. Cancer. 2016, 7, 555–568. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K.; Banerjee, S.; Acosta, E.P.; Lillard, J.W.; Singh, R. Resveratrol induces cell cycle arrest and apoptosis with docetaxel in prostate cancer cells via a p53/p21WAF1/CIP1 and p27KIP1 pathway. Oncotarget 2017, 8, 17216–17228. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; Fang, S.; Chen, Y.; Yang, Z.; Yuan, Y.; Zhang, J.; Ye, L.; Gu, W. Inhibition of FASN suppresses the malignant biological behavior of non-small cell lung cancer cells via deregulating glucose metabolism and AKT/ERK pathway. Lipids Health Dis. 2019, 18, 118. [Google Scholar] [CrossRef]
- Su, Y.C.; Feng, Y.H.; Wu, H.T.; Huang, Y.S.; Tung, C.L.; Wu, P.; Chang, C.J.; Shiau, A.L.; Wu, C.L. Elovl6 is a negative clinical predictor for liver cancer and knockdown of Elovl6 reduces murine liver cancer progression. Sci. Rep. 2018, 8, 6586. [Google Scholar] [CrossRef]
- Park, J.H.; Woo, Y.M.; Youm, E.M.; Hamad, N.; Won, H.H.; Naka, K.; Park, E.J.; Park, J.H.; Kim, H.J.; Kim, S.H.; et al. HMGCLL1 is a predictive biomarker for deep molecular response to imatinib therapy in chronic myeloid leukemia. Leukemia 2019, 33, 1439–1450. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Zha, J.; Yang, X.; Li, Q.; Zhang, Q.; Yin, A.; Beharry, Z.; Huang, H.; Huang, J.; Bartlett, M.; et al. Long-chain fatty acyl-CoA synthetase 1 promotes prostate cancer progression by elevation of lipogenesis and fatty acid beta-oxidation. Oncogene 2021, 40, 1806–1820. [Google Scholar] [CrossRef] [PubMed]




| Sr. # | Gene Name | Primer Sequence (5′–3′) | Tm | GC Content | Product Size (bp) |
|---|---|---|---|---|---|
| 1 | CD 24 | Forward GTCCAGAAAGGAGAATACAG | 56.4 | 45 | 277 |
| Reverse GAAATGGTGCTGGAGATAA | 53 | 42.11 | |||
| 2 | CD29 | Forward CAGAGGCTCCAAAGATATAA | 54.3 | 40 | 213 |
| Reverse GAGTAAGACAGGTCCATAAG | 56.4 | 45 | |||
| 3 | CD44 | Forward GTCCAGAAAGGAGAATACAG | 56.4 | 42.11 | 332 |
| Reverse GAAATGGTGCTGGAGATAA | 53 | 45 | |||
| 4 | Caspase- 3 | Forward GAACTGGACTGTGGCATTGA | 58.4 | 50 | 133 |
| Reverse CCTTTGAATTTCGCCAAGAA | 54.3 | 40 | |||
| 5 | FAS | Forward TGCAGAAGATGTAGATTGTGTGATGA | 63.1 | 50 | 67 |
| Reverse GGGTCCGGGTGCAGTTTATT | 63.3 | 50 | |||
| 6 | Ki67 | Forward CCTGACAGTGGAAAACCTCT | 57 | 50 | 219 |
| Reverse CACAATTTCCTCTGGTGCTG | 57 | 50 | |||
| 7 | Cyclin A | Forward GATGCTGACCCATACCTCAA | 57 | 50 | 250 |
| Reverse GGTTGAGGAGAGAAACACCA | 57 | 50 | |||
| 8 | CDK2 | Forward ACCAGCTCTTCCGGATCTTT | 58 | 50 | 186 |
| Reverse TAGGGTCGTAGTGCAGCATT | 58 | 50 | |||
| 9 | P21 | Forward GGAAGACCATGTGGACCTGT | 60.5 | 55 | 146 |
| Reverse -GGCGTTTGGAGTGGTAGAAA | 58.4 | 50 | |||
| 10 | P53 | Forward GTTCCGAGAGCTGAATGAGG | 60.5 | 55 | 123 |
| Reverse TTATGGCGGGAGGTAGACTG | 55 | 60.5 | |||
| 11 | FASN | Forward TTCCGAGATTCCATCCTA | 57 | 44.4 | 107 |
| Reverse TGGACGATGTCATCAAAG | 57 | 44.4 | |||
| 12 | HMGCLL1 | Forward ATCGACTTTCCCAAACTG | 57 | 44.4 | 144 |
| Reverse TAGGAGTAAGGACAGGATAG | 57 | 45 | |||
| 13 | ELOVL6 | Forward CTGATCTTCCTGCACTGGTATC | 62 | 50 | 113 |
| Reverse TGCACGCCATAGTTCATAGTC | 62 | 47.6 | |||
| 14 | ACSL1 | Forward CCTTGCCCAGATGATACTTT | 56.8 | 47.6 | 88 |
| Reverse CTCCATGACACAGCATTACA | 55.5 | 47.6 | |||
| 15 | GAPDH | Forward CTCTGATTTGGTCGTATTG | 53 | 42.1 | 112 |
| Reverse TGTAAACCATGTAGTTGAGG | 54 | 40 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rasool, S.; Tayyeb, A.; Raza, M.A.; Ashfaq, H.; Perveen, S.; Kanwal, Z.; Riaz, S.; Naseem, S.; Abbas, N.; Ahmad, N.; et al. Citrullus colocynthis-Mediated Green Synthesis of Silver Nanoparticles and Their Antiproliferative Action against Breast Cancer Cells and Bactericidal Roles against Human Pathogens. Nanomaterials 2022, 12, 3781. https://doi.org/10.3390/nano12213781
Rasool S, Tayyeb A, Raza MA, Ashfaq H, Perveen S, Kanwal Z, Riaz S, Naseem S, Abbas N, Ahmad N, et al. Citrullus colocynthis-Mediated Green Synthesis of Silver Nanoparticles and Their Antiproliferative Action against Breast Cancer Cells and Bactericidal Roles against Human Pathogens. Nanomaterials. 2022; 12(21):3781. https://doi.org/10.3390/nano12213781
Chicago/Turabian StyleRasool, Shafqat, Asima Tayyeb, Muhammad Akram Raza, Hanfa Ashfaq, Sadia Perveen, Zakia Kanwal, Saira Riaz, Shahzad Naseem, Nadeem Abbas, Naushad Ahmad, and et al. 2022. "Citrullus colocynthis-Mediated Green Synthesis of Silver Nanoparticles and Their Antiproliferative Action against Breast Cancer Cells and Bactericidal Roles against Human Pathogens" Nanomaterials 12, no. 21: 3781. https://doi.org/10.3390/nano12213781
APA StyleRasool, S., Tayyeb, A., Raza, M. A., Ashfaq, H., Perveen, S., Kanwal, Z., Riaz, S., Naseem, S., Abbas, N., Ahmad, N., & Alomar, S. Y. (2022). Citrullus colocynthis-Mediated Green Synthesis of Silver Nanoparticles and Their Antiproliferative Action against Breast Cancer Cells and Bactericidal Roles against Human Pathogens. Nanomaterials, 12(21), 3781. https://doi.org/10.3390/nano12213781






