Catalytically Active Amyloids as Future Bionanomaterials
Abstract
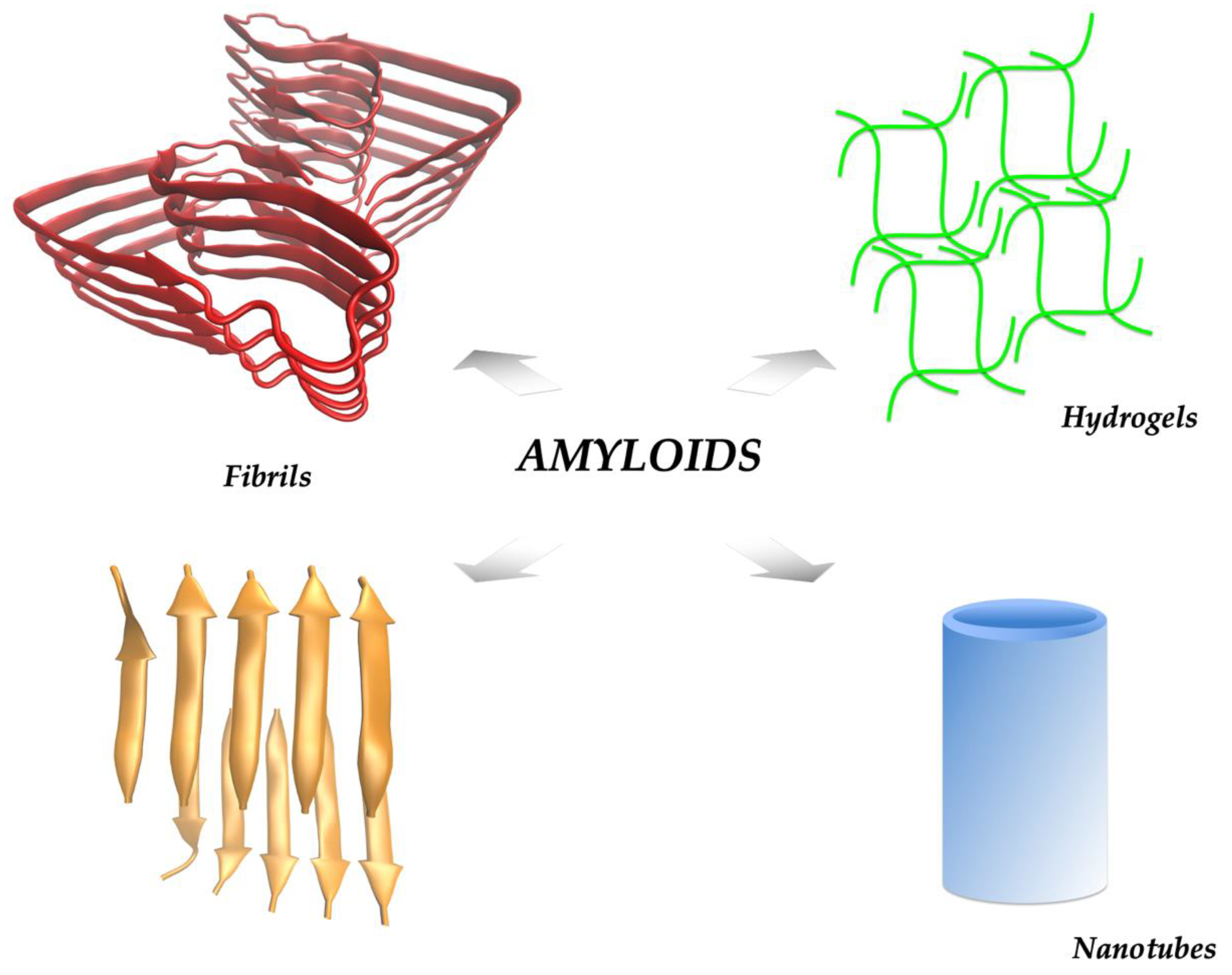
:1. The Amyloid State
2. Catalytic Amyloids
3. Peroxidase-like Activity
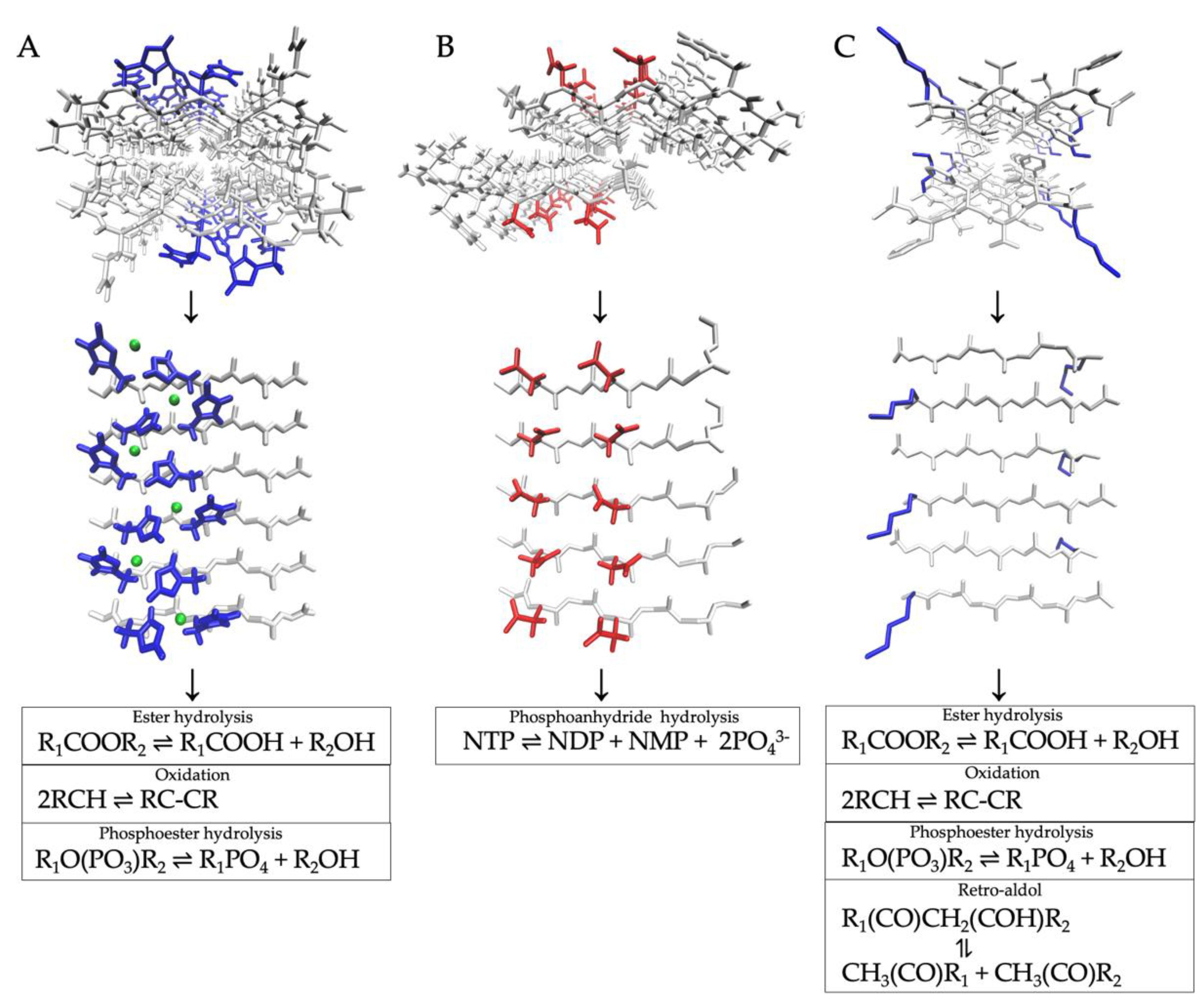
3.1. Esterase Activities
3.2. Phosphoesterase Activities
3.3. Redox Activities
3.4. Catalytic Amyloids Derived from Ab42
3.5. Stability of Catalytic Amyloids
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Kyle, R.A. Amyloidosis: A Convoluted Story: Historical Review. Br. J. Haematol. 2001, 114, 529–538. [Google Scholar] [CrossRef] [PubMed]
- Ke, P.C.; Zhou, R.; Serpell, L.C.; Riek, R.; Knowles, T.P.J.; Lashuel, H.A.; Gazit, E.; Hamley, I.W.; Davis, T.P.; Fändrich, M.; et al. Half a century of amyloids: Past, present and future. Chem. Soc. Rev. 2020, 49, 5473–5509. [Google Scholar] [CrossRef] [PubMed]
- Riek, R. The Three-Dimensional Structures of Amyloids. Cold Spring Harb. Perspect. Biol. 2017, 9, a023572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maggio, J.E.; Mantyh, P.W. Brain Amyloid—A Physicochemical Perspective. Brain Pathol. 1996, 6, 147–162. [Google Scholar] [CrossRef]
- Greenwald, J.; Riek, R. Biology of Amyloid: Structure, Function, and Regulation. Structure 2010, 18, 1244–1260. [Google Scholar] [CrossRef]
- Diaz-Espinoza, R. Recent High-Resolution Structures of Amyloids Involved in Neurodegenerative Diseases. Front. Aging Neurosci. 2021, 13, 782617. [Google Scholar] [CrossRef]
- Chiti, F.; Dobson, C.M. Protein Misfolding, Amyloid Formation, and Human Disease: A Summary of Progress over the Last Decade. Annu. Rev. Biochem. 2017, 86, 27–68. [Google Scholar] [CrossRef]
- Sawaya, M.R.; Hughes, M.P.; Rodriguez, J.A.; Riek, R.; Eisenberg, D.S. The Expanding Amyloid Family: Structure, Stability, Function, and Pathogenesis. Cell 2021, 184, 4857–4873. [Google Scholar] [CrossRef]
- Hartl, F.U.; Hayer-Hartl, M. Converging Concepts of Protein Folding In Vitro and In Vivo. Nat. Struct. Mol. Biol. 2009, 16, 574–581. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Adamcik, J.; Diener, M.; Kumita, J.R.; Mezzenga, R. Different Folding States from the Same Protein Sequence Determine Reversible vs Irreversible Amyloid Fate. J. Am. Chem. Soc. 2021, 143, 11473–11481. [Google Scholar] [CrossRef]
- Guijarro, J.I.; Sunde, M.; Jones, J.A.; Campbell, I.D.; Dobson, C.M. Amyloid Fibril Formation by an SH3 Domain. Proc. Natl. Acad. Sci. USA 1998, 95, 4224–4228. [Google Scholar] [CrossRef] [Green Version]
- Buell, A.K. Stability Matters, Too—The Thermodynamics of Amyloid Fibril Formation. Chem. Sci. 2022, 13, 10177–10192. [Google Scholar] [CrossRef] [PubMed]
- Gallardo, R.; Ramakers, M.; De Smet, F.; Claes, F.; Khodaparast, L.; Khodaparast, L.; Couceiro, J.R.; Langenberg, T.; Siemons, M.; Nyström, S.; et al. De Novo Design of a Biologically Active Amyloid. Science 2016, 354, aah4949. [Google Scholar] [CrossRef] [PubMed]
- Akbey, Ü.; Andreasen, M. Functional Amyloids from Bacterial Biofilms—Structural Properties and Interaction Partners. Chem. Sci. 2022, 13, 6457–6477. [Google Scholar] [CrossRef] [PubMed]
- Levkovich, S.A.; Gazit, E.; Bar-Yosef, D.L. Two Decades of Studying Functional Amyloids in Microorganisms. Trends Microbiol. 2021, 29, 251–265. [Google Scholar] [CrossRef]
- Otzen, D.; Riek, R. Functional Amyloids. Cold Spring Harb. Perspect. Biol. 2019, 11, a033860. [Google Scholar] [CrossRef]
- Hardy, J.A.; Higgins, G.A. Alzheimer’s Disease: The Amyloid Cascade Hypothesis. Science 1992, 256, 184–185. [Google Scholar] [CrossRef]
- Fraser, P.E.; Nguyen, J.T.; Surewicz, W.K.; Kirschner, D.A. PH-Dependent Structural Transitions of Alzheimer Amyloid Peptides. Biophys. J. 1991, 60, 1190–1201. [Google Scholar] [CrossRef] [Green Version]
- Ke, P.C.; Sani, M.-A.; Ding, F.; Kakinen, A.; Javed, I.; Separovic, F.; Davis, T.P.; Mezzenga, R. Implications of Peptide Assemblies in Amyloid Diseases. Chem. Soc. Rev. 2017, 46, 6492–6531. [Google Scholar] [CrossRef]
- Harrison, R.S.; Sharpe, P.C.; Singh, Y.; Fairlie, D.P. Amyloid Peptides and Proteins in Review. In Reviews of Physiology, Biochemistry and Pharmacology; Amara, S.G., Bamberg, E., Fleischmann, B., Gudermann, T., Hebert, S.C., Jahn, R., Lederer, W.J., Lill, R., Miyajima, A., Offermanns, S., et al., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 1–77. ISBN 978-3-540-73800-8. [Google Scholar]
- Nelson, R.; Eisenberg, D. Recent Atomic Models of Amyloid Fibril Structure. Curr. Opin. Struct. Biol. 2006, 16, 260–265. [Google Scholar] [CrossRef]
- Sawaya, M.R.; Sambashivan, S.; Nelson, R.; Ivanova, M.I.; Sievers, S.A.; Apostol, M.I.; Thompsaon, M.J.; Balbirnie, M.; Wiltzius, J.J.W.; McFarlane, H.T.; et al. Atomic Structures of Amyloid Cross-Beta Spines Reveal Varied Steric Zippers. Nature 2007, 447, 453–457. [Google Scholar] [CrossRef] [PubMed]
- Friedmann, M.P.; Torbeev, V.; Zelenay, V.; Sobol, A.; Greenwald, J.; Riek, R. Towards Prebiotic Catalytic Amyloids Using High Throughput Screening. PLoS ONE 2015, 10, e0143948. [Google Scholar] [CrossRef]
- Chiti, F.; Dobson, C.M. Amyloid Formation by Globular Proteins under Native Conditions. Nat. Chem. Biol. 2009, 5, 15–22. [Google Scholar] [CrossRef]
- Frederix, P.W.J.M.; Scott, G.G.; Abul-Haija, Y.M.; Kalafatovic, D.; Pappas, C.G.; Javid, N.; Hunt, N.T.; Ulijn, R.V.; Tuttle, T. Exploring the Sequence Space for (Tri-)Peptide Self-Assembly to Design and Discover New Hydrogels. Nat. Chem. 2015, 7, 30–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xuan, Q.; Wang, Y.; Chen, C.; Wang, P. Rational Biological Interface Engineering: Amyloidal Supramolecular Microstructure-Inspired Hydrogel. Front. Bioeng. Biotechnol. 2021, 9, 718883. [Google Scholar] [CrossRef]
- Belwal, V.K.; Chaudhary, N. Amyloids and Their Untapped Potential as Hydrogelators. Soft Matter 2020, 16, 10013–10028. [Google Scholar] [CrossRef]
- Das, S.; Zhou, K.; Ghosh, D.; Jha, N.N.; Singh, P.K.; Jacob, R.S.; Bernard, C.C.; Finkelstein, D.I.; Forsythe, J.S.; Maji, S.K. Implantable Amyloid Hydrogels for Promoting Stem Cell Differentiation to Neurons. NPG Asia Mater. 2016, 8, e304. [Google Scholar] [CrossRef] [Green Version]
- Lu, K.; Jacob, J.; Thiyagarajan, P.; Conticello, V.P.; Lynn, D.G. Exploiting Amyloid Fibril Lamination for Nanotube Self-Assembly. J. Am. Chem. Soc. 2003, 125, 6391–6393. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Guo, P.; Pingali, S.V.; Pabit, S.; Thiyagarajan, P.; Berland, K.M.; Lynn, D.G. Light Harvesting Antenna on an Amyloid Scaffold. Chem. Commun. 2008, 48, 6522–6524. [Google Scholar] [CrossRef]
- Taheri, R.A.; Akhtari, Y.; Tohidi Moghadam, T.; Ranjbar, B. Assembly of Gold Nanorods on HSA Amyloid Fibrils to Develop a Conductive Nanoscaffold for Potential Biomedical and Biosensing Applications. Sci. Rep. 2018, 8, 9333. [Google Scholar] [CrossRef]
- Reches, M.; Gazit, E. Casting Metal Nanowires within Discrete Self-Assembled Peptide Nanotubes. Science 2003, 300, 625–627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faller, P.; Hureau, C.; Berthoumieu, O. Role of Metal Ions in the Self-Assembly of the Alzheimer’s Amyloid-β Peptide. Inorg. Chem. 2013, 52, 12193–12206. [Google Scholar] [CrossRef]
- Alies, B.; Hureau, C.; Faller, P. The Role of Metal Ions in Amyloid Formation: General Principles from Model Peptides. Metallomics 2013, 5, 183–192. [Google Scholar] [CrossRef]
- Diaz-Espinoza, R.; Nova, E.; Monasterio, O. Overcoming Electrostatic Repulsions during Amyloid Assembly: Effect of pH and Interaction with Divalent Metals Using Model Peptides. Arch. Biochem. Biophys. 2017, 621, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Soon, W.L.; Peydayesh, M.; Mezzenga, R.; Miserez, A. Plant-Based Amyloids from Food Waste for Removal of Heavy Metals from Contaminated Water. Chem. Eng. J. 2022, 445, 136513. [Google Scholar] [CrossRef]
- Bolisetty, S.; Mezzenga, R. Amyloid–Carbon Hybrid Membranes for Universal Water Purification. Nat. Nanotechnol. 2016, 11, 365–371. [Google Scholar] [CrossRef]
- Gremer, L.; Schölzel, D.; Schenk, C.; Reinartz, E.; Labahn, J.; Ravelli, R.B.G.; Tusche, M.; Lopez-Iglesias, C.; Hoyer, W.; Heise, H.; et al. Fibril Structure of Amyloid-β(1–42) by Cryo–Electron Microscopy. Science 2017, 358, 116–119. [Google Scholar] [CrossRef] [Green Version]
- Castillo-Caceres, C.; Duran-Meza, E.; Nova, E.; Araya-Secchi, R.; Monasterio, O.; Diaz-Espinoza, R. Functional Characterization of the ATPase-like Activity Displayed by a Catalytic Amyloid. Biochim. Biophys. Acta Gen. Subj. 2021, 1865, 129729. [Google Scholar] [CrossRef]
- Rufo, C.M.; Moroz, Y.S.; Moroz, O.V.; Stöhr, J.; Smith, T.A.; Hu, X.; DeGrado, W.F.; Korendovych, I.V. Short Peptides Self-Assemble to Produce Catalytic Amyloids. Nat. Chem. 2014, 6, 303–309. [Google Scholar] [CrossRef] [Green Version]
- West, M.W.; Wang, W.; Patterson, J.; Mancias, J.D.; Beasley, J.R.; Hecht, M.H. De Novo Amyloid Proteins from Designed Combinatorial Libraries. Proc. Natl. Acad. Sci. USA 1999, 96, 11211–11216. [Google Scholar] [CrossRef] [PubMed]
- Aumüller, T.; Fändrich, M. Protein Chemistry: Catalytic Amyloid Fibrils. Nat. Chem. 2014, 6, 273–274. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Wang, T.; Makhlynets, O.V.; Wu, Y.; Polizzi, N.F.; Wu, H.; Gosavi, P.M.; Stöhr, J.; Korendovych, I.V.; DeGrado, W.F. Zinc-Binding Structure of a Catalytic Amyloid from Solid-State NMR. Proc. Natl. Acad. Sci. USA 2017, 114, 6191–6196. [Google Scholar]
- Liang, S.; Wu, X.-L.; Zong, M.-H.; Lou, W.-Y. Construction of Zn-Heptapeptide Bionanozymes with Intrinsic Hydrolase-like Activity for Degradation of Di(2-Ethylhexyl) Phthalate. J. Colloid Interface Sci. 2022, 622, 860–870. [Google Scholar] [CrossRef]
- Colletier, J.-P.; Laganowsky, A.; Landau, M.; Zhao, M.; Soriaga, A.B.; Goldschmidt, L.; Flot, D.; Cascio, D.; Sawaya, M.R.; Eisenberg, D. Molecular Basis for Amyloid-β Polymorphism. Proc. Natl. Acad. Sci. USA 2011, 108, 16938–16943. [Google Scholar] [CrossRef] [Green Version]
- Makam, P.; Yamijala, S.S.R.K.C.; Tao, K.; Shimon, L.J.W.; Eisenberg, D.S.; Sawaya, M.R.; Wong, B.M.; Gazit, E. Non-Proteinaceous Hydrolase Comprised of a Phenylalanine Metallo-Supramolecular Amyloid-like Structure. Nat. Catal. 2019, 2, 977–985. [Google Scholar] [CrossRef] [PubMed]
- Omosun, T.O.; Hsieh, M.-C.; Childers, W.S.; Das, D.; Mehta, A.K.; Anthony, N.R.; Pan, T.; Grover, M.A.; Berland, K.M.; Lynn, D.G. Catalytic Diversity in Self-Propagating Peptide Assemblies. Nat. Chem. 2017, 9, 805–809. [Google Scholar] [PubMed]
- Zhang, C.; Xue, X.; Luo, Q.; Li, Y.; Yang, K.; Zhuang, X.; Jiang, Y.; Zhang, J.; Liu, J.; Zou, G.; et al. Self-Assembled Peptide Nanofibers Designed as Biological Enzymes for Catalyzing Ester Hydrolysis. ACS Nano 2014, 8, 11715–11723. [Google Scholar] [CrossRef]
- Díaz-Caballero, M.; Navarro, S.; Nuez-Martínez, M.; Peccati, F.; Rodríguez-Santiago, L.; Sodupe, M.; Teixidor, F.; Ventura, S. PH-Responsive Self-Assembly of Amyloid Fibrils for Dual Hydrolase-Oxidase Reactions. ACS Catal. 2021, 11, 595–607. [Google Scholar] [CrossRef]
- Garcia, A.M.; Kurbasic, M.; Kralj, S.; Melchionna, M.; Marchesan, S. A Biocatalytic and Thermoreversible Hydrogel from a Histidine-Containing Tripeptide. Chem. Commun. 2017, 53, 8110–8113. [Google Scholar] [CrossRef]
- Carlomagno, T.; Cringoli, M.C.; Kralj, S.; Kurbasic, M.; Fornasiero, P.; Pengo, P.; Marchesan, S. Biocatalysis of D,L-Peptide Nanofibrillar Hydrogel. Molecules 2020, 25, 2995. [Google Scholar] [CrossRef]
- Kurbasic, M.; Garcia, A.M.; Viada, S.; Marchesan, S. Tripeptide Self-Assembly into Bioactive Hydrogels: Effects of Terminus Modification on Biocatalysis. Molecules 2021, 26, 173. [Google Scholar] [CrossRef]
- Arad, E.; Baruch Leshem, A.; Rapaport, H.; Jelinek, R. β-Amyloid Fibrils Catalyze Neurotransmitter Degradation. Chem. Catal. 2021, 1, 908–922. [Google Scholar] [CrossRef]
- Arad, E.; Yosefi, G.; Kolusheva, S.; Bitton, R.; Rapaport, H.; Jelinek, R. Native Glucagon Amyloids Catalyze Key Metabolic Reactions. ACS Nano 2022, 16, 12889–12899. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Kumar, M.; Mittal, A.; Mehta, P.K. Microbial Enzymes: Industrial Progress in 21st Century. 3 Biotech 2016, 6, 174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Houde, A.; Kademi, A.; Leblanc, D. Lipases and Their Industrial Applications: An Overview. Appl. Biochem. Biotechnol. 2004, 118, 155–170. [Google Scholar] [CrossRef]
- Lengyel, Z.; Rufo, C.M.; Moroz, Y.S.; Makhlynets, O.V.; Korendovych, I.V. Copper-Containing Catalytic Amyloids Promote Phosphoester Hydrolysis and Tandem Reactions. ACS Catal. 2018, 8, 59–62. [Google Scholar] [CrossRef] [PubMed]
- Al-Garawi, Z.S.; McIntosh, B.A.; Neill-Hall, D.; Hatimy, A.A.; Sweet, S.M.; Bagley, M.C.; Serpell, L.C. The Amyloid Architecture Provides a Scaffold for Enzyme-like Catalysts. Nanoscale 2017, 9, 10773–10783. [Google Scholar] [CrossRef]
- Monasterio, O.; Nova, E.; Diaz-Espinoza, R. Development of a Novel Catalytic Amyloid Displaying a Metal-Dependent ATPase-like Activity. Biochem. Biophys. Res. Commun. 2017, 482, 1194–1200. [Google Scholar] [CrossRef]
- Hureau, C.; Faller, P. Aβ-Mediated ROS Production by Cu Ions: Structural Insights, Mechanisms and Relevance to Alzheimer’s Disease. Biochimie 2009, 91, 1212–1217. [Google Scholar] [CrossRef]
- Makhlynets, O.V.; Gosavi, P.M.; Korendovych, I.V. Short Self-Assembling Peptides Are Able to Bind to Copper and Activate Oxygen. Angew. Chem. Int. Ed. 2016, 55, 9017–9020. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; Yin, J.-H.; Lan, C.; Meng, L.; Xu, N. Supramolecule Self-Assembly Synthesis of Amyloid Phenylalanine-Cu Fibrils with Laccase-like Activity and Their Application for Dopamine Determination. Microchim. Acta 2022, 189, 98. [Google Scholar] [CrossRef]
- Makam, P.; Yamijala, S.S.R.K.C.; Bhadram, V.S.; Shimon, L.J.W.; Wong, B.M.; Gazit, E. Single Amino Acid Bionanozyme for Environmental Remediation. Nat. Commun. 2022, 13, 1505. [Google Scholar] [CrossRef]
- Liu, Q.; Wan, K.; Shang, Y.; Wang, Z.-G.; Zhang, Y.; Dai, L.; Wang, C.; Wang, H.; Shi, X.; Liu, D.; et al. Cofactor-Free Oxidase-Mimetic Nanomaterials from Self-Assembled Histidine-Rich Peptides. Nat. Mater. 2021, 20, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Zozulia, O.; Marshall, L.R.; Kim, I.; Kohn, E.M.; Korendovych, I.V. Self-Assembling Catalytic Peptide Nanomaterials Capable of Highly Efficient Peroxidase Activity. Chem. Eur. J. 2021, 27, 5388–5392. [Google Scholar]
- Zozulia, O.; Korendovych, I.V. Semi-Rationally Designed Short Peptides Self-Assemble and Bind Hemin to Promote Cyclopropanation. Angew. Chem. Int. Ed. 2020, 59, 8108–8112. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, A.; Afrose, S.P.; Ahmed, S.; Venugopal, A.; Das, D. Cross-β Amyloid Nanotubes for Hydrolase–Peroxidase Cascade Reactions. Chem. Commun. 2020, 56, 7869–7872. [Google Scholar] [CrossRef]
- Sarkhel, B.; Chatterjee, A.; Das, D. Covalent Catalysis by Cross β Amyloid Nanotubes. J. Am. Chem. Soc. 2020, 142, 4098–4103. [Google Scholar] [CrossRef]
- Reja, A.; Afrose, S.P.; Das, D. Aldolase Cascade Facilitated by Self-Assembled Nanotubes from Short Peptide Amphiphiles. Angew. Chem. Int. Ed. 2020, 59, 4329–4334. [Google Scholar] [CrossRef]
- Pelin, J.N.B.D.; Gerbelli, B.B.; Soares, B.M.; Aguilar, A.M.; Alves, W.A. Amyloidogenic Model Peptides as Catalysts for Stereoselective Aldol Reactions. Catal. Sci. Technol. 2019, 9, 4304–4313. [Google Scholar] [CrossRef]
- Tena-Solsona, M.; Nanda, J.; Díaz-Oltra, S.; Chotera, A.; Ashkenasy, G.; Escuder, B. Emergent Catalytic Behavior of Self-Assembled Low Molecular Weight Peptide-Based Aggregates and Hydrogels. Chem. Eur. J. 2016, 22, 6687–6694. [Google Scholar] [CrossRef]
- Mahato, C.; Menon, S.; Singh, A.; Afrose, S.P.; Mondal, J.; Das, D. Short Peptide-Based Cross-β Amyloids Exploit Dual Residues for Phosphoesterase like Activity. Chem. Sci. 2022, 13, 9225–9231. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, A.; Ghosh, S.; Ghosh, C.; Das, D. Fluorescent Microswimmers Based on Cross-β Amyloid Nanotubes and Divergent Cascade Networks. Angew. Chem. Int. Ed. 2022, 61, e202201547. [Google Scholar] [CrossRef]
- Chatterjee, A.; Mahato, C.; Das, D. Complex Cascade Reaction Networks via Cross β Amyloid Nanotubes. Angew. Chem. Int. Ed. 2021, 60, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Foguel, D.; Suarez, M.C.; Ferrão-Gonzales, A.D.; Porto, T.C.R.; Palmieri, L.; Einsiedler, C.M.; Andrade, L.R.; Lashuel, H.A.; Lansbury, P.T.; Kelly, J.W.; et al. Dissociation of Amyloid Fibrils of α-Synuclein and Transthyretin by Pressure Reveals Their Reversible Nature and the Formation of Water-Excluded Cavities. Proc. Natl. Acad. Sci. USA 2003, 100, 9831–9836. [Google Scholar] [CrossRef] [Green Version]
- Ostermeier, L.; de Oliveira, G.A.P.; Dzwolak, W.; Silva, J.L.; Winter, R. Exploring the Polymorphism, Conformational Dynamics and Function of Amyloidogenic Peptides and Proteins by Temperature and Pressure Modulation. Biophys. Chem. 2021, 268, 106506. [Google Scholar] [CrossRef] [PubMed]
- Piccirilli, F.; Plotegher, N.; Spinozzi, F.; Bubacco, L.; Mariani, P.; Beltramini, M.; Tessari, I.; Militello, V.; Perucchi, A.; Amenitsch, H.; et al. Pressure Effects on α-Synuclein Amyloid Fibrils: An Experimental Investigation on Their Dissociation and Reversible Nature. Arch. Biochem. Biophys. 2017, 627, 46–55. [Google Scholar] [CrossRef] [Green Version]
- Sasahara, K.; Naiki, H.; Goto, Y. Kinetically Controlled Thermal Response of Β2-Microglobulin Amyloid Fibrils. J. Mol. Biol. 2005, 352, 700–711. [Google Scholar] [CrossRef]
- Smith, J.F.; Knowles, T.P.J.; Dobson, C.M.; MacPhee, C.E.; Welland, M.E. Characterization of the Nanoscale Properties of Individual Amyloid Fibrils. Proc. Natl. Acad. Sci. USA 2006, 103, 15806–15811. [Google Scholar] [CrossRef] [Green Version]
- Knowles, T.P.; Fitzpatrick, A.W.; Meehan, S.; Mott, H.R.; Vendruscolo, M.; Dobson, C.M.; Welland, M.E. Role of Intermolecular Forces in Defining Material Properties of Protein Nanofibrils. Science 2007, 318, 1900–1903. [Google Scholar] [CrossRef]
- Lamour, G.; Nassar, R.; Chan, P.H.W.; Bozkurt, G.; Li, J.; Bui, J.M.; Yip, C.K.; Mayor, T.; Li, H.; Wu, H.; et al. Mapping the Broad Structural and Mechanical Properties of Amyloid Fibrils. Biophys. J. 2017, 112, 584–594. [Google Scholar] [CrossRef] [Green Version]
- Schleeger, M.; vandenAkker, C.C.; Deckert-Gaudig, T.; Deckert, V.; Velikov, K.P.; Koenderink, G.; Bonn, M. Amyloids: From Molecular Structure to Mechanical Properties. Polymer 2013, 54, 2473–2488. [Google Scholar] [CrossRef] [Green Version]
- Bortolini, C.; Jones, N.C.; Hoffmann, S.V.; Wang, C.; Besenbacher, F.; Dong, M. Mechanical Properties of Amyloid-like Fibrils Defined by Secondary Structures. Nanoscale 2015, 7, 7745–7752. [Google Scholar] [CrossRef] [PubMed]
- Rebek, J. On the Structure of Histidine and Its Role in Enzyme Active Sites. Struct. Chem. 1990, 1, 129–131. [Google Scholar] [CrossRef]
| Peptide 1,2 | Catalytic Activity | Cofactor | kcat 2 (s−1) | kcat/KM2 (M−1s−1) |
|---|---|---|---|---|
| Ac-IHIHIQI-Am | Ester hydrolysis 3 | Zn | 2.6 × 10−2 | 62 |
| Oxidation | Cu | - | - | |
| Ac-IHIHIYI-Am | Ester hydrolysis 3 | Zn | 8.3 × 10−3 | 355 |
| Phosphoester hydrolysis | Cu | 8 × 10−5 | 2.8 × 10−2 | |
| Ac-IHVHLQI-Am | Ester hydrolysis 3 | Zn | 1.76 | 127.7 |
| Phe | Ester hydrolysis | Zn | - | 76.5 |
| Oxidation | Cu | 11.9 | 63 × 10−3 | |
| Ac-HSGQQKFQFQFEQQ-Am | Ester hydrolysis | None | 1.95 × 10−3 | 9 × 10−2 |
| Ac-HYHYHYHYH-Am | Ester hydrolysis | None | 3.5 × 10−3 | 1.64 |
| Oxidation | Cu | - | - | |
| HLDLIHLDL | Ester hydrolysis | None | 2.8 × 10−3 | 2.9 |
| HFDFD 4 | Ester hydrolysis | None | 8.7 × 10−3 | - |
| Ab42 | Ester hydrolysis | None | 1.9 × 10−3 | 0.66 |
| Oxidation | None | - | - | |
| Glucagon | Ester hydrolysis 3 | None | 2.5 × 10−3 | 0.57 |
| Phosphoanhydride hydrolysis | None | 1.6 × 10−5 | 2.7 × 10−1 | |
| Phosphoester hydrolysis | None | 7 × 10−3 | 59.3 | |
| Ac-NADFDGDQMAVHV-Am | Phosphoanhydride hydrolysis | Mn | 2.3 × 10−4 | 4.2 × 10−2 |
| Ac-SDIDVFI-Am | Phosphoanhydride hydrolysis | Mn | 4.2 × 10−6 | 6.4 × 10−2 |
| Ac-Oligohistidine-Am | Oxidation 5 | None | 1.3 × 10−4 | 0.7 |
| Ac-LALHLFL-Am | Oxidation 5 | Hemin | 1.3 | 300 |
| Ac-LMLHLFL-Am | Oxidation 5 | Hemin | 7.8 | 565 |
| Ac-KLVFFAL-Am | Retro-aldol | None | 6.2 × 10−5 | - |
| Ac-HLVFFAL-Am | Oxidation 5 | Hemin | 0.24 | - |
| Im-KLVFFAL-Am | Ester hydrolysis | None | 1.5 × 10−3 | 2.1 |
| C10-FFVK-Am | Retro-aldol | None | 1.7 × 10−5 | - |
| Ac-PRFRFRFRF-Am | Retro-aldol | None | - | - |
| Ac-RLVFFAH-Am | Phosphoester hydrolysis | None | 10.9 × 10−5 | 1.58 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diaz-Espinoza, R. Catalytically Active Amyloids as Future Bionanomaterials. Nanomaterials 2022, 12, 3802. https://doi.org/10.3390/nano12213802
Diaz-Espinoza R. Catalytically Active Amyloids as Future Bionanomaterials. Nanomaterials. 2022; 12(21):3802. https://doi.org/10.3390/nano12213802
Chicago/Turabian StyleDiaz-Espinoza, Rodrigo. 2022. "Catalytically Active Amyloids as Future Bionanomaterials" Nanomaterials 12, no. 21: 3802. https://doi.org/10.3390/nano12213802
APA StyleDiaz-Espinoza, R. (2022). Catalytically Active Amyloids as Future Bionanomaterials. Nanomaterials, 12(21), 3802. https://doi.org/10.3390/nano12213802






