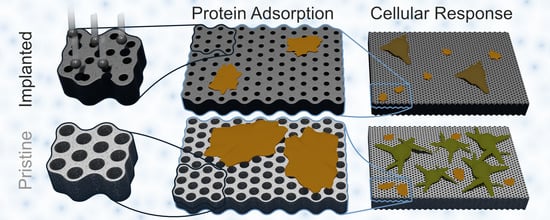
Laminin Adsorption and Adhesion of Neurons and Glial Cells on Carbon Implanted Titania Nanotube Scaffolds for Neural Implant Applications
Abstract
:1. Introduction
2. Materials and Methods
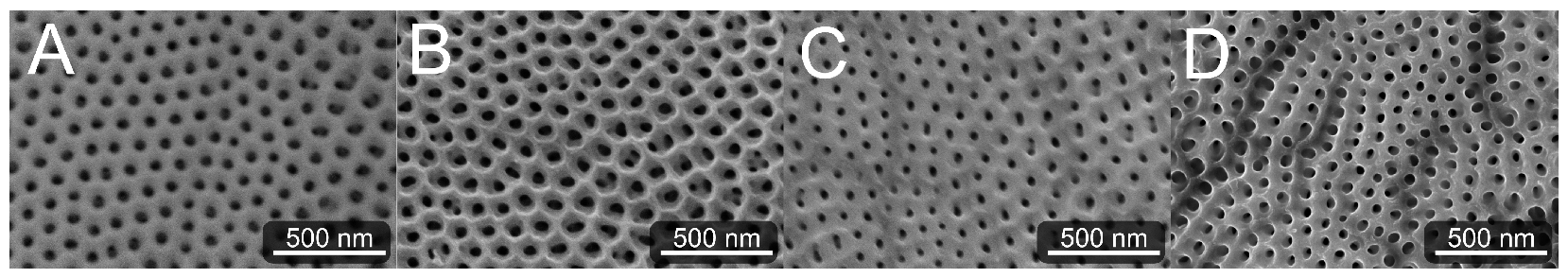
2.1. Titania Nanotube Scaffolds
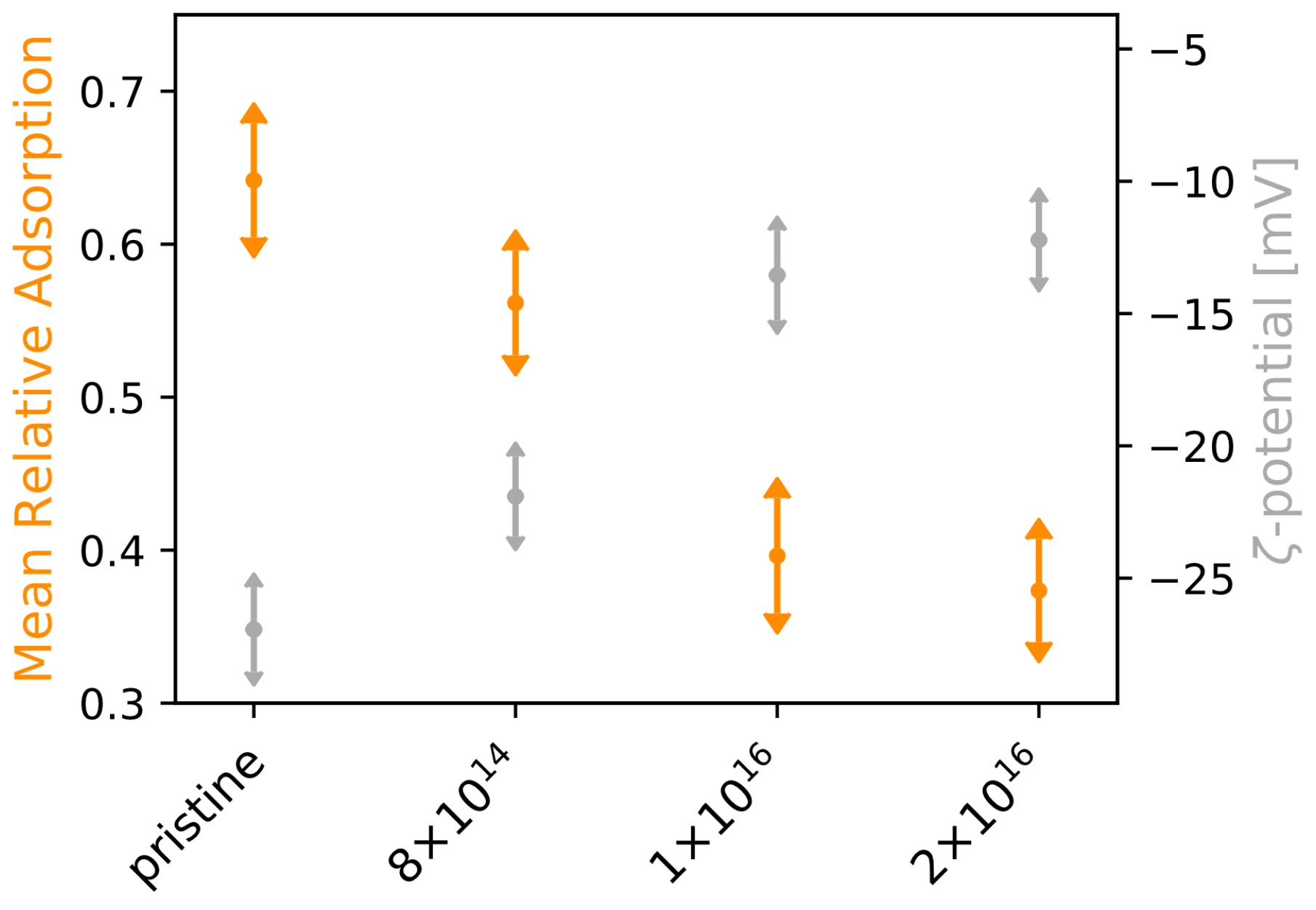
2.2. -Potential
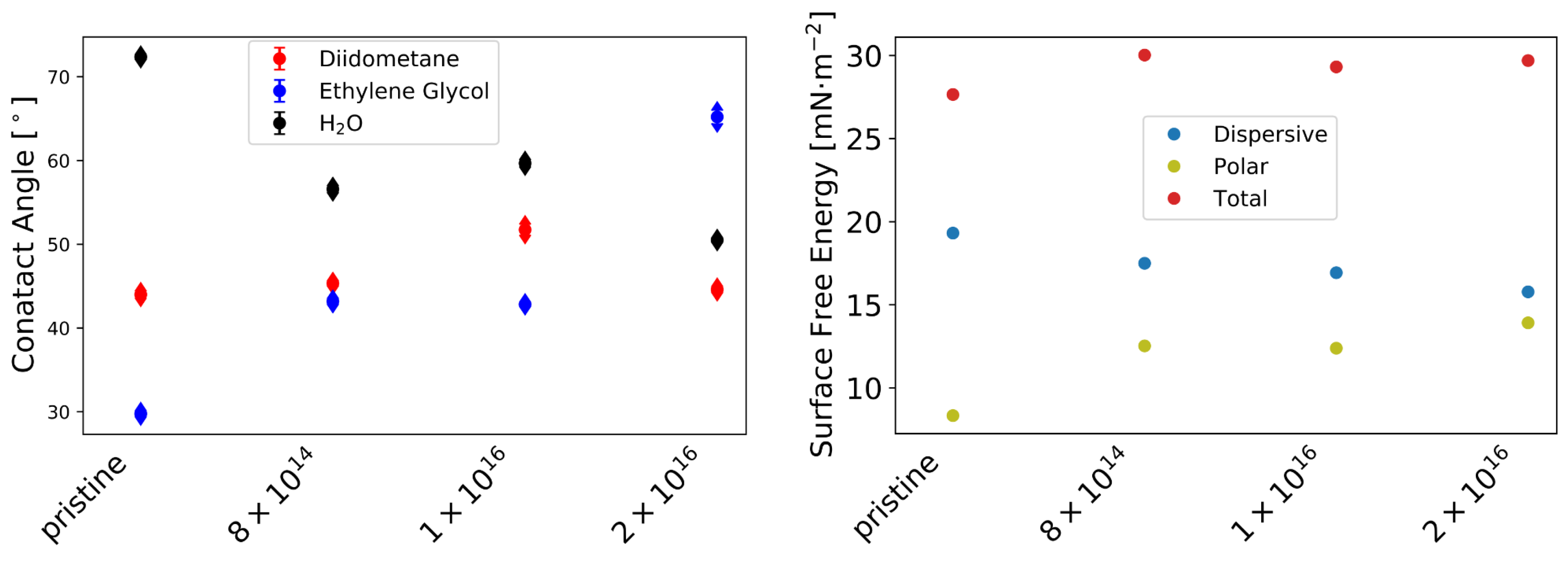
2.3. Surface Free Energy
2.4. Protein Adsorption
2.5. Cell Culture
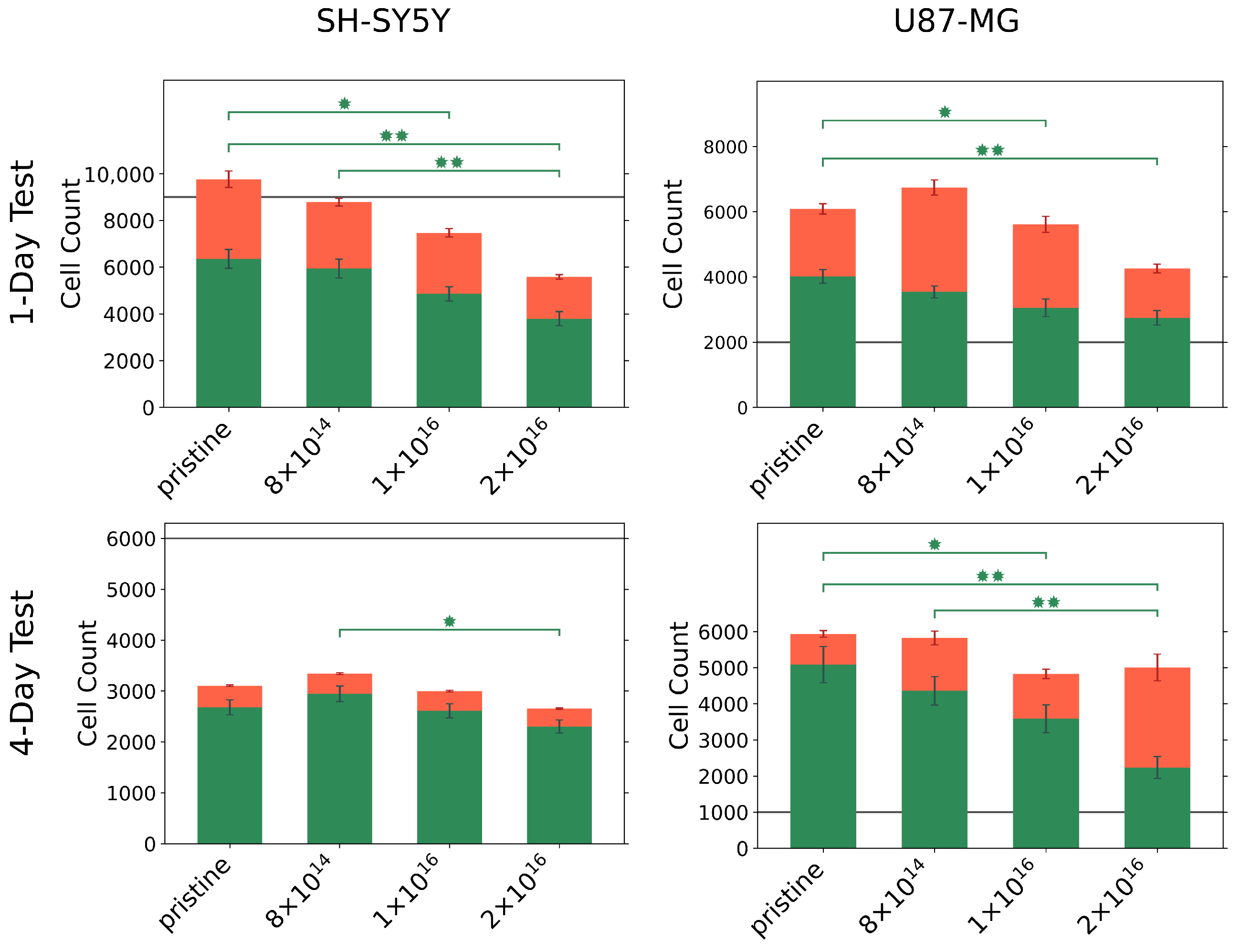
2.6. Cell Viability/Toxicity
2.7. Fluorescent Staining
2.8. Image Processing and Cell Detection
2.9. Atomic Force Microscope
2.10. Scanning Electron Microscope
2.11. Environmental Scanning Electron Microscope
2.12. Statistical Significance
3. Results
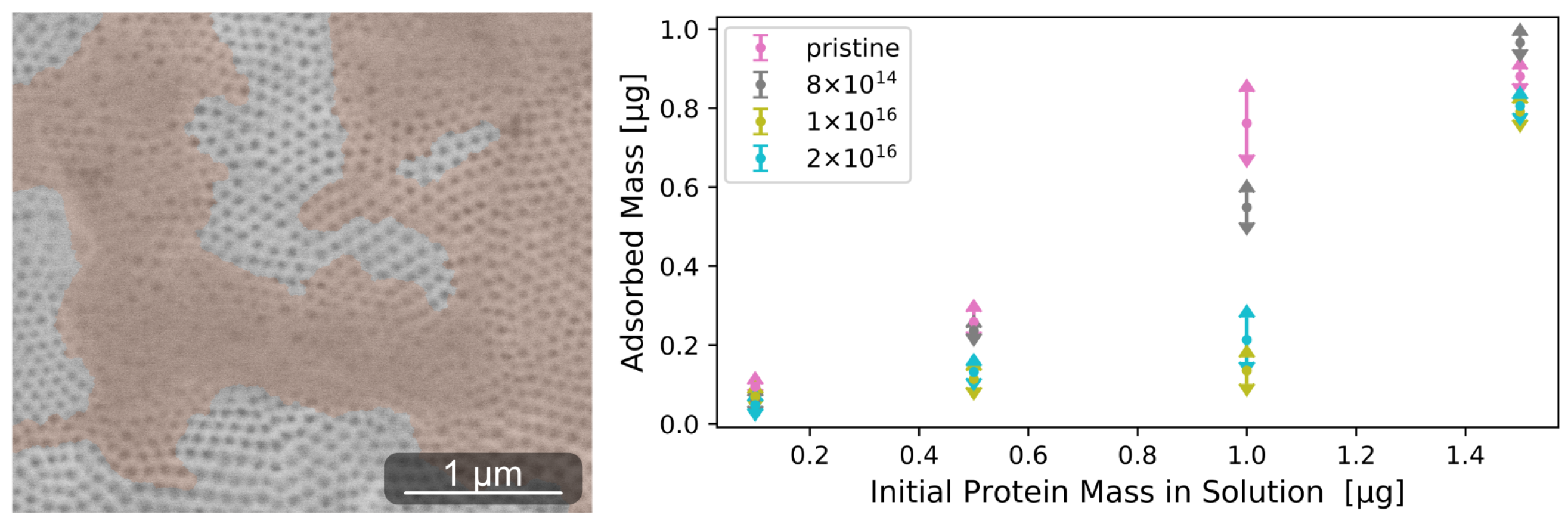
3.1. Protein Adsorption
3.2. Cell Viability and Cytotoxicity
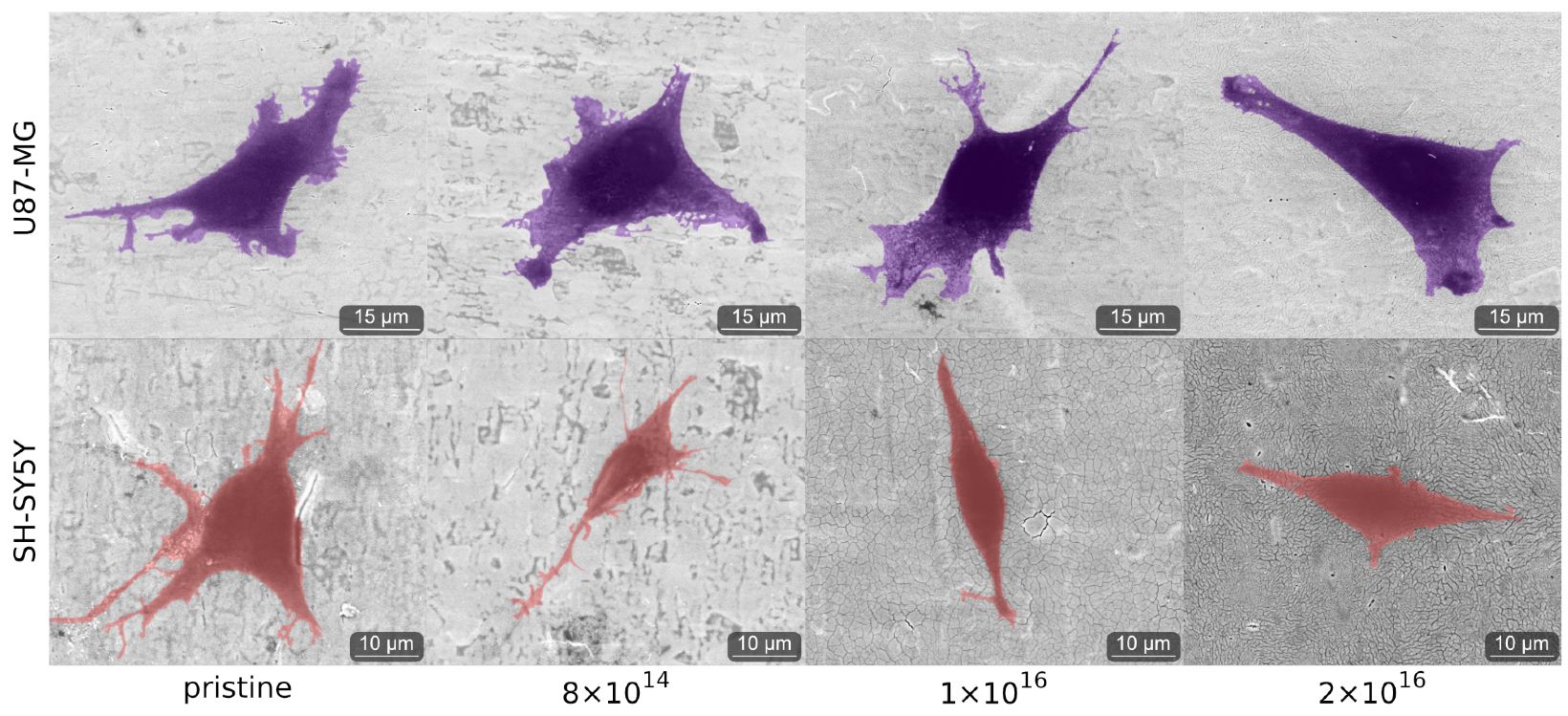
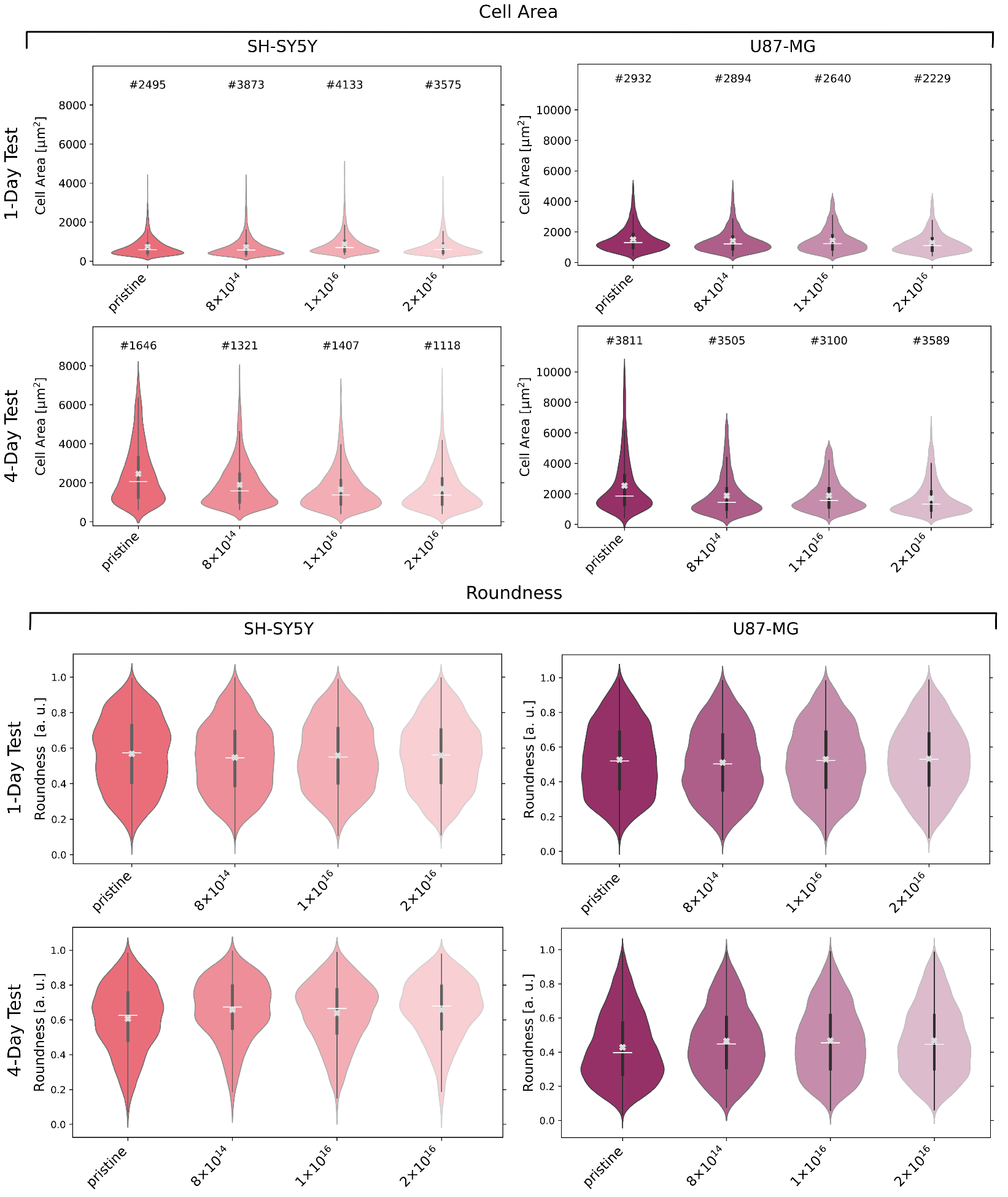
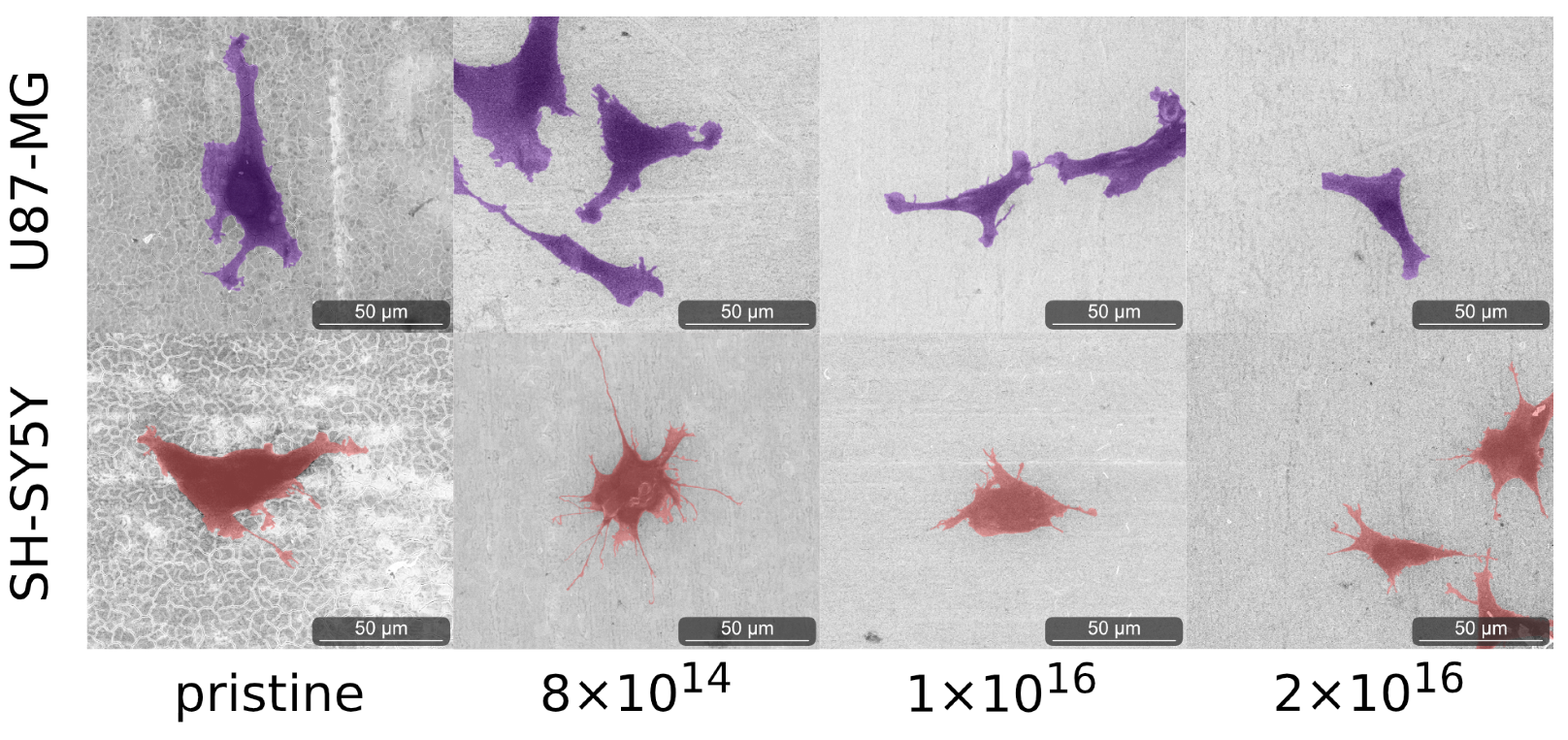
3.3. Cell Morphology
3.4. Cell Adhesion
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Results and Discussion
Appendix A.1. Surface Characterization
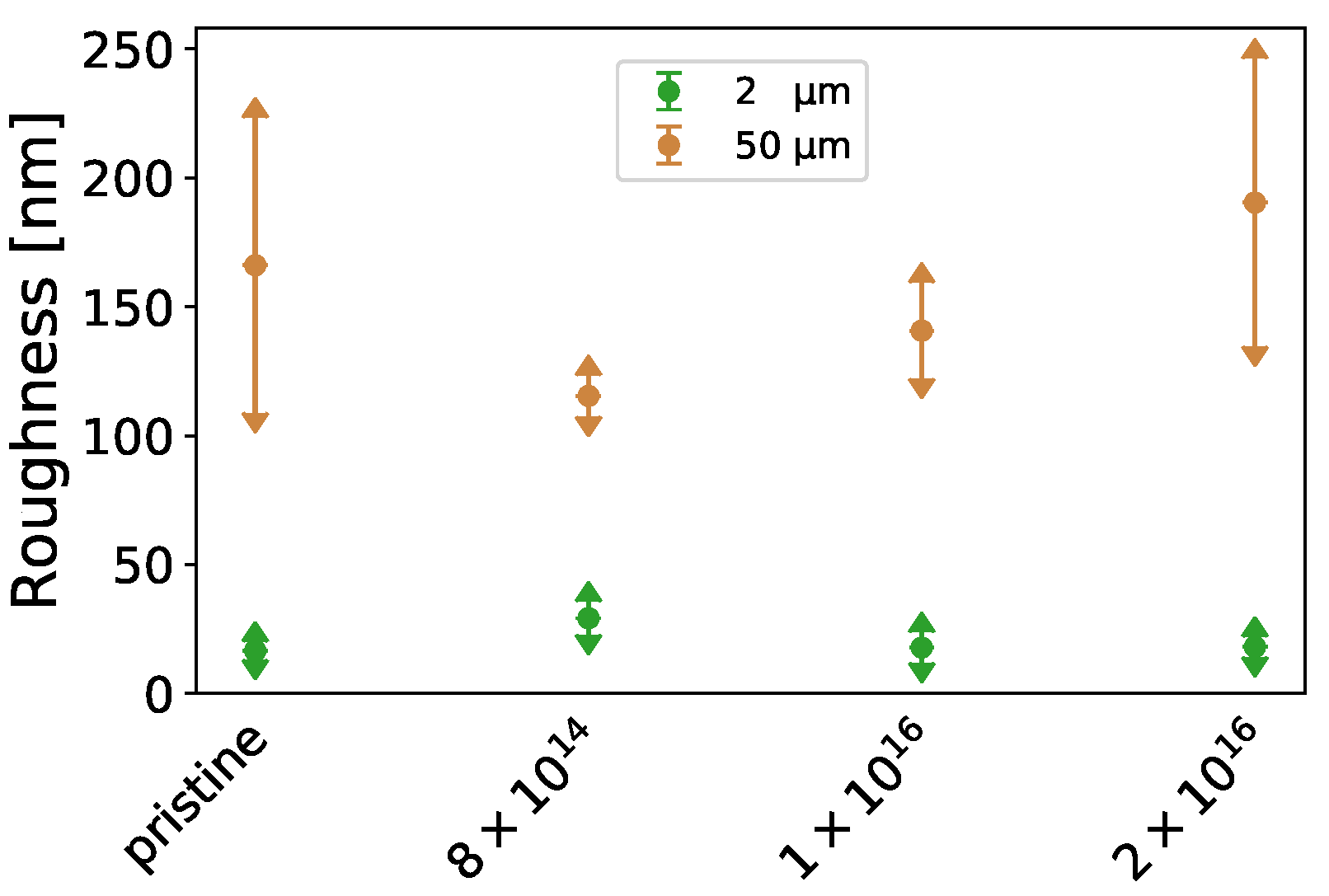
| Fluence [ions·cm] | -Potential [mV] |
|---|---|
| pristine | |
| 8 10 | |
| 1 10 | |
| 2 10 |
Appendix A.2. Protein Adsorption
Appendix A.3. Cell Adhesion
Appendix B. Sample Cleaning
References
- Dorsey, E.R.; Sherer, T.; Okun, M.S.; Bloem, B.R. The Emerging Evidence of the Parkinson Pandemic. J. Park. Dis. 2018, 8, S3–S8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roser, M.; Ortiz-Ospina, E.; Ritchie, H. Life Expectancy. Our World Data, 2013. Available online: https://ourworldindata.org/life-expectancy#citation(accessed on 1 September 2022).
- Kumar, A.; Bagchi, D. (Eds.) Antioxidants and Functional Foods for Neurodegenerative Disorders: Uses in Prevention and Therapy, 1st ed.; CRC Press: Boca Raton, FL, USA, 2021. [Google Scholar] [CrossRef]
- Lee, W.; Someya, T. Emerging Trends in Flexible Active Multielectrode Arrays. Chem. Mater. 2019, 31, 6347–6358. [Google Scholar] [CrossRef]
- Hong, N.; Nam, Y. Neurons-on-a-Chip: In Vitro NeuroTools. Mol. Cells 2022, 45, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Susloparova, A.; Halliez, S.; Begard, S.; Colin, M.; Buée, L.; Pecqueur, S.; Alibart, F.; Thomy, V.; Arscott, S.; Pallecchi, E.; et al. Low impedance and highly transparent microelectrode arrays (MEA) for in vitro neuron electrical activity probing. Sens. Actuators B Chem. 2021, 327, 128895. [Google Scholar] [CrossRef]
- Craighead, H. Future lab-on-a-chip technologies for interrogating individual molecules. Nature 2006, 442, 387–393. [Google Scholar] [CrossRef]
- Ferguson, M.; Sharma, D.; Ross, D.; Zhao, F. A Critical Review of Microelectrode Arrays and Strategies for Improving Neural Interfaces. Adv. Healthc. Mater. 2019, 8, e1900558. [Google Scholar] [CrossRef]
- Lozano, A.M.; Lipsman, N.; Bergman, H.; Brown, P.; Chabardes, S.; Chang, J.W.; Matthews, K.; McIntyre, C.C.; Schlaepfer, T.E.; Schulder, M.; et al. Deep brain stimulation: Current challenges and future directions. Nat. Rev. Neurol. 2019, 15, 148–160. [Google Scholar] [CrossRef]
- Salman, M.M.; Al-Obaidi, Z.; Kitchen, P.; Loreto, A.; Bill, R.M.; Wade-Martins, R. Advances in Applying Computer-Aided Drug Design for Neurodegenerative Diseases. Int. J. Mol. Sci. 2021, 22, 4688. [Google Scholar] [CrossRef]
- Wang, M.; Mi, G.; Shi, D.; Bassous, N.; Hickey, D.; Webster, T.J. Nanotechnology and Nanomaterials for Improving Neural Interfaces. Adv. Funct. Mater. 2018, 28, 1700905. [Google Scholar] [CrossRef]
- Dong, R.; Liu, X.; Cheng, S.; Tang, L.; Chen, M.; Zhong, L.; Chen, Z.; Liu, S.; Jiang, X. Highly Stretchable Metal-Polymer Conductor Electrode Array for Electrophysiology. Adv. Healthc. Mater. 2021, 10, e2000641. [Google Scholar] [CrossRef]
- Colachis, S.C.; Dunlap, C.F.; Annetta, N.V.; Tamrakar, S.M.; Bockbrader, M.A.; Friedenberg, D.A. Long-term intracortical microelectrode array performance in a human: A 5 year retrospective analysis. J. Neural Eng. 2021, 18, 0460d7. [Google Scholar] [CrossRef] [PubMed]
- Kolaya, E.; Firestein, B.L. Deep brain stimulation: Challenges at the tissue-electrode interface and current solutions. Biotechnol. Prog. 2021, 37, e3179. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Shin, H.; Lee, D.; Choi, S.; Cho, I.J.; Seo, J. A Lubricated Nonimmunogenic Neural Probe for Acute Insertion Trauma Minimization and Long-Term Signal Recording. Adv. Sci. 2021, 8, e2100231. [Google Scholar] [CrossRef] [PubMed]
- Branner, A.; Stein, R.B.; Fernandez, E.; Aoyagi, Y.; Normann, R.A. Long-term stimulation and recording with a penetrating microelectrode array in cat sciatic nerve. IEEE Trans.-Bio-Med. Eng. 2004, 51, 146–157. [Google Scholar] [CrossRef]
- Limousin, P.; Foltynie, T. Long-term outcomes of deep brain stimulation in Parkinson disease. Nat. Rev. Neurol. 2019, 15, 234–242. [Google Scholar] [CrossRef] [Green Version]
- Cogan, S.F. Neural stimulation and recording electrodes. Annu. Rev. Biomed. Eng. 2008, 10, 275–309. [Google Scholar] [CrossRef] [Green Version]
- Merrill, E.G.; Ainsworth, A. Glass-coated platinum-plated tungsten microelectrodes. Med. Biol. Eng. 1972, 10, 662–672. [Google Scholar] [CrossRef]
- Chatard, C.; Meiller, A.; Marinesco, S. Microelectrode Biosensors for in vivo Analysis of Brain Interstitial Fluid. Electroanalysis 2018, 30, 977–998. [Google Scholar] [CrossRef]
- Reader, T.A. A simplified method of preparing and filling multibarreled glass microelectrodes. Brain Res. Bull. 1978, 3, 719–720. [Google Scholar] [CrossRef]
- Kennedy, P.R. The cone electrode: A long-term electrode that records from neurites grown onto its recording surface. J. Neurosci. Methods 1989, 29, 181–193. [Google Scholar] [CrossRef]
- Chen, B.; Zhang, B.; Chen, C.; Hu, J.; Qi, J.; He, T.; Tian, P.; Zhang, X.; Ni, G.; Cheng, M.M.C. Penetrating glassy carbon neural electrode arrays for brain-machine interfaces. Biomed. Microdevices 2020, 22, 43. [Google Scholar] [CrossRef]
- Bennett, C.; Samikkannu, M.; Mohammed, F.; Dietrich, W.D.; Rajguru, S.M.; Prasad, A. Blood brain barrier (BBB)-disruption in intracortical silicon microelectrode implants. Biomaterials 2018, 164, 1–10. [Google Scholar] [CrossRef]
- Chatard, C.; Sabac, A.; Moreno-Velasquez, L.; Meiller, A.; Marinesco, S. Minimally Invasive Microelectrode Biosensors Based on Platinized Carbon Fibers for in Vivo Brain Monitoring. ACS Cent. Sci. 2018, 4, 1751–1760. [Google Scholar] [CrossRef]
- Fiáth, R.; Raducanu, B.C.; Musa, S.; Andrei, A.; Lopez, C.M.; van Hoof, C.; Ruther, P.; Aarts, A.; Horváth, D.; Ulbert, I. A silicon-based neural probe with densely-packed low-impedance titanium nitride microelectrodes for ultrahigh-resolution in vivo recordings. Biosens. Bioelectron. 2018, 106, 86–92. [Google Scholar] [CrossRef] [Green Version]
- Xu, S.; Deng, Y.; Luo, J.; He, E.; Liu, Y.; Zhang, K.; Yang, Y.; Xu, S.; Sha, L.; Song, Y.; et al. High-Throughput PEDOT:PSS/PtNPs-Modified Microelectrode Array for Simultaneous Recording and Stimulation of Hippocampal Neuronal Networks in Gradual Learning Process. ACS Appl. Mater. Interfaces 2022, 14, 15736–15746. [Google Scholar] [CrossRef]
- Kim, S.; Jang, L.K.; Jang, M.; Lee, S.; Hardy, J.G.; Lee, J.Y. Electrically Conductive Polydopamine-Polypyrrole as High Performance Biomaterials for Cell Stimulation in Vitro and Electrical Signal Recording in Vivo. ACS Appl. Mater. Interfaces 2018, 10, 33032–33042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aydın, M.; Aydın, E.B.; Sezgintürk, M.K. A Highly Selective Poly(thiophene)-graft-Poly(methacrylamide) Polymer Modified ITO Electrode for Neuron Specific Enolase Detection in Human Serum. Macromol. Biosci. 2019, 19, e1900109. [Google Scholar] [CrossRef] [PubMed]
- Renz, A.F.; Reichmuth, A.M.; Stauffer, F.; Thompson-Steckel, G.; Vörös, J. A guide towards long-term functional electrodes interfacing neuronal tissue. J. Neural Eng. 2018, 15, 061001. [Google Scholar] [CrossRef]
- Rossetti, N.; Hagler, J.; Kateb, P.; Cicoira, F. Neural and electromyography PEDOT electrodes for invasive stimulation and recording. J. Mater. Chem. C 2021, 9, 7243–7263. [Google Scholar] [CrossRef]
- Silvaragi, T.G.B.; Vigneswari, S.; Murugaiyah, V.; Al-Ashraf, A.; Ramakrishna, S. Exploring polymeric biomaterials in developing neural prostheses. J. Bioact. Compat. Polym. 2022, 37, 75–84. [Google Scholar] [CrossRef]
- Barberi, J.; Spriano, S. Titanium and Protein Adsorption: An Overview of Mechanisms and Effects of Surface Features. Materials 2021, 14, 1590. [Google Scholar] [CrossRef] [PubMed]
- Sidambe, A.T. Biocompatibility of Advanced Manufactured Titanium Implants-A Review. Materials 2014, 7, 8168–8188. [Google Scholar] [CrossRef] [Green Version]
- Pałka, K.; Pokrowiecki, R. Porous Titanium Implants: A Review. Adv. Eng. Mater. 2018, 20, 1700648. [Google Scholar] [CrossRef]
- Markowska-Szczupak, A.; Endo-Kimura, M.; Paszkiewicz, O.; Kowalska, E. Are Titania Photocatalysts and Titanium Implants Safe? Review on the Toxicity of Titanium Compounds. Nanomaterials 2020, 10, 2065. [Google Scholar] [CrossRef]
- Weidt, A.; Mayr, S.G.; Zink, M. Influence of Topological Cues on Fibronectin Adsorption and Contact Guidance of Fibroblasts on Microgrooved Titanium. ACS Appl. Bio Mater. 2019, 2, 1066–1077. [Google Scholar] [CrossRef]
- Wei, Y.; Ma, Y.; Chen, J.; Yang, X.; Ni, S.; Hong, F.; Ni, S. Novel ordered TiO2 nanodot array on 316LSS with enhanced antibacterial properties. Mater. Lett. 2020, 266, 127503. [Google Scholar] [CrossRef]
- Guo, T.; Oztug, N.A.K.; Han, P.; Ivanovski, S.; Gulati, K. Old is Gold: Electrolyte Aging Influences the Topography, Chemistry, and Bioactivity of Anodized TiO2 Nanopores. ACS Appl. Mater. Interfaces 2021, 13, 7897–7912. [Google Scholar] [CrossRef]
- Fu, Y.; Mo, A. A Review on the Electrochemically Self-organized Titania Nanotube Arrays: Synthesis, Modifications, and Biomedical Applications. Nanoscale Res. Lett. 2018, 13, 187. [Google Scholar] [CrossRef]
- Dvorak, F.; Zazpe, R.; Krbal, M.; Sopha, H.; Prikryl, J.; Ng, S.; Hromadko, L.; Bures, F.; Macak, J.M. One-dimensional anodic TiO2 nanotubes coated by atomic layer deposition: Towards advanced applications. Appl. Mater. Today 2019, 14, 1–20. [Google Scholar] [CrossRef]
- Tsuchiya, H.; Schmuki, P. Less known facts and findings about TiO2 nanotubes. Nanoscale 2020, 12, 8119–8132. [Google Scholar] [CrossRef]
- Ozkan, S.; Mazare, A.; Schmuki, P. Critical parameters and factors in the formation of spaced TiO2 nanotubes by self-organizing anodization. Electrochim. Acta 2018, 268, 435–447. [Google Scholar] [CrossRef]
- Mohan, L.; Dennis, C.; Padmapriya, N.; Anandan, C.; Rajendran, N. Effect of Electrolyte Temperature and Anodization Time on Formation of TiO2 Nanotubes for Biomedical Applications. Mater. Today Commun. 2020, 23, 101103. [Google Scholar] [CrossRef]
- Dallacasagrande, V.; Zink, M.; Huth, S.; Jakob, A.; Müller, M.; Reichenbach, A.; Käs, J.A.; Mayr, S.G. Tailoring substrates for long-term organotypic culture of adult neuronal tissue. Adv. Mater. 2012, 24, 2399–2403. [Google Scholar] [CrossRef] [PubMed]
- Kallendrusch, S.; Merz, F.; Bechmann, I.; Mayr, S.G.; Zink, M. Long-Term Tissue Culture of Adult Brain and Spleen Slices on Nanostructured Scaffolds. Adv. Healthc. Mater. 2017, 6, 1601336. [Google Scholar] [CrossRef] [PubMed]
- Nah, Y.C.; Paramasivam, I.; Schmuki, P. Doped TiO2 and TiO2 nanotubes: Synthesis and applications. Chemphyschem A Eur. J. Chem. Phys. Phys. Chem. 2010, 11, 2698–2713. [Google Scholar] [CrossRef] [PubMed]
- Nycz, M.; Arkusz, K.; Pijanowska, D.G. Influence of the Silver Nanoparticles (AgNPs) Formation Conditions onto Titanium Dioxide (TiO2) Nanotubes Based Electrodes on Their Impedimetric Response. Nanomaterials 2019, 9, 1072. [Google Scholar] [CrossRef] [Green Version]
- Kupferer, A.; Holm, A.; Lotnyk, A.; Mändl, S.; Mayr, S.G. Compositional Patterning in Carbon Implanted Titania Nanotubes. Adv. Funct. Mater. 2021, 31, 2104250. [Google Scholar] [CrossRef]
- Kupferer, A.; Mändl, S.; Mayr, S.G. Tailoring morphology in titania nanotube arrays by implantation: Experiments and modelling on designed pore size—and beyond. Mater. Res. Lett. 2021, 9, 483–489. [Google Scholar] [CrossRef]
- Breite, D.; Went, M.; Prager, A.; Schulze, A. The critical zeta potential of polymer membranes: How electrolytes impact membrane fouling. RSC Adv. 2016, 6, 98180–98189. [Google Scholar] [CrossRef]
- German Institute for Standardisation Registered Association. Beuth Verlag DIN 55660-2:2011-12 Paints and varnishes-Wettability-Part 2: Determination of the Free Surface Energy of Solid Surfaces by Measuring the Contact Angle; Beuth Verlag: Berlin, Germany, 2011. [Google Scholar]
- Kluyver, T.; Ragan-Kelley, B.; Pérez, F.; Granger, B.; Bussonnier, M.; Frederic, J.; Kelley, K.; Hamrick, J.; Grout, J.; Corlay, S.; et al. Jupyter Notebooks—A publishing format for reproducible computational workflows. Position. Power Acad. Publ. Play. Agents Agendas 2016, 87–90. [Google Scholar] [CrossRef]
- Virtanen, P.; Gommers, R.; Oliphant, T.E.; Haberland, M.; Reddy, T.; Cournapeau, D.; Burovski, E.; Peterson, P.; Weckesser, W.; Bright, J.; et al. SciPy 1.0: Fundamental Algorithms for Scientific Computing in Python. Nat. Methods 2020, 17, 261–272. [Google Scholar] [CrossRef] [Green Version]
- McKinney, W. Data structures for statistical computing in python. In Proceedings of the 9th Python in Science Conference, Austin, TX, USA, 28 June–3 July 2010; van der Walt, S., Millman, J., Eds.; Volume 445, pp. 51–56. [Google Scholar]
- Harris, C.R.; Millman, K.J.; Van Der Walt, S.J.; Gommers, R.; Virtanen, P.; Cournapeau, D.; Wieser, E.; Taylor, J.; Berg, S.; Smith, N.J.; et al. Array programming with NumPy. Nature 2020, 585, 357–362. [Google Scholar] [CrossRef]
- Hunter, J.D. Matplotlib: A 2D graphics environment. Comput. Sci. Eng. 2007, 9, 90–95. [Google Scholar] [CrossRef]
- Waskom, M.; Botvinnik, O.; O’Kane, D.; Hobson, P.; Lukauskas, S.; Gemperline, D.C.; Augspurger, T.; Halchenko, Y.; Cole, J.B.; Warmenhoven, J.; et al. mwaskom/seaborn: v0.8.1 (September 2017), 2017. Zenodo 2017, v0.8.1. [Google Scholar] [CrossRef]
- Nečas, D.; Klapetek, P. Gwyddion: An open-source software for SPM data analysis. Open Phys. 2012, 10, 181–188. [Google Scholar] [CrossRef]
- Seabold, S.; Perktold, J. Statsmodels: Econometric and Statistical Modeling with Python. In Proceedings of the 9th Python in Science Conference, Austin, TX, USA, 28 June–3 July 2010; pp. 92–96. [Google Scholar] [CrossRef] [Green Version]
- Kupferer, A.; Mensing, M.; Lehnert, J.; Mändl, S.; Mayr, S.G. Carbon and Neon Ion Bombardment Induced Smoothing and Surface Relaxation of Titania Nanotubes. Nanomaterials 2021, 11, 2458. [Google Scholar] [CrossRef]
- Akpek, A. Analysis of Surface Properties of Ag and Ti Ion-Treated Medical Textiles by Metal Vapor Vacuum Arc Ion Implantation. Coatings 2021, 11, 102. [Google Scholar] [CrossRef]
- Vishnu, J.; Manivasagam, G. Surface Modification and Biological Approaches for Tackling Titanium Wear-Induced Aseptic Loosening. J. Bio-Tribo-Corros. 2021, 7, 1–19. [Google Scholar] [CrossRef]
- Wang, Q.; Wu, L.; Liu, S.; Cao, P.; Yang, J.; Wang, L. Nanostructured Titanium Alloys Surface Modification Technology for Antibacterial and Osteogenic Properties. Curr. Nanosci. 2021, 17, 175–193. [Google Scholar] [CrossRef]
- Giamblanco, N.; Martines, E.; Marletta, G. Laminin adsorption on nanostructures: Switching the molecular orientation by local curvature changes. Langmuir 2013, 29, 8335–8342. [Google Scholar] [CrossRef]
- Lin, J.H.; Chang, H.Y.; Kao, W.L.; Lin, K.Y.; Liao, H.Y.; You, Y.W.; Kuo, Y.T.; Kuo, D.Y.; Chu, K.J.; Chu, Y.H.; et al. Effect of surface potential on extracellular matrix protein adsorption. Langmuir ACS J. Surfaces Colloids 2014, 30, 10328–10335. [Google Scholar] [CrossRef] [PubMed]
- Mulheran, P.A.; Pellenc, D.; Bennett, R.A.; Green, R.J.; Sperrin, M. Mechanisms and dynamics of protein clustering on a solid surface. Phys. Rev. Lett. 2008, 100, 068102. [Google Scholar] [CrossRef] [PubMed]
- Rabe, M.; Verdes, D.; Seeger, S. Surface-induced spreading phenomenon of protein clusters. Soft Matter 2009, 5, 1039. [Google Scholar] [CrossRef]
- Pellenc, D.; Bennett, R.A.; Green, R.J.; Sperrin, M.; Mulheran, P.A. New insights on growth mechanisms of protein clusters at surfaces: An AFM and simulation study. Langmuir 2008, 24, 9648–9655. [Google Scholar] [CrossRef] [PubMed]
- Onuma, K.; Kanzaki, N. Size Distribution and Intermolecular Interaction of Laminin-1 in Physiological Solutions. J. Phys. Chem. B 2003, 107, 11799–11804. [Google Scholar] [CrossRef]
- Pal, A.; Gope, A.; Athair, A.S.; Iannacchione, G.S. A comparative study of the drying evolution and dried morphology of two globular proteins in de-ionized water solutions. RSC Adv. 2020, 10, 16906–16916. [Google Scholar] [CrossRef]
- Tarafdar, S.; Tarasevich, Y.Y.; Dutta Choudhury, M.; Dutta, T.; Zang, D. Droplet Drying Patterns on Solid Substrates: From Hydrophilic to Superhydrophobic Contact to Levitating Drops. Adv. Condens. Matter Phys. 2018, 2018, 1–24. [Google Scholar] [CrossRef] [Green Version]
- Young, B.; Pitt, W.; Cooper, S. Protein adsorption on polymeric biomaterials I. Adsorption isotherms. J. Colloid Interface Sci. 1988, 124, 28–43. [Google Scholar] [CrossRef]
- Chen, J.; Cramer, S.M. Protein adsorption isotherm behavior in hydrophobic interaction chromatography. J. Chromatogr. A 2007, 1165, 67–77. [Google Scholar] [CrossRef]
- Fujita, H.; Kudo, T.A.; Kanetaka, H.; Miyazaki, T.; Hashimoto, M.; Kawashita, M. Adsorption of Laminin on Hydroxyapatite and Alumina and the MC3T3-E1 Cell Response. ACS Biomater. Sci. Eng. 2016, 2, 1162–1168. [Google Scholar] [CrossRef] [Green Version]
- Kulkarni, M.; Flašker, A.; Lokar, M.; Mrak-Poljšak, K.; Mazare, A.; Artenjak, A.; Čučnik, S.; Kralj, S.; Velikonja, A.; Schmuki, P.; et al. Binding of plasma proteins to titanium dioxide nanotubes with different diameters. Int. J. Nanomed. 2015, 10, 1359–1373. [Google Scholar] [CrossRef] [Green Version]
- Mayazur Rahman, S.; Reichenbach, A.; Zink, M.; Mayr, S.G. Mechanical spectroscopy of retina explants at the protein level employing nanostructured scaffolds. Soft Matter 2016, 12, 3431–3441. [Google Scholar] [CrossRef] [Green Version]
- Yoon, S.B.; Lee, G.; Park, S.B.; Cho, H.; Lee, J.O.; Koh, B. Properties of differentiated SH-SY5Y grown on carbon-based materials. RSC Adv. 2020, 10, 19382–19389. [Google Scholar] [CrossRef]
- Brunetti, V.; Maiorano, G.; Rizzello, L.; Sorce, B.; Sabella, S.; Cingolani, R.; Pompa, P.P. Neurons sense nanoscale roughness with nanometer sensitivity. Proc. Natl. Acad. Sci. USA 2010, 107, 6264–6269. [Google Scholar] [CrossRef] [Green Version]
- Abend, A.; Steele, C.; Schmidt, S.; Frank, R.; Jahnke, H.G.; Zink, M. Proliferation and Cluster Analysis of Neurons and Glial Cell Organization on Nanocolumnar TiN Substrates. Int. J. Mol. Sci. 2020, 21, 6249. [Google Scholar] [CrossRef]
- Hallab, N.J.; Bundy, K.J.; O’Connor, K.; Moses, R.L.; Jacobs, J.J. Evaluation of metallic and polymeric biomaterial surface energy and surface roughness characteristics for directed cell adhesion. Tissue Eng. 2001, 7, 55–71. [Google Scholar] [CrossRef] [Green Version]
- Tian, A.; Qin, X.; Wu, A.; Zhang, H.; Xu, Q.; Xing, D.; Yang, H.; Qiu, B.; Xue, X.; Zhang, D.; et al. Nanoscale TiO2 nanotubes govern the biological behavior of human glioma and osteosarcoma cells. Int. J. Nanomed. 2015, 10, 2423–2439. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Qin, X.; Tian, A.; Zhang, D.; Xue, X.; Wu, A. Nano size effects of TiO2 nanotube array on the glioma cells behavior. Int. J. Mol. Sci. 2012, 14, 244–254. [Google Scholar] [CrossRef]
- Choi, W.J.; Jung, J.; Lee, S.; Chung, Y.J.; Yang, C.S.; Lee, Y.K.; Lee, Y.S.; Park, J.K.; Ko, H.W.; Lee, J.O. Effects of substrate conductivity on cell morphogenesis and proliferation using tailored, atomic layer deposition-grown ZnO thin films. Sci. Rep. 2015, 5, 9974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abend, A.; Steele, C.; Jahnke, H.G.; Zink, M. Adhesion of Neurons and Glial Cells with Nanocolumnar TiN Films for Brain-Machine Interfaces. Int. J. Mol. Sci. 2021, 22, 8588. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frenzel, J.; Kupferer, A.; Zink, M.; Mayr, S.G. Laminin Adsorption and Adhesion of Neurons and Glial Cells on Carbon Implanted Titania Nanotube Scaffolds for Neural Implant Applications. Nanomaterials 2022, 12, 3858. https://doi.org/10.3390/nano12213858
Frenzel J, Kupferer A, Zink M, Mayr SG. Laminin Adsorption and Adhesion of Neurons and Glial Cells on Carbon Implanted Titania Nanotube Scaffolds for Neural Implant Applications. Nanomaterials. 2022; 12(21):3858. https://doi.org/10.3390/nano12213858
Chicago/Turabian StyleFrenzel, Jan, Astrid Kupferer, Mareike Zink, and Stefan G. Mayr. 2022. "Laminin Adsorption and Adhesion of Neurons and Glial Cells on Carbon Implanted Titania Nanotube Scaffolds for Neural Implant Applications" Nanomaterials 12, no. 21: 3858. https://doi.org/10.3390/nano12213858
APA StyleFrenzel, J., Kupferer, A., Zink, M., & Mayr, S. G. (2022). Laminin Adsorption and Adhesion of Neurons and Glial Cells on Carbon Implanted Titania Nanotube Scaffolds for Neural Implant Applications. Nanomaterials, 12(21), 3858. https://doi.org/10.3390/nano12213858