Investigation of the Adsorption Process of Chromium (VI) Ions from Petrochemical Wastewater Using Nanomagnetic Carbon Materials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Instruments
2.2. Preparation of Adsorbent
2.3. Characterization
2.4. Adsorption and Desorption Study
2.5. Data Processing
3. Results and Discussion
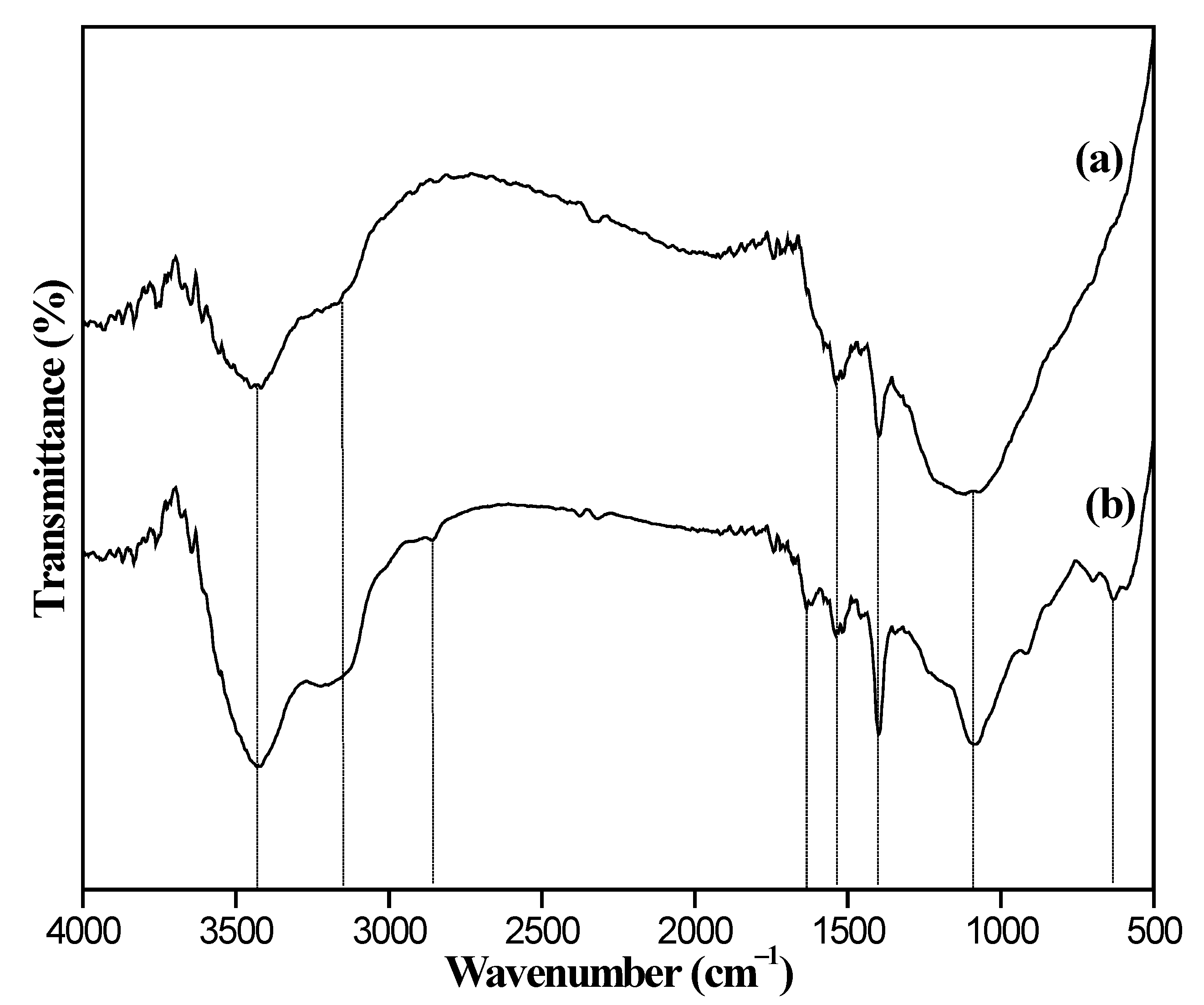
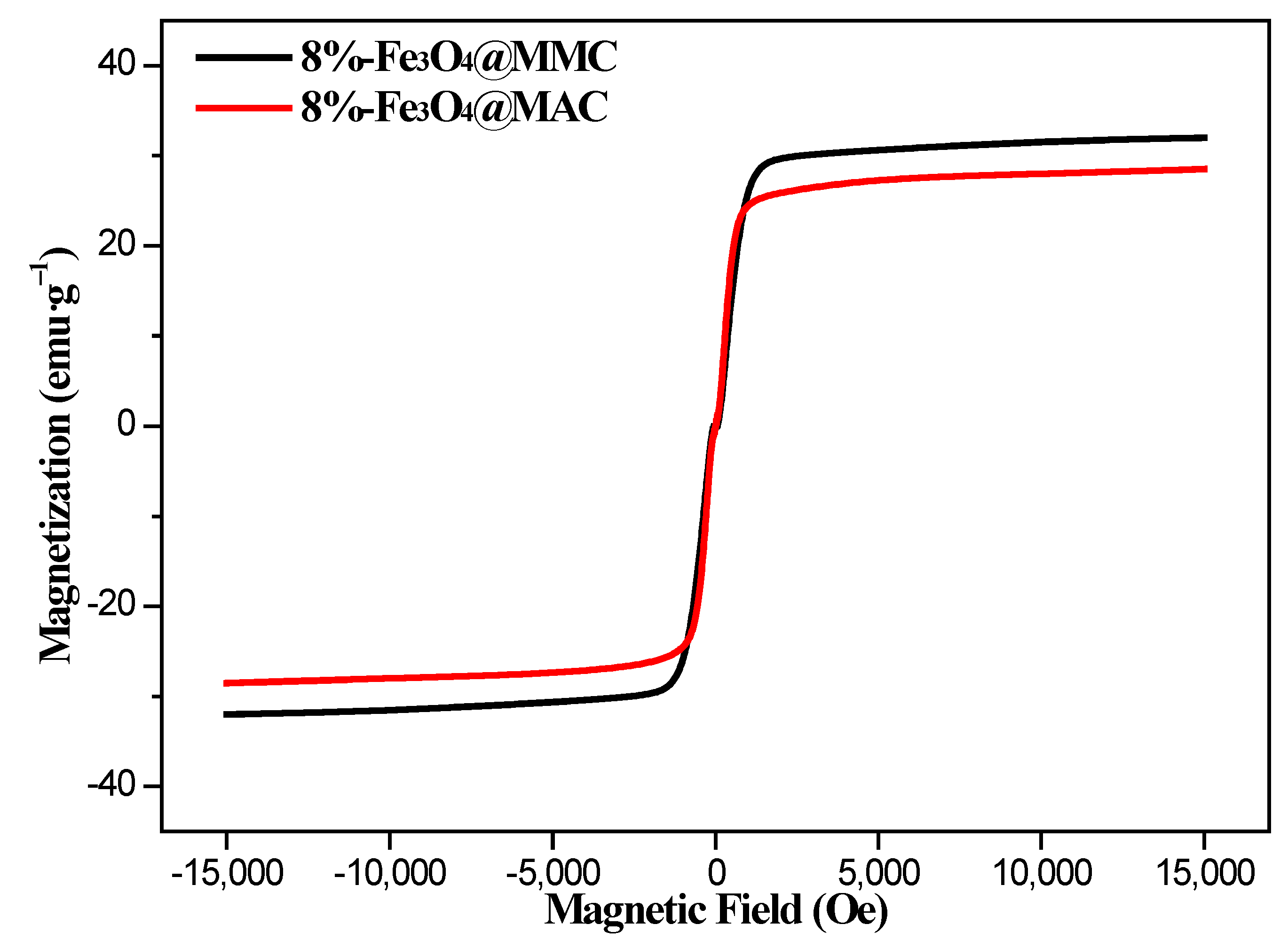
3.1. Characterization of Adsorbents
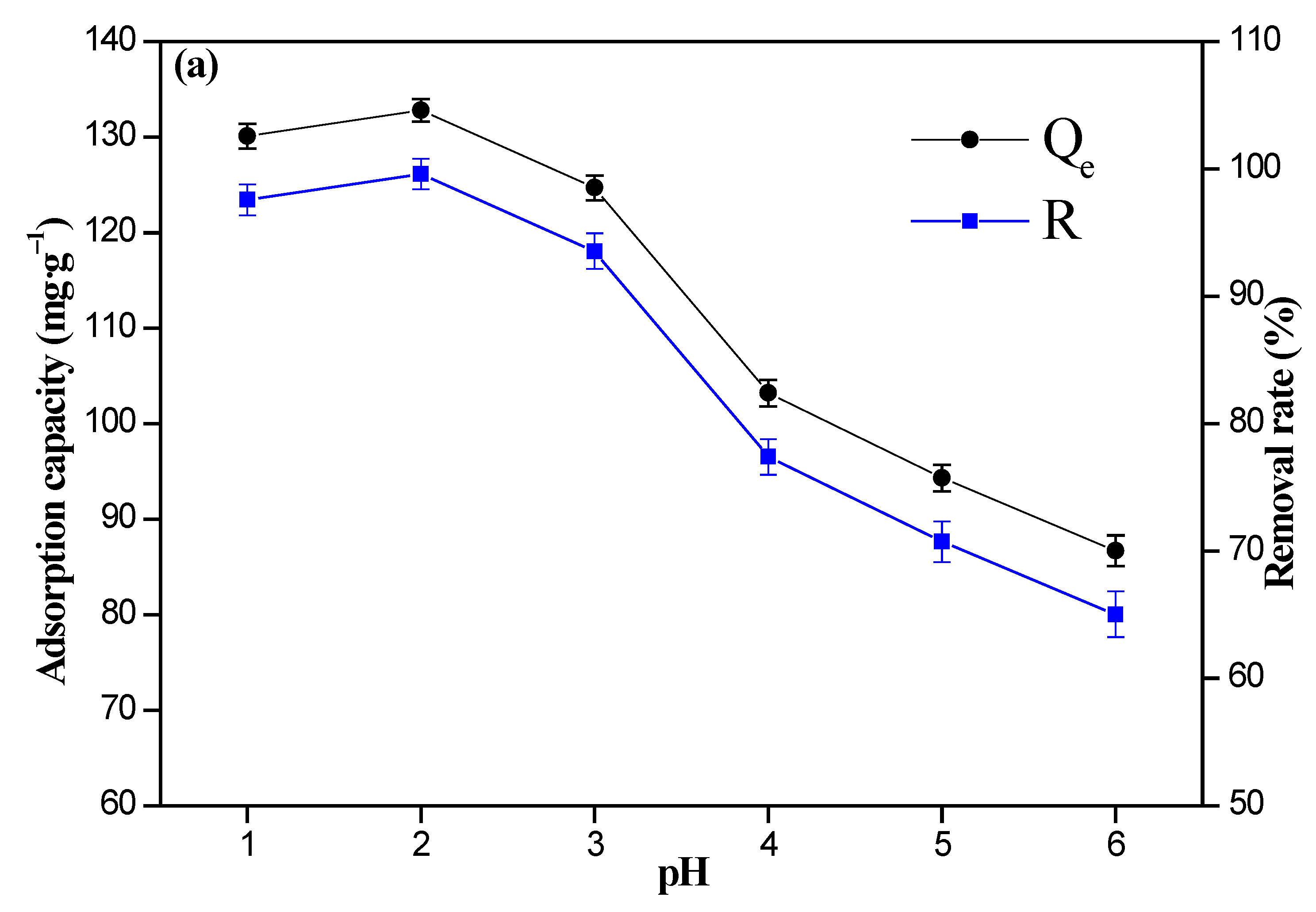
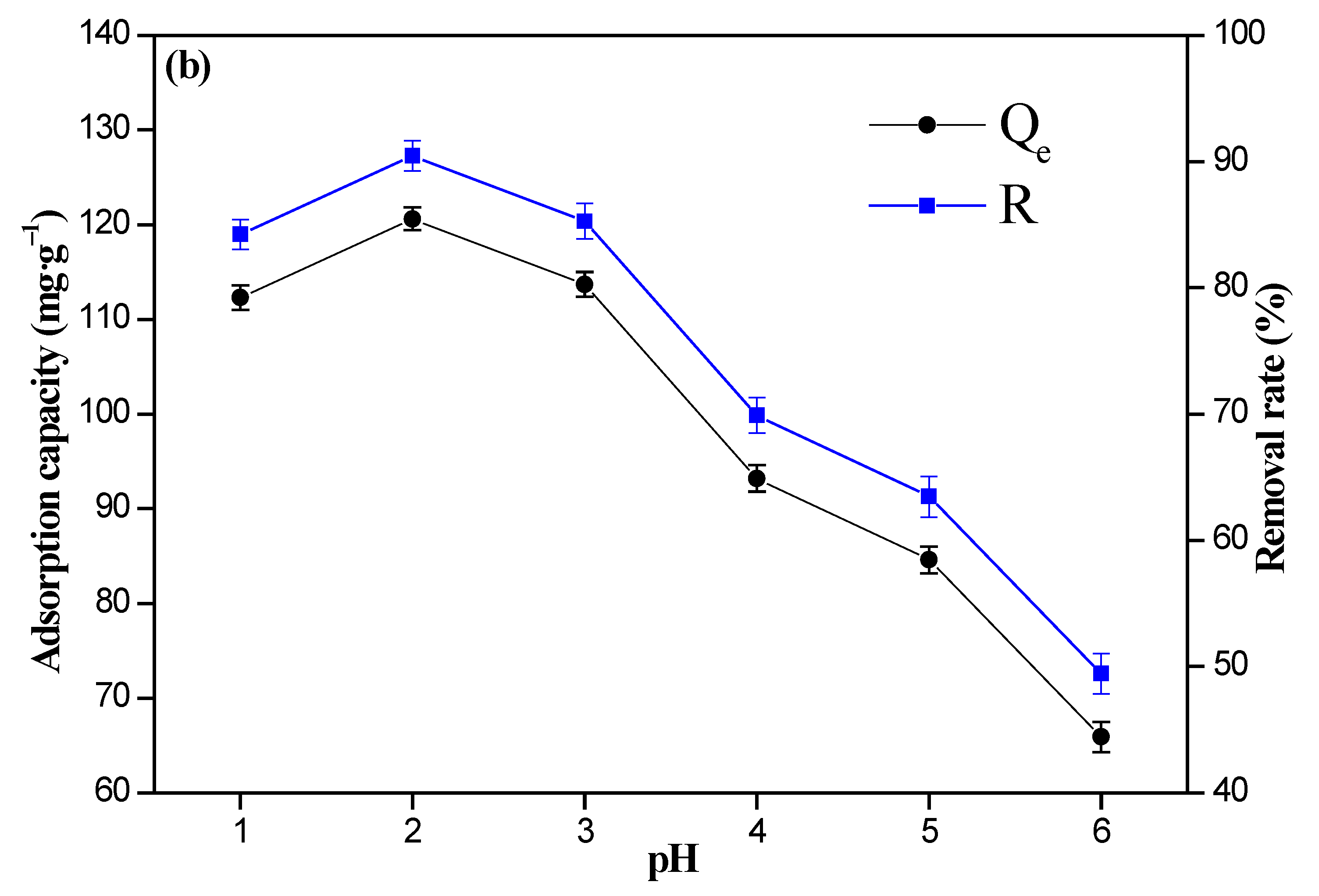
3.2. Effect of pH Value and Initial Cr(VI) Ions’ Concentration
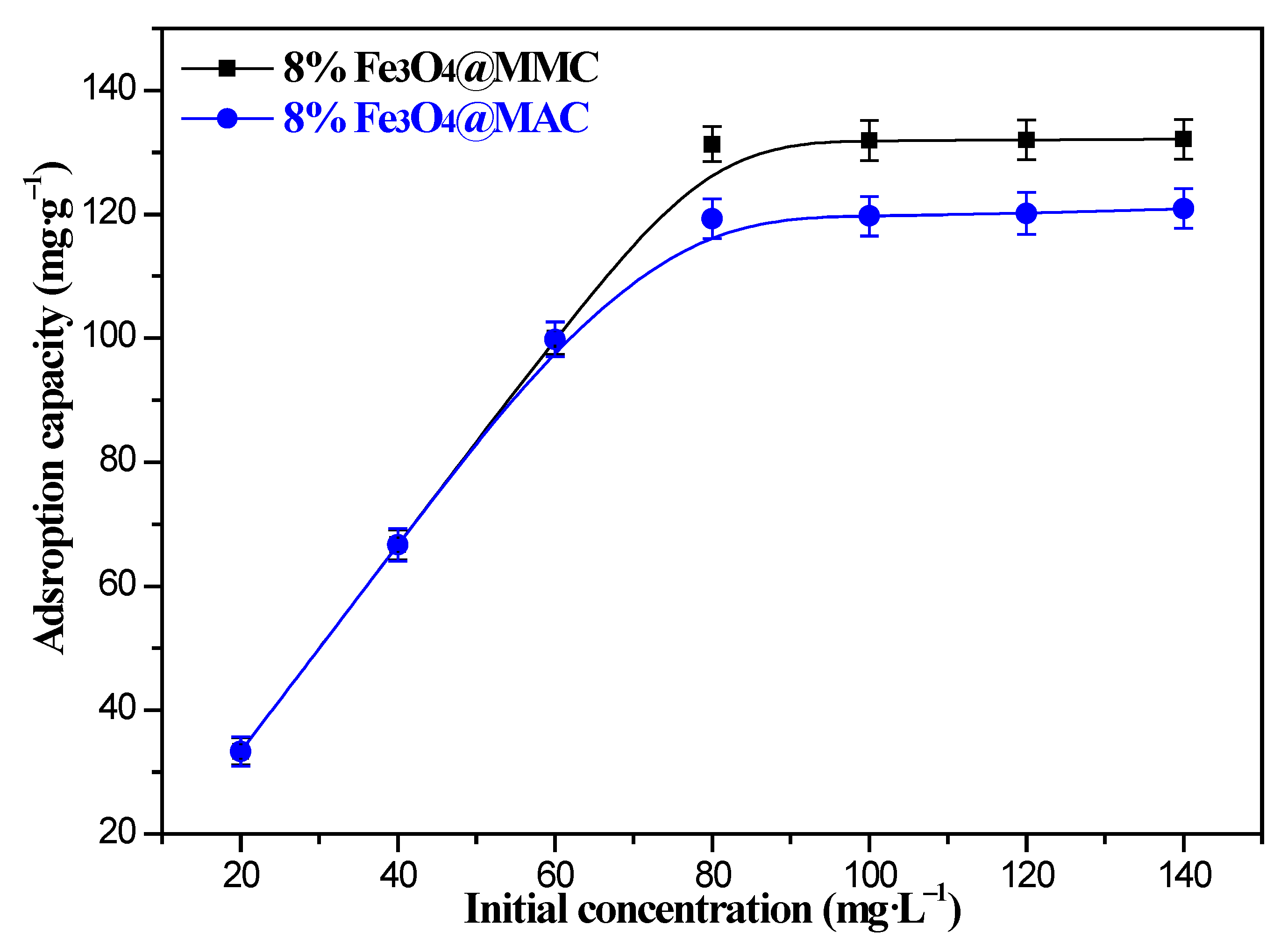
3.3. Effect of Fe3O4 Loading
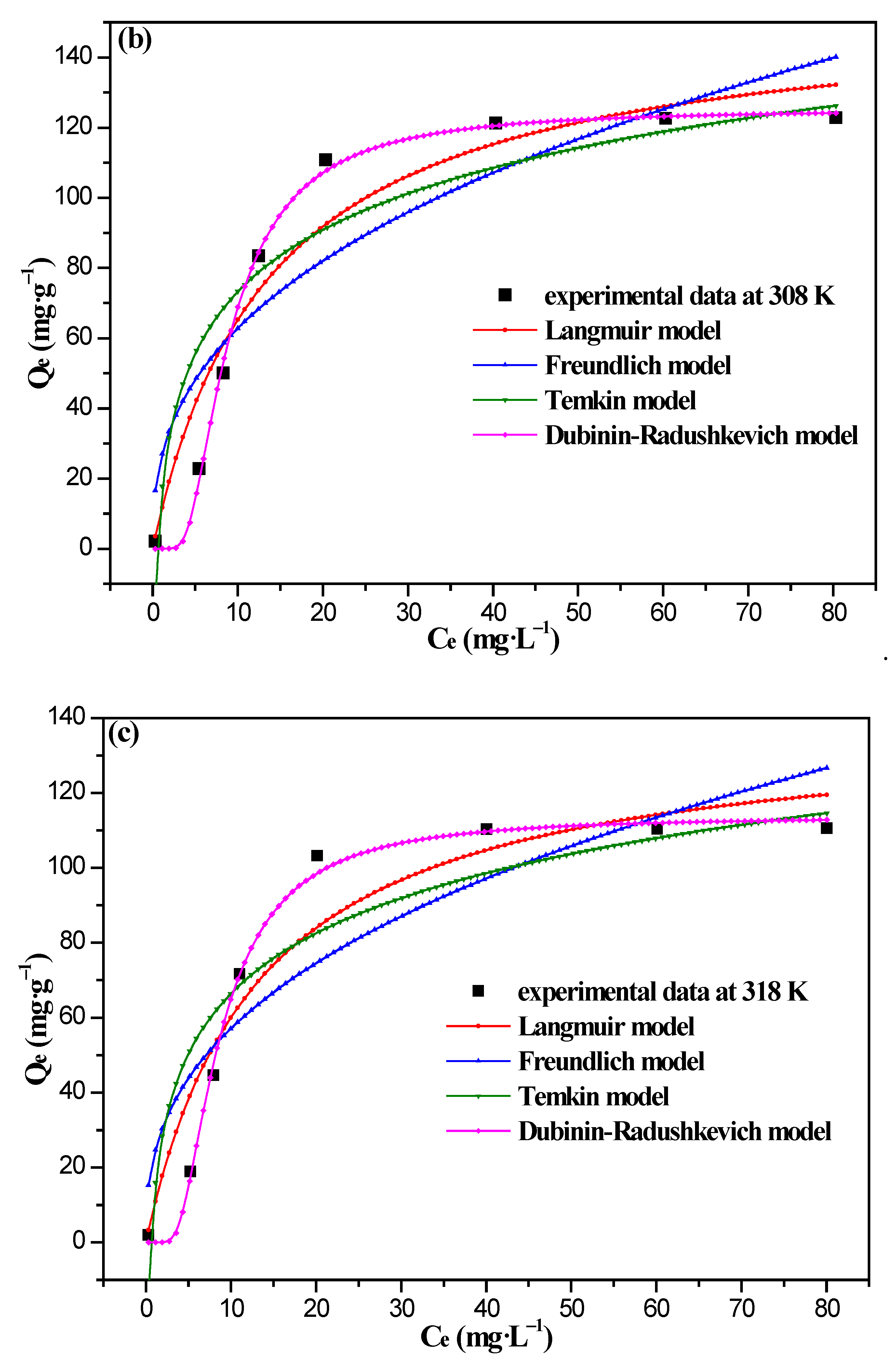
3.4. Adsorption Isotherm Analysis
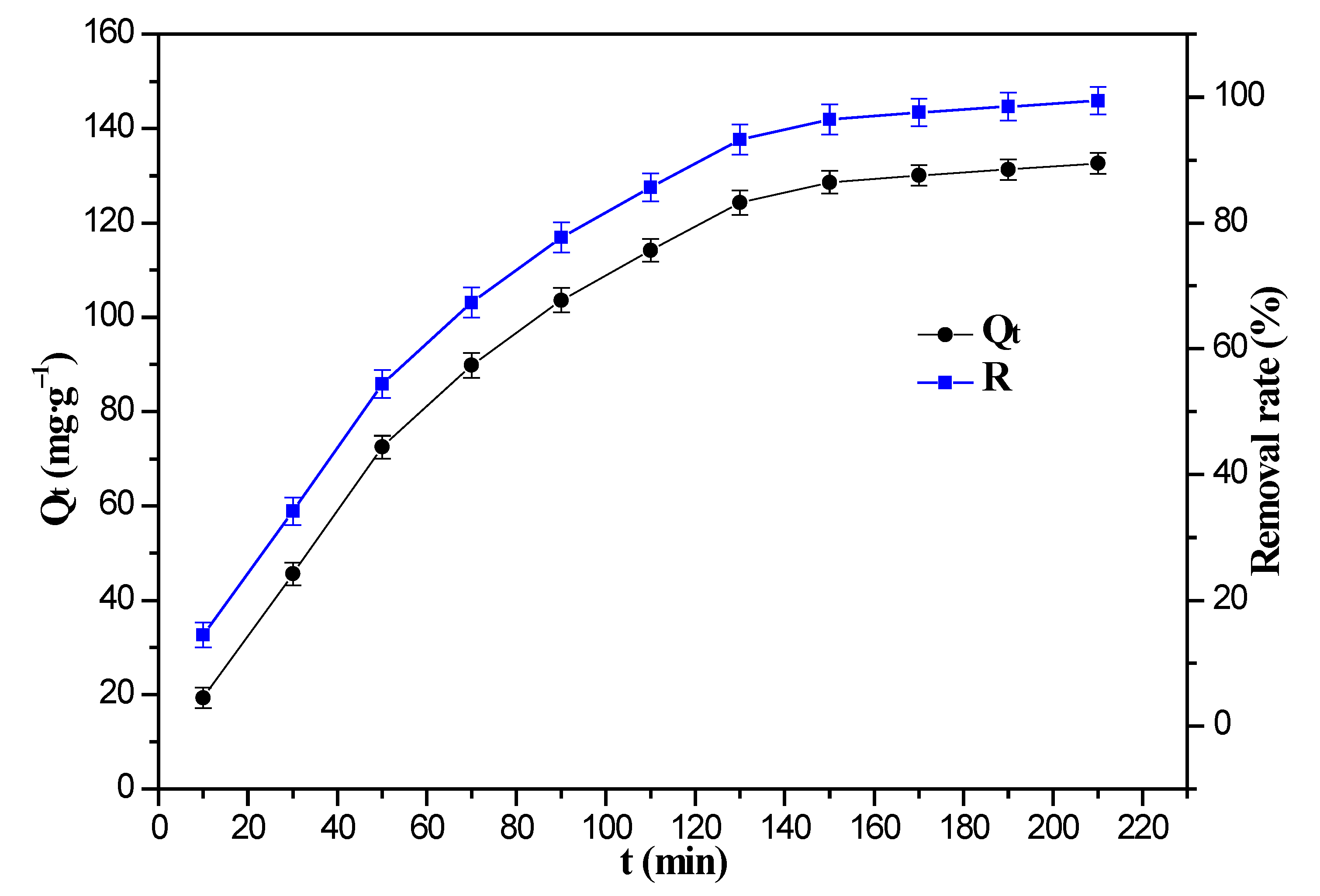
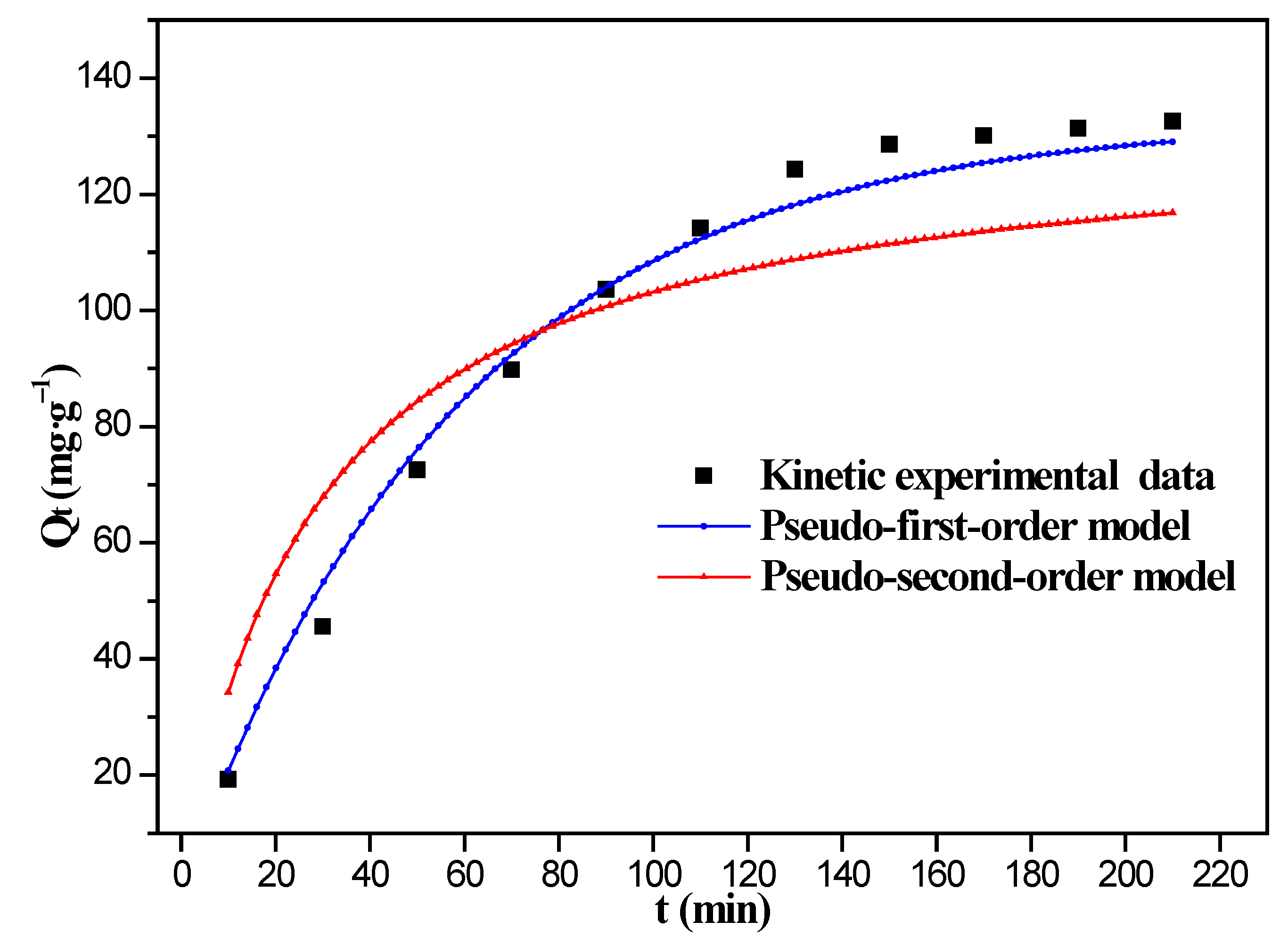
3.5. Effect of Adsorption Time and Adsorption Kinetics
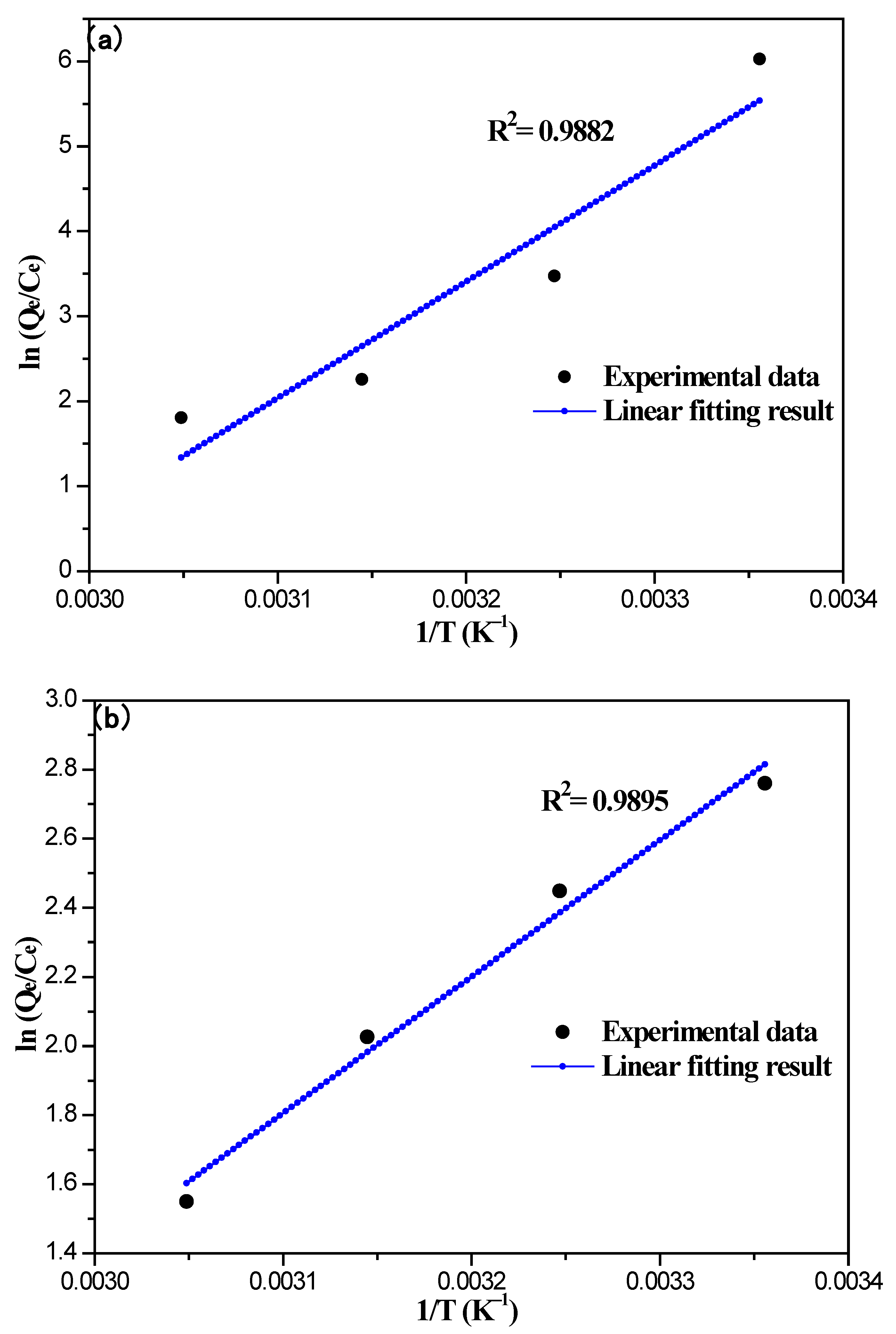
3.6. Thermodynamic Analysis
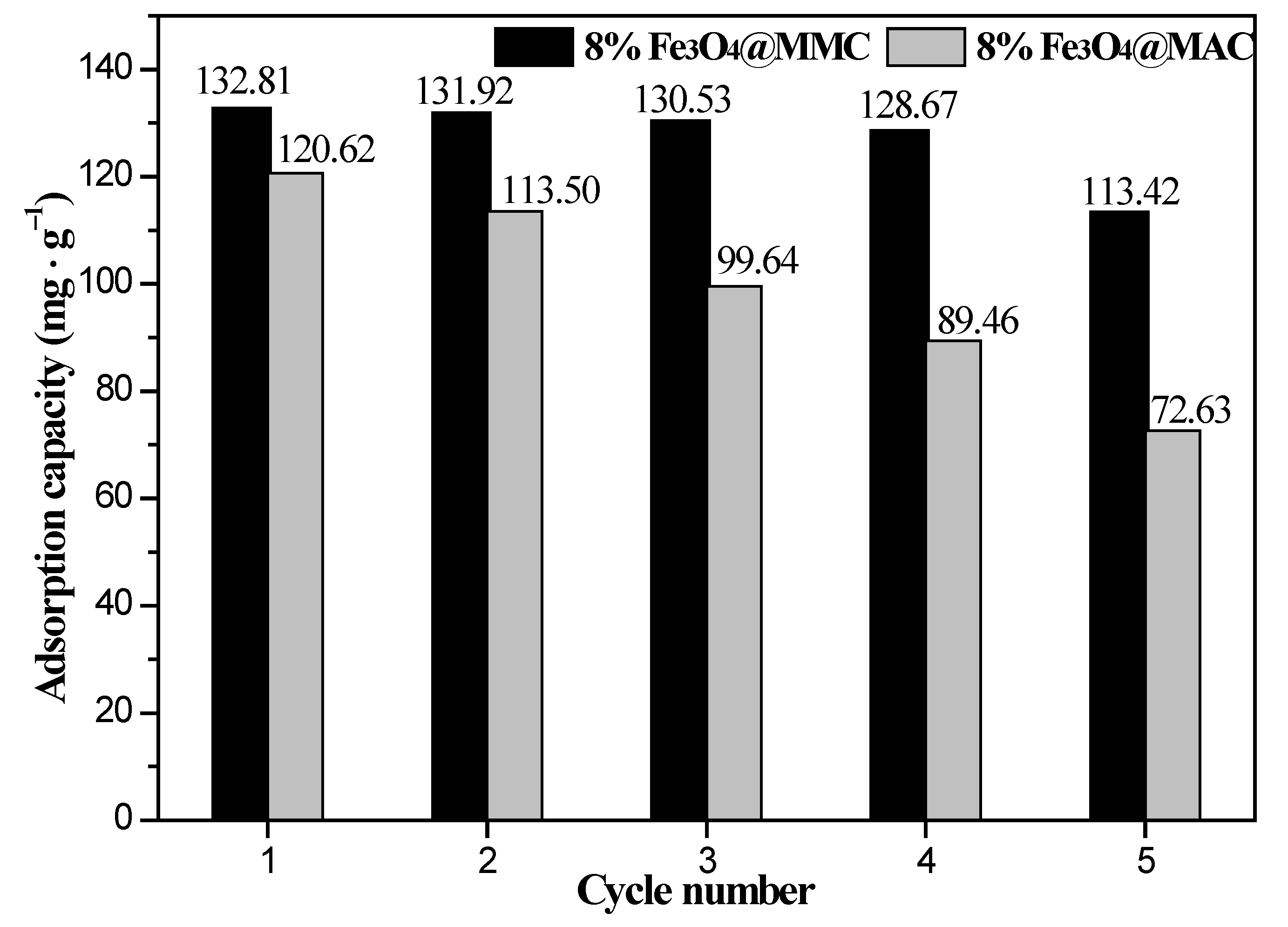
3.7. Reutilization of Adsorbent
3.8. Possible Adsorption Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Meng, M.; Yu, J. Chinese nuclear energy politics: Viewpoint on energy. Energ. Source Part B 2018, 13, 72–75. [Google Scholar] [CrossRef]
- Valdiglesias, V.; Pásaro, E.; Méndez, J.; Laffon, B. In vitro evaluation of selenium genotoxic, cytotoxic, and protective effects: A review. Arch. Toxicol. 2010, 84, 337–351. [Google Scholar] [CrossRef] [PubMed]
- Azimi, A.; Azari, A.; Rezakazemi, M.; Ansarpour, M. Removal of heavy metals from industrial wastewaters: A review. ChemBioEng Rev. 2017, 4, 37–59. [Google Scholar] [CrossRef]
- Kadirvelu, K.; Faur-Brasquet, C.; Cloirec, P.L. Removal of Cu(II), Pb(II), and Ni(II) by adsorption onto activated carbon cloths. Langmuir 2000, 16, 8404–8409. [Google Scholar] [CrossRef]
- Zhang, L.; Jiang, X.Q.; Xu, T.C.; Yang, L.J.; Zhang, Y.Y.; Jin, H.J. Sorption characteristics and separation of rhenium ions from aqueous solutions using modified nano-Al2O3. Ind. Eng. Chem. Res. 2012, 51, 5577–5584. [Google Scholar] [CrossRef]
- Soares, E.V.; Soares, H.M.V.M. Bioremediation of industrial effluents containing heavy metals using brewing cells of saccharomyces cerevisiae as a green technology: A review. Environ. Sci. Pollut. Res. 2012, 19, 1066–1083. [Google Scholar] [CrossRef]
- Verma, S.; Prasad, B.; Mishra, I.M. Pretreatment of petrochemical wastewater by coagulation and flocculation and the sludge characteristics. J. Hazard. Mater. 2010, 178, 1055–1064. [Google Scholar] [CrossRef] [PubMed]
- Ding, P.; Chu, L.; Wang, J. Advanced treatment of petrochemical wastewater by combined ozonation biological aerated filter. Environ. Sci. Pollut. Res. 2018, 25, 9673–9682. [Google Scholar] [CrossRef]
- Babel, S.; Kurniawan, T.A. Low-cost adsorbents for heavy metals uptake from contaminated water: A review. J. Hazard. Mater. 2003, 97, 219–243. [Google Scholar] [CrossRef]
- Cruz, V.C.; Pagnanelli, F.; Cibati, A.; Uccelletti, D.; Palleschi, C.; Toro, L. Biotreatment and bioassessment of heavy metal removal by sulphate reducing bacteria in fixed bed reactors. Water Res. 2010, 44, 151–158. [Google Scholar] [CrossRef]
- Habibi, S.; Nematollahzadeh, A.; Mousavi, S.A. Nano-scale modification of polysulfone membrane matrix and the surface for the separation of chromium ions from water. Chem. Eng. J. 2015, 267, 306–316. [Google Scholar] [CrossRef]
- Zhang, S.; Li, X.Y.; Chen, J.P. Preparation and evaluation of a magnetite-doped activated carbon fiber for enhanced arsenic removal. Carbon 2010, 48, 60–67. [Google Scholar] [CrossRef]
- Li, W.G.; Gong, X.J.; Li, X.; Zhang, D.Y.; Gong, H.N. Removal of Cr(VI) from low temperature micro-polluted surface water by tannic acid immobilized powdered activated carbon. Bioresour. Technol. 2012, 113, 106–113. [Google Scholar] [CrossRef]
- Cazetta, A.L.; Pezoti, O.; Bedin, K.C.; Silva, T.L.; Junior, A.P.; Asefa, T.; Almeida, V.C. Magnetic activated carbon derived from biomass waste by concurrent synthesis: Efficient adsorbent for toxic dyes. ACS Sustain. Chem. Eng. 2016, 4, 1058–1068. [Google Scholar] [CrossRef]
- Norouzi, S.; Heidari, M.; Alipour, V.; Rahmanian, O.; Fazlzadeh, M.; Mohammadi-Moghadam, F.; Nourmoradi, H.; Goudarzi, B.; Dindarloo, K. Preparation, characterization and Cr(VI) adsorption evaluation of NaOH-activated carbon produced from date press cake; an agro-industrial waste. Bioresour. Technol. 2018, 258, 48–56. [Google Scholar] [CrossRef]
- Yu, X.; Wang, S.H.; Zhang, J. Preparation of high adsorption performance activated carbon by pyrolysis of waste polyester fabric. J. Mater. Sci. 2018, 53, 5458–5466. [Google Scholar] [CrossRef]
- Przepiórski, J.; Karolczyk, J.; Takeda, K.; Tsumura, T.; Toyoda, M.; Morawski, A. Porous carbon obtained by carbonization of PET mixed with basic magnesium carbonate: Pore structure and pore creation mechanism. Ind. Eng. Chem. Res. 2009, 48, 7110–7116. [Google Scholar] [CrossRef]
- Chen, F.; Guo, S.H.; Wang, Y.H.; Ma, L.L.; Li, B.; Song, Z.M.; Huang, L.; Zhang, W. Concurrent adsorption and reduction of chromium(VI) to chromium(III) using nitrogen-doped porous carbon adsorbent derived form loofah sponge. Front. Environ. Sci. Eng. 2022, 16, 57–68. [Google Scholar] [CrossRef]
- Liu, W.J.; Jiang, H.; Tian, K.; Ding, Y.W.; Yu, H.Q. Mesoporous carbon stabilized MgO nanoparticles synthes ized by pyrolysis of MgCl2 preloaded waste biomass for highly efficient CO2 capture. Environ. Sci. Technol. 2013, 47, 9397–9403. [Google Scholar] [CrossRef]
- Fan, H.; Liu, H.; Hu, X.; Lv, G.; Zheng, Y.; He, F.; Ma, D.; Liu, Q.; Lu, Y.; Shen, W. Fe2P@mesoporous carbon nanosheets synthesized via an organic template method as a cathode electrocatalyst for Zn-Air batteries. J. Mater. Chem. A. 2019, 7, 11321–11330. [Google Scholar] [CrossRef]
- Hamadi, N.K.; Chen, X.D.; Farid, M.M.; Lu, M.G.Q. Adsorption kinetics for the removal of chromium (VI) from aqueous solution by adsorbents derived from used tyres and sawdust. Chem. Eng. J. 2001, 84, 95–105. [Google Scholar] [CrossRef]
- El-Sikaily, A.; Nemr, A.E.; Khaled, A.; Abdelwehab, O. Removal of toxic chromium from wastewater using green alga Ulva lactuca and its activated carbon. J. Hazard. Mater. 2007, 148, 216–228. [Google Scholar] [CrossRef] [PubMed]
- Nemr, A.E. Potential of pomegranate husk carbon for Cr(VI) removal from wastewater: Kinetic and isotherm studies. J. Hazard. Mater. 2009, 161, 132–141. [Google Scholar] [CrossRef]
- Gupta, V.K.; Rastogi, A.; Nayak, A. Adsorption studies on the removal of hexavalent chromium from aqueous solution using a low cost fertilizer industry waste material. J. Coll. Inter. Sci. 2010, 342, 135–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atieh, M.A. Removal of Chromium (VI) from polluted water using carbon nanotubes supported with activated carbon. Proc. Environ. Sci. 2011, 4, 281–293. [Google Scholar] [CrossRef] [Green Version]
- Zhu, J.H.; Wei, S.Y.; Gu, H.B.; Rapole, S.B.; Wang, Q.; Luo, Z.; Haldolaarachchige, N.; Young, D.P.; Guo, Z. One-pot synthesis of magnetic graphene nanocomposites decorated with Core@double-shell nanoparticles for fast chromium removal. Environ. Sci. Technol. 2012, 46, 977–985. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.X.; Qiu, B.; Gu, H.B.; Yang, X.R.; Wei, H.G.; Huang, X.H.; Wang, Y.R.; Rutman, D.; Cao, D.M.; Bhana, S. Synergistic Interactions between activated carbon fabrics and toxic hexavalent chromium. ECS J. Solid Stat. Sci. Technol. 2014, 3, M1–M9. [Google Scholar] [CrossRef] [Green Version]
- Tytlak, A.; Oleszczuk, P.; Dobrowolski, R. Sorption and desorption of Cr(VI) ions from water by biochars in different environmental conditions. Environ. Sci. Pollut. Res. 2015, 22, 5985–5994. [Google Scholar] [CrossRef] [Green Version]
- Peng, H.; Guo, J.; Li, B.; Liu, Z.H.; Tao, C.Y. High-efficient recovery of chromium (VI) with lead sulfate. J. Taiwan Inst. Chem. Eng. 2018, 85, 149–154. [Google Scholar] [CrossRef]
- Xu, Z.H.; Yuan, Z.H.; Zhang, D.F.; Huang, Y.X.; Chen, W.F.; Sun, Z.H.; Zhou, Y.W. Cr(VI) removal with rapid and superior performance utilizing cost-efficient waste-polyester-textile-based mesoporous carbon: Behavior and mechanism. J. Mol. Liq. 2019, 278, 496–504. [Google Scholar] [CrossRef]
- Chen, H.; Yan, T.T.; Jiang, F. Adsorption of Cr(VI) from aqueous solution on mesoporous carbon nitride. J. Taiwan Inst. Chem. Eng. 2014, 45, 1842–1849. [Google Scholar] [CrossRef]
- Zhang, N.; Eric, M.; Zhang, C.G.; Zhang, J.; Feng, K.; Li, Y.C.; Wang, S.S. ZVI impregnation altered arsenic sorption by ordered mesoporous carbon in presence of Cr(VI): A mechanistic investigation. J. Hazard. Mater. 2021, 414, 125507–125516. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.W.; Fu, F.L.; Zhang, L.; Tang, B. Insight into efficient co-removal of Se(IV) and Cr(VI) by magnetic mesoporous carbon microspheres: Performance and mechanism. Chem. Eng. J. 2018, 346, 590–599. [Google Scholar] [CrossRef]
- Huang, S.H.; Chen, D.H. Rapid removal of heavy metal cations and anions from aqueous solutions by an amino-functionalized magnetic nano-adsorbent. J. Hazard. Mater. 2009, 163, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.H.; Gu, H.B.; Rapole, S.B.; Luo, Z.P.; Pallavkar, S.; Haldolaarachchige, N.; Benson, T.J.; Ho, T.C.; Hopper, J.; Young, D.P.; et al. Looped carbon capturing and environmental remediation: Case study of magnetic polypropylene nanocomposites. RSC Adv. 2012, 2, 4844–4856. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, S.M.; Liu, Q.L.; Chen, Z.X.; Gu, J.J.; Zhu, C.L.; Lu, T.; Zhang, D.; Ma, J. N-doped porous carbon with magnetic particles formed in situ for enhanced Cr(VI) removal. Water Res. 2013, 47, 4188–4197. [Google Scholar] [CrossRef] [PubMed]
- Vinu, A.; Murugesan, C.S.V.; Hartmann, M. Adsorption of cytochrome C on new mesoporous carbon molecular sieves. J. Phys. Chem. B 2003, 107, 8297–8299. [Google Scholar] [CrossRef]
- Knöfel, C.; Descarpentries, J.; Benzaouia, A.; Zeleňák, V.; Mornet, S.; Llewellyn, P.L.; Hornebecq, V. Functiona lised micro-/mesoporous silica for the adsorption of carbon dioxide. Micro. Mesop. Mater. 2007, 99, 79–85. [Google Scholar] [CrossRef]
- Kennedy, L.J.; Vijaya, J.J.; Kayalvizhi, K.; Sekaran, G. Adsorption of phenol from aqueous solutions using mesoporous carbon prepared by two-stage process. Chem. Eng. J. 2017, 1–3, 279–287. [Google Scholar] [CrossRef]
- Tanthapanichakoon, W.; Ariyadejwanich, P.; Japthong, P.; Nakagawa, K.; Mukai, S.R.; Tamon, H. Adsorption-desorption character istics of phenol and reactive dyes from aqueous solution on mesoporous activated carbon prepared from waste tires. Water Res. 2005, 39, 1347–1353. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.P.; Qi, J.R.; Yang, S.F.; Yu, K.F.; Wang, Z.C.; Xu, H.D. Adsorption of Cr(VI) on micro- and mesoporous rice husk-based active carbon. Mater. Chem. Phys. 2003, 78, 132–137. [Google Scholar] [CrossRef]
- Walter, W.G. APHA standard methods for the examination of water and wastewater. Am. J. Public Health 1961, 51, 940–957. [Google Scholar] [CrossRef] [Green Version]
- Piccin, J.S.; Feris, L.A.; Copper, F.M.; Gutterres, M. Dye adsorption by leather waste: Mechanism diffusion, nature studies, and thermodynamic data. J. Chem. Eng. Data 2013, 58, 873–882. [Google Scholar] [CrossRef]
- Qu, H.; Lai, Y.; Niu, D.; Sun, S. Surface-enhanced Raman scattering from magneto-metal nano particle assemblies. Anal. Chim. Acta 2013, 763, 38–42. [Google Scholar] [CrossRef]
- Zak, A.K.; Shirmadh, H.; Mohammadi, S.; Banihashemian, S.M. Solvothermal synthesis of porous Fe3O4 nano particles for humidity sensor application. Mater. Res. Express 2020, 7, 025001–025009. [Google Scholar] [CrossRef]
- Muniz, F.T.L.; Miranda, M.A.R.; Santos, C.M.; Sasaki, J.M. The scherrer equation and the dynamical theory of X-ray diffraction. Acta. Cryst. 2016, A72, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhou, F.Z.; Wang, W.J.; Guo, H.L.; Liu, M.X.; Zhu, H.D.; Sun, H.M. Recyclable adsorbents based on Fe3O4 nanoparticles on lanthanum-modified montmorillonite for the efficient phosphate removal. IET Nanobiotechnol. 2020, 14, 527–536. [Google Scholar] [CrossRef]
- Demirbas, E.; Kobya, M.; Sulak, M.T. Adsorption kinetics of a basic dye from aqueous solutions onto apricot stone activated carbon. Bioresour. Technol. 2008, 99, 5368–5373. [Google Scholar] [CrossRef]
- Lyubchik, S.I.; Lyubchik, A.I.; Galushko, O.L.; Tikhonova, L.P.; Vital, J.; Fonseca, I.M.; Lyubchik, S.B. Kinetics and thermodynamics of the Cr(III) adsorption on the activated carbon from co-mingled wastes. Colloids Surf. A 2004, 242, 151–158. [Google Scholar] [CrossRef]
- Qi, H.X.; Niu, X.J.; Wu, H.P.; Liu, X.P.; Chen, Y.Q. Adsorption of Chromium (VI) by Cu(I)-MOF in water: Optimization, kinetics, and thermodynamics. J. Chem. 2021, 2021, 4413095. [Google Scholar] [CrossRef]
- Bagheri, A.R.; Ghaedi, M.; Asfaram, A.; Bazrafshan, A.A.; Jannesar, R. Comparative study on ultrasonic assisted adsorption of dyes from single system onto Fe3O4 magnetite nanoparticles loaded on activated carbon: Experimental design methodology. Ultrason. Sonochem. 2017, 34, 294–304. [Google Scholar] [CrossRef] [PubMed]
- Saha, N.; Volpe, M.; Fiori, L.; Volpe, R.; Messineo, A.; Reza, M.T. Cationic dye adsorption on hydrochars of winery and citrus juice industries residues: Performance, mechanism, and thermodynamics. Energies 2020, 13, 4686–4702. [Google Scholar] [CrossRef]
- Fang, J.; Gu, Z.M.; Gang, D.C.; Liu, C.X.; Ilton, E.S.; Deng, B.L. Cr(VI) removal from aqueous solution by activated carbon coated with quaternized poly(4-vinylpyridine). Environ. Sci. Technol. 2007, 41, 4748–4753. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.H.; Gu, H.B.; Guo, J.; Chen, M.J.; Wei, H.G.; Luo, Z.P.; Colorado, H.A.; Yerra, N.; Ding, D.W.; Ho, T.C.; et al. Mesoporous magnetic carbon nanocomposite fabrics for highly efficient Cr(VI) removal. J. Mater. Chem. A 2014, 2, 2256–2265. [Google Scholar] [CrossRef]
- Qiu, B.; Wang, Y.R.; Sun, D.Z.; Wang, Q.; Zhang, X.; Weeks, B.L.; O’Connor, R.; Huang, X.H.; Wei, S.Y.; Guo, Z.H. Cr(VI) removal by magnetic carbon nanocomposites derived from cellulose at different carbonization temperatures. J. Mater. Chem. A 2015, 3, 9817–9825. [Google Scholar] [CrossRef]
- Yang, T.T.; Meng, L.R.; Han, S.W.; Hou, J.H.; Wang, S.S.; Wang, X.Z. Simultaneous reductive and sorptive removal of Cr(VI) by activated carbon supported β-FeOOH. RSC Adv. 2017, 7, 34687–34693. [Google Scholar] [CrossRef] [Green Version]
- Su, Q.H.; Lin, Z.; Tian, C.; Su, X.T.; Xue, X.G.; Su, Z. Improved removal of Cr(VI) using Fe3O4/C magnetic nanocomposites derived from potassium fulvic acid. ChemistrySelect 2019, 4, 13656–13662. [Google Scholar] [CrossRef]
- Nogueira, H.P.; Toma, S.H.; Silveira, A.T.; Carvalho, A.A.C.; Fioroto, A.M.; Araka, K. Efficient Cr(VI) removal from wastewater by activated carbon superparamagnetic composites. Microchem. J. 2019, 149, 104025–104030. [Google Scholar] [CrossRef]
- Abuzalat, Q.; Wong, D.; Elsayed, M.A. Nano-porous composites of activated carbon-metal organic frameworks (Fe-BDC@AC) for rapid removal of Cr(VI): Synthesis, adsorption, mechanism, and kinetics studies. J. Inorg. Organ. Poly. Mater. 2022, 32, 1924–1934. [Google Scholar] [CrossRef]
- Shahawy, A.E.; Mubarak, M.F.; Shafie, M.E.; Abdulla, H.M. Fe(III) and Cr(VI) ions’ removal using AgNPs/GO/chitosan nanocomposite as an adsorbent for wastewater treatment. RSC Adv. 2022, 12, 17065–17084. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, G.; Tian, J.; Wang, D.; Cai, D.; Wu, Z. Functionalized Fe3O4@C nanospheres with adjustable structure for efficient hexavalent chromium removal. ACS Sus. Chem. Eng. 2017, 5, 11042–11050. [Google Scholar] [CrossRef]
- Caravelli, A.H.; Giannuzzi, L.; Zaritzky, N.E.J.J.o.H.M. Reduction of hexavalent chromium by sphaerotilus natans a filamentous micro-organism present in activated sludges. J. Hazard. Mater. 2008, 156, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.D.; Xu, J.; Liu, J.; Liu, J.; Xia, F.; Wang, C.C.; Dahlgren, R.A.; Liu, W. Mechanism of Cr(VI) removal by magnetic greigite/biochar composites. Sci. Total Environ. 2020, 700, 134414–134423. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yuan, X.Z.; Wu, Y.; Chen, X.H.; Leng, L.J.; Wang, H.; Li, H.; Zeng, G.M. Facile synthesis of polypyrrole decorated reduced graphene oxide-Fe3O4 magnetic composites and its application for the Cr(VI) removal. Chem. Eng. J. 2015, 262, 597–606. [Google Scholar] [CrossRef]






| Adsorbents | Q max (mg·g−1) | Optimum pH | Years | References |
|---|---|---|---|---|
| Activated carbon from tires | 48.1 | 2.0 | 2001 | [21] |
| Activated carbon | 112.36 | 1.0 | 2007 | [22] |
| Pomegranate husk carbon | 35.2 | 1.0 | 2009 | [23] |
| Carbon slurry | 15.24 | 2.0 | 2010 | [24] |
| Carbon nanotube | 9.0 | 2.0 | 2011 | [25] |
| Graphene nanocomposites | 1.03 | 2.0 | 2012 | [26] |
| Carbon fabrics | 5.59 | 1.0 | 2014 | [27] |
| Biochar | 24.6 | 2.0 | 2015 | [28] |
| Rubberwood sawdust activated carbon | 44.05 | 13.9 | 2018 | [29] |
| Models | Equation | Parameters |
|---|---|---|
| Langmuir | Qe = | QL (mg·g−1) maximum adsorption capacity; KL (L·mg−1) Langmuir constant. |
| Freundlich | Qe = KF | KF (mg·g−1)(L·mg−1)−1/n Freundlich constant; 1/nF heterogeneity factor. |
| Temkin | Qe = AT + BTlnCe | AT Temkin constant;BT adsorption energy factor. |
| Dubinin–Radushkevich | Qe = | QD (mg·g−1) adsorption capacity; AD Dubinin–Radushkevich free energy constant. |
| Absorbent | Surface Area (m2·g−1) | Average Pore size (nm) | Pore Volume (cm3·g−1) | Average Particle Size (nm) |
|---|---|---|---|---|
| MAC | 1303.0 | 1.82 | 2.41 | 17.45 |
| 6%Fe3O4@MAC | 1306.3 | 1.82 | 2.43 | 19.27 |
| 8%Fe3O4@MAC | 1311.3 | 1.81 | 2.45 | 21.23 |
| 10%Fe3O4@MAC | 1307.6 | 1.79 | 2.39 | 23.46 |
| 15%Fe3O4@MAC | 1304.3 | 1.78 | 2.30 | 26.33 |
| MMC | 436.7 | 6.82 | 0.35 | 16.34 |
| 6%Fe3O4@MMC | 441.6 | 6.81 | 0.36 | 17.83 |
| 8%Fe3O4@MMC | 452.3 | 6.80 | 0.38 | 18.37 |
| 10%Fe3O4@MMC | 450.7 | 6.67 | 0.31 | 19.42 |
| 15%Fe3O4@MMC | 448.4 | 6.63 | 0.25 | 20.06 |
| Element | Weight Percentage (%) | Atom Percentage (%) |
|---|---|---|
| C | 41.10 | 53.01 |
| O | 45.04 | 43.69 |
| Fe | 11.31 | 3.12 |
| Cr | 2.55 | 0.18 |
| Total | 100 | 100 |
| Absorbents | Adsorption Capacity (mg·g−1) | Removal Rate of Cr(VI) Ions (%) | Adsorption Capacity a (mg·g−1) | Fe3O4 Loading b (wt.%) |
|---|---|---|---|---|
| 6%Fe3O4@MMC | 126.37 | 94.78 | 126.51 | 5.97 |
| 6%Fe3O4@MAC | 116.15 | 87.11 | 116.33 | 5.96 |
| 8%Fe3O4@MMC | 132.80 | 99.60 | 132.94 | 7.99 |
| 8%Fe3O4@MAC | 120.61 | 90.46 | 120.72 | 7.98 |
| 10%Fe3O4@MMC | 124.03 | 93.02 | 124.15 | 9.97 |
| 10%Fe3O4@MAC | 114.76 | 86.07 | 115.01 | 9.96 |
| 15%Fe3O4@MMC | 116.49 | 87.37 | 116.82 | 14.98 |
| 15%Fe3O4@MAC | 108.54 | 81.41 | 109.05 | 14.96 |
| Temperature/K | 298 | 308 | 318 |
|---|---|---|---|
| Langmuir model | KL = 0.079 | KL = 0.073 | KL = 0.076 |
| QL = 166.788 | QL = 154.770 | QL = 139.171 | |
| R2 = 0.892 | R2 = 0.923 | R2 = 0.912 | |
| Freundlich model | KF = 29.844 | KF = 29.296 | KF = 23.694 |
| nF = 2.689 | nF = 2.596 | nF = 2.614 | |
| R2 = 0.779 | R2 = 0.821 | R2 = 0.807 | |
| Temkin model | AT = 17.109 | AT = 14.672 | AT = 13.200 |
| BT = 27.723 | BT = 25.431 | BT = 23.132 | |
| R2 = 0.802 | R2 = 0.829 | R2 = 0.820 | |
| Dubinin–Radushkevich Model | QD = 137.954 | QD = 125.430 | QD = 113.884 |
| AD = 64.682 | AD = 66.275 | AD = 61.638 | |
| R2 = 0.988 | R2 = 0.997 | R2 = 0.996 |
| Temperature /K | 8%Fe3O4@MMC | 8%Fe3O4@MAC | ||||
|---|---|---|---|---|---|---|
| Adsorption Capacity (mg·g−1) | Removal Rate (%) | ΔG (kJ·mol−1) | Adsorption Capacity (mg·g−1) | Removal Rate (%) | ΔG (kJ·mol−1) | |
| 288 | 128.93 | 96.70 | −9.31 | 118.33 | 88.75 | −6.17 |
| 298 | 132.80 | 99.60 | −14.94 | 120.61 | 90.46 | −6.84 |
| 308 | 126.77 | 95.08 | −8.89 | 116.54 | 87.41 | −6.27 |
| 318 | 113.52 | 85.14 | −5.97 | 109.32 | 81.99 | −5.36 |
| 328 | 104.68 | 78.51 | −4.93 | 98.49 | 73.87 | −4.23 |
| Adsorbents | Carbon Form | Q max (mg·g−1) | Years | References |
|---|---|---|---|---|
| GAC-QPVP | granular activated carbon | 53.7 | 2007 | [53] |
| MN | mesoporous magnetic carbon | 3.74 | 2014 | [54] |
| ZVI/carbon | magnetic carbon | 275.6 | 2015 | [55] |
| β-FeOOH/SYBK | activated carbon | 37.04 | 2017 | [56] |
| Fe3O4/C | synthesize magnetite carbon from potassium fulvic acid | 64.0 | 2019 | [57] |
| AC/SPION | activated carbon | 16.82 | 2019 | [58] |
| Fe-BDC@AC | activated carbon | 100 | 2022 | [59] |
| AgNPs/GO/Chitosan | graphene | 46.45 | 2022 | [60] |
| 8%Fe3O4@MMC | mesoporous magnetic carbon | 132.80 | in this work | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Long, W.; Chen, Z.; Chen, X.; Zhong, Z. Investigation of the Adsorption Process of Chromium (VI) Ions from Petrochemical Wastewater Using Nanomagnetic Carbon Materials. Nanomaterials 2022, 12, 3815. https://doi.org/10.3390/nano12213815
Long W, Chen Z, Chen X, Zhong Z. Investigation of the Adsorption Process of Chromium (VI) Ions from Petrochemical Wastewater Using Nanomagnetic Carbon Materials. Nanomaterials. 2022; 12(21):3815. https://doi.org/10.3390/nano12213815
Chicago/Turabian StyleLong, Wei, Zhilong Chen, Xiwen Chen, and Zhanye Zhong. 2022. "Investigation of the Adsorption Process of Chromium (VI) Ions from Petrochemical Wastewater Using Nanomagnetic Carbon Materials" Nanomaterials 12, no. 21: 3815. https://doi.org/10.3390/nano12213815






