Spinel CoFe2O4 Nanoflakes: A Path to Enhance Energy Generation and Environmental Remediation Potential of Waste-Derived rGO
Abstract
:1. Introduction
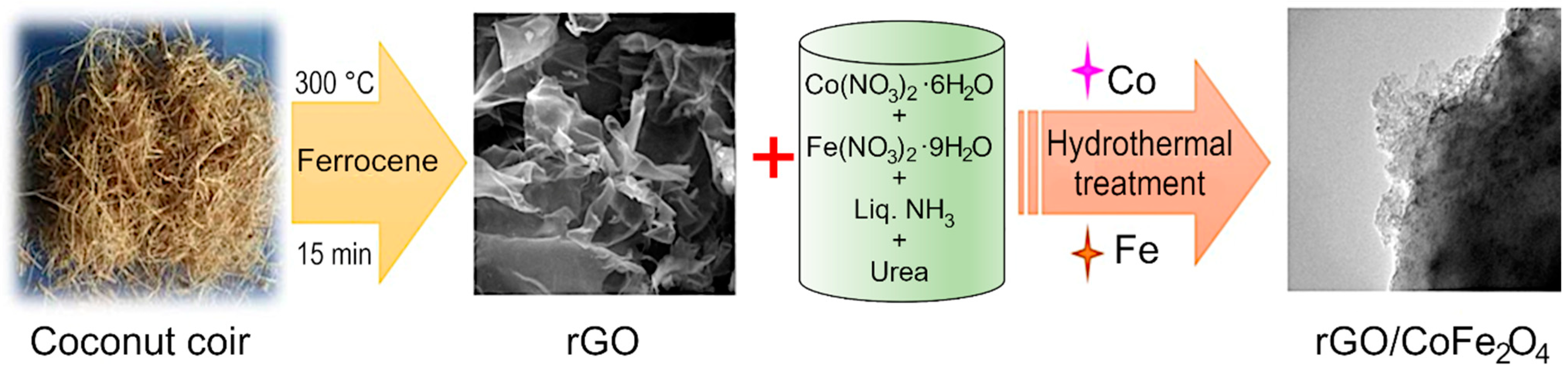
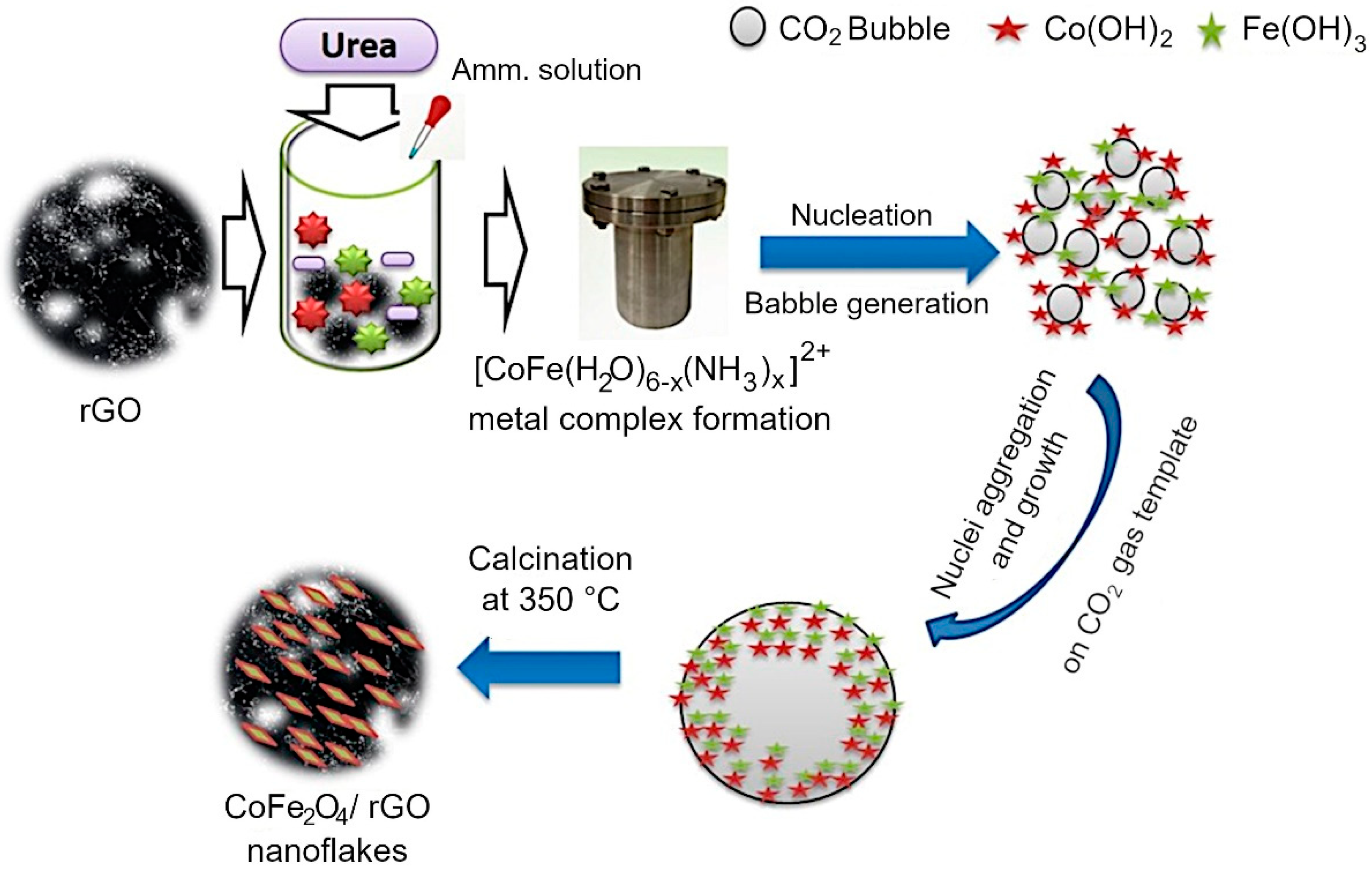
2. Materials and Synthesis Methods
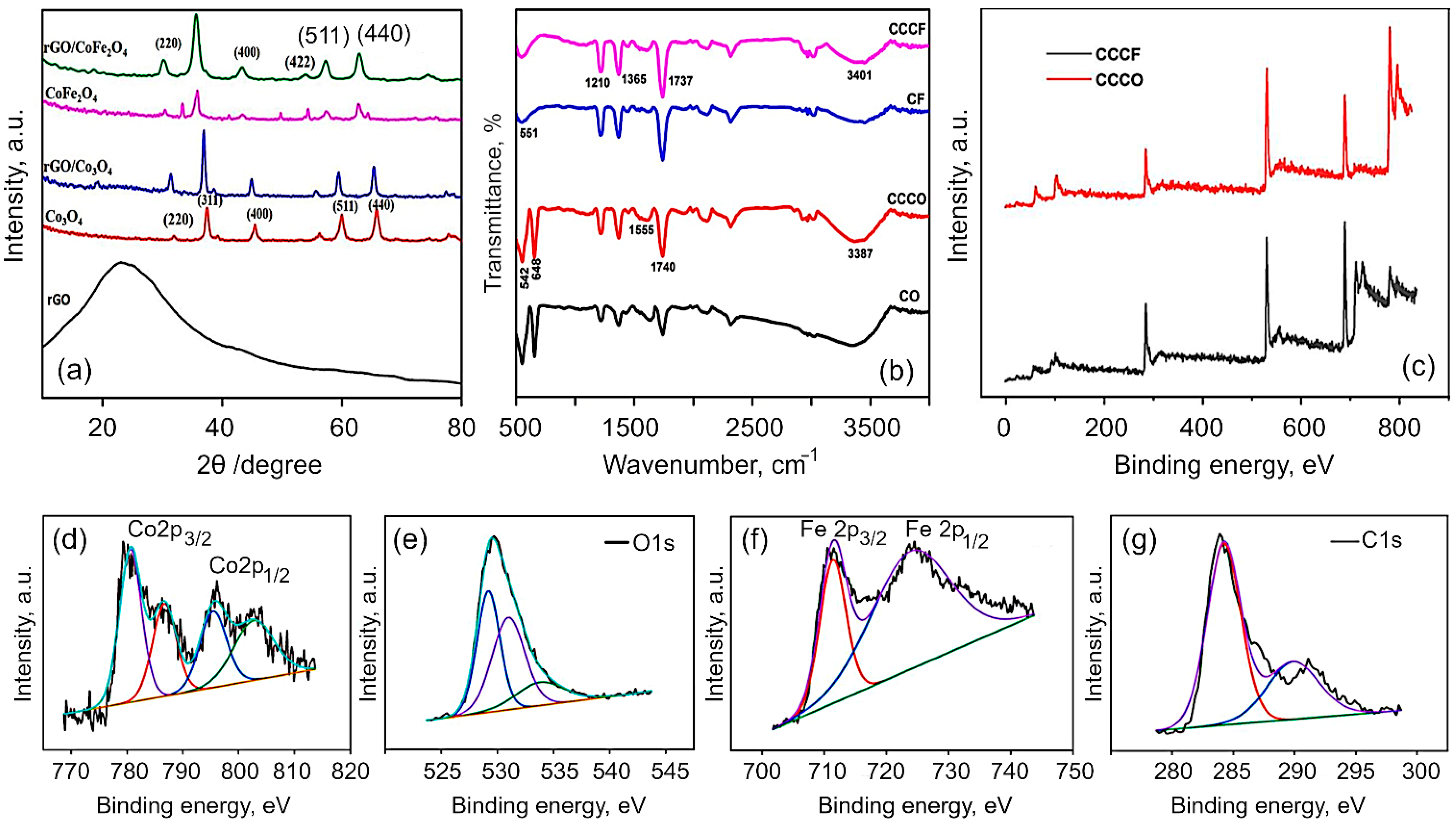
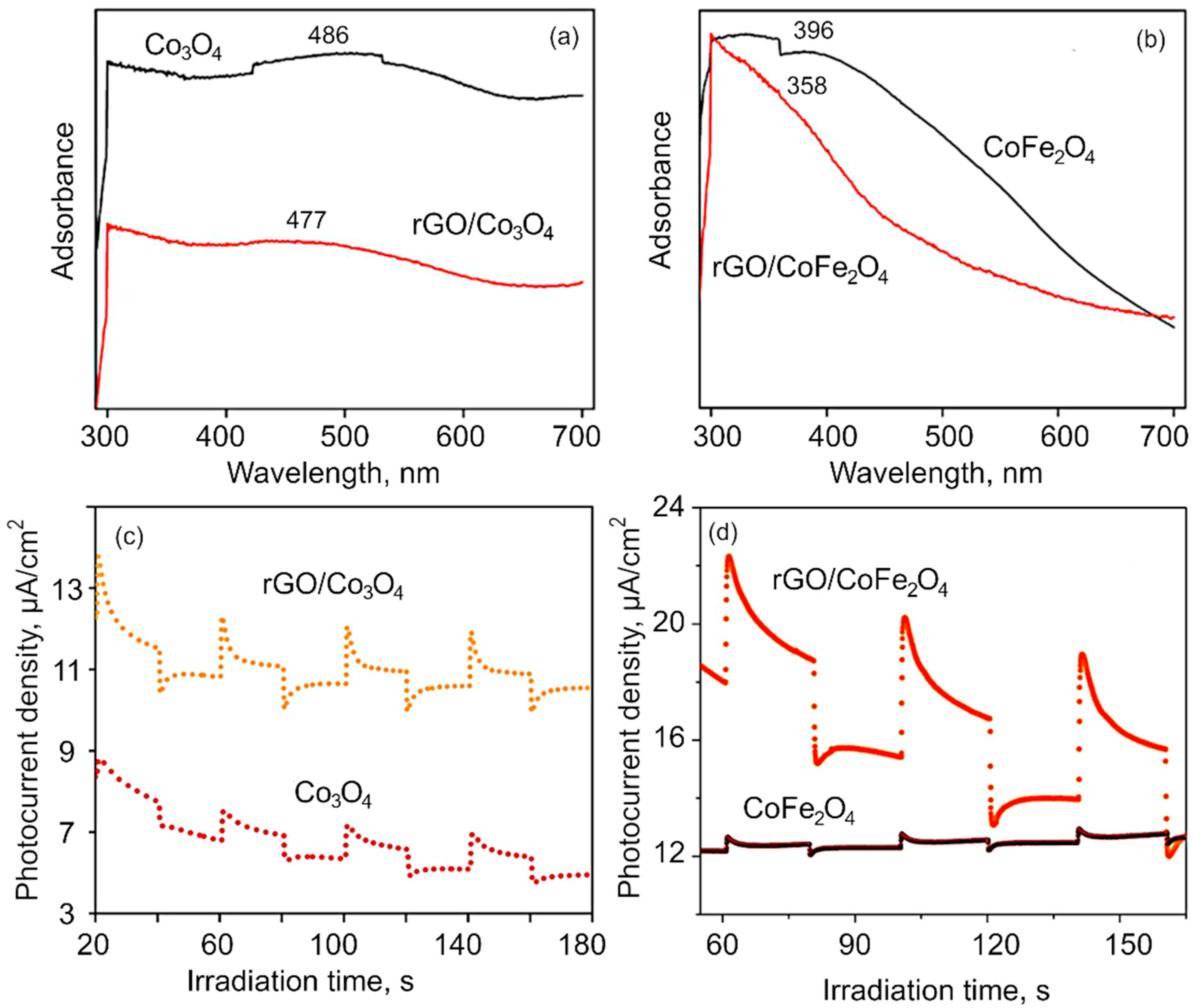
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yrjälä, K.; Ramakrishnan, M.; Salo, E. Agricultural waste streams as resource in circular economy for biochar production towards carbon neutrality. Curr. Opin. Environ. Sci. Health 2022, 26, 100339. [Google Scholar] [CrossRef]
- Bazaka, O.; Prasad, K.; Levchenko, I.; Jacob, M.V.; Bazaka, K.; Kingshott, P.; Crawford, R.J.; Ivanova, E.P. Decontamination-induced modification of bioactivity in essential oil-based plasma polymer coatings. Molecules 2021, 26, 7133. [Google Scholar] [CrossRef] [PubMed]
- El-Deeb, Z.M.; Aboutaleb, W.A.; Dhmees, A.S.; El Naggar, A.M.A.; Emara, K.; Elgendy, A.T.; Ahmed, A.I. Bio-fuels production through waste tires pyrolytic oil upgrading over Ni-W/zeolite composites derived from blast furnace slag. Int. J. Energy Res. 2022, 46, 17376–17390. [Google Scholar] [CrossRef]
- Bhattarai, R.M.; Chhetri, K.; Saud, S.; Teke, S.; Kim, S.J.; Mok, Y.S. Eco-friendly synthesis of cobalt molybdenum hydroxide 3d nanostructures on carbon fabric coupled with cherry flower waste-derived activated carbon for quasi-solid-state flexible asymmetric supercapacitors. ACS Appl. Nano Mater. 2022, 5, 160–175. [Google Scholar] [CrossRef]
- Piferi, C.; Carra, C.; Bazaka, C.; Roman, H.E.; Dell’Orto, E.C.; Morandi, V.; Levchenko, I.; Riccardi, C. Controlled deposition of nanostructured hierarchical TiO2 thin films by low pressure supersonic plasma jets. Nanomaterials 2022, 12, 533. [Google Scholar] [CrossRef]
- Liu, Z.; Zheng, J.; Duan, L.; Zhu, Z. Biomass-assisted synthesis of CeO2 nanorods for CO2 photoreduction under visible light. Appl. Nano Mater. 2021, 4, 4226–4237. [Google Scholar] [CrossRef]
- Levchenko, I.; Mandhakini, M.; Prasad, K.; Bazaka, O.; Ivanova, E.P.; Jacob, M.V.; Baranov, O.; Riccardi, C.; Roman, H.E.; Xu, S.; et al. Functional nanomaterials from waste and low-value natural products: A technological approach level. Adv. Mater. Technol. 2022, 7, 2101471. [Google Scholar] [CrossRef]
- Levchenko, I.; Xu, S.; Baranov, O.; Bazaka, O.; Ivanova, E.P.; Bazaka, K. Plasma and polymers: Recent progress and trends. Molecules 2021, 26, 4091. [Google Scholar] [CrossRef]
- Tahir, M.H.; Mubashir, M.H.; Schulze, M.; Irfan, R.M. Thermochemical conversion of cabbage waste to bioenergy and bio-chemicals production. Int. J. Energy Res. 2022; in press. [Google Scholar] [CrossRef]
- Zheng, J.; Yan, B.; Feng, L.; Zhang, Q.; Zhang, C.; Yang, W.; Han, J.; Jiang, S.; He, S. Potassium citrate assisted synthesis of hierarchical porous carbon materials for high performance supercapacitors. Diamond Relat. Mater. 2022, 128, 109247. [Google Scholar] [CrossRef]
- Zheng, S.; Zhang, J.; Deng, H.; Du, Y.; Shi, X. Chitin derived nitrogen-doped porous carbons with ultrahigh specific surface area and tailored hierarchical porosity for high performance supercapacitors. J. Biores. Bioprod. 2021, 6, 142–151. [Google Scholar] [CrossRef]
- Chen, S.; Jiang, S.; Jiang, H. A review on conversion of crayfish-shell derivatives to functional materials and their environmental applications. J. Biores. Bioprod. 2020, 5, 238–247. [Google Scholar] [CrossRef]
- Carra, C.; Medvids, A.; Litvinas, D.; Sčajev, P.; Malinauskas, T.; Selskis, A.; Roman, H.E.; Bazaka, K.; Levchenko, I.; Riccardi, C. Hierarchical carbon nanocone-silica metamaterials: Implications for white light photoluminescence. ACS Appl. Nano Mater. 2022, 5, 4787–4800. [Google Scholar] [CrossRef]
- Li, F.; Yu, Y.; Xu, C.; Li, Y.; He, Z.; Bi, X. Atomic-layer-deposited ZnO/Al2O3 nanolaminates for white-light-emitting diodes. ACS Appl. Nano Mater. 2022, 5, 8730–8734. [Google Scholar] [CrossRef]
- Alancherry, S.; Bazaka, K.; Levchenko, I.; Al-Jumaili, A.; Kandel, B.; Alex, A.; Hernandez, F.C.R.; Varghese, O.K.; Jacob, M.V. Fabrication of nano-onion-structured graphene films from citrus sinensis extract and their wetting and sensing characteristics. ACS Appl. Mater. Interfaces 2020, 12, 29594–29604. [Google Scholar] [CrossRef]
- Chen, T.; Wang, B.; Qi, Z.; Guo, Z.; Tian, Y.; Meng, F. Coatings comprised of graphene oxide decorated with helical polypyrrole nanofibers for microwave absorption and corrosion protection. ACS Appl. Nano Mater. 2022, 5, 9780–9791. [Google Scholar] [CrossRef]
- Kumar, A.; Aljumaili, A.; Bazaka, O.; Ivanova, E.P.; Levchenko, I.; Bazaka, K.; Jacob, M. Functional nanomaterials, synergism and biomimicry for environmentally benign marine antifouling technology. Mater. Horiz. 2021, 8, 3201–3238. [Google Scholar] [CrossRef]
- Kharisov, B.I.; Dias, H.V.R.; Kharissova, O.V. Mini-review: Ferrite nanoparticles in the catalysis. Arab. J. Chem. 2019, 12, 1234–1246. [Google Scholar] [CrossRef] [Green Version]
- Joseph, H.M.; Sugunan, S.; Gurrala, L.; Mohan, M.K.; Gopi, S. New insights into surface functionalization and preparation methods of MWCNT based semiconductor photocatalyst. Ceram. Int. 2019, 45, 14490–14499. [Google Scholar] [CrossRef]
- Tamilselvi, R.; Lekshmi, G.S.; Padmanathan, N.; Selvaraj, V.; Bazaka, O.; Levchenko, I.; Bazaka, K.; Mandhakini, M. NiFe2O4/rGO nanocomposites produced by soft bubble assembly for energy storage and environmental remediation. Renew. Energy 2022, 181, 1386–1401. [Google Scholar] [CrossRef]
- Sree, G.V.; Rajasekaran, P.; Bazaka, O.; Levchenko, I.; Bazaka, K.; Mandhakini, M. Biowaste Valorization by conversion to nanokeratin-urea composite fertilizers for sustainable and controllable release of carbon and nitrogen. Carbon Trends 2021, 5, 100083. [Google Scholar] [CrossRef]
- Deshmukh, S.; Jakobczyk, P.; Ficek, M.; Ryl, J.; Geng, D.; Bogdanowicz, R. Tuning the laser-induced processing of 3D porous graphenic nanostructures by boron-doped diamond particles for flexible microsupercapacitors. Adv. Funct. Mater. 2022, 32, 2206097. [Google Scholar] [CrossRef]
- Lei, J.; Liu, J.; Tang, N.; Han, H.; Li, Z.; Li, K.; Zhai, T.; Chen, H.; Xia, H. Novel gram-scale synthesis of carbon nano-onions from heavy oil for supercapacitors. Adv. Mater. Interfaces 2021, 8, 2101208. [Google Scholar] [CrossRef]
- Tamilselvi, R.; Padmanathan, N.; Mani Rahulan, K.; Mohana Priya, P.; Sasikumar, R.; Mandhakini, M. Reduced graphene oxide (rGO): Supported NiO, Co3O4 and NiCo2O4 hybrid composite on carbon cloth (CC)—Bi-functional electrode/catalyst for energy storage and conversion devices. J. Mater. Sci. Mater. Electron. 2017, 29, 4869–4880. [Google Scholar] [CrossRef]
- Yan, B.; Zheng, J.; Feng, L.; Du, C.; Jian, S.; Yang, W.; Wu, Y.A.; Jiang, S.; He, S.; Chen, W. Wood-derived biochar as thick electrodes for high-rate performance supercapacitors. Biochar 2022, 4, 50. [Google Scholar] [CrossRef]
- Fahmi, F.; Dewayanti, N.A.A.; Widiyastuti, W.; Setyawan, H. Preparation of porous graphene-like material from coconut shell charcoals for supercapacitors. Cogent Eng. 2020, 7, 1748962. [Google Scholar] [CrossRef]
- Singh, P.; Minh, N.Q. Solid Oxide Fuel Cells: Technology Status. Int. J. Appl. Ceram. Technol. 2004, 1, 5–15. [Google Scholar] [CrossRef]
- Yadav, K.K.; Singh, H.; Rana, S.; Suniana; Sammi, H.; Nishanthi, S.T.; Wadhwa, R.; Khan, N.; Jha, M. Utilization of waste coir fibre architecture to synthesize porous graphene oxide and their derivatives: An efficient energy storage material. J. Clean. Prod. 2020, 276, 124240. [Google Scholar] [CrossRef]
- Younes, H.; Zou, L. Asymmetric configuration of pseudocapacitive composite and rGO electrodes for enhanced capacitive deionization. Environ. Sci. Water Res. Technol. 2020, 6, 392–403. [Google Scholar] [CrossRef]
- Younes, H.; Ravaux, F.; Hadri, N.E.; Zou, L. Nanostructuring of pseudocapacitive MnFe2O4/Porous rGO electrodes in capacitive deionization. Electrochim. Acta 2019, 306, 1–8. [Google Scholar] [CrossRef]
- Tamilselvi, R.; Ramesh, M.; Lekshmi, G.S.; Bazaka, O.; Levchenko, I.; Bazaka, K.; Mandhakini, M. Graphene oxide–based supercapacitors from agricultural wastes: A step to mass production of highly efficient electrodes for electrical transportation systems. Renew. Energy 2020, 151, 731–739. [Google Scholar] [CrossRef]
- Li, X.; Yu, J.; Wageh, S.; Al-Ghamdi, A.A.; Xie, J. Graphene in photocatalysis: A review. Small 2016, 12, 6640–6696. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhang, G.; Wang, L.; Huang, T.; Qin, L. Highly active S-modified ZnFe2O4 heterogeneous catalyst and its photo Fenton behavior under UVA visible irradiation. Ind. Eng. Chem. Res. 2011, 50, 7219. [Google Scholar] [CrossRef]
- Zafar, Q.; Azmer, M.I.; Al-Sehemi, A.G.; Al-Assiri, M.S.; Kalam, A.; Sulaiman, K. Evaluation of humidity sensing properties of TMBHPET thin film embedded with spinel cobalt ferrite nanoparticles. J. Nanopart. Res. 2016, 18, 186. [Google Scholar] [CrossRef]
- Paulsen, J.A.; Ring, A.P.; Lo, C.C.H.; Snyder, J.E.; Jiles, D.C. Manganese substituted cobalt ferrite magnetostrictive materials for magnetic stress sensor applications. J. Appl. Phys. 2005, 97, 044502. [Google Scholar] [CrossRef] [Green Version]
- Li, H.S.; Zhang, Y.P.; Wang, S.Y.; Wu, Q.; Liu, C.H. Study on nanomagnets supported TiO2 photocatalysts prepared by a sol–gel process in reverse microemulsion combining with solventthermal technique. J. Hazard. Mater. 2009, 169, 1045. [Google Scholar] [CrossRef]
- Yu, L.; Peng, X.; Ni, F.; Li, J.; Wang, D.; Luan, Z. Arsenite removal from aqueous solutions by c-Fe2O3-TiO2 magnetic nanoparticles through simultaneous photocatalytic oxidation and adsorption. J. Hazard. Mater. 2013, 246, 10. [Google Scholar] [CrossRef]
- Xin, T.; Ma, M.; Zhang, H.; Gu, J.; Wang, S.; Liu, M.; Zhang, Q. A facile approach for the synthesis of magnetic separable Fe3O4@TiO2, core–shell nanocomposites as highly recyclable photocatalysts. Appl. Surf. Sci. 2014, 288, 51. [Google Scholar] [CrossRef]
- Levchenko, I.; Baranov, O.; Riccardi, C.; Roman, H.E.; Cvelbar, U.; Ivanova, E.; Mandhakini, M.; Ščajev, P.; Malinauskas, T.; Xu, S.; et al. Nanoengineered carbon-based interfaces for advanced energy and photonics applications: A recent progress and innovations. Adv. Mater. Interfaces, 2022; in press. [Google Scholar] [CrossRef]
- Piferi, C.; Bazaka, K.; D’Aversa, D.L.; Di Girolamo, R.; De Rosa, C.; Roman, H.E.; Riccardi, C.; Levchenko, I. Hydrophilicity and hydrophobicity control of plasma-treated surfaces via fractal parameters. Adv. Mater. Interfaces 2021, 8, 2100724. [Google Scholar] [CrossRef]
- Casbeer, E.; Sharma, V.K.; Li, X.-Z. Synthesis and photocatalytic activity of ferrites under visible light: A review. Separ. Purif. Technol. 2012, 87, 1–14. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, W.; Goebl, J.; Yina, Y. Self-templated synthesis of hollow nanostructures. Nano Today 2009, 4, 494–507. [Google Scholar] [CrossRef]
- Zeng, W.; Huang, Y.; Xiong, Y.; Wang, N.; Xu, C.; Huang, L. Gas bubble templated synthesis of Mn3O4-embedded hollow carbon nanospheres in ethanol flame for elastic supercapacitor. J. Alloys Compd. 2018, 731, 210–221. [Google Scholar] [CrossRef]
- Wang, H.; Liu, X.; Hu, O.S.W.; Wang, J. Robust amino-functionalized mesoporous silica hollow spheres templated by CO2 bubbles. Molecules 2022, 27, 53. [Google Scholar] [CrossRef]
- Yu, L.; Yu, X.Y.; Lou, X.W.D. The design and synthesis of hollow micro-/nanostructures: Present and future trends. Adv. Mater. 2018, 30, 1800939. [Google Scholar] [CrossRef]
- Pileni, M.P. Magnetic fluids: Fabrication, magnetic properties and organization of nanocrystals. Adv. Funct. Mater. 2001, 11, 323. [Google Scholar] [CrossRef]
- Kim, D.H.; Nikles, D.E.; Jhonson, D.T.; Brazel, C.S. Heat generation of aqueously dispersed CoFe2O4 nanoparticles as heating agents for magnetically activated drug delivery and hyperthermia. J. Magn. Magn. Mater. 2008, 320, 2390. [Google Scholar] [CrossRef]
- Erdem, D.; Bingham, N.S.; Heiligtag, F.J.; Pilet, N.; Warnicke, P.; Heyderman, L.J.; Niederberger, M. CoFe2O4 and CoFe2O4-SiO2 nanoparticle thin films with perpendicular magnetic anisotropy for magnetic and magneto-optical applications. Adv. Funct. Mater. 2016, 26, 1954. [Google Scholar] [CrossRef]
- Ahmed, J.; Alshehri, S.M.; Alhabarah, A.N.; Ahmad, T.; Ahmad, T. Nitrogen doped cobalt ferrite/carbon (NCFC) nanocomposites for supercapacitor application. ChemElectroChem 2017, 4, 2952. [Google Scholar] [CrossRef]
- Samavati, A.; Ismail, A.F. Antibacterial properties of copper-substituted cobalt ferrite nanoparticles synthesized by co-precipitation method. Particuology 2017, 30, 158. [Google Scholar] [CrossRef]
- Ren, H.; Li, Y.; Ni, Q.; Bai, Y.; Zhao, H.; Wu, C. Unraveling anionic redox for sodium layered oxide cathodes: Breakthroughs and perspectives. Adv. Mater. 2022, 34, 2106171. [Google Scholar] [CrossRef] [PubMed]
- Mahala, C.; Sharma, M.D.; Basu, M. 2D nanostructures of CoFe2O4 and NiFe2O4: Efficient oxygen evolution catalyst. Electrochem. Acta 2018, 273, 462–473. [Google Scholar] [CrossRef]
- Lavela, P.; Tirado, J.L. CoFe2O4 and NiFe2O4 synthesized by sol–gel procedures for their use as anode materials for Li ion batteries. J. Power Sources 2007, 172, 379–387. [Google Scholar] [CrossRef]
- Karthigayan, N.; Manimuthu, P.; Priya, M.; Sagadevan, S. Synthesis and characterization of NiFe2O4, CoFe2O4 and CuFe2O4 thin films for anode material in Li-ion batteries. Nanomater. Nanotechnol. 2017, 7, 1847980417711084. [Google Scholar] [CrossRef] [Green Version]
- Geng, S.J.; Zhu, J.H. Promising alloys for intermediate-temperature solid oxide fuel cell interconnect application. J. Power Sources 2006, 160, 1009–1016. [Google Scholar] [CrossRef]
- Fu, M.; Zhu, Z.; Zhang, Z.; Zhuang, Q.; Chen, W.; Liu, Q. Microwave deposition synthesis of Ni(OH)2/sorghum stalk biomass carbon electrode materials for supercapacitors. J. Alloys Compd. 2019, 782, 952–960. [Google Scholar] [CrossRef]
- Shafi, P.M.; Bose, A.C. Impact of crystalline defects and size on X-ray line broadening: A phenomenological approach for tetragonal SnO2 nanocrystals. AIP Adv. 2015, 5, 057137. [Google Scholar] [CrossRef]
- Xu, H.; Hai, Z.; Diwu, J.; Zhang, Q.; Gao, L.; Cui, D.; Zang, J.; Liu, J.; Xue, C. Synthesis and microwave absorption properties of core-shell structured Co3O4-PANI nanocomposites. J. Nanomater. 2015, 2015, 845983. [Google Scholar] [CrossRef] [Green Version]
- Bhargava, R.; Khana, S.; Ahmad, N.; Ansari, M.M.N. Investigation of structural, optical and electrical properties of Co3O4 nanoparticles. AIP Conf. Proc. 2018, 1953, 030034. [Google Scholar] [CrossRef]
- Zhang, K.; Lee, T.H.; Cha, J.H.; Jang, H.W.; Choi, J.-W.; Mahmoudi, M.; Shokouhimehr, M. Metal-organic framework-derived metal oxide nanoparticles@reduced graphene oxide composites as cathode materials for rechargeable aluminium-ion batteries. Sci. Rep. 2019, 9, 13739. [Google Scholar] [CrossRef]
- Yue, X.M.; Liu, Z.J.; Xiao, C.-C.; Ye, M.; Ge, Z.-P.; Peng, C.; Gu, Z.-Y.; Zhu, J.-S.; Zhang, S.-Q. Synthesis of Co3O4/reduced graphene oxide by one step-hydrothermal and calcination method for high-performance supercapacitors. Ionics 2021, 27, 339–349. [Google Scholar] [CrossRef]
- Anamika, S.; Himanshu, G.; Dehiya, B.S. Synthesis and microstructural characterization of pure cobalt ferrite for D.C. Electrical study. J. Mater. Sci. Mechan. Eng. 2017, 4, 136–141. [Google Scholar]
- Ravindra, A.V.; Padhan, P.; Prellier, W. Electronic structure and optical band gap of CoFe2O4 thin films. Appl. Phys. Lett. 2012, 101, 161902. [Google Scholar] [CrossRef] [Green Version]
- Khalil, L.; Eid, C.; Bechelany, M.; Abboud, N.; Khoury, A.; Miele, P. Design of CoFe2O4/Co3O4 nanofibers with tunable morphology by Electrospinning. Mater. Lett. 2015, 140, 27–30. [Google Scholar] [CrossRef]
- Bo, W.; Songmei, L.; Jianhua, L.; Mei, Y.; Bin, L.; Xiaoyu, W. An efficient route to a hierarchical CoFe2O4@graphene hybrid films with superior cycling stability and rate capability for lithium storage. Electrochim. Acta 2014, 146, 679–687. [Google Scholar] [CrossRef]
- Quadakkers, W.J.; Piron-Abellan, J.; Shemet, V.; Singheiser, L. Metallic interconnectors for solid oxide fuel cells–A review. Mater. High Temp. 2003, 20, 115–127. [Google Scholar] [CrossRef]
- Niveditha, C.V.; Aswini, R.; Jabeen, F.M.J.; Ramanarayan, R.; Pullanjiyot, N.; Swaminathan, S. Feather like highly active Co3O4 electrode for supercapacitor application: A potentiodynamic approach. Mater. Res. Exp. 2018, 5, 065501. [Google Scholar] [CrossRef]
- Hafeez, H.Y.; Lakhera, S.K.; Narayanan, N.; Harish, S.; Hayakawa, Y.; Lee, B.K.; Neppolian, B. Environmentally sustainable synthesis of a CoFe2O4–TiO2/rGO ternary photocatalyst: A highly efficient and stable photocatalyst for high production of hydrogen (solar fuel). ACS Omega 2019, 4, 880–891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Wang, G.; Wang, L.; Feng, S.; Fang, D.; Xu, W.; Wei, H.; Qi, L.; Ren, W. Novel urea-assisted hydrothermal synthesis of tetrametallic Co6Fe4Mo12Bi1.5Ox phase for the selective oxidation of tert-butyl alcohol to methacrolein. Catal. Commun. 2019, 130, 105762. [Google Scholar] [CrossRef]
- Tsoncheva, T.; Ivanova, R.; Henych, J.; Dimitrov, M.; Kormunda, M.; Kovacheva, D.; Scotti, N.; Santo, V.D.; Štengl, V. Effect of preparation procedure on the formation of nanostructured ceria–zirconia mixed oxide catalysts for ethyl acetate oxidation: Homogeneous precipitation with urea vs template-assisted hydrothermal synthesis. Appl. Catal. A Gen. 2015, 502, 418–432. [Google Scholar] [CrossRef]
- Pallavolu, M.R.; Banerjee, A.N.; Nallapureddy, R.R.; Joo, S.W. Urea-assisted hydrothermal synthesis of MnMoO4/MnCO3 hybrid electrochemical electrode and fabrication of high-performance asymmetric supercapacitor. J. Mater. Sci.Technol. 2022, 96, 332–344. [Google Scholar] [CrossRef]


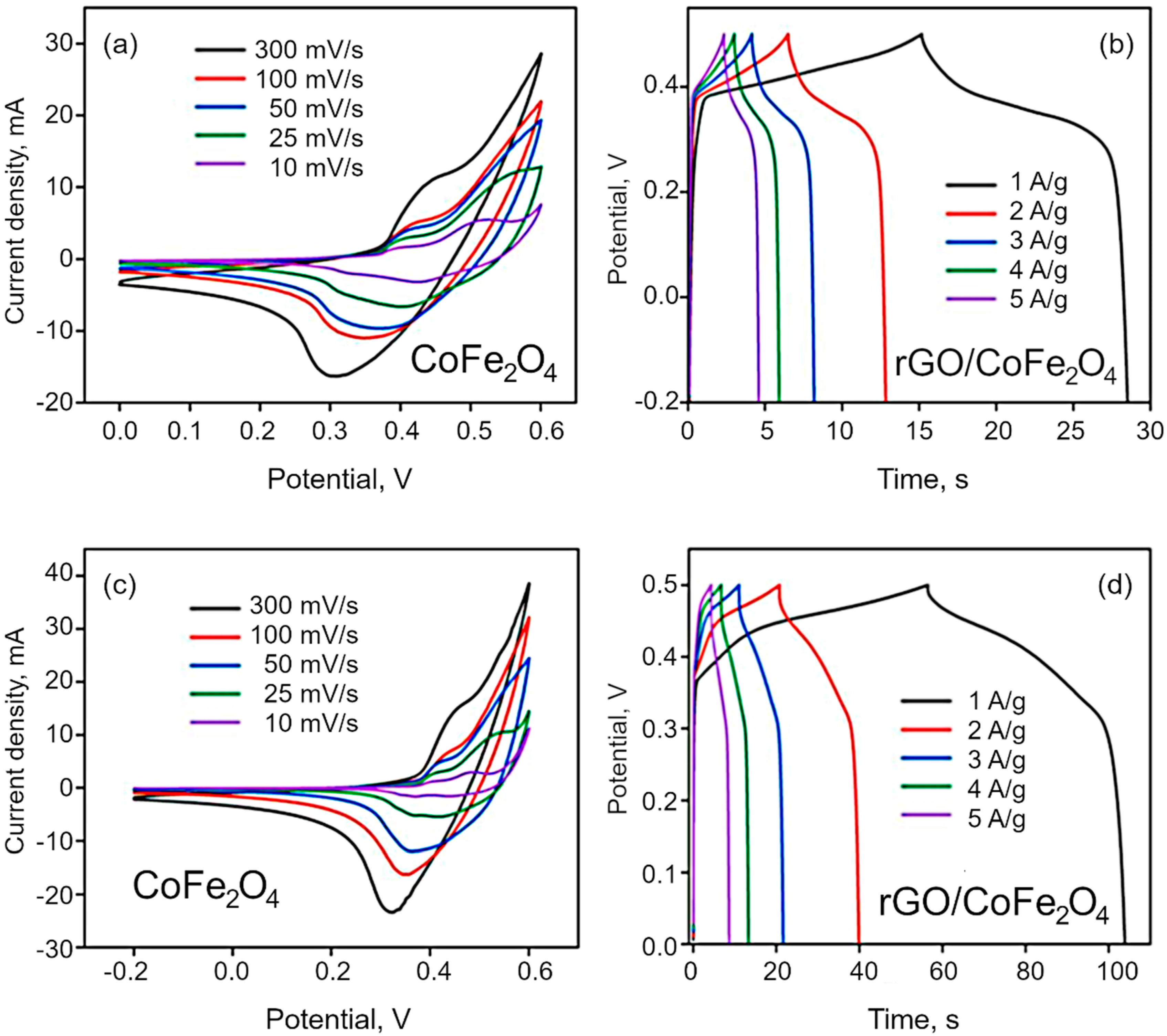
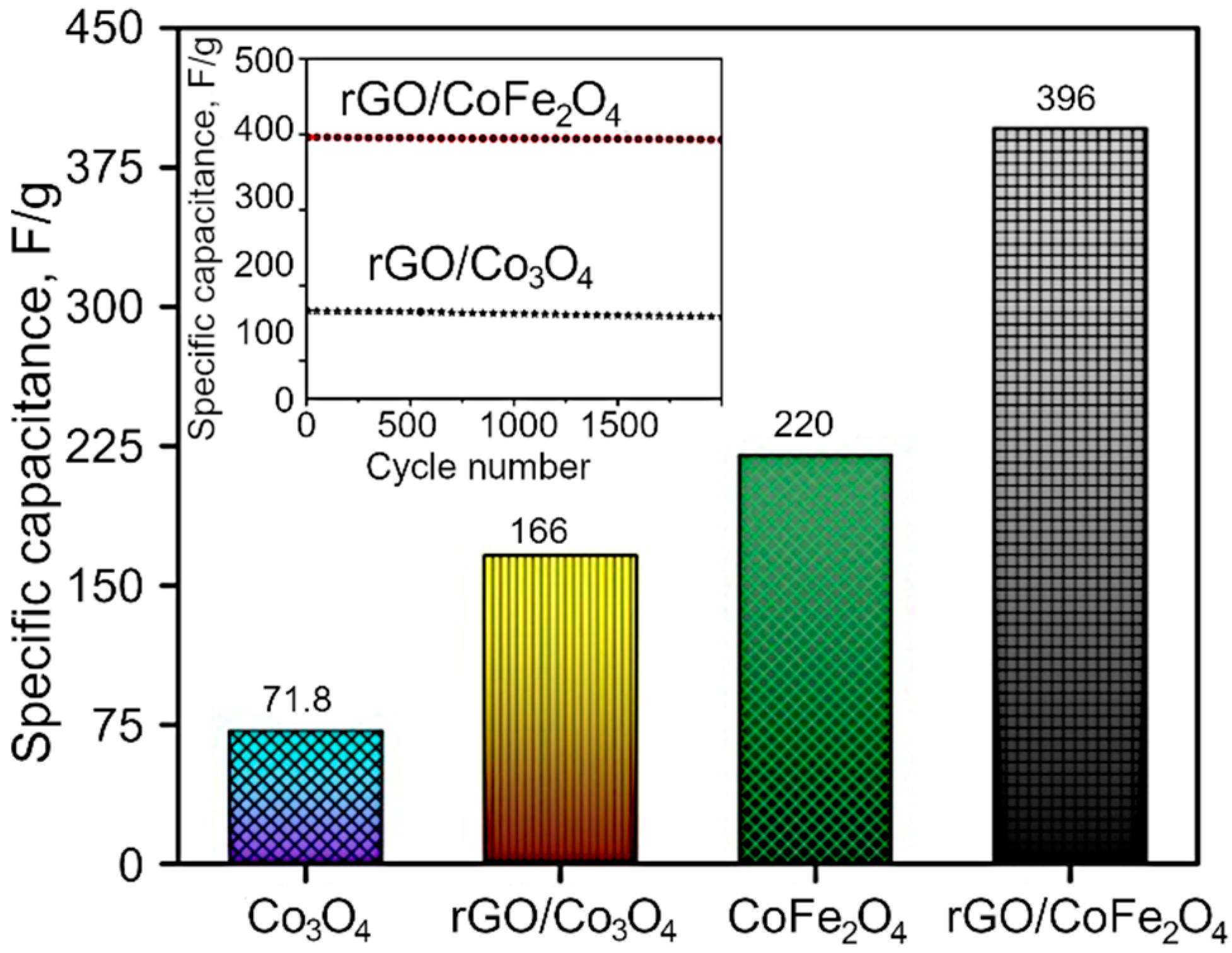

| Sample Type | Lattice Parameter Å | Scherrer Crystallite Size D, nm | WH Crystallite Size D, nm | Strain ε, % |
|---|---|---|---|---|
| Co3O4 | 8.01 | 1.5854 | 4.7 ± 0.009 | 3 |
| rGO/Co3O4 | 8.07 | 1.115 | 11.6 ± 0.002 | 2 |
| CoFe2O4 | 5.83 | 1.601 | 9.2 ± 0.002 | 0 |
| rGO/CoFe2O4 | 5.85 | 1.315 | 8.7 ± 0.002 | 0.4 |
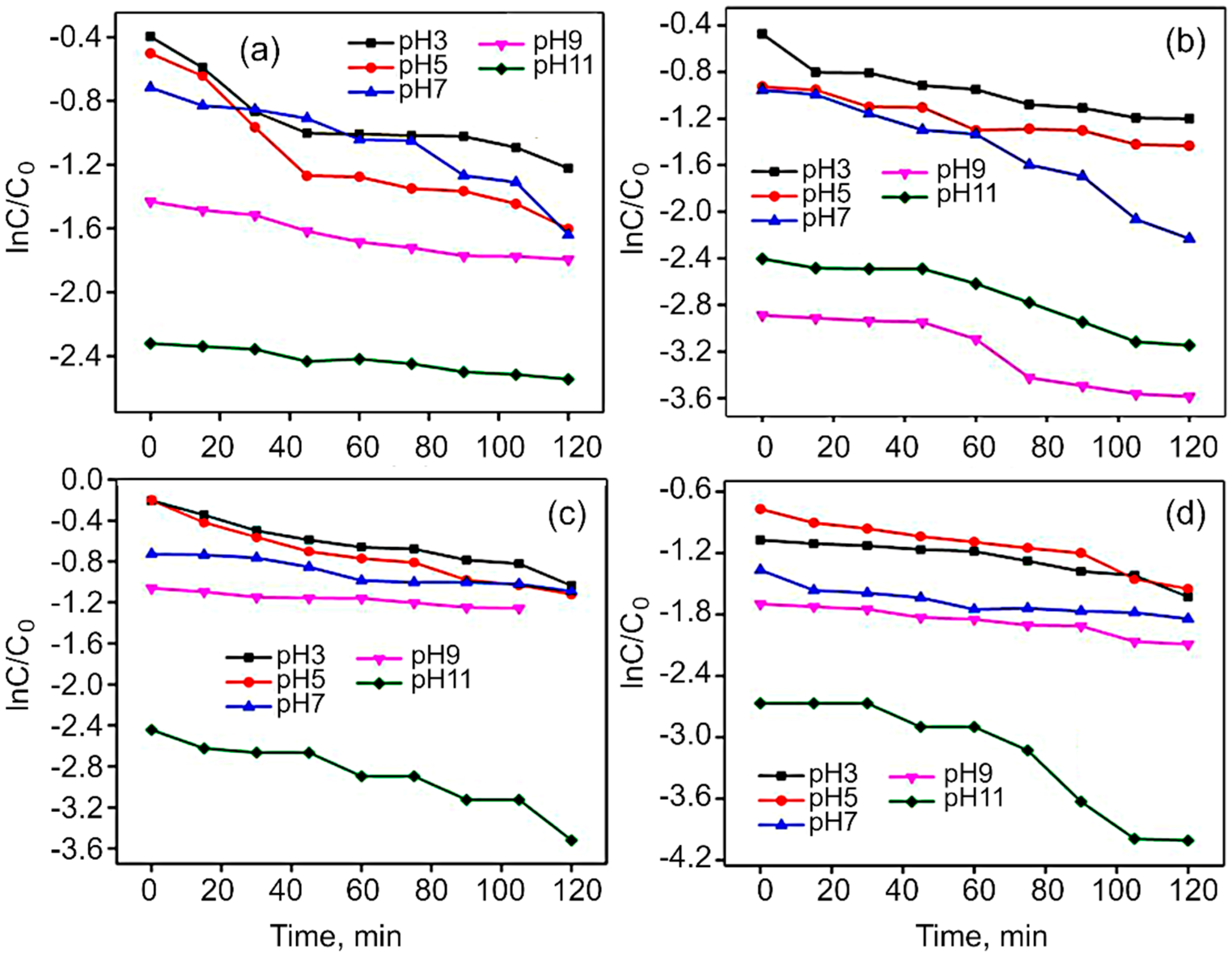
| Catalysts | Rate Constant (k), min−1 | Degradation Efficiency, % |
|---|---|---|
| Co3O4 | 0.00436 | 66.2 |
| rGO/Co3O4 | 0.00592 | 68.1 |
| CoFe2O4 | 0.00513 | 72.7 |
| rGO/CoFe2O4 | 0.00667 | 80.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramasamy, T.; Satheesh, L.G.; Selvaraj, V.; Bazaka, O.; Levchenko, I.; Bazaka, K.; Mandhakini, M. Spinel CoFe2O4 Nanoflakes: A Path to Enhance Energy Generation and Environmental Remediation Potential of Waste-Derived rGO. Nanomaterials 2022, 12, 3822. https://doi.org/10.3390/nano12213822
Ramasamy T, Satheesh LG, Selvaraj V, Bazaka O, Levchenko I, Bazaka K, Mandhakini M. Spinel CoFe2O4 Nanoflakes: A Path to Enhance Energy Generation and Environmental Remediation Potential of Waste-Derived rGO. Nanomaterials. 2022; 12(21):3822. https://doi.org/10.3390/nano12213822
Chicago/Turabian StyleRamasamy, Tamilselvi, Lekshmi Gopakumari Satheesh, Vaithilingam Selvaraj, Olha Bazaka, Igor Levchenko, Kateryna Bazaka, and Mohandas Mandhakini. 2022. "Spinel CoFe2O4 Nanoflakes: A Path to Enhance Energy Generation and Environmental Remediation Potential of Waste-Derived rGO" Nanomaterials 12, no. 21: 3822. https://doi.org/10.3390/nano12213822







