Star Polycation Mediated dsRNA Improves the Efficiency of RNA Interference in Phytoseiulus persimilis
Abstract
:1. Introduction
2. Methods and Materials
2.1. Mite Colony
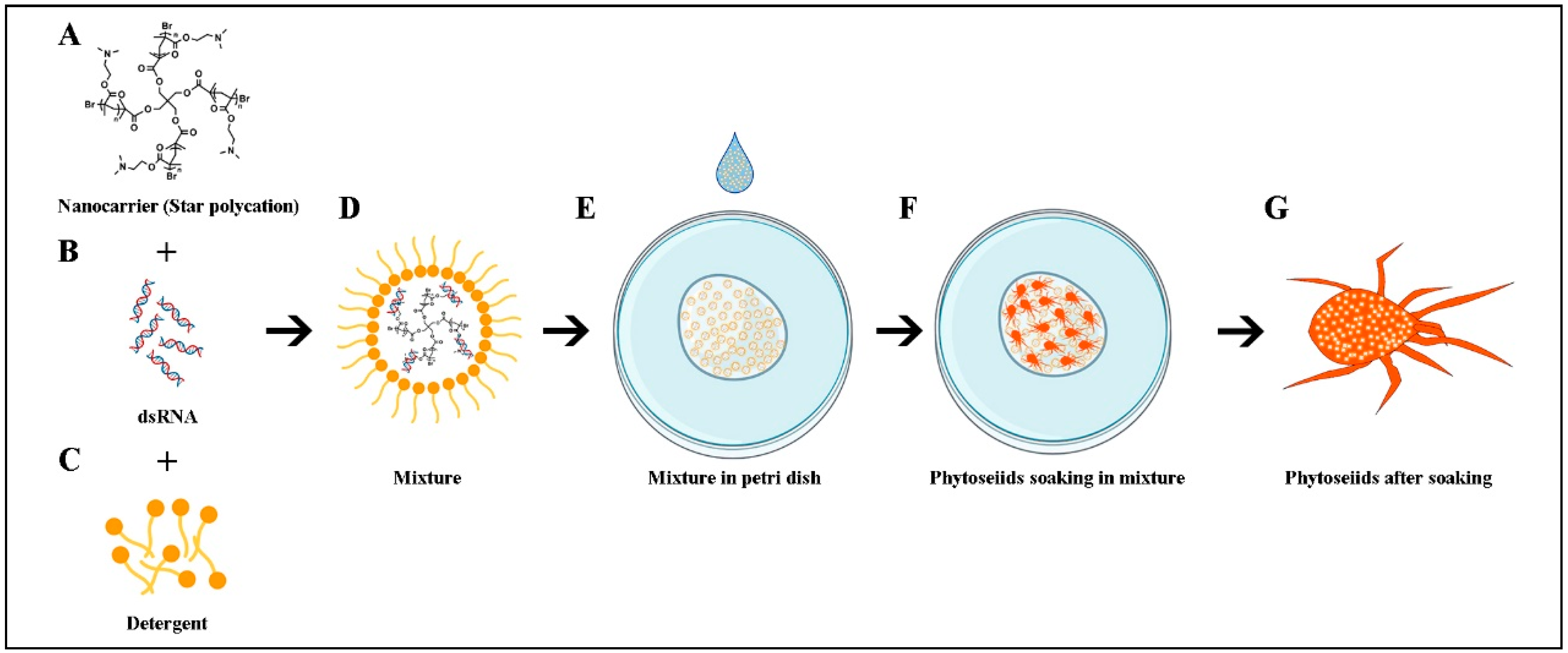
2.2. Acquisition of the Five Genes and the SPc Mediated dsRNA Complexes
Gene Selection
2.3. Gene Cloning and cDNA Synthesis
2.4. dsRNA Synthesis
2.5. SPc Synthesis and RNA Interference
2.6. Change in Reproductive Capabilities of P. persimilis as Affected by RNAi
2.7. Relative Expression of the Five Genes in P. persimilis When Interfered
3. Results
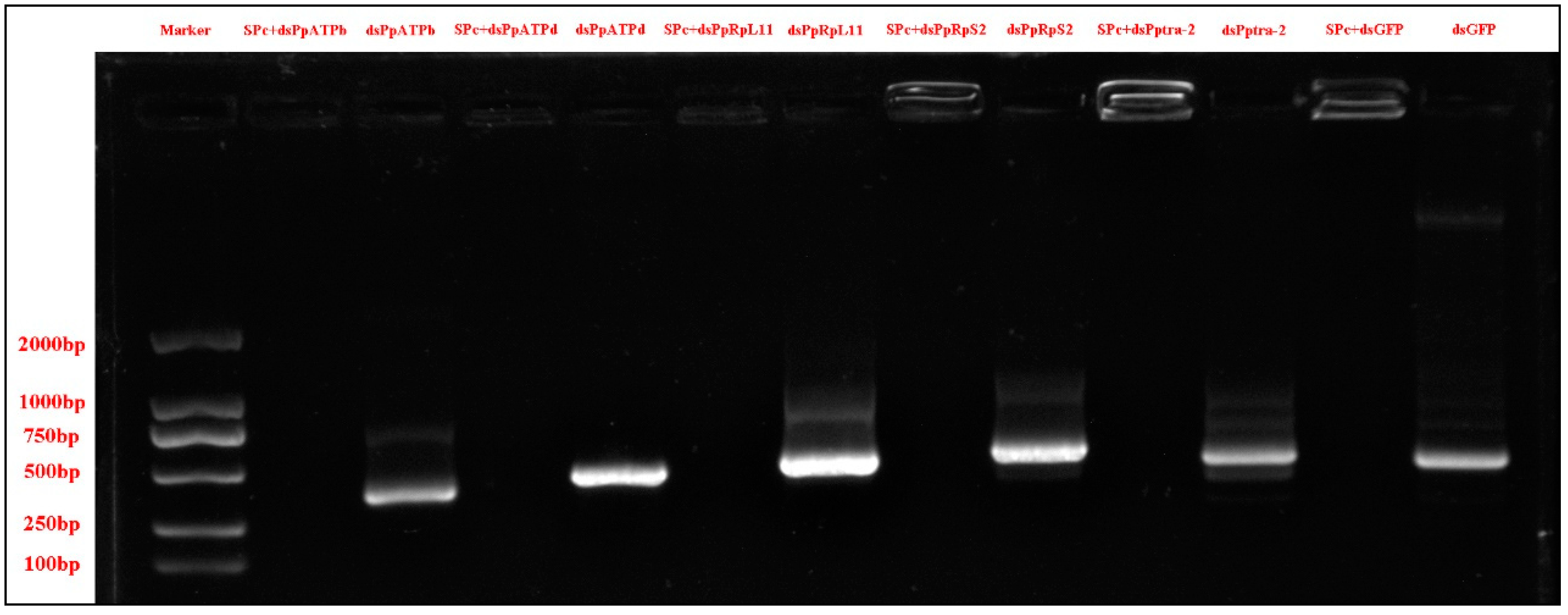
3.1. Acquisition of the Five Genes and the SPc Mediated dsRNA Complexes
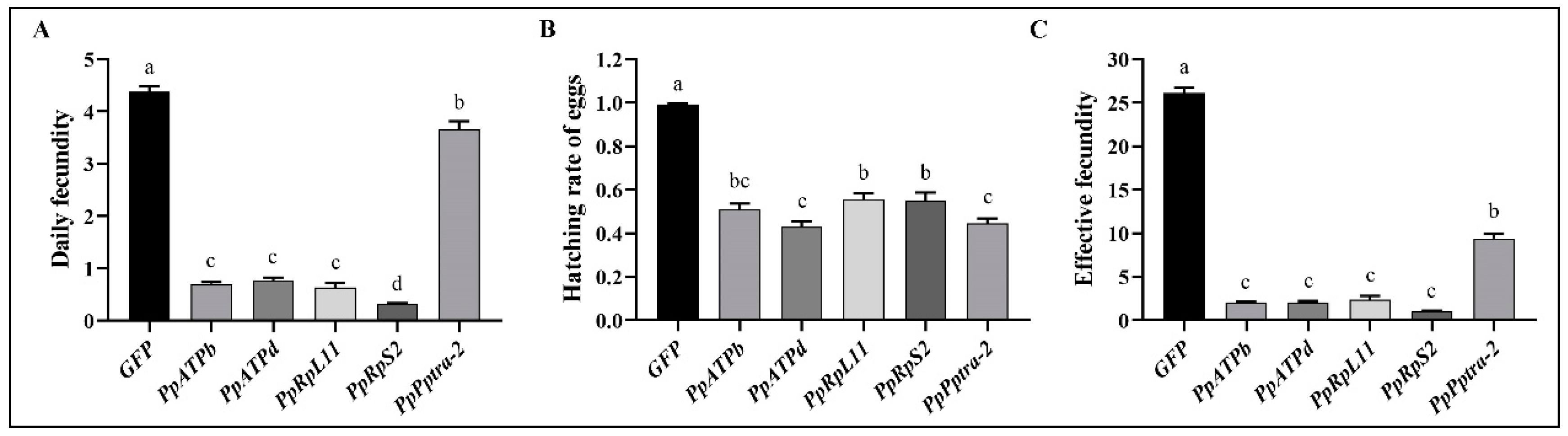
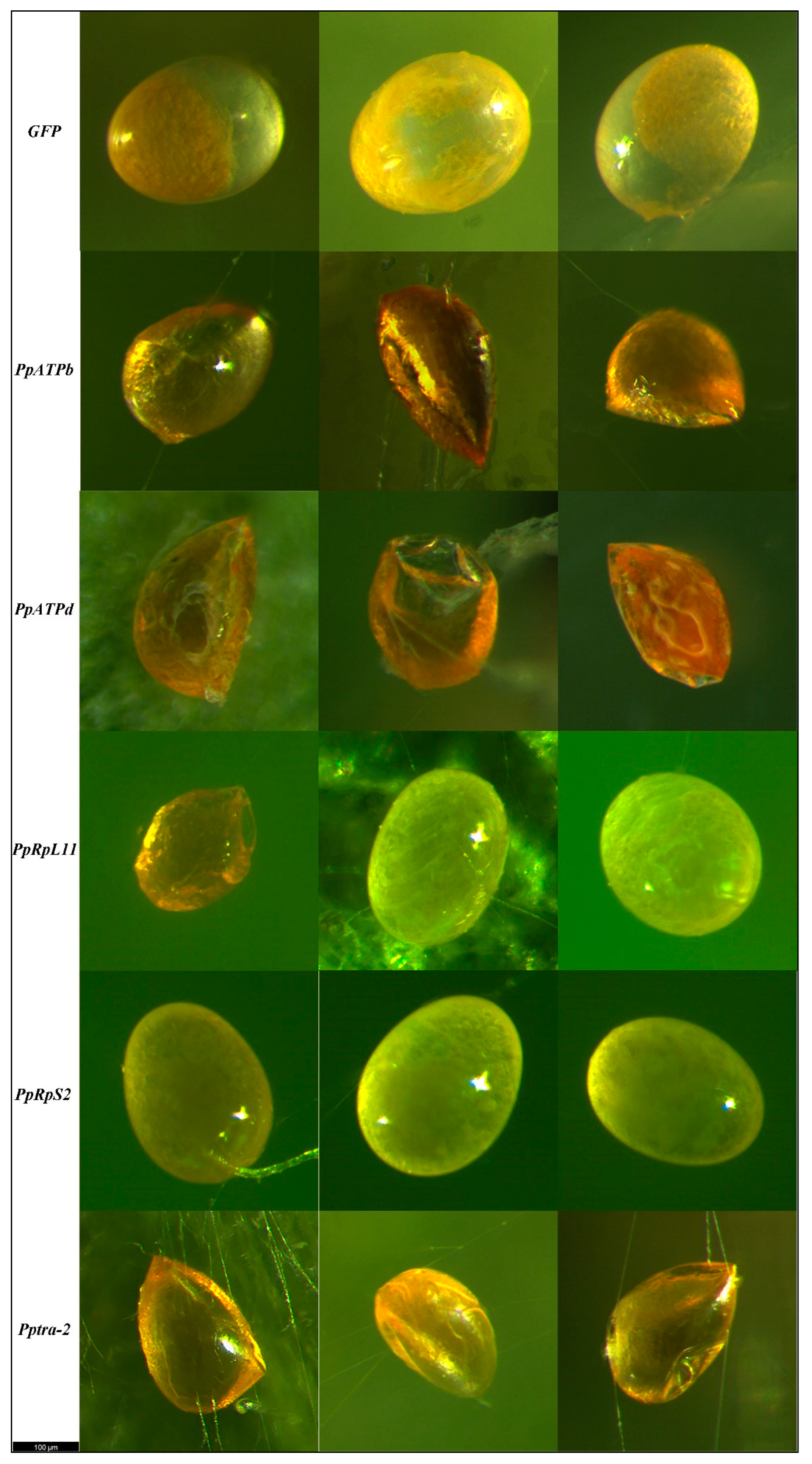
3.2. Change in Reproductive Capabilities of P. persimilis as Affected by RNAi
3.3. Relative Expression of the Five Genes in P. persimilis When Interfered
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Moraes, G.J.; Mcmurtry, J.A.; Denmark, H.A.; Campos, C.B. A revised catalog of the mite family Phytoseiidae. Zootaxa 2004, 434, 1–494. [Google Scholar] [CrossRef]
- McMurtry, J.A.; Gilberto, J.; Moraes, G.; Sourassou, N.F. Revision of the lifestyles of phytoseiid mites (Acari: Phytoseiidae) and implications for biological control strategies. Syst. Appl. Acarol. 2013, 18, 297–320. [Google Scholar] [CrossRef] [Green Version]
- van Lenteren, J.C. The state of commercial augmentative biological control: Plenty of natural enemies, but a frustrating lack of uptake. Biocontrol 2012, 57, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Helle, W.; Bolland, H.R.; Arendonk, R.J.; Boer, R.D.; Schulten, G.G.; Russell, V.M. Genetic evidence for biparental males in haplo-diploid predator mites (Acarina: Phytoseiidae). Genetica 1978, 49, 165–171. [Google Scholar] [CrossRef]
- Gardner, A.; Ross, L. Mating ecology explains patterns of genome elimination. Ecol. Lett. 2014, 17, 1602–1612. [Google Scholar] [CrossRef] [Green Version]
- Cabrera, A.R.; Donohue, K.V.; Khalil, S.M.; Scholl, E.; Opperman, C.; Sonenshine, D.E.; Roe, R.M. New approach for the study of mite reproduction: The first transcriptome analysis of a mite, Phytoseiulus persimilis (Acari: Phytoseiidae). J. Insect Physiol. 2011, 57, 52–61. [Google Scholar] [CrossRef]
- Zhao, Y.-L.; Li, D.-S.; Zhang, M.; Chen, W.; Zhang, G.-R. Food source affects the expression of vitellogenin and fecundity of a biological control agent, Neoseiulus cucumeris. Exp. Appl. Acarol. 2014, 63, 333–347. [Google Scholar] [CrossRef]
- Pomerantz, A.F.; Hoy, M.A. Expression analysis of Drosophila doublesex, transformer-2, intersex, fruitless-like, and vitellogenin homologs in the parahaploid predator Metaseiulus occidentalis (Chelicerata: Acari: Phytoseiidae). Exp. Appl. Acarol. 2015, 65, 1–16. [Google Scholar] [CrossRef]
- Ding, L.; Chen, F.; Luo, R.; Pan, Q.; Wang, C.; Yu, S.; Cong, L.; Liu, H.; Li, H.; Ran, C. Gene cloning and difference analysis of vitellogenin in Neoseiulus barkeri (Hughes). Bull. Entomol. Res. 2018, 108, 141–149. [Google Scholar] [CrossRef]
- Jiang, J.-Q.; Zhang, Y.; Ma, L.; Niu, T.-T.; Dong, T.-T.; Sheng, R.-R.; Li, L.; Xu, Y.-Y.; Xi, L.-Y.; Li, G.-T. Molecular characterization of Neoseiulus barkeri vitellogenin genes and vitellogenin receptor during reproductive diapause. Insects 2020, 11, 203. [Google Scholar] [CrossRef]
- Zamore, P.D. RNA interference: Listening to the sound of silence. Nat. Struct. Biol. 2001, 8, 746–750. [Google Scholar] [CrossRef] [PubMed]
- Meister, G.; Tuschl, T. Mechanisms of gene silencing by double-stranded RNA. Nature 2004, 431, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.Y.; Palli, S.R. Mechanisms, Applications, and Challenges of Insect RNA Interference. Annu. Rev. Entomol. 2020, 65, 293–311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, S.; Ren, B.-Y.; Shen, J. Nanoparticle-mediated double-stranded RNA delivery system: A promising approach for sustainable pest management. Insect Sci. 2021, 28, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Pomerantz, A.F.; Hoy, M.A. RNAi-mediated knockdown of transformer-2 in the predatory mite Metaseiulus occidentalis via oral delivery of double-stranded RNA. Exp. Appl. Acarol. 2015, 65, 17–27. [Google Scholar] [CrossRef]
- Bi, S.-J.; Lv, J.-L.; Xu, J.; Shi, D.-Y.; Wang, E.-D.; Li, G.-T.; Xu, X.-N. RNAi mediated knockdown of RpL11, RpS2, and tra-2 led to reduced reproduction of Phytoseiulus persimilis. Exp. Appl. Acarol. 2019, 78, 505–520. [Google Scholar] [CrossRef]
- Kunte, N.; McGraw, E.; Bell, S.; Held, D.; Avila, L.A. Prospects, challenges, and current status of RNAi through insect feeding. Pest Manag. Sci. 2020, 76, 26–41. [Google Scholar] [CrossRef]
- Silver, K.; Cooper, A.M.; Zhu, K.Y. Strategies for enhancing the efficiency of RNA interference in insects. Pest Manag. Sci. 2021, 77, 2645–2658. [Google Scholar] [CrossRef]
- Dolez, P. Nanomaterials Definitions, Classifications, and Applications. In Nanoengineering: Global Approaches to Health and Safety Issues; Elsevier: Amsterdam, The Netherlands, 2015; pp. 3–40. [Google Scholar]
- Miele, E.; Spinelli, G.P.; Miele, E.; Di Fabrizio, E.; Ferretti, E.; Tomao, S.; Gulino, A. Nanoparticle-based delivery of small interfering RNA: Challenges for cancer therapy. Int. J. Nanomed. 2012, 7, 3637–3657. [Google Scholar] [CrossRef] [Green Version]
- Ponnuswamy, N.; Bastings, M.M.C.; Nathwani, B.; Ryu, J.H.; Chou, L.Y.T.; Vinther, M.; Li, W.A.; Anastassacos, F.M.; Mooney, D.J.; Shih, W.M. Oligolysine-based coating protects DNA nanostructures from low-salt denaturation and nuclease degradation. Nat. Commun. 2017, 8, 15654. [Google Scholar] [CrossRef]
- Leung, H.M.; Chan, M.S.; Liu, L.-S.; Wong, S.W.; Lo, T.W.; Lau, C.-H.; Tin, C.; Lo, P.K. Dual-function, cationic, peptide-coated nanodiamond systems: Facilitating nuclear-targeting delivery for enhanced gene therapy applications. ACS Sustain. Chem. Eng. 2018, 6, 9671–9681. [Google Scholar] [CrossRef]
- Niu, J.-Z.; Shen, G.-M.; Christiaens, O.; Smagghe, G.; He, L.; Wang, J.-J. Beyond insects: Current status and achievements of RNA interference in mite pests and future perspectives. Pest Manag. Sci. 2018, 74, 2680–2687. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-H.; Qian, J.; Xu, Y.-Y.; Yan, S.; Shen, J.; Yin, M.-Z. A facile-synthesized star polycation is constructed as a highly efficient gene vector in pest management. ACS Sustain. Chem. Eng. 2019, 7, 6316–6322. [Google Scholar] [CrossRef]
- Ma, Z.-Z.; Zheng, Y.; Chao, Z.-J.; Chen, H.-T.; Zhang, Y.-H.; Yin, M.-Z.; Shen, J.; Yan, S. Visualization of the process of a nanocarrier-mediated gene delivery: Stabilization, endocytosis and endosomal escape of genes for intracellular spreading. J. Nanobiotechnol. 2022, 20, 124. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Qian, J.; Cai, C.; Ma, Z.-Z.; Li, J.-H.; Yin, M.-Z.; Ren, B.-Y.; Shen, J. Spray method application of transdermal dsRNA delivery system for efficient gene silencing and pest control on soybean aphid Aphis glycines. J. Pest Sci. 2019, 93, 449–459. [Google Scholar] [CrossRef]
- Ma, Z.-Z.; Zhang, Y.-H.; Li, M.-S.; Chao, Z.-J.; Du, X.-G.; Yan, S.; Shen, J. A first greenhouse application of bacteria-expressed and nanocarrier-delivered RNA pesticide for Myzus persicae control. J. Pest Sci. 2022, 95, 1–13. [Google Scholar] [CrossRef]
- Zhang, Y.-H.; Ma, Z.-Z.; Zhou, H.; Chao, Z.-J.; Yan, S.; Shen, J. Nanocarrier-delivered dsRNA suppresses wing development of green peach aphids. Insect Sci. 2022, 29, 669–682. [Google Scholar] [CrossRef]
- Zheng, Y.; Hu, Y.-S.; Yan, S.; Zhou, H.; Song, D.-L.; Yin, M.-Z.; Shen, J. A polymer/detergent formulation improves dsRNA penetration through the body wall and RNAi-induced mortality in the soybean aphid Aphis glycines. Pest Manag. Sci. 2019, 75, 1993–1999. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yan, S.; Li, M.-J.; Wang, Y.; Shi, X.-Y.; Liang, P.; Yin, M.-Z.; Shen, J.; Gao, X.-W. Nanodelivery System Alters an Insect Growth Regulator’s Action Mode: From Oral Feeding to Topical Application. ACS Appl. Mater. Interfaces 2022, 14, 65105–65113. [Google Scholar] [CrossRef]
- Li, M.-S.; Ma, Z.-Z.; Peng, M.; Li, L.; Yin, M.-Z.; Yan, S.; Shen, J. A gene and drug co-delivery application helps to solve the short life disadvantage of RNA drug. Nano Today 2022, 43, 101452. [Google Scholar] [CrossRef]
- Jiang, Q.-H.; Xie, Y.-H.; Peng, M.; Wang, Z.-J.; Li, T.-H.; Yin, M.-Z.; Shen, J.; Yan, S. A nanocarrier pesticide delivery system with promising benefits in the case of dinotefuran: Strikingly enhanced bioactivity and reduced pesticide residue. Environ. Sci. Nano 2022, 9, 988–999. [Google Scholar] [CrossRef]
- Dong, M.; Chen, D.-M.; Che, L.; Gu, N.; Yin, M.-Z.; Du, X.-G.; Shen, J.; Yan, S. Biotoxicity evaluation of a cationic star polymer on a predatory ladybird and cooperative pest control by polymer-delivered pesticides and ladybird. ACS Appl. Mater. Interfaces 2022, 14, 6083–6092. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Nehra, M.; Dilbaghi, N.; Marrazza, G.; Hassan, A.A.; Kim, K.H. Nano-based smart pesticide formulations: Emerging opportunities for agriculture. J. Control. Release 2019, 294, 131–153. [Google Scholar] [CrossRef]
- Wang, Z.-H.; Cai, Q.; Yan, S.; Yang, S.-Y.; Lu, Q.; Wang, E.-D.; Zhang, B.; Lv, J.-L.; Xu, X.-N. Molecular characterization, expression, and function of Vitellogenin genes in Phytoseiulus persimilis. Exp. Appl. Acarol. 2022, 86, 343–356. [Google Scholar] [CrossRef]
- Zhang, X.-X.; Lv, J.-L.; Hu, Y.; Wang, B.-M.; Chen, X.; Xu, X.-N.; Wang, E.-D. Prey Preference and Life Table of Amblyseius orientalis on Bemisia tabaci and Tetranychus cinnabarinus. PLoS ONE 2015, 10, e0138820. [Google Scholar] [CrossRef]
- Vasanthakumar, T.; Rubinstein, J.L. Structure and Roles of V-type ATPases. Trends Biochem. Sci. 2020, 45, 295–307. [Google Scholar] [CrossRef]
- Wieczorek, H.; Beyenbach, K.W.; Huss, M.; Vitavska, O. Vacuolar-type proton pumps in insect epithelia. J. Exp. Biol. 2009, 212, 1611–1619. [Google Scholar] [CrossRef] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Arrese, E.L.; Soulages, J.L. Insect fat body: Energy, metabolism, and regulation. Annu. Rev. Entomol. 2010, 55, 207–225. [Google Scholar] [CrossRef] [Green Version]
- Flatt, T. Paying the costs of reproduction. Elife 2015, 4, e09556. [Google Scholar] [CrossRef]
- Ruhland, F.; Pétillon, J.; Trabalon, M. Physiological costs during the first maternal care in the wolf spider Pardosa saltans (Araneae, Lycosidae). J. Insect Physiol. 2016, 95, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Laing, J.E. Life history and life table of Phytoseiulus persimilis Athias-Henriot. Acarologia 1968, 10, 578–588. [Google Scholar]
- Ibrahim, A.B.; Monteiro, T.R.; Cabral, G.B.; Aragão, F.J.L. RNAi-mediated resistance to whitefly (Bemisia tabaci) in genetically engineered lettuce (Lactuca sativa). Transgenic Res. 2017, 26, 613–624. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Nunes, M.A.; España, M.U.; Namin, H.H.; Jin, P.; Bensoussan, N.; Zhurov, V.; Rahman, T.; De Clercq, R.; Hilson, P.; et al. RNAi-based reverse genetics in the chelicerate model Tetranychus urticae: A comparative analysis of five methods for gene silencing. PLoS ONE 2017, 12, e0180654. [Google Scholar] [CrossRef]
- Hooper, S.D.; Boué, S.; Krause, R.; Jensen, L.J.; Mason, C.E.; Ghanim, M.; White, K.P.; Furlong, E.E.; Bork, P. Identification of tightly regulated groups of genes during Drosophila melanogaster embryogenesis. Mol. Syst. Biol. 2007, 3, 72. [Google Scholar] [CrossRef]
- Cusanovich, D.A.; Reddington, J.P.; Garfield, D.A.; Daza, R.M.; Aghamirzaie, D.; Marco-Ferreres, R.; Pliner, H.A.; Christiansen, L.; Qiu, X.; Steemers, F.J.; et al. The cis-regulatory dynamics of embryonic development at single-cell resolution. Nature 2018, 555, 538–542. [Google Scholar] [CrossRef] [PubMed]
- Donoughe, S. Insect egg morphology: Evolution, development, and ecology. Curr. Opin. Insect Sci. 2022, 50, 100868. [Google Scholar] [CrossRef]

| Primers | Forward (5′–3′) | Reverse (5′–3′) |
|---|---|---|
| dsRNA synthesis | ||
| dsGFP | TAATACGACTCACTATAGGG TGAGCAAGGGCGAGGAG | TAATACGACTCACTATAGGG CGGCGGTCACGAACTCCAG |
| dsPpATPb | TAATACGACTCACTATAGGG CCCACTCACTGTAGCCAAT | TAATACGACTCACTATAGGG TGTCGTTTACGGAACTCGG |
| dsPpATPd | TAATACGACTCACTATAGGG ACTGGGTGAAGTTGGCTGA | TAATACGACTCACTATAGGG ATTGCTGAGTCTCGTGGTC |
| dsPpRpL11 | TAATACGACTCACTATAGGG CCGGCAGAGTTCAGAAAGAC | TAATACGACTCACTATAGGG CTACGGTGAGGCACGTTGTA |
| dsPpRpS2 | TAATACGACTCACTATAGGG GACGCTTTTCTTGGAACGAC | TAATACGACTCACTATAGGG CCACAAGTCCGGAGTCAGAT |
| dsPptra-2 | TAATACGACTCACTATAGGG GGAGACGAAGGAAAACGTCA | TAATACGACTCACTATAGGG CGAGTATATCTCCGGCTTCG |
| RT-qPCR | ||
| PpATPb | GAGGATGGGCTTCATACCT | ACGGCAACTCCTGAGAAGA |
| PpATPd | GGTTCGGAAAGAGGAAATG | TCGGCAAGTTTGGGATTC |
| PpRpl11 | CGGGAATACGAACTACGC | TCTGCTGGAACCATTTGAT |
| PpRpS20 | CAAGGAAGGCGAGAAGG | TGACACCGAGACCAACG |
| Pptra-2 | AGATCGGCGTAGCAGGAGT | TCTGGGCATCGTAGACAACC |
| actin | TGGTCGGTATGGGTCAGA | TGGCAGGAGTGTTGAAGGTC |
| Gene | Daily Fecundity (Mean ± SEM) | |||||
|---|---|---|---|---|---|---|
| Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 | |
| GFP | 4.03 ± 0.16 a (n = 79) | 5.04 ± 0.13 a (n = 79) | 4.98 ± 0.17 a (n = 79) | 4.36 ± 0.15 a (n = 77) | 3.92 ± 0.15 a (n = 77) | 3.89 ± 0.24 a (n = 77) |
| PpATPb | 3.32 ± 0.16 a (n = 79) | 0.79 ± 0.11 b (n = 77) | 0.05 ± 0.05 b (n = 74) | 0 b (n = 74) | 0 b (n = 74) | 0.10 ± 0.07 b (n = 71) |
| PpATPd | 3.49 ± 0.14 a (n = 73) | 0.48 ± 0.10 b (n = 73) | 0.38 ± 0.08 b (n = 73) | 0.11 ± 0.06 b (n = 73) | 0.06 ± 0.03 b (n = 73) | 0.11 ± 0.04 b (n = 72) |
| PpRpL11 | 2.25 ± 0.11 a (n = 92) | 0.61 ± 0.14 b (n = 92) | 0.26 ± 0.09 bc (n = 92) | 0.21 ± 0.10 bc (n = 91) | 0.16 ± 0.09 bc (n = 92) | 0.29 ± 0.13 c (n = 91) |
| PpRpS2 | 1.89 ± 0.10 a (n = 88) | 0.02 ± 0.02 b (n = 88) | 0.01± 0.01 b (n = 88) | 0 b (n = 88) | 0 b (n = 88) | 0 b (n = 88) |
| Pptra-2 | 3.60 ± 0.18 a (n = 79) | 3.52 ± 0.15 a (n = 79) | 2.98 ± 0.20 a (n = 79) | 4.32 ± 0.27 a (n = 79) | 3.51 ± 0.22 a (n = 79) | 4.01 ± 0.27 a (n = 77) |
| Gene | Hatching Rate (Mean ± SEM) | |||||
|---|---|---|---|---|---|---|
| Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 | |
| GFP | 0.99 ± 0.01 a (n = 77) | 0.99 ± 0.01 a (n = 79) | 0.99 ± 0.01 a (n = 78) | 1.00 ± 0.00 a (n = 74) | 0.98 ± 0.01 a (n = 74) | 0.99 ± 0.003 a (n = 72) |
| PpATPb | 0.62 ± 0.03 a (n = 74) | 0.10 ± 0.04 b (n = 41) | 0 b (n = 1) | 0 b (n = 5) | - | 0 b (n = 1) |
| PpATPd | 0.56 ± 0.03 a (n = 71) | 0.04 ± 0.02 b (n = 46) | 0 b (n = 16) | - | 0 b (n = 3) | 0 b (n = 1) |
| PpRpL11 | 0.67 ± 0.03 a (n = 83) | 0.18 ± 0.07 b (n = 28) | 0.08 ± 0.13 b (n = 10) | 0 b (n = 5) | 0 b (n = 4) | 0 b (n = 5) |
| PpRpS2 | 0.56 ± 0.04 a (n = 79) | 0 b (n = 1) | 0 b (n = 1) | - | - | - |
| Pptra-2 | 0.96 ± 0.02 a (n = 69) | 0.87 ± 0.03 a (n = 71) | 0.40 ± 0.05 b (n = 71) | 0.19 ± 0.0 c (n = 63) | 0.03 ± 0.02 d (n = 63) | 0.07 ± 0.03 d (n = 60) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Li, M.; Kong, Z.; Wang, E.; Zhang, B.; Lv, J.; Xu, X. Star Polycation Mediated dsRNA Improves the Efficiency of RNA Interference in Phytoseiulus persimilis. Nanomaterials 2022, 12, 3809. https://doi.org/10.3390/nano12213809
Wang Z, Li M, Kong Z, Wang E, Zhang B, Lv J, Xu X. Star Polycation Mediated dsRNA Improves the Efficiency of RNA Interference in Phytoseiulus persimilis. Nanomaterials. 2022; 12(21):3809. https://doi.org/10.3390/nano12213809
Chicago/Turabian StyleWang, Zhenhui, Mingxia Li, Ziyi Kong, Endong Wang, Bo Zhang, Jiale Lv, and Xuenong Xu. 2022. "Star Polycation Mediated dsRNA Improves the Efficiency of RNA Interference in Phytoseiulus persimilis" Nanomaterials 12, no. 21: 3809. https://doi.org/10.3390/nano12213809
APA StyleWang, Z., Li, M., Kong, Z., Wang, E., Zhang, B., Lv, J., & Xu, X. (2022). Star Polycation Mediated dsRNA Improves the Efficiency of RNA Interference in Phytoseiulus persimilis. Nanomaterials, 12(21), 3809. https://doi.org/10.3390/nano12213809






