Alloying Iron into Palladium Nanoparticles for an Efficient Catalyst in Acetylene Dicarbonylation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Catalyst Synthesis
2.3. Catalyst Characterization
2.4. Computational Methods
2.5. Catalyst Evaluation
3. Results and Discussion
3.1. Structure and Morphology of Nanocatalyst
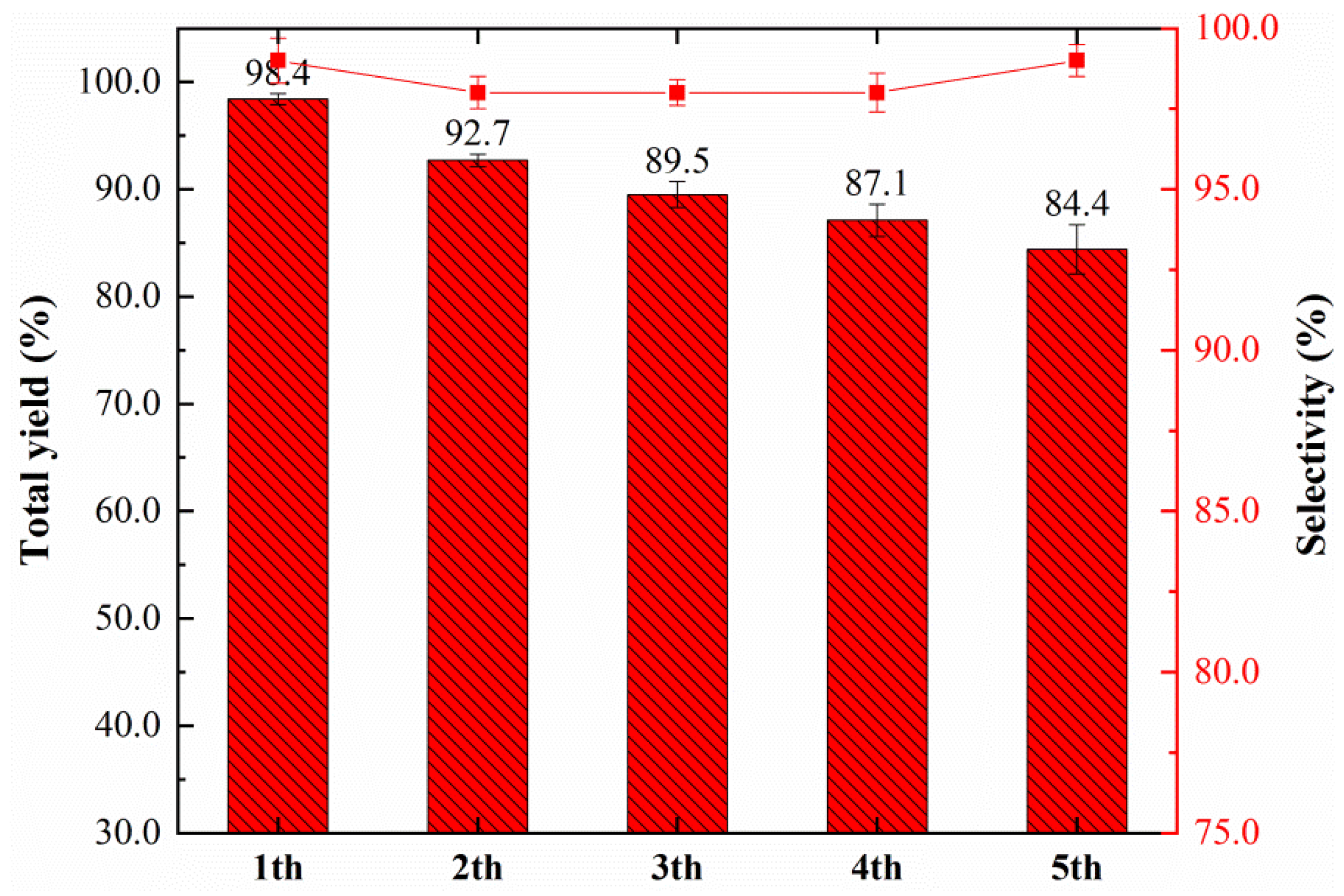
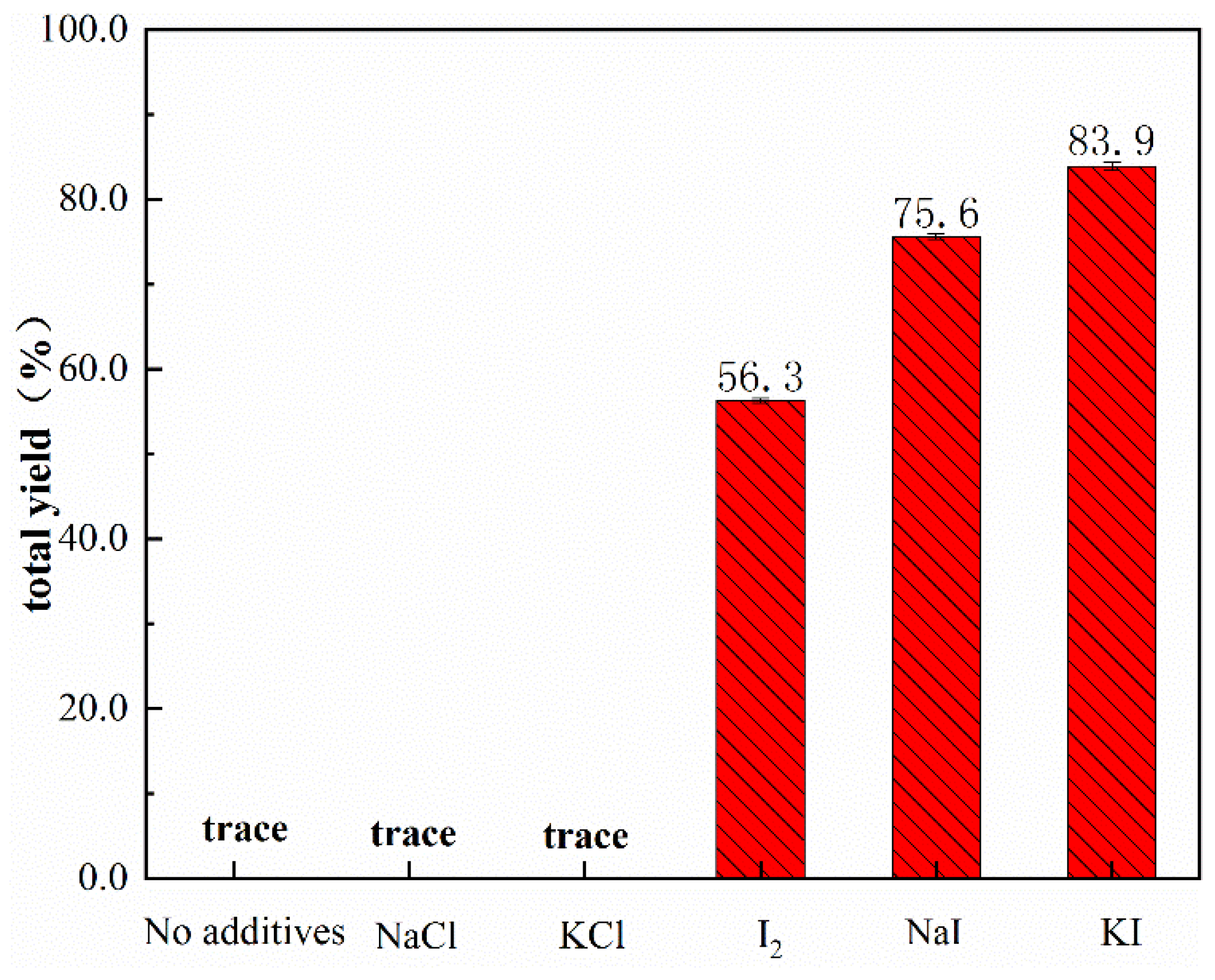
3.2. Catalyst Performance and Evaluation
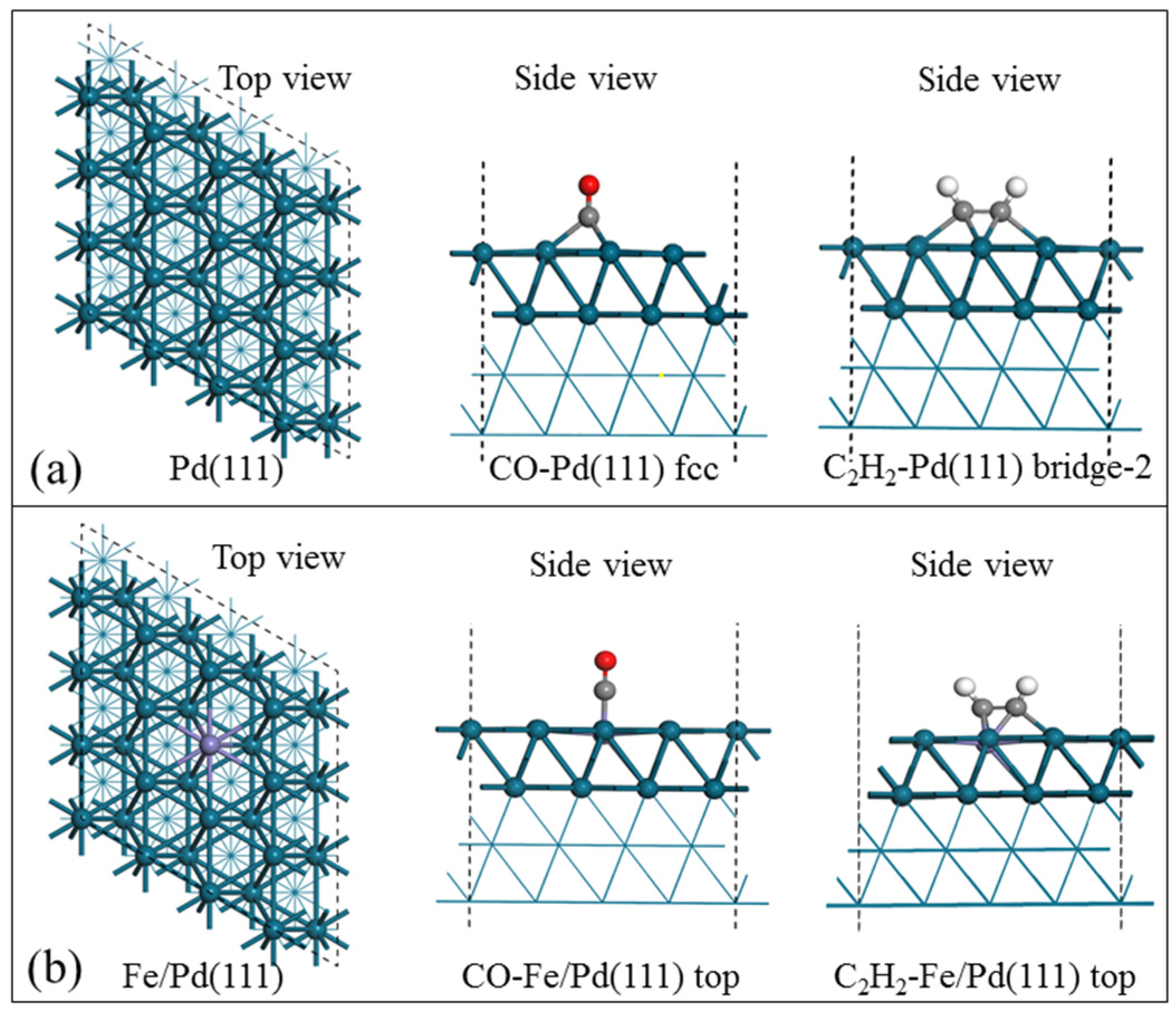
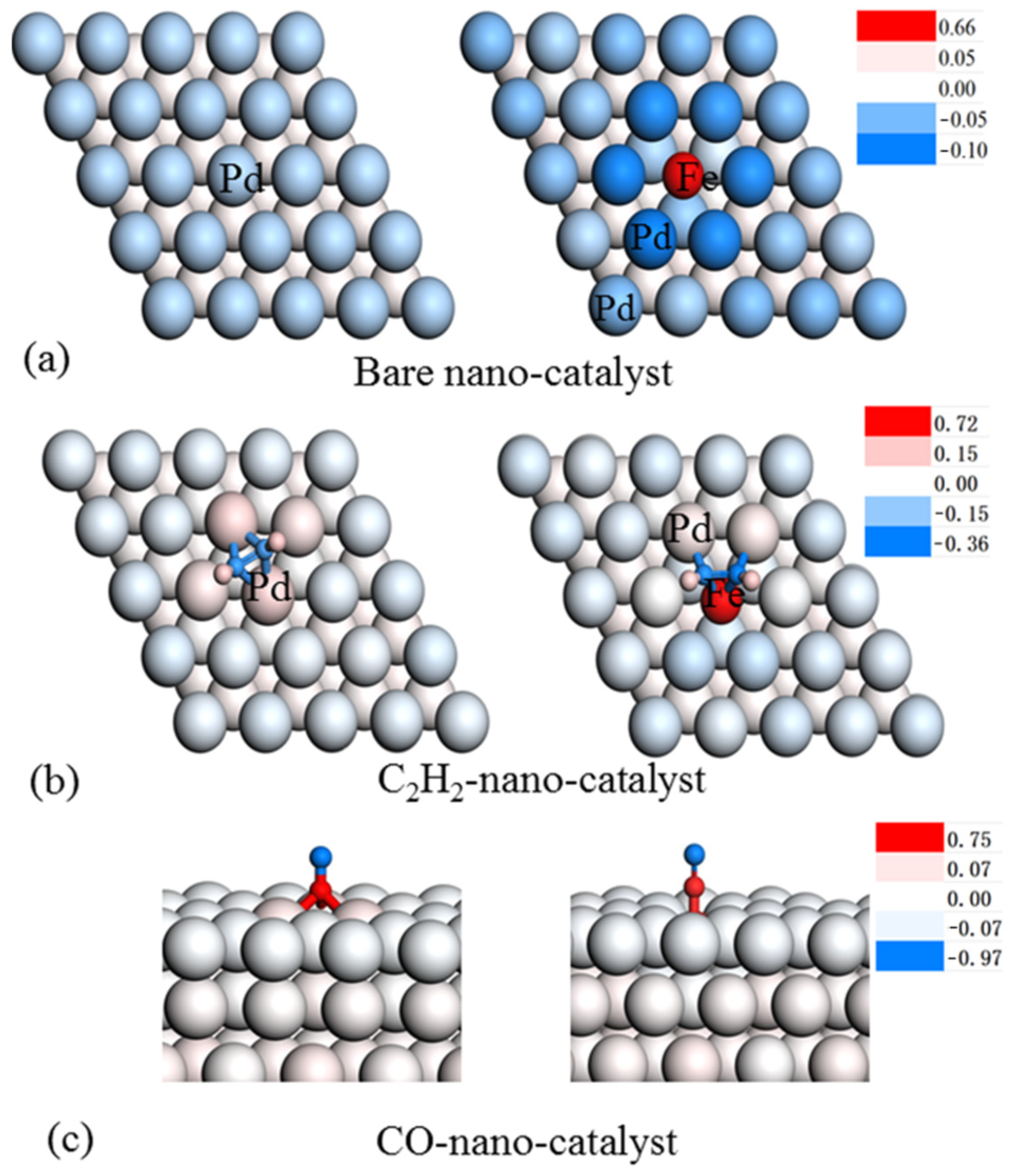
3.3. Mechanism Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Trotuş, I.-T.; Zimmermann, T.; Schüth, F. Catalytic Reactions of Acetylene: A Feedstock for the Chemical Industry Revisited. Chem. Rev. 2013, 114, 1761–1782. [Google Scholar] [CrossRef]
- Wei, X.; Ma, Z.; Mu, X.; Lu, J.; Hu, B. Catalyst in Acetylene Carbonylation: From Homogeneous to Heterogeneous. Prog. Chem. 2021, 33, 243. [Google Scholar] [CrossRef]
- Sun, Y.; Zeng, J.; Zhang, J.; Yang, J.; Qian, W.; Yu, F.; Dai, B.; Li, J. Combustion Products of Calcium Carbide Reused by Cu-Based Catalysts for Acetylene Carbonylation. ACS Omega 2020, 5, 27692–27701. [Google Scholar] [CrossRef]
- Putin, A.Y.; Katsman, E.A.; Bruk, L.G. State of Palladium Complexes in the PdBr2–LiBr–CH3CN–H2O Catalytic System, Used to Obtain Succinic Anhydride. Russ. J. Phys. Chem. A 2019, 93, 222–230. [Google Scholar] [CrossRef]
- Li, X.; Feng, S.; Song, X.; Yuan, Q.; Li, B.; Ning, L.; Chen, W.; Li, J.; Ding, Y. The evolution of single-site Pd1/AC catalyst during the process of acetylene dialkoxycarbonylation. J. Catal. 2022, 413, 762–768. [Google Scholar] [CrossRef]
- Wei, X.; Ma, Z.; Lu, J.; Mu, X.; Hu, B. The highly efficient and selective dicarbonylation of acetylene catalysed by palladium nanosheets supported on activated carbon. New J. Chem. 2020, 44, 11835–11840. [Google Scholar] [CrossRef]
- Wei, X.; Ma, Z.; Lu, J.; Mu, X.; Hu, B. Strong metal–support interactions between palladium nanoclusters and hematite toward enhanced acetylene dicarbonylation at low temperature. New J. Chem. 2020, 44, 1221–1227. [Google Scholar] [CrossRef]
- Wei, X.; Ma, Z.; Mu, X.; Zhang, Q.; Hu, B. Synergistic effect of hematite facet and Pd nanocluster for enhanced acetylene dicarbonylation. Mol. Catal. 2021, 499, 111303. [Google Scholar] [CrossRef]
- Chang, C.-R.; Zhao, Z.-J.; Köhler, K.; Genest, A.; Li, J.; Rösch, N. Theoretical study on the leaching of palladium in a CO atmosphere. Catal. Sci. Technol. 2012, 2, 2238. [Google Scholar] [CrossRef]
- Sankar, M.; Dimitratos, N.; Miedziak, P.J.; Wells, P.P.; Kiely, C.J.; Hutchings, G.J. Designing bimetallic catalysts for a green and sustainable future. Chem. Soc. Rev. 2012, 41, 8099–8139. [Google Scholar] [CrossRef]
- Wu, C.H.; Liu, C.; Su, D.; Xin, H.L.; Fang, H.-T.; Eren, B.; Zhang, S.; Murray, C.B.; Salmeron, M.B. Bimetallic synergy in cobalt–palladium nanocatalysts for CO oxidation. Nat. Catal. 2018, 2, 78–85. [Google Scholar] [CrossRef] [Green Version]
- Mauriello, F.; Paone, E.; Pietropaolo, R.; Balu, A.M.; Luque, R. Catalytic transfer hydrogenolysis of lignin-derived aromatic ethers promoted by bimetallic Pd/Ni systems. ACS Sustain. Chem. Eng. 2018, 6, 9269–9276. [Google Scholar] [CrossRef]
- Gholinejad, M.; Bahrami, M.; Nájera, C.; Pullithadathil, B. Magnesium oxide supported bimetallic Pd/Cu nanoparticles as an efficient catalyst for Sonogashira reaction. J. Catal. 2018, 363, 81–91. [Google Scholar] [CrossRef] [Green Version]
- Sun, Q.; Chen, B.W.J.; Wang, N.; He, Q.; Chang, A.; Yang, C.M.; Asakura, H.; Tanaka, T.; Hulsey, M.J.; Wang, C.H.; et al. Zeolite-Encaged Pd-Mn Nanocatalysts for CO2 Hydrogenation and Formic Acid Dehydrogenation. Angew. Chem. 2020, 59, 20183–20191. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Chung, Y.; Lee, K.-S.; Kwon, Y. Enhancements in catalytic activity and duration of PdFe bimetallic catalysts and their use in direct formic acid fuel cells. J. Ind. Eng. Chem. 2020, 90, 351–357. [Google Scholar] [CrossRef]
- Wan, J.; Liu, Z.; Yang, X.; Cheng, P.; Yan, C. Cyanogel-Derived Synthesis of Porous PdFe Nanohydrangeas as Electrocatalysts for Oxygen Reduction Reaction. Nanomaterials 2021, 11, 3382. [Google Scholar] [CrossRef]
- Guczi, L. Structural Effect of Bimetallic Catalysts in the CO + H2 Reaction. In Studies in Surface Science and Catalysis; Elsevier: Amsterdam, The Netherlands, 1988; Volume 38, pp. 85–96. [Google Scholar] [CrossRef]
- Hafner, J. Ab-initio simulations of materials using VASP: Density-functional theory and beyond. J. Comput. Chem. 2008, 29, 2044–2078. [Google Scholar] [CrossRef]
- Wang, C.; Wang, L.; Zhang, J.; Wang, H.; Lewis, J.P.; Xiao, F.-S. Product selectivity controlled by zeolite crystals in biomass hydrogenation over a palladium catalyst. J. Am. Chem. Soc. 2016, 138, 7880–7883. [Google Scholar] [CrossRef]
- Li, H.; Zhu, Z.; Li, H.; Li, P.; Zhou, X. Recyclable hollow Pd-Fe nanospheric catalyst for Sonogashira-, Heck-, and Ullmann-type coupling reactions of aryl halide in aqueous media. J. Colloid Interface Sci 2010, 349, 613–619. [Google Scholar] [CrossRef]
- He, Q.; Ji, J.; Zhang, Q.; Yang, X.; Lin, Y.; Meng, Q.; Zhang, Y.; Xu, M. PdMn and PdFe nanoparticles over a reduced graphene oxide carrier for methanol electro-oxidation under alkaline conditions. Ionics 2020, 26, 2421–2433. [Google Scholar] [CrossRef]
- Téllez, V.C.; Portillo, M.C.; Santiesteban, H.J.; Castillo, M.P.; Santiago, A.C.; Mora-Ramírez, M.; Coyotecatl, H.A.; Moreno, O.P. Green synthesis of palladium mixed with PdO nanoparticles by chemical bath deposition. Opt. Mater. 2021, 112, 110747. [Google Scholar] [CrossRef]
- Wu, P.; Tan, S.; Moon, J.; Yan, Z.; Fung, V.; Li, N.; Yang, S.Z.; Cheng, Y.; Abney, C.W.; Wu, Z.; et al. Harnessing strong metal-support interactions via a reverse route. Nat. Commun. 2020, 11, 3042. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, T.; Hayes, P. Analysis of XPS spectra of Fe2+ and Fe3+ ions in oxide materials. Appl. Surf. Sci. 2008, 254, 2441–2449. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, D.; Zhao, P.; Niu, Z.; Peng, Q.; Li, Y. Monodispersed Pd-Ni nanoparticles: Composition control synthesis and catalytic properties in the Miyaura-Suzuki reaction. Inorg. Chem. 2011, 50, 2046–2048. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.K.; Lee, J.K.; Kang, K.H.; Song, J.C.; Song, I.K. Selective cleavage of C-O bond in benzyl phenyl ether to aromatics over Pd–Fe bimetallic catalyst supported on ordered mesoporous carbon. Appl. Catal. A Gen. 2015, 498, 142–149. [Google Scholar] [CrossRef]
- Babu, N.S.; Lingaiah, N.; Kumar, J.V.; Prasad, P.S.S. Studies on alumina supported Pd–Fe bimetallic catalysts prepared by deposition–precipitation method for hydrodechlorination of chlorobenzene. Appl. Catal. A Gen. 2009, 367, 70–76. [Google Scholar] [CrossRef]
- Zhao, S.; Zhang, Q.; Song, C.; Pei, X.; Xiong, X.; Hu, B. Palladium-catalyzed Dicarbonylation of Acetylene: Efficient Synthesis of Dicarboxylic Acid Dimethyl Esters. J. Mol. Catal. (China) 2017, 31, 411–418. [Google Scholar] [CrossRef]
- Zhao, J.; He, Y.; Wang, F.; Yang, Y.; Zheng, W.; Huo, C.; Jiao, H.; Yang, Y.; Li, Y.; Wen, X. A recyclable CoGa intermetallic compound catalyst for the hydroformylation reaction. J. Catal. 2021, 404, 244–249. [Google Scholar] [CrossRef]
- Zhao, J.; He, Y.; Wang, F.; Zheng, W.; Huo, C.; Liu, X.; Jiao, H.; Yang, Y.; Li, Y.; Wen, X. Suppressing Metal Leaching in a Supported Co/SiO2 Catalyst with Effective Protectants in the Hydroformylation Reaction. ACS Catal. 2019, 10, 914–920. [Google Scholar] [CrossRef]
- Li, X.; Feng, S.; Hemberger, P.; Bodi, A.; Song, X.; Yuan, Q.; Mu, J.; Li, B.; Jiang, Z.; Ding, Y. Iodide-Coordinated Single-Site Pd Catalysts for Alkyne Dialkoxycarbonylation. ACS Catal. 2021, 11, 9242–9251. [Google Scholar] [CrossRef]
- Murata, K.; Eleeda, E.; Ohyama, J.; Yamamoto, Y.; Arai, S.; Satsuma, A. Identification of active sites in CO oxidation over a Pd/Al2O3 catalyst. Phys. Chem. Chem. Phys. 2019, 21, 18128–18137. [Google Scholar] [CrossRef] [PubMed]
- Jang, E.J.; Lee, J.; Oh, D.G.; Kwak, J.H. CH4 Oxidation Activity in Pd and Pt–Pd Bimetallic Catalysts: Correlation with Surface PdOx Quantified from the DRIFTS Study. ACS Catal. 2021, 11, 5894–5905. [Google Scholar] [CrossRef]
- Jiang, Y.; Chen, W.; Liao, H.; Jin, L.; Sun, S. Special IR properties of palladium nanoparticles and their aggregations in CO molecular probe infrared spectroscopy. Chin. Sci. Bull. 2004, 49, 1581–1585. [Google Scholar] [CrossRef] [Green Version]
- Crespo-Quesada, M.; Andanson, J.M.; Yarulin, A.; Lim, B.; Xia, Y.; Kiwi-Minsker, L. UV-ozone cleaning of supported poly(vinylpyrrolidone)-stabilized palladium nanocubes: Effect of stabilizer removal on morphology and catalytic behavior. Langmuir 2011, 27, 7909–7916. [Google Scholar] [CrossRef]
- Sun, W.; Wu, S.; Lu, Y.; Wang, Y.; Cao, Q.; Fang, W. Effective control of particle size and electron density of Pd/C and Sn-Pd/C nanocatalysts for vanillin production via base-free oxidation. ACS Catal. 2020, 10, 7699–7709. [Google Scholar] [CrossRef]
- Yang, J.; Li, S.; Zhang, L.; Liu, X.; Wang, J.; Pan, X.; Li, N.; Wang, A.; Cong, Y.; Wang, X.; et al. Hydrodeoxygenation of furans over Pd-FeOx/SiO2 catalyst under atmospheric pressure. Appl. Catal. B Environ. 2017, 201, 266–277. [Google Scholar] [CrossRef]
- Henkelman, G.; Arnaldsson, A.; Jónsson, H. A fast and robust algorithm for Bader decomposition of charge density. Comput. Mater. Sci. 2006, 36, 354–360. [Google Scholar] [CrossRef]
- Wang, S.-B.; Zhu, W.; Ke, J.; Lin, M.; Zhang, Y.-W. Pd–Rh Nanocrystals with Tunable Morphologies and Compositions as Efficient Catalysts toward Suzuki Cross-Coupling Reactions. ACS Catal. 2014, 4, 2298–2306. [Google Scholar] [CrossRef]
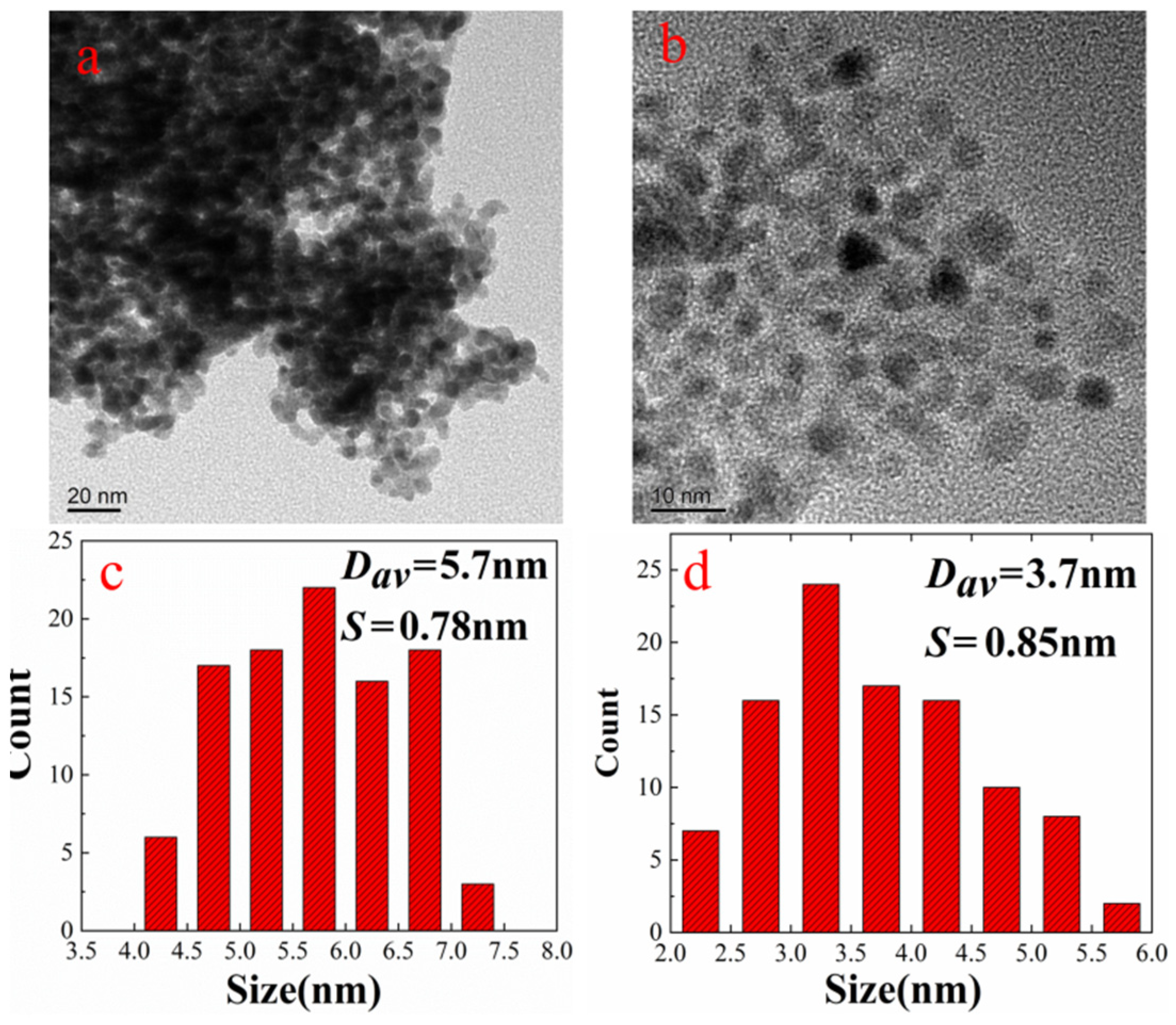
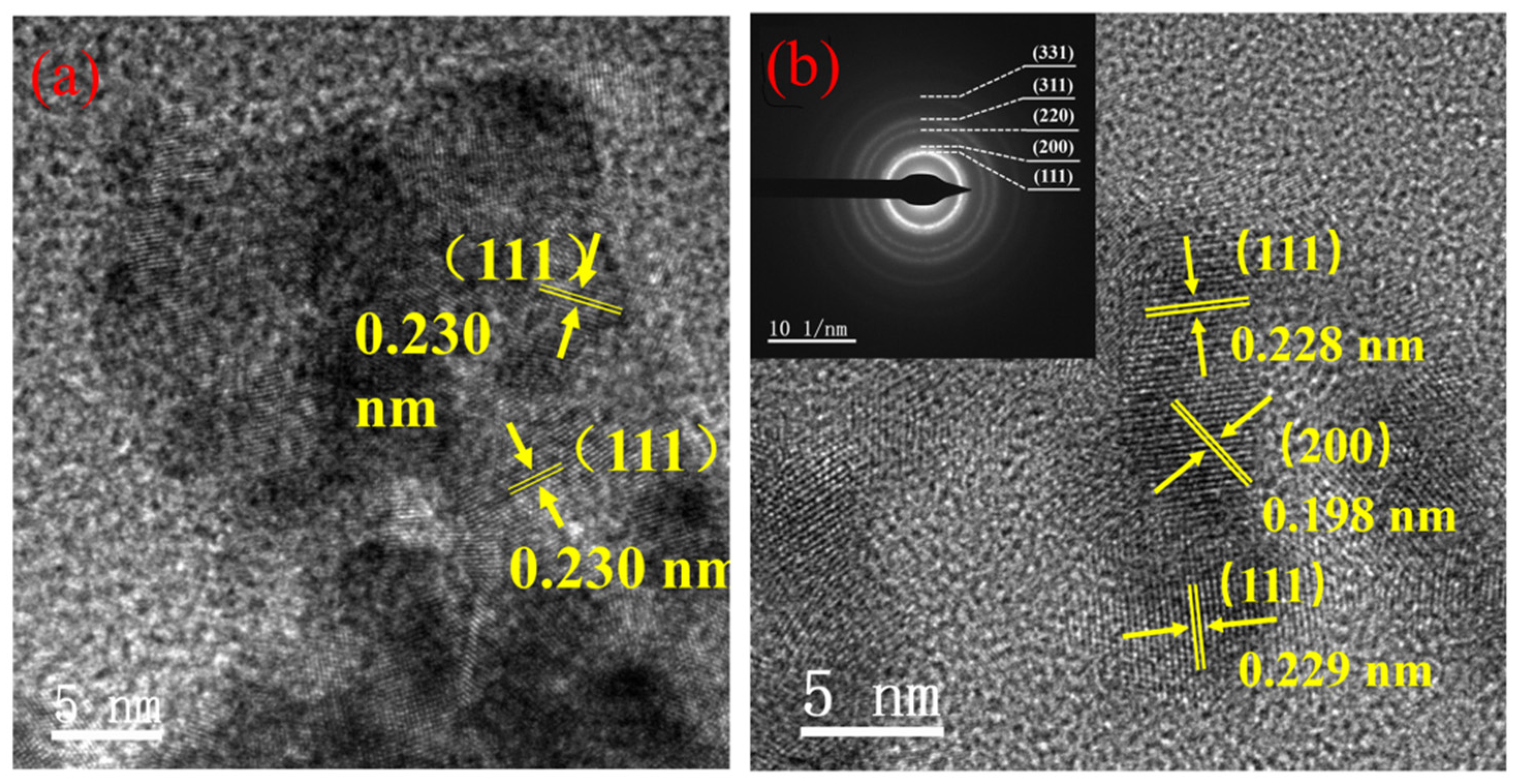
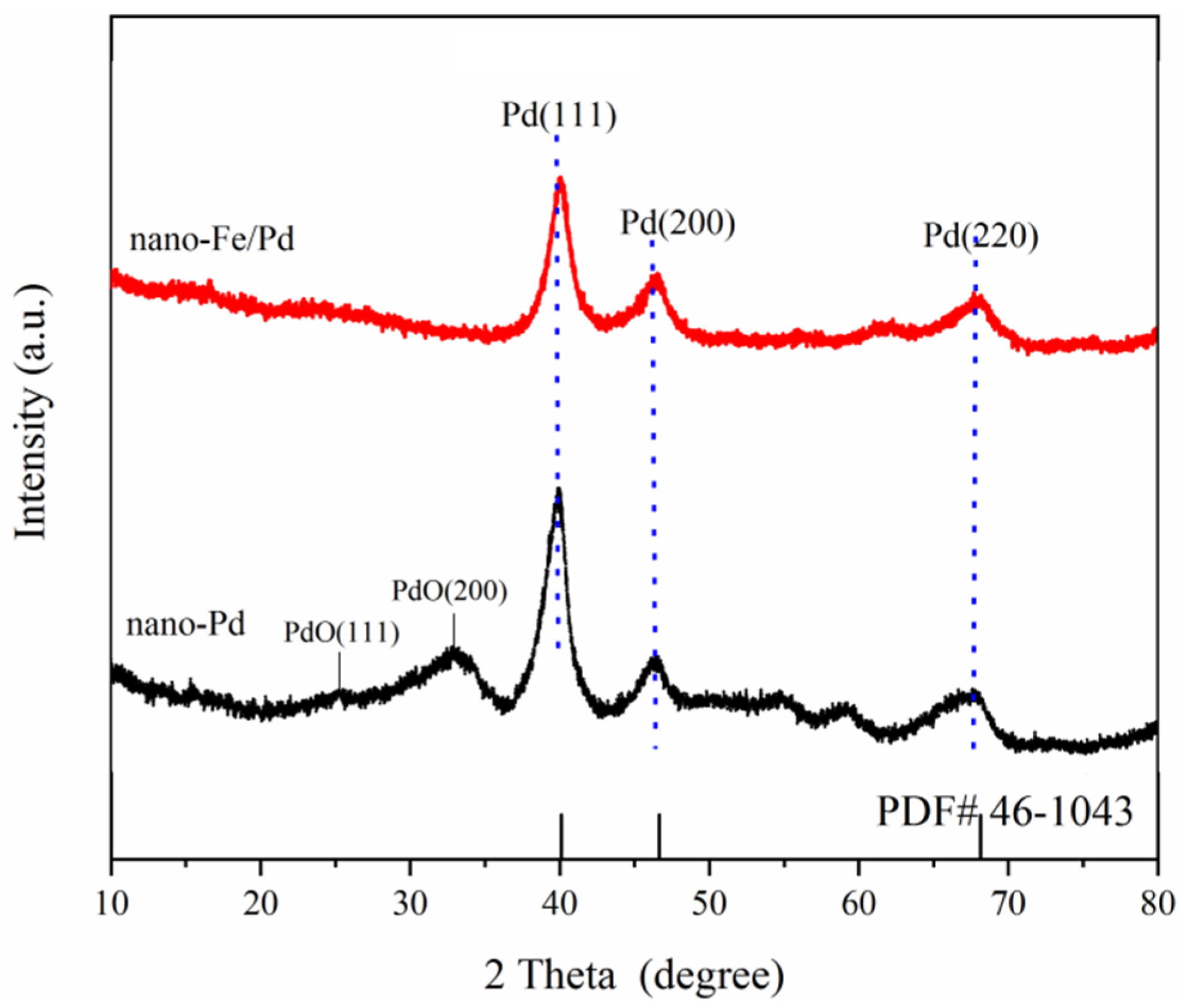
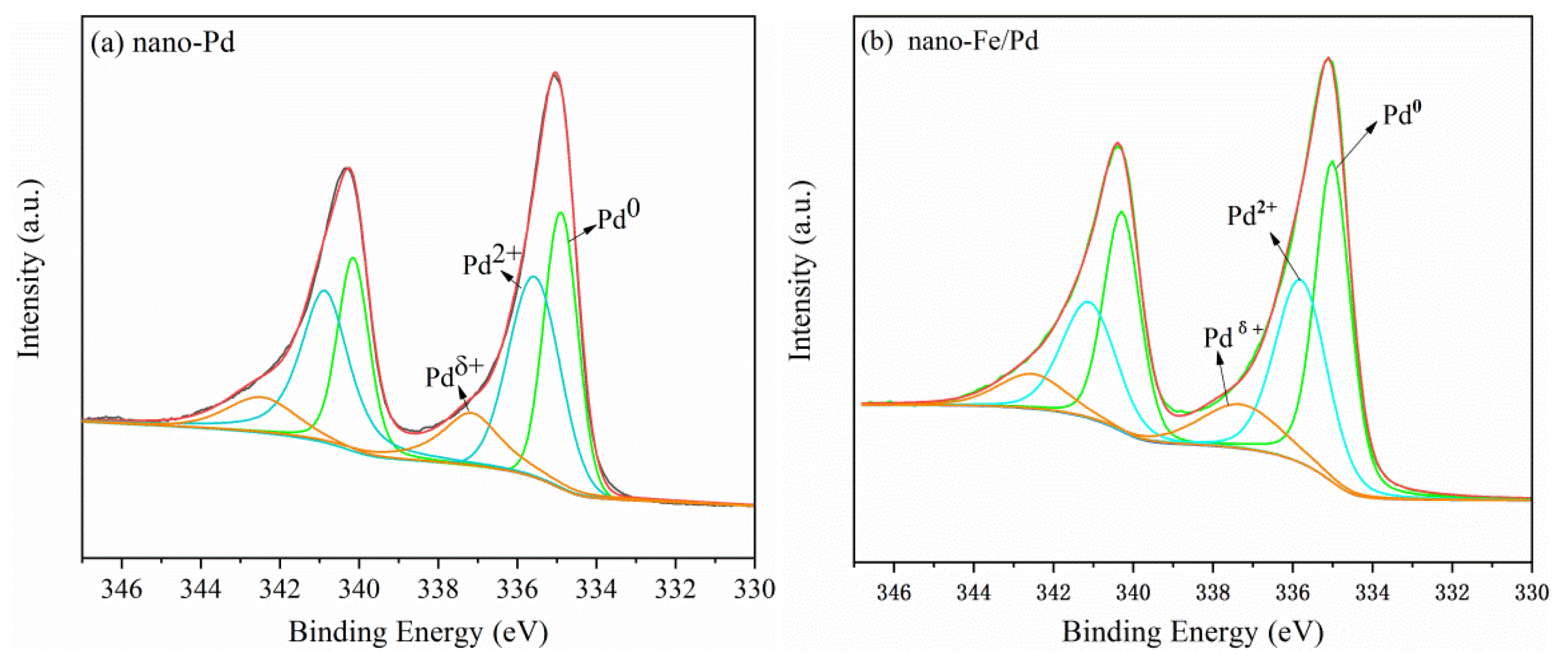

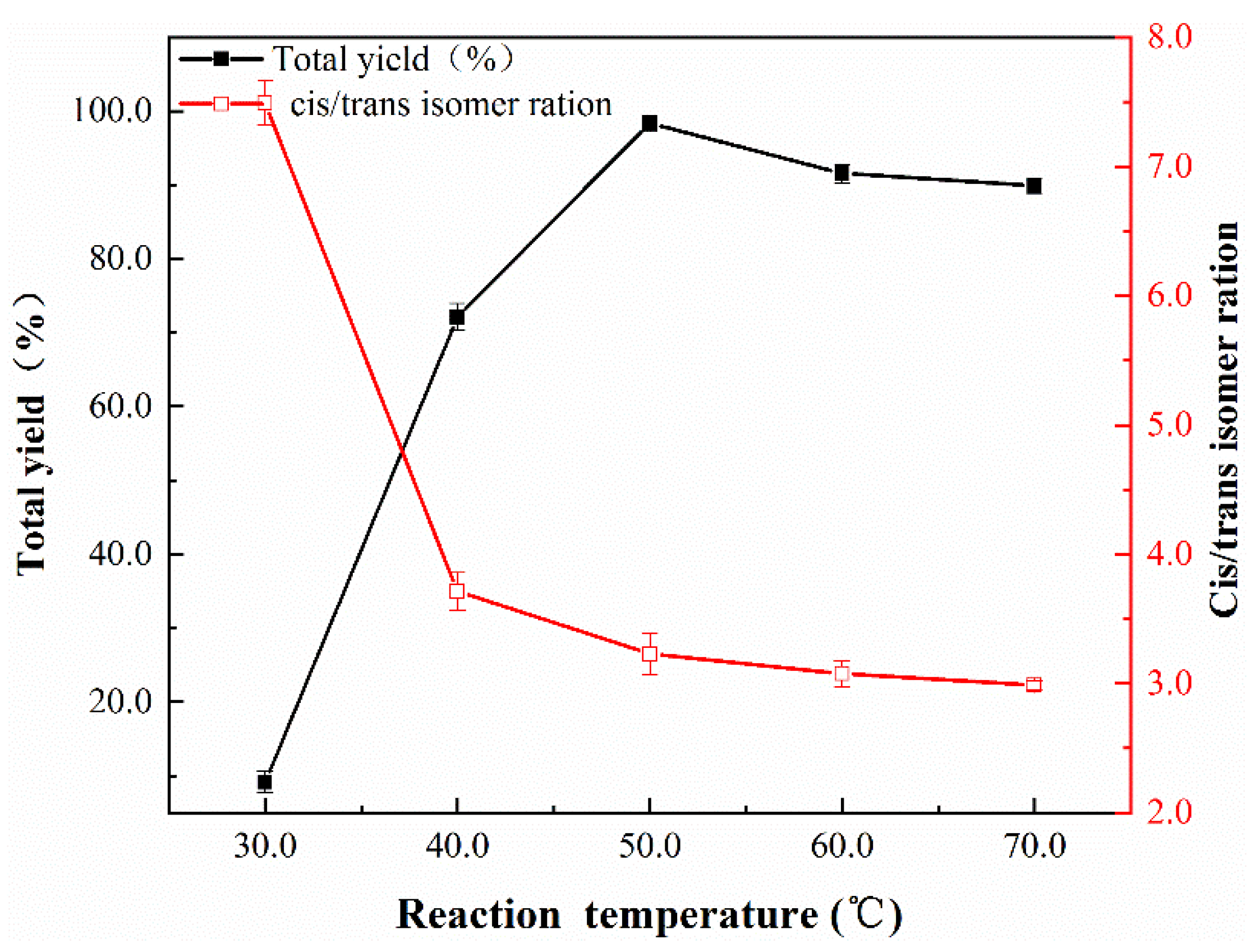
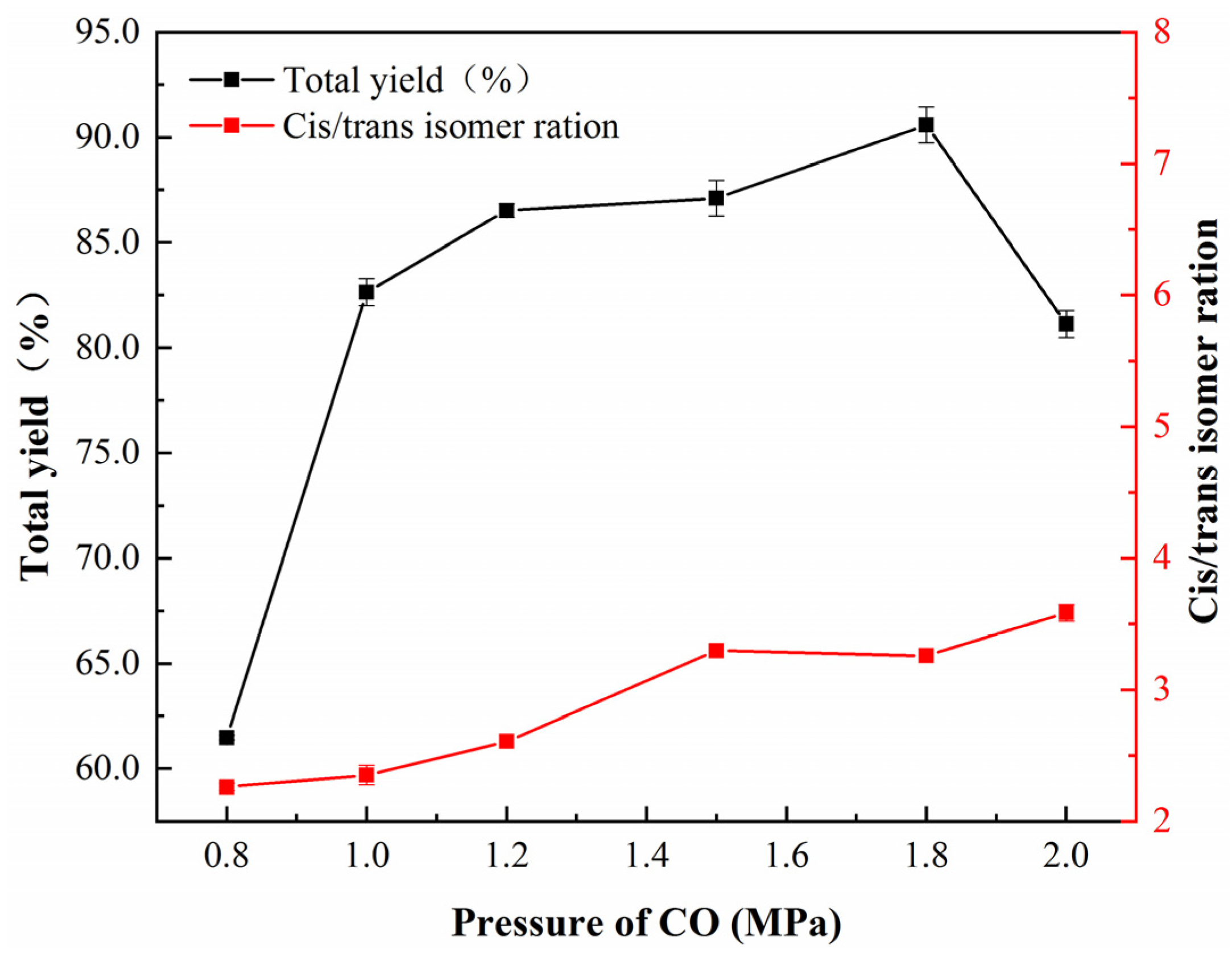
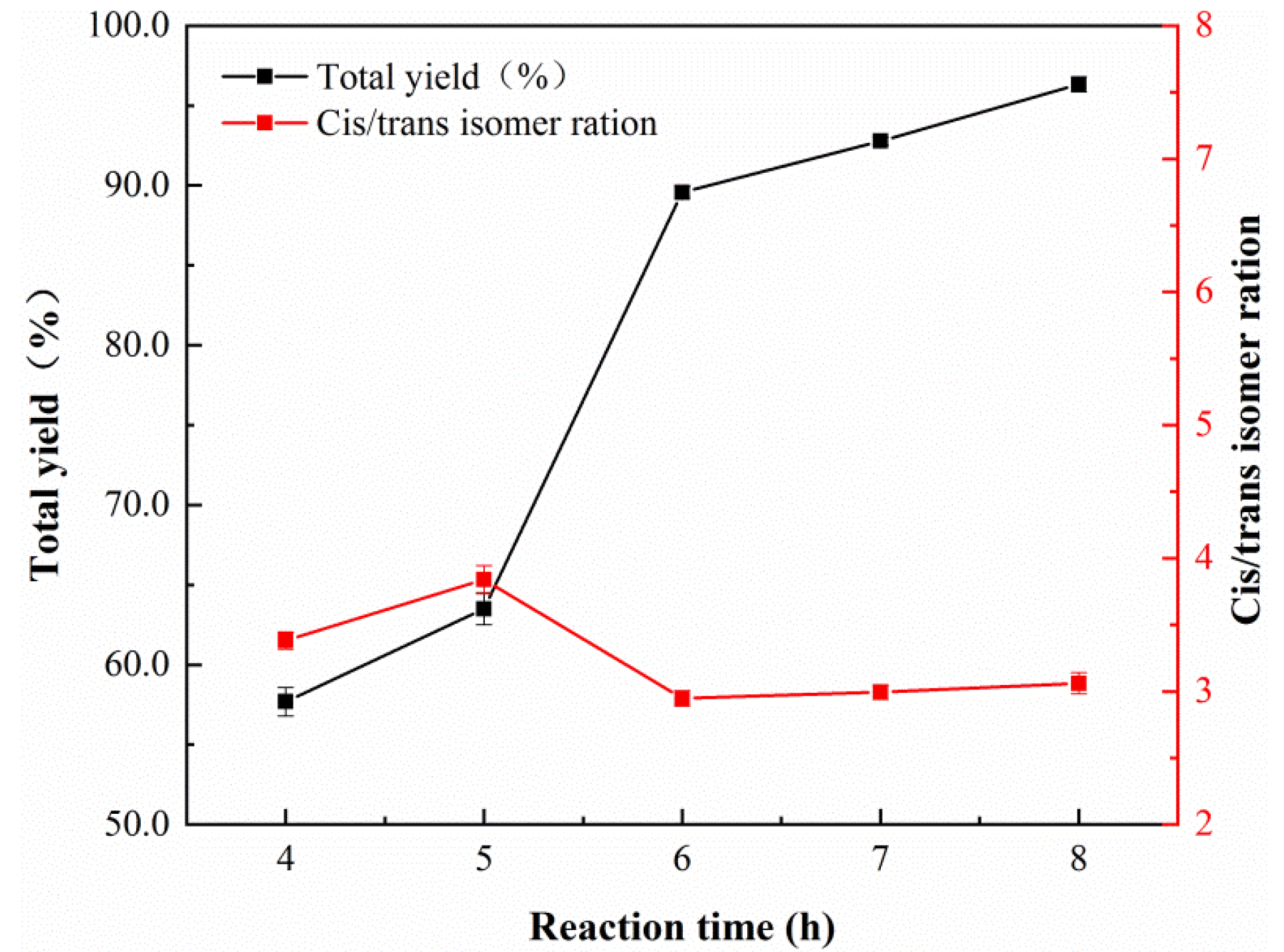
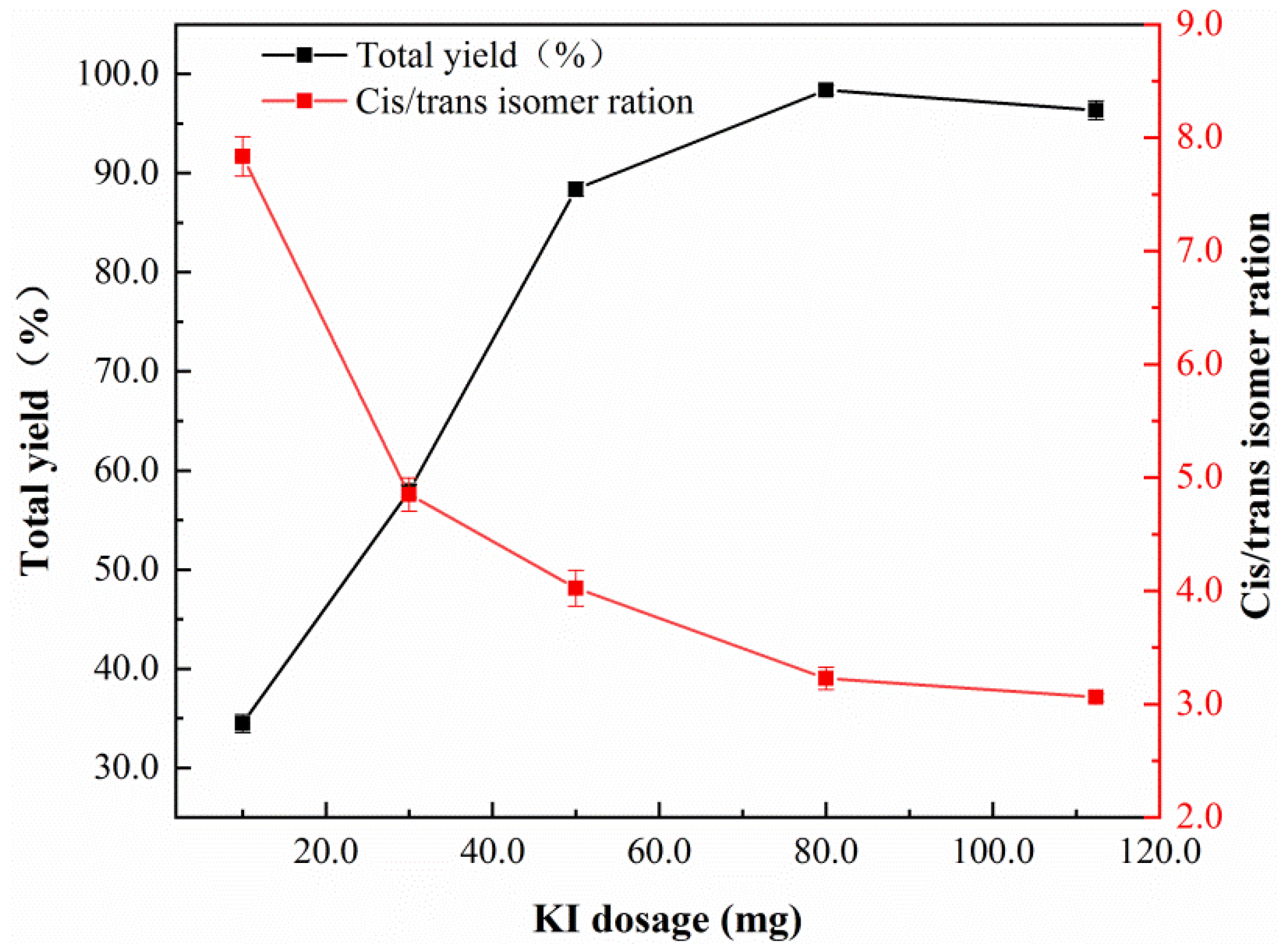

| Sample | Nano-Pd | Nano-Fe/Pd | ||
|---|---|---|---|---|
| Binding Energy (eV) | Atom% | Binding Energy (eV) | Atom% | |
| Pd0 | 334.9 | 38.67 | 334.9 | 47.17 |
| Pd2+ | 335.6 | 43.14 | 335.8 | 38.00 |
| Pdδ+ | 337.2 | 18.2 | 337.5 | 14.82 |
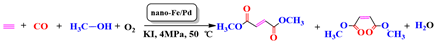 | |||||
|---|---|---|---|---|---|
| Entry | Catalyst | Sel (%) | Total Yield (%) | ||
| DMO | DMF | DMM | |||
| 1 | Pd | 0.5 | 29.0 | 70.5 | 66.8 |
| 2 | Fe/Pd | 0.3 | 26.2 | 73.5 | 83.9 |
| 3 | Co/Pd | 0.3 | 17.3 | 83.4 | 10.4 |
| 4 | Cu/Pd | 0.1 | 28.2 | 71.7 | 79.2 |
| 5 | PdCl2 | 0.1 | 26.6 | 73.3 | 82.8 |
| Adsorption Sites | Eads (KJ/mol) | Bond-Length (Å) | |||
|---|---|---|---|---|---|
| Eads (CO) | Eads (C2H2) | C≡O Bond | C≡C Bond | ||
| p (4 × 4) Pd (111) | |||||
| 1 | hcp | −199.1 | −185.2 | 1.19 | 1.37 |
| 2 | fcc | −200.7 | −186.6 | 1.19 | 1.37 |
| 3 | top | −139.4 | −77.6 | 1.16 | 1.26 |
| 4 | Bridge | −182.4 | −152.4 | 1.17 | 1.32 |
| 5 | Bridge-2 | - | −191.2 | - | 1.39 |
| p (4 × 4) Fe/Pd (111) | |||||
| 6 | hcp | −43.5 | −77.4 | 1.20 | 1.39 |
| 7 | fcc | −23.9 | −102.9 | 1.18 | 1.41 |
| 8 | top | −52.3 | −106.9 | 1.17 | 1.41 |
| 9 | bridge | −50.0 | −78.7 | 1.90 | 1.39 |
| 10 | Bridge-2 | - | −103.2 | - | 1.41 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Zhang, J.; Liu, Z.; Wu, Y.; Lv, Y.; Xie, Y.; Wang, H. Alloying Iron into Palladium Nanoparticles for an Efficient Catalyst in Acetylene Dicarbonylation. Nanomaterials 2022, 12, 3803. https://doi.org/10.3390/nano12213803
Zhang Y, Zhang J, Liu Z, Wu Y, Lv Y, Xie Y, Wang H. Alloying Iron into Palladium Nanoparticles for an Efficient Catalyst in Acetylene Dicarbonylation. Nanomaterials. 2022; 12(21):3803. https://doi.org/10.3390/nano12213803
Chicago/Turabian StyleZhang, Yuchen, Jianhui Zhang, Zongcheng Liu, Yiyi Wu, Yu Lv, Yadian Xie, and Huanjiang Wang. 2022. "Alloying Iron into Palladium Nanoparticles for an Efficient Catalyst in Acetylene Dicarbonylation" Nanomaterials 12, no. 21: 3803. https://doi.org/10.3390/nano12213803







