Challenges of Periodontal Tissue Engineering: Increasing Biomimicry through 3D Printing and Controlled Dynamic Environment
Abstract
1. Introduction
2. The Role of PDL Cells
3. Biomimetic Scaffolds to Reproduce the Micro-Environment of PDL
3.1. Cell Sheet Technology
3.2. The 3D Printing
3.2.1. Synthetic Polymers and Surface Modifications of Printed Scaffolds
3.2.2. Natural Polymers
3.2.3. Hydrogels
4. Mimicking the Physical Micro-Environment of PDL
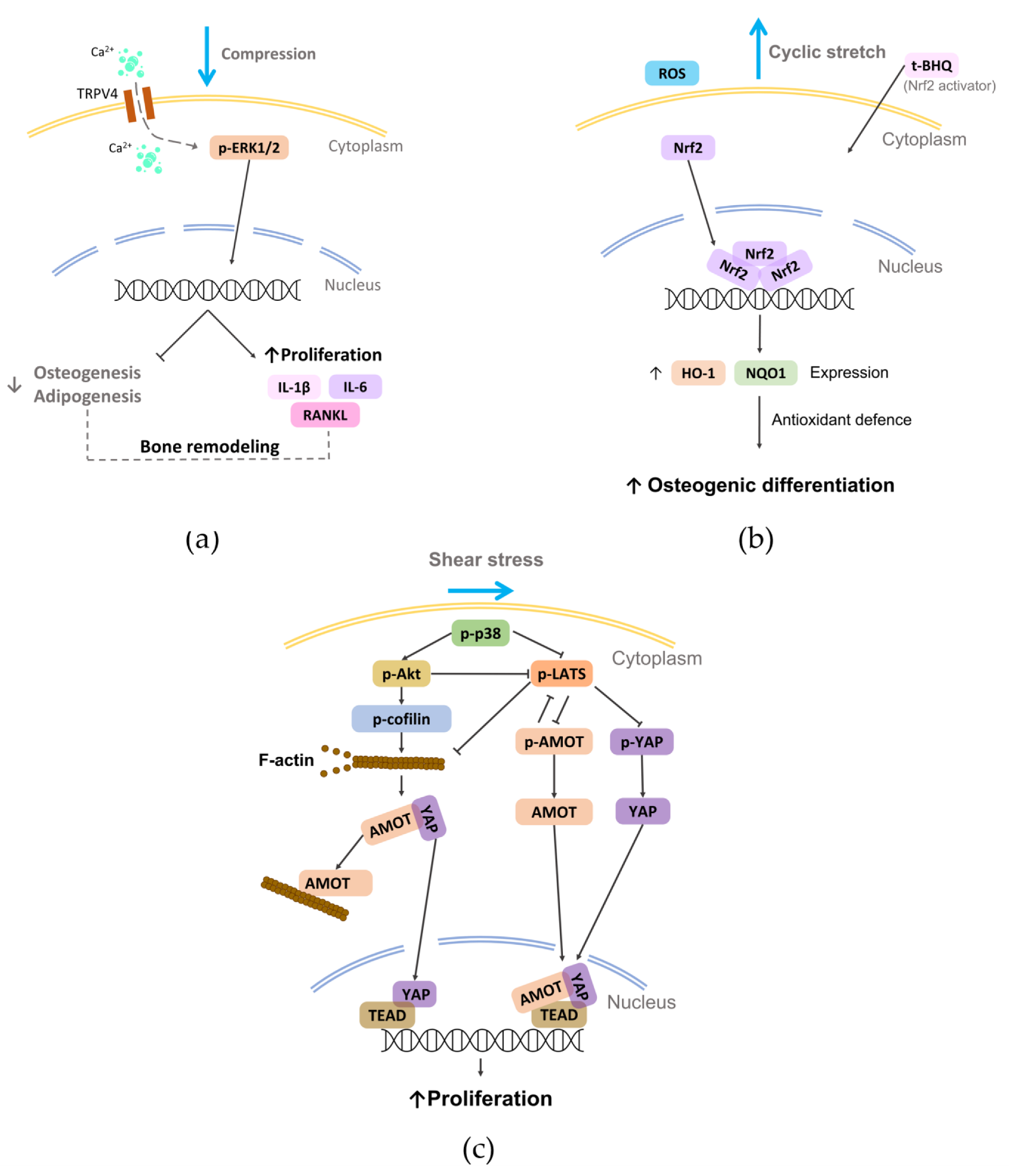
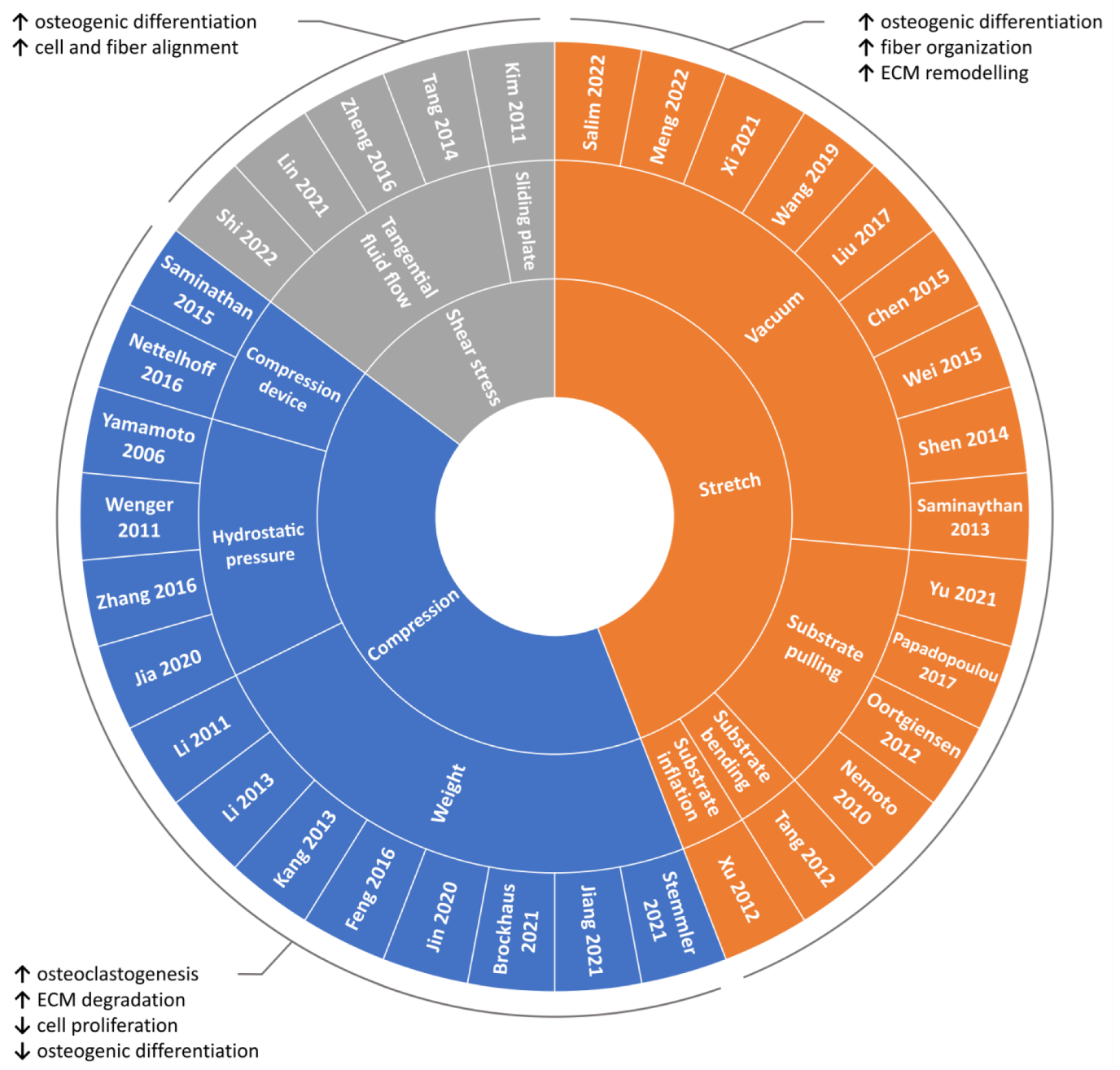
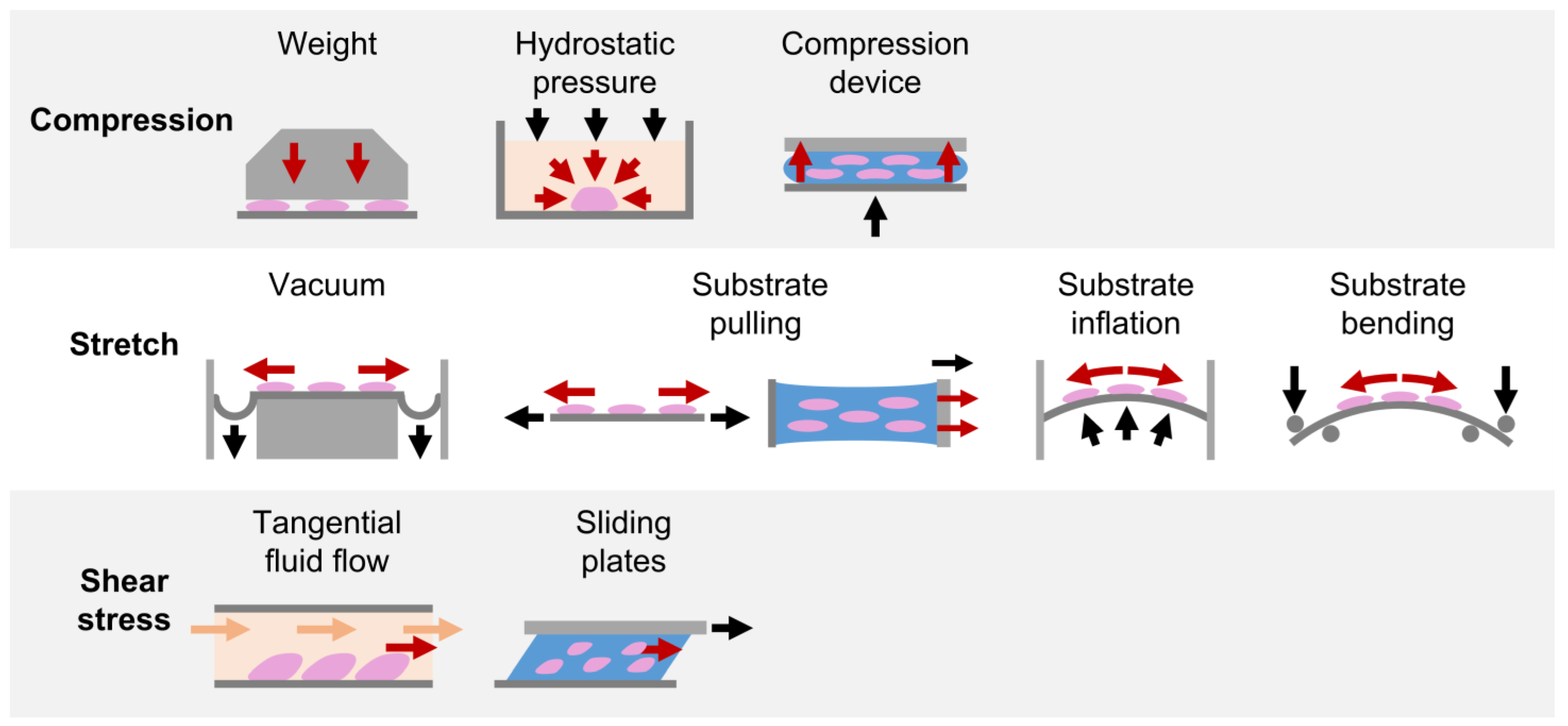
4.1. Compression
4.1.1. Weight Method
4.1.2. Hydrostatic Pressure Method
4.1.3. Substrate Deformation Methods
4.2. Stretch
4.2.1. Substrate Deformation—Vacuum Approach
4.2.2. Substrate Deformation—Pulling Approach
4.2.3. Substrate Deformation—Inflation and Bending Approaches
4.3. Shear Stress
5. Future Perspectives
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Menicanin, D.; Hynes, K.; Han, J.; Gronthos, S.; Bartold, P.M. Cementum and Periodontal Ligament Regeneration. Adv. Exp. Med. Biol. 2015, 881, 207–236. [Google Scholar] [CrossRef] [PubMed]
- Socransky, S.S.; Haffajee, A.D. Periodontal microbial ecology. Periodontology 2000 2005, 38, 135–187. [Google Scholar] [CrossRef]
- Beertsen, W.; McCulloch, C.A.; Sodek, J. The periodontal ligament: A unique, multifunctional connective tissue. Periodontology 2000 1997, 13, 20–40. [Google Scholar] [CrossRef] [PubMed]
- Itaya, S.; Oka, K.; Ogata, K.; Tamura, S.; Kira-Tatsuoka, M.; Fujiwara, N.; Otsu, K.; Tsuruga, E.; Ozaki, M.; Harada, H. Hertwig’s epithelial root sheath cells contribute to formation of periodontal ligament through epithelial-mesenchymal transition by TGF-beta. Biomed. Res. 2017, 38, 61–69. [Google Scholar] [CrossRef] [PubMed]
- de Jong, T.; Bakker, A.D.; Everts, V.; Smit, T.H. The intricate anatomy of the periodontal ligament and its development: Lessons for periodontal regeneration. J. Periodontal Res. 2017, 52, 965–974. [Google Scholar] [CrossRef]
- Ortun-Terrazas, J.; Cegonino, J.; Santana-Penin, U.; Santana-Mora, U.; Perez Del Palomar, A. Approach towards the porous fibrous structure of the periodontal ligament using micro-computerized tomography and finite element analysis. J. Mech. Behav. Biomed. Mater. 2018, 79, 135–149. [Google Scholar] [CrossRef]
- Gathercole, L.J.; Keller, A. Crimp morphology in the fibre-forming collagens. Matrix 1991, 11, 214–234. [Google Scholar] [CrossRef]
- Maceri, F.; Marino, M.; Vairo, G. A unified multiscale mechanical model for soft collagenous tissues with regular fiber arrangement. J. Biomech. 2010, 43, 355–363. [Google Scholar] [CrossRef]
- Szczesny, S.E.; Driscoll, T.P.; Tseng, H.Y.; Liu, P.C.; Heo, S.J.; Mauck, R.L.; Chao, P.G. Crimped Nanofibrous Biomaterials Mimic Microstructure and Mechanics of Native Tissue and Alter Strain Transfer to Cells. ACS Biomater. Sci. Eng. 2017, 3, 2869–2876. [Google Scholar] [CrossRef]
- Cho, M.I.; Garant, P.R. Development and general structure of the periodontium. Periodontology 2000 2000, 24, 9–27. [Google Scholar] [CrossRef]
- Bosshardt, D.D.; Bergomi, M.; Vaglio, G.; Wiskott, A. Regional structural characteristics of bovine periodontal ligament samples and their suitability for biomechanical tests. J. Anat. 2008, 212, 319–329. [Google Scholar] [CrossRef]
- Tsuruga, E.; Irie, K.; Sakakura, Y.; Yajima, T. Expression of fibrillins and tropoelastin by human gingival and periodontal ligament fibroblasts in vitro. J. Periodontal Res. 2002, 37, 23–28. [Google Scholar] [CrossRef]
- Peres, M.A.; Macpherson, L.M.D.; Weyant, R.J.; Daly, B.; Venturelli, R.; Mathur, M.R.; Listl, S.; Celeste, R.K.; Guarnizo-Herreno, C.C.; Kearns, C.; et al. Oral diseases: A global public health challenge. Lancet 2019, 394, 249–260. [Google Scholar] [CrossRef]
- Lourenco, T.G.; Heller, D.; Silva-Boghossian, C.M.; Cotton, S.L.; Paster, B.J.; Colombo, A.P. Microbial signature profiles of periodontally healthy and diseased patients. J. Clin. Periodontol. 2014, 41, 1027–1036. [Google Scholar] [CrossRef]
- Feres, M.; Teles, F.; Teles, R.; Figueiredo, L.C.; Faveri, M. The subgingival periodontal microbiota of the aging mouth. Periodontology 2000 2016, 72, 30–53. [Google Scholar] [CrossRef]
- Sowmya, S.; Mony, U.; Jayachandran, P.; Reshma, S.; Kumar, R.A.; Arzate, H.; Nair, S.V.; Jayakumar, R. Tri-Layered Nanocomposite Hydrogel Scaffold for the Concurrent Regeneration of Cementum, Periodontal Ligament, and Alveolar Bone. Adv. Healthc. Mater. 2017, 6, 1601251. [Google Scholar] [CrossRef]
- Kawaguchi, H.; Hirachi, A.; Hasegawa, N.; Iwata, T.; Hamaguchi, H.; Shiba, H.; Takata, T.; Kato, Y.; Kurihara, H. Enhancement of periodontal tissue regeneration by transplantation of bone marrow mesenchymal stem cells. J. Periodontol. 2004, 75, 1281–1287. [Google Scholar] [CrossRef]
- Du, J.; Shan, Z.; Ma, P.; Wang, S.; Fan, Z. Allogeneic bone marrow mesenchymal stem cell transplantation for periodontal regeneration. J. Dent. Res. 2014, 93, 183–188. [Google Scholar] [CrossRef]
- Mohammed, E.; Khalil, E.; Sabry, D. Effect of Adipose-Derived Stem Cells and Their Exo as Adjunctive Therapy to Nonsurgical Periodontal Treatment: A Histologic and Histomorphometric Study in Rats. Biomolecules 2018, 8, 167. [Google Scholar] [CrossRef]
- Seo, B.M.; Miura, M.; Gronthos, S.; Bartold, P.M.; Batouli, S.; Brahim, J.; Young, M.; Robey, P.G.; Wang, C.Y.; Shi, S. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet 2004, 364, 149–155. [Google Scholar] [CrossRef]
- Bartold, P.M.; Gronthos, S.; Ivanovski, S.; Fisher, A.; Hutmacher, D.W. Tissue engineered periodontal products. J. Periodontal. Res. 2016, 51, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Iwata, T.; Yamato, M.; Zhang, Z.; Mukobata, S.; Washio, K.; Ando, T.; Feijen, J.; Okano, T.; Ishikawa, I. Validation of human periodontal ligament-derived cells as a reliable source for cytotherapeutic use. J. Clin. Periodontol. 2010, 37, 1088–1099. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.C.; Bae, S.H.; Lee, G.; Ryu, C.J.; Jang, Y.J. Activation of beta-catenin by TGF-beta1 promotes ligament-fibroblastic differentiation and inhibits cementoblastic differentiation of human periodontal ligament cells. Stem Cells 2020. Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.; Lee, J.H.; Son, C.; Lee, D.S.; Park, J.C. CPNE7 regenerates periodontal ligament via TAU-mediated alignment and cementum attachment protein-mediated attachment. J. Clin. Periodontol. 2022, 49, 609–620. [Google Scholar] [CrossRef] [PubMed]
- Park, C.H.; Oh, J.H.; Jung, H.M.; Choi, Y.; Rahman, S.U.; Kim, S.; Kim, T.I.; Shin, H.I.; Lee, Y.S.; Yu, F.H.; et al. Effects of the incorporation of epsilon-aminocaproic acid/chitosan particles to fibrin on cementoblast differentiation and cementum regeneration. Acta Biomater. 2017, 61, 134–143. [Google Scholar] [CrossRef]
- Iwasaki, K.; Komaki, M.; Akazawa, K.; Nagata, M.; Yokoyama, N.; Watabe, T.; Morita, I. Spontaneous differentiation of periodontal ligament stem cells into myofibroblast during ex vivo expansion. J. Cell. Physiol. 2019, 234, 20377–20391. [Google Scholar] [CrossRef]
- Zhu, Y.; Song, X.; Han, F.; Li, Y.; Wei, J.; Liu, X. Alteration of histone acetylation pattern during long-term serum-free culture conditions of human fetal placental mesenchymal stem cells. PLoS ONE 2015, 10, e0117068. [Google Scholar] [CrossRef]
- Shinagawa-Ohama, R.; Mochizuki, M.; Tamaki, Y.; Suda, N.; Nakahara, T. Heterogeneous Human Periodontal Ligament-Committed Progenitor and Stem Cell Populations Exhibit a Unique Cementogenic Property Under In Vitro and In Vivo Conditions. Stem Cells Dev. 2017, 26, 632–645. [Google Scholar] [CrossRef]
- Abuarqoub, D.A.; Aslam, N.; Barham, R.B.; Ababneh, N.A.; Shahin, D.A.; Al-Oweidi, A.A.; Jafar, H.D.; Al-Salihi, M.A.; Awidi, A.S. The effect of platelet lysate in culture of PDLSCs: An in vitro comparative study. PeerJ 2019, 7, e7465. [Google Scholar] [CrossRef]
- Zheng, W.; Wang, S.; Ma, D.; Tang, L.; Duan, Y.; Jin, Y. Loss of proliferation and differentiation capacity of aged human periodontal ligament stem cells and rejuvenation by exposure to the young extrinsic environment. Tissue Eng. Part A 2009, 15, 2363–2371. [Google Scholar] [CrossRef]
- Du, T.; Liu, N.; Gu, B.; Li, L.; Yuan, Y.; Zhang, W.; Zhang, T. Effects of Aging on the Proliferation and Differentiation Capacity of Human Periodontal Ligament Stem Cells. Chin. Med. Sci. J. 2017, 32, 81–83. [Google Scholar] [CrossRef]
- Silverio, K.G.; Rodrigues, T.L.; Coletta, R.D.; Benevides, L.; Da Silva, J.S.; Casati, M.Z.; Sallum, E.A.; Nociti, F.H., Jr. Mesenchymal stem cell properties of periodontal ligament cells from deciduous and permanent teeth. J. Periodontol. 2010, 81, 1207–1215. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, G.H.; Kim, J.W.; Pyeon, H.J.; Lee, J.C.; Lee, G.; Nam, H. In Vivo Angiogenic Capacity of Stem Cells from Human Exfoliated Deciduous Teeth with Human Umbilical Vein Endothelial Cells. Mol. Cells 2016, 39, 790–796. [Google Scholar] [CrossRef]
- Song, J.S.; Kim, S.O.; Kim, S.H.; Choi, H.J.; Son, H.K.; Jung, H.S.; Kim, C.S.; Lee, J.H. In vitro and in vivo characteristics of stem cells derived from the periodontal ligament of human deciduous and permanent teeth. Tissue Eng. Part A 2012, 18, 2040–2051. [Google Scholar] [CrossRef]
- Kim, K.; Jeon, M.; Lee, H.S.; Park, J.C.; Moon, S.J.; Kim, S.O.; Cho, S.W.; Song, J.S. Comparative analysis of secretory factors from permanent- and deciduous-teeth periodontal ligament cells. Arch. Oral Biol. 2016, 71, 65–79. [Google Scholar] [CrossRef]
- Sonoyama, W.; Liu, Y.; Fang, D.; Yamaza, T.; Seo, B.M.; Zhang, C.; Liu, H.; Gronthos, S.; Wang, C.Y.; Wang, S.; et al. Mesenchymal stem cell-mediated functional tooth regeneration in swine. PLoS ONE 2006, 1, e79. [Google Scholar] [CrossRef]
- Shi, H.; Zong, W.; Xu, X.; Chen, J. Improved biphasic calcium phosphate combined with periodontal ligament stem cells may serve as a promising method for periodontal regeneration. Am. J. Transl. Res. 2018, 10, 4030–4041. [Google Scholar]
- Chen, F.M.; Gao, L.N.; Tian, B.M.; Zhang, X.Y.; Zhang, Y.J.; Dong, G.Y.; Lu, H.; Chu, Q.; Xu, J.; Yu, Y.; et al. Treatment of periodontal intrabony defects using autologous periodontal ligament stem cells: A randomized clinical trial. Stem Cell Res. Ther. 2016, 7, 33. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Bartold, P.M.; Gronthos, S. Standardization of Criteria Defining Periodontal Ligament Stem Cells. J. Dent. Res. 2017, 96, 487–490. [Google Scholar] [CrossRef]
- Menicanin, D.; Mrozik, K.M.; Wada, N.; Marino, V.; Shi, S.; Bartold, P.M.; Gronthos, S. Periodontal-ligament-derived stem cells exhibit the capacity for long-term survival, self-renewal, and regeneration of multiple tissue types in vivo. Stem Cells Dev. 2014, 23, 1001–1011. [Google Scholar] [CrossRef] [PubMed]
- Trubiani, O.; Zalzal, S.F.; Paganelli, R.; Marchisio, M.; Giancola, R.; Pizzicannella, J.; Buhring, H.J.; Piattelli, M.; Caputi, S.; Nanci, A. Expression profile of the embryonic markers nanog, OCT-4, SSEA-1, SSEA-4, and frizzled-9 receptor in human periodontal ligament mesenchymal stem cells. J. Cell. Physiol. 2010, 225, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Pelaez, D.; Huang, C.Y.; Cheung, H.S. Isolation of pluripotent neural crest-derived stem cells from adult human tissues by connexin-43 enrichment. Stem Cells Dev. 2013, 22, 2906–2914. [Google Scholar] [CrossRef] [PubMed]
- Kawanabe, N.; Murata, S.; Murakami, K.; Ishihara, Y.; Hayano, S.; Kurosaka, H.; Kamioka, H.; Takano-Yamamoto, T.; Yamashiro, T. Isolation of multipotent stem cells in human periodontal ligament using stage-specific embryonic antigen-4. Differentiation 2010, 79, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Komaki, M. Pericytes in the Periodontal Ligament. Adv. Exp. Med. Biol. 2019, 1122, 169–186. [Google Scholar] [CrossRef]
- Huang, C.Y.; Pelaez, D.; Dominguez-Bendala, J.; Garcia-Godoy, F.; Cheung, H.S. Plasticity of stem cells derived from adult periodontal ligament. Regen. Med. 2009, 4, 809–821. [Google Scholar] [CrossRef]
- Fortino, V.R.; Chen, R.S.; Pelaez, D.; Cheung, H.S. Neurogenesis of neural crest-derived periodontal ligament stem cells by EGF and bFGF. J. Cell. Physiol. 2014, 229, 479–488. [Google Scholar] [CrossRef]
- Lekovic, V.; Kenney, E.B.; Kovacevic, K.; Carranza, F.A., Jr. Evaluation of guided tissue regeneration in Class II furcation defects. A clinical re-entry study. J. Periodontol. 1989, 60, 694–698. [Google Scholar] [CrossRef]
- Nasajpour, A.; Mandla, S.; Shree, S.; Mostafavi, E.; Sharifi, R.; Khalilpour, A.; Saghazadeh, S.; Hassan, S.; Mitchell, M.J.; Leijten, J.; et al. Nanostructured Fibrous Membranes with Rose Spike-Like Architecture. Nano Lett. 2017, 17, 6235–6240. [Google Scholar] [CrossRef]
- Iwata, T.; Yamato, M.; Tsuchioka, H.; Takagi, R.; Mukobata, S.; Washio, K.; Okano, T.; Ishikawa, I. Periodontal regeneration with multi-layered periodontal ligament-derived cell sheets in a canine model. Biomaterials 2009, 30, 2716–2723. [Google Scholar] [CrossRef]
- Vaquette, C.; Fan, W.; Xiao, Y.; Hamlet, S.; Hutmacher, D.W.; Ivanovski, S. A biphasic scaffold design combined with cell sheet technology for simultaneous regeneration of alveolar bone/periodontal ligament complex. Biomaterials 2012, 33, 5560–5573. [Google Scholar] [CrossRef]
- Panduwawala, C.P.; Zhan, X.; Dissanayaka, W.L.; Samaranayake, L.P.; Jin, L.; Zhang, C. In vivo periodontal tissue regeneration by periodontal ligament stem cells and endothelial cells in three-dimensional cell sheet constructs. J. Periodontal Res. 2017, 52, 408–418. [Google Scholar] [CrossRef]
- Pilipchuk, S.P.; Monje, A.; Jiao, Y.; Hao, J.; Kruger, L.; Flanagan, C.L.; Hollister, S.J.; Giannobile, W.V. Integration of 3D Printed and Micropatterned Polycaprolactone Scaffolds for Guidance of Oriented Collagenous Tissue Formation In Vivo. Adv. Healthc. Mater. 2016, 5, 676–687. [Google Scholar] [CrossRef]
- Park, C.H.; Rios, H.F.; Jin, Q.; Sugai, J.V.; Padial-Molina, M.; Taut, A.D.; Flanagan, C.L.; Hollister, S.J.; Giannobile, W.V. Tissue engineering bone-ligament complexes using fiber-guiding scaffolds. Biomaterials 2012, 33, 137–145. [Google Scholar] [CrossRef]
- Zhou, T.; Liu, X.; Sui, B.; Liu, C.; Mo, X.; Sun, J. Development of fish collagen/bioactive glass/chitosan composite nanofibers as a GTR/GBR membrane for inducing periodontal tissue regeneration. Biomed. Mater. 2017, 12, 055004. [Google Scholar] [CrossRef]
- Cho, H.; Tarafder, S.; Fogge, M.; Kao, K.; Lee, C.H. Periodontal ligament stem/progenitor cells with protein-releasing scaffolds for cementum formation and integration on dentin surface. Connect. Tissue Res. 2016, 57, 488–495. [Google Scholar] [CrossRef]
- Ding, T.; Li, J.; Zhang, X.; Du, L.; Li, Y.; Li, D.; Kong, B.; Ge, S. Super-assembled core/shell fibrous frameworks with dual growth factors for in situ cementum-ligament-bone complex regeneration. Biomater. Sci. 2020, 8, 2459–2471. [Google Scholar] [CrossRef]
- Owaki, T.; Shimizu, T.; Yamato, M.; Okano, T. Cell sheet engineering for regenerative medicine: Current challenges and strategies. Biotechnol. J. 2014, 9, 904–914. [Google Scholar] [CrossRef]
- Okano, T.; Yamada, N.; Sakai, H.; Sakurai, Y. A novel recovery system for cultured cells using plasma-treated polystyrene dishes grafted with poly(N-isopropylacrylamide). J. Biomed. Mater. Res. 1993, 27, 1243–1251. [Google Scholar] [CrossRef]
- Hasegawa, M.; Yamato, M.; Kikuchi, A.; Okano, T.; Ishikawa, I. Human periodontal ligament cell sheets can regenerate periodontal ligament tissue in an athymic rat model. Tissue Eng. 2005, 11, 469–478. [Google Scholar] [CrossRef]
- Takahashi, H.; Nakayama, M.; Itoga, K.; Yamato, M.; Okano, T. Micropatterned thermoresponsive polymer brush surfaces for fabricating cell sheets with well-controlled orientational structures. Biomacromolecules 2011, 12, 1414–1418. [Google Scholar] [CrossRef] [PubMed]
- Raju, R.; Oshima, M.; Inoue, M.; Morita, T.; Huijiao, Y.; Waskitho, A.; Baba, O.; Inoue, M.; Matsuka, Y. Three-dimensional periodontal tissue regeneration using a bone-ligament complex cell sheet. Sci. Rep. 2020, 10, 1656. [Google Scholar] [CrossRef] [PubMed]
- Tsumanuma, Y.; Iwata, T.; Washio, K.; Yoshida, T.; Yamada, A.; Takagi, R.; Ohno, T.; Lin, K.; Yamato, M.; Ishikawa, I.; et al. Comparison of different tissue-derived stem cell sheets for periodontal regeneration in a canine 1-wall defect model. Biomaterials 2011, 32, 5819–5825. [Google Scholar] [CrossRef] [PubMed]
- Son, H.; Jeon, M.; Choi, H.J.; Lee, H.S.; Kim, I.H.; Kang, C.M.; Song, J.S. Decellularized human periodontal ligament for periodontium regeneration. PLoS ONE 2019, 14, e0221236. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Han, Q.; Wang, C.; Liu, Y.; Chen, B.; Wang, J. Porous Scaffold Design for Additive Manufacturing in Orthopedics: A Review. Front. Bioeng. Biotechnol. 2020, 8, 609. [Google Scholar] [CrossRef] [PubMed]
- Park, C.H.; Kim, K.H.; Rios, H.F.; Lee, Y.M.; Giannobile, W.V.; Seol, Y.J. Spatiotemporally controlled microchannels of periodontal mimic scaffolds. J. Dent. Res. 2014, 93, 1304–1312. [Google Scholar] [CrossRef]
- Oliveira, N.K.; Salles, T.H.C.; Pedroni, A.C.; Miguita, L.; D’Avila, M.A.; Marques, M.M.; Deboni, M.C.Z. Osteogenic potential of human dental pulp stem cells cultured onto poly-epsilon-caprolactone/poly (rotaxane) scaffolds. Dent. Mater. 2019, 35, 1740–1749. [Google Scholar] [CrossRef]
- Thattaruparambil Raveendran, N.; Vaquette, C.; Meinert, C.; Samuel Ipe, D.; Ivanovski, S. Optimization of 3D bioprinting of periodontal ligament cells. Dent. Mater. 2019, 35, 1683–1694. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, G.; Johnson, B.N.; Jia, X. Three-dimensional (3D) printed scaffold and material selection for bone repair. Acta Biomater. 2019, 84, 16–33. [Google Scholar] [CrossRef]
- Park, C.H. Biomaterial-Based Approaches for Regeneration of Periodontal Ligament and Cementum Using 3D Platforms. Int. J. Mol. Sci. 2019, 20, 4364. [Google Scholar] [CrossRef]
- Niinomi, M. Metallic biomaterials. J. Artif. Organs 2008, 11, 105–110. [Google Scholar] [CrossRef]
- Park, J.; Park, S.; Kim, J.E.; Jang, K.J.; Seonwoo, H.; Chung, J.H. Enhanced Osteogenic Differentiation of Periodontal Ligament Stem Cells Using a Graphene Oxide-Coated Poly(epsilon-caprolactone) Scaffold. Polymers 2021, 13, 797. [Google Scholar] [CrossRef]
- Peng, W.; Ren, S.; Zhang, Y.; Fan, R.; Zhou, Y.; Li, L.; Xu, X.; Xu, Y. MgO Nanoparticles-Incorporated PCL/Gelatin-Derived Coaxial Electrospinning Nanocellulose Membranes for Periodontal Tissue Regeneration. Front. Bioeng. Biotechnol. 2021, 9, 668428. [Google Scholar] [CrossRef]
- Jeon, H.; Lee, H.; Kim, G. A surface-modified poly(varepsilon-caprolactone) scaffold comprising variable nanosized surface-roughness using a plasma treatment. Tissue Eng. Part C Methods 2014, 20, 951–963. [Google Scholar] [CrossRef]
- Vera-Sanchez, M.; Aznar-Cervantes, S.; Jover, E.; Garcia-Bernal, D.; Onate-Sanchez, R.E.; Hernandez-Romero, D.; Moraleda, J.M.; Collado-Gonzalez, M.; Rodriguez-Lozano, F.J.; Cenis, J.L. Silk-Fibroin and Graphene Oxide Composites Promote Human Periodontal Ligament Stem Cell Spontaneous Differentiation into Osteo/Cementoblast-Like Cells. Stem Cells Dev. 2016, 25, 1742–1754. [Google Scholar] [CrossRef]
- Fu, C.; Yang, X.; Tan, S.; Song, L. Enhancing Cell Proliferation and Osteogenic Differentiation of MC3T3-E1 Pre-osteoblasts by BMP-2 Delivery in Graphene Oxide-Incorporated PLGA/HA Biodegradable Microcarriers. Sci. Rep. 2017, 7, 12549. [Google Scholar] [CrossRef]
- Rasperini, G.; Pilipchuk, S.P.; Flanagan, C.L.; Park, C.H.; Pagni, G.; Hollister, S.J.; Giannobile, W.V. 3D-printed Bioresorbable Scaffold for Periodontal Repair. J. Dent. Res. 2015, 94, 153S–157S. [Google Scholar] [CrossRef]
- Kade, J.C.; Dalton, P.D. Polymers for Melt Electrowriting. Adv. Healthc. Mater. 2021, 10, e2001232. [Google Scholar] [CrossRef]
- Latimer, J.M.; Maekawa, S.; Yao, Y.; Wu, D.T.; Chen, M.; Giannobile, W.V. Regenerative Medicine Technologies to Treat Dental, Oral, and Craniofacial Defects. Front. Bioeng. Biotechnol. 2021, 9, 704048. [Google Scholar] [CrossRef]
- Eichholz, K.F.; Hoey, D.A. Mediating human stem cell behaviour via defined fibrous architectures by melt electrospinning writing. Acta Biomater. 2018, 75, 140–151. [Google Scholar] [CrossRef]
- Vaquette, C.; Pilipchuk, S.P.; Bartold, P.M.; Hutmacher, D.W.; Giannobile, W.V.; Ivanovski, S. Tissue Engineered Constructs for Periodontal Regeneration: Current Status and Future Perspectives. Adv. Healthc. Mater. 2018, 7, e1800457. [Google Scholar] [CrossRef] [PubMed]
- Ivanovski, S.; Vaquette, C.; Gronthos, S.; Hutmacher, D.W.; Bartold, P.M. Multiphasic scaffolds for periodontal tissue engineering. J. Dent. Res. 2014, 93, 1212–1221. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Fernandez, T.; Rathan, S.; Hobbs, C.; Pitacco, P.; Freeman, F.E.; Cunniffe, G.M.; Dunne, N.J.; McCarthy, H.O.; Nicolosi, V.; O’Brien, F.J.; et al. Pore-forming bioinks to enable spatio-temporally defined gene delivery in bioprinted tissues. J. Control. Release 2019, 301, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, N.; Abdal-Hay, A.; Hamlet, S.; Graham, E.; Ivanovski, S. Effects of Gradient and Offset Architectures on the Mechanical and Biological Properties of 3-D Melt Electrowritten (MEW) Scaffolds. ACS. Biomater. Sci. Eng. 2019, 5, 3448–3461. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Raymond, J.E.; Kauffmann, F.; Maekawa, S.; Sugai, J.V.; Lahann, J.; Giannobile, W.V. Multicompartmental Scaffolds for Coordinated Periodontal Tissue Engineering. J. Dent. Res. 2022, 101, 1457–1466. [Google Scholar] [CrossRef]
- Lee, C.H.; Singla, A.; Lee, Y. Biomedical applications of collagen. Int. J. Pharm. 2001, 221, 1–22. [Google Scholar] [CrossRef]
- van den Bos, T.; Tonino, G.J. Composition and metabolism of the extracellular matrix in the periodontal ligament of impeded and unimpeded rat incisors. Arch. Oral. Biol. 1984, 29, 893–897. [Google Scholar] [CrossRef]
- Bozec, L.; Odlyha, M. Thermal denaturation studies of collagen by microthermal analysis and atomic force microscopy. Biophys. J. 2011, 101, 228–236. [Google Scholar] [CrossRef]
- Hinton, T.J.; Jallerat, Q.; Palchesko, R.N.; Park, J.H.; Grodzicki, M.S.; Shue, H.J.; Ramadan, M.H.; Hudson, A.R.; Feinberg, A.W. Three-dimensional printing of complex biological structures by freeform reversible embedding of suspended hydrogels. Sci. Adv. 2015, 1, e1500758. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.; Hudson, A.R.; Shiwarski, D.J.; Tashman, J.W.; Hinton, T.J.; Yerneni, S.; Bliley, J.M.; Campbell, P.G.; Feinberg, A.W. 3D bioprinting of collagen to rebuild components of the human heart. Science 2019, 365, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Mota, J.; Yu, N.; Caridade, S.G.; Luz, G.M.; Gomes, M.E.; Reis, R.L.; Jansen, J.A.; Walboomers, X.F.; Mano, J.F. Chitosan/bioactive glass nanoparticle composite membranes for periodontal regeneration. Acta Biomater. 2012, 8, 4173–4180. [Google Scholar] [CrossRef]
- Qasim, S.B.; Delaine-Smith, R.M.; Fey, T.; Rawlinson, A.; Rehman, I.U. Freeze gelated porous membranes for periodontal tissue regeneration. Acta Biomater. 2015, 23, 317–328. [Google Scholar] [CrossRef]
- Ge, S.; Zhao, N.; Wang, L.; Yu, M.; Liu, H.; Song, A.; Huang, J.; Wang, G.; Yang, P. Bone repair by periodontal ligament stem cellseeded nanohydroxyapatite-chitosan scaffold. Int. J. Nanomed. 2012, 7, 5405–5414. [Google Scholar] [CrossRef]
- Varoni, E.M.; Vijayakumar, S.; Canciani, E.; Cochis, A.; De Nardo, L.; Lodi, G.; Rimondini, L.; Cerruti, M. Chitosan-Based Trilayer Scaffold for Multitissue Periodontal Regeneration. J. Dent. Res. 2018, 97, 303–311. [Google Scholar] [CrossRef]
- Maturavongsadit, P.; Narayanan, L.K.; Chansoria, P.; Shirwaiker, R.; Benhabbour, S.R. Cell-Laden Nanocellulose/Chitosan-Based Bioinks for 3D Bioprinting and Enhanced Osteogenic Cell Differentiation. ACS Appl. Bio Mater. 2021, 4, 2342–2353. [Google Scholar] [CrossRef]
- Trubiani, O.; Orsini, G.; Zini, N.; Di Iorio, D.; Piccirilli, M.; Piattelli, A.; Caputi, S. Regenerative potential of human periodontal ligament derived stem cells on three-dimensional biomaterials: A morphological report. J. Biomed. Mater. Res. A 2008, 87, 986–993. [Google Scholar] [CrossRef]
- Ma, Y.; Ji, Y.; Huang, G.; Ling, K.; Zhang, X.; Xu, F. Bioprinting 3D cell-laden hydrogel microarray for screening human periodontal ligament stem cell response to extracellular matrix. Biofabrication 2015, 7, 044105. [Google Scholar] [CrossRef]
- Xu, Q.; Li, B.; Yuan, L.; Dong, Z.; Zhang, H.; Wang, H.; Sun, J.; Ge, S.; Jin, Y. Combination of platelet-rich plasma within periodontal ligament stem cell sheets enhances cell differentiation and matrix production. J. Tissue Eng. Regen. Med. 2017, 11, 627–636. [Google Scholar] [CrossRef]
- Pan, J.; Deng, J.; Luo, Y.; Yu, L.; Zhang, W.; Han, X.; You, Z.; Liu, Y. Thermosensitive Hydrogel Delivery of Human Periodontal Stem Cells Overexpressing Platelet-Derived Growth Factor-BB Enhances Alveolar Bone Defect Repair. Stem Cells Dev. 2019, 28, 1620–1631. [Google Scholar] [CrossRef]
- Safi, I.N.; Al-Shammari, A.M.; Ul-Jabbar, M.A.; Hussein, B.M.A. Preparing polycaprolactone scaffolds using electrospinning technique for construction of artificial periodontal ligament tissue. J. Taibah. Univ. Med. Sci. 2020, 15, 363–373. [Google Scholar] [CrossRef]
- Duan, X.; Ji, M.; Deng, F.; Sun, Z.; Lin, Z. Effects of connective tissue growth factor on human periodontal ligament fibroblasts. Arch. Oral Biol. 2017, 84, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Thomadakis, G.; Ramoshebi, L.N.; Crooks, J.; Rueger, D.C.; Ripamonti, U. Immunolocalization of Bone Morphogenetic Protein-2 and -3 and Osteogenic Protein-1 during murine tooth root morphogenesis and in other craniofacial structures. Eur. J. Oral Sci. 1999, 107, 368–377. [Google Scholar] [CrossRef] [PubMed]
- Pitaru, S.; Pritzki, A.; Bar-Kana, I.; Grosskopf, A.; Savion, N.; Narayanan, A.S. Bone morphogenetic protein 2 induces the expression of cementum attachment protein in human periodontal ligament clones. Connect. Tissue Res. 2002, 43, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Kono, K.; Maeda, H.; Fujii, S.; Tomokiyo, A.; Yamamoto, N.; Wada, N.; Monnouchi, S.; Teramatsu, Y.; Hamano, S.; Koori, K.; et al. Exposure to transforming growth factor-beta1 after basic fibroblast growth factor promotes the fibroblastic differentiation of human periodontal ligament stem/progenitor cell lines. Cell Tissue Res. 2013, 352, 249–263. [Google Scholar] [CrossRef]
- Kang, W.; Liang, Q.; Du, L.; Shang, L.; Wang, T.; Ge, S. Sequential application of bFGF and BMP-2 facilitates osteogenic differentiation of human periodontal ligament stem cells. J. Periodontal Res. 2019, 54, 424–434. [Google Scholar] [CrossRef]
- Ding, T.; Kang, W.; Li, J.; Yu, L.; Ge, S. An in situ tissue engineering scaffold with growth factors combining angiogenesis and osteoimmunomodulatory functions for advanced periodontal bone regeneration. J. Nanobiotechnology 2021, 19, 247. [Google Scholar] [CrossRef]
- James, A.W.; LaChaud, G.; Shen, J.; Asatrian, G.; Nguyen, V.; Zhang, X.; Ting, K.; Soo, C. A Review of the Clinical Side Effects of Bone Morphogenetic Protein-2. Tissue Eng. Part B Rev. 2016, 22, 284–297. [Google Scholar] [CrossRef]
- Qu, M.; Jiang, X.; Zhou, X.; Wang, C.; Wu, Q.; Ren, L.; Zhu, J.; Zhu, S.; Tebon, P.; Sun, W.; et al. Stimuli-Responsive Delivery of Growth Factors for Tissue Engineering. Adv. Healthc. Mater. 2020, 9, e1901714. [Google Scholar] [CrossRef]
- Yuk, H.; Lu, B.; Lin, S.; Qu, K.; Xu, J.; Luo, J.; Zhao, X. 3D printing of conducting polymers. Nat. Commun. 2020, 11, 1604. [Google Scholar] [CrossRef]
- Shen, Z.; Zhang, Z.; Zhang, N.; Li, J.; Zhou, P.; Hu, F.; Rong, Y.; Lu, B.; Gu, G. High-Stretchability, Ultralow-Hysteresis ConductingPolymer Hydrogel Strain Sensors for Soft Machines. Adv. Mater. 2022, 34, e2203650. [Google Scholar] [CrossRef]
- Zhao, W.; Cao, J.; Wang, F.; Tian, F.; Zheng, W.; Bao, Y.; Zhang, K.; Zhang, Z.; Yu, J.; Xu, J.; et al. 3D Printing of Stretchable, Adhesive and Conductive Ti3C2Tx-Polyacrylic Acid Hydrogels. Polymers 2022, 14, 1992. [Google Scholar] [CrossRef]
- Gauthier, R.; Jeannin, C.; Attik, N.; Trunfio-Sfarghiu, A.M.; Gritsch, K.; Grosgogeat, B. Tissue Engineering for Periodontal Ligament Regeneration: Biomechanical Specifications. J. Biomech. Eng. 2021, 143, 030801. [Google Scholar] [CrossRef]
- Yang, L.; Yang, Y.; Wang, S.; Li, Y.; Zhao, Z. In vitro mechanical loading models for periodontal ligament cells: From two-dimensional to three-dimensional models. Arch. Oral Biol. 2015, 60, 416–424. [Google Scholar] [CrossRef]
- Natali, A.N.; Pavan, P.G.; Scarpa, C. Numerical analysis of tooth mobility: Formulation of a non-linear constitutive law for the periodontal ligament. Dent. Mater. 2004, 20, 623–629. [Google Scholar] [CrossRef]
- Martino, F.; Perestrelo, A.R.; Vinarsky, V.; Pagliari, S.; Forte, G. Cellular Mechanotransduction: From Tension to Function. Front. Physiol. 2018, 9, 824. [Google Scholar] [CrossRef]
- Schiller, H.B.; Fassler, R. Mechanosensitivity and compositional dynamics of cell-matrix adhesions. EMBO Rep. 2013, 14, 509–519. [Google Scholar] [CrossRef]
- Webster, K.D.; Ng, W.P.; Fletcher, D.A. Tensional homeostasis in single fibroblasts. Biophys. J. 2014, 107, 146–155. [Google Scholar] [CrossRef]
- Brown, R.A.; Prajapati, R.; McGrouther, D.A.; Yannas, I.V.; Eastwood, M. Tensional homeostasis in dermal fibroblasts: Mechanical responses to mechanical loading in three-dimensional substrates. J. Cell. Physiol. 1998, 175, 323–332. [Google Scholar] [CrossRef]
- Kim, C.; Ye, F.; Ginsberg, M.H. Regulation of integrin activation. Annu. Rev. Cell. Dev. Biol. 2011, 27, 321–345. [Google Scholar] [CrossRef]
- Calderwood, D.A.; Zent, R.; Grant, R.; Rees, D.J.; Hynes, R.O.; Ginsberg, M.H. The Talin head domain binds to integrin beta subunit cytoplasmic tails and regulates integrin activation. J. Biol. Chem. 1999, 274, 28071–28074. [Google Scholar] [CrossRef]
- Haining, A.W.; von Essen, M.; Attwood, S.J.; Hytonen, V.P.; Del Rio Hernandez, A. All Subdomains of the Talin Rod Are Mechanically Vulnerable and May Contribute to Cellular Mechanosensing. ACS Nano. 2016, 10, 6648–6658. [Google Scholar] [CrossRef] [PubMed]
- Carisey, A.; Tsang, R.; Greiner, A.M.; Nijenhuis, N.; Heath, N.; Nazgiewicz, A.; Kemkemer, R.; Derby, B.; Spatz, J.; Ballestrem, C. Vinculin regulates the recruitment and release of core focal adhesion proteins in a force-dependent manner. Curr. Biol. 2013, 23, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Kiema, T.; Lad, Y.; Jiang, P.; Oxley, C.L.; Baldassarre, M.; Wegener, K.L.; Campbell, I.D.; Ylanne, J.; Calderwood, D.A. The molecular basis of filamin binding to integrins and competition with talin. Mol. Cell 2006, 21, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Das, M.; Ithychanda, S.S.; Qin, J.; Plow, E.F. Migfilin and filamin as regulators of integrin activation in endothelial cells and neutrophils. PLoS ONE 2011, 6, e26355. [Google Scholar] [CrossRef] [PubMed]
- Shifrin, Y.; Pinto, V.I.; Hassanali, A.; Arora, P.D.; McCulloch, C.A. Force-induced apoptosis mediated by the Rac/Pak/p38 signalling pathway is regulated by filamin A. Biochem. J. 2012, 445, 57–67. [Google Scholar] [CrossRef]
- Li, Y.; Zheng, W.; Liu, J.S.; Wang, J.; Yang, P.; Li, M.L.; Zhao, Z.H. Expression of osteoclastogenesis inducers in a tissue model of periodontal ligament under compression. J. Dent. Res. 2011, 90, 115–120. [Google Scholar] [CrossRef]
- Li, Y.; Li, M.; Tan, L.; Huang, S.; Zhao, L.; Tang, T.; Liu, J.; Zhao, Z. Analysis of time-course gene expression profiles of a periodontal ligament tissue model under compression. Arch. Oral Biol. 2013, 58, 511–522. [Google Scholar] [CrossRef]
- Kang, K.L.; Lee, S.W.; Ahn, Y.S.; Kim, S.H.; Kang, Y.G. Bioinformatic analysis of responsive genes in two-dimension and three-dimension cultured human periodontal ligament cells subjected to compressive stress. J. Periodontal Res. 2013, 48, 87–97. [Google Scholar] [CrossRef]
- Feng, L.; Yang, R.; Liu, D.; Wang, X.; Song, Y.; Cao, H.; He, D.; Gan, Y.; Kou, X.; Zhou, Y. PDL Progenitor-Mediated PDL Recovery Contributes to Orthodontic Relapse. J. Dent. Res. 2016, 95, 1049–1056. [Google Scholar] [CrossRef]
- Jin, S.S.; He, D.Q.; Wang, Y.; Zhang, T.; Yu, H.J.; Li, Z.X.; Zhu, L.S.; Zhou, Y.H.; Liu, Y. Mechanical force modulates periodontal ligament stem cell characteristics during bone remodelling via TRPV4. Cell Prolif. 2020, 53, e12912. [Google Scholar] [CrossRef]
- Brockhaus, J.; Craveiro, R.B.; Azraq, I.; Niederau, C.; Schroder, S.K.; Weiskirchen, R.; Jankowski, J.; Wolf, M. In Vitro Compression Model for Orthodontic Tooth Movement Modulates Human Periodontal Ligament Fibroblast Proliferation, Apoptosis and Cell Cycle. Biomolecules 2021, 11, 932. [Google Scholar] [CrossRef]
- Stemmler, A.; Symmank, J.; Steinmetz, J.; von Brandenstein, K.; Hennig, C.L.; Jacobs, C. GDF15 Supports the Inflammatory Response of PdL Fibroblasts Stimulated by P. gingivalis LPS and Concurrent Compression. Int. J. Mol. Sci. 2021, 22, 13608. [Google Scholar] [CrossRef]
- Jiang, N.; He, D.; Ma, Y.; Su, J.; Wu, X.; Cui, S.; Li, Z.; Zhou, Y.; Yu, H.; Liu, Y. Force-Induced Autophagy in Periodontal Ligament Stem Cells Modulates M1 Macrophage Polarization via AKT Signaling. Front. Cell Dev. Biol. 2021, 9, 666631. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, W.; Zhao, J.; Ma, X.; Shen, L.; Zhang, Y.; Jin, F.; Jin, Y. Mechanical stress regulates osteogenic differentiation and RANKL/OPG ratio in periodontal ligament stem cells by the Wnt/beta-catenin pathway. Biochim. Biophys. Acta 2016, 1860, 2211–2219. [Google Scholar] [CrossRef]
- Yamamoto, T.; Kita, M.; Kimura, I.; Oseko, F.; Terauchi, R.; Takahashi, K.; Kubo, T.; Kanamura, N. Mechanical stress induces expression of cytokines in human periodontal ligament cells. Oral Dis. 2006, 12, 171–175. [Google Scholar] [CrossRef]
- Wenger, K.H.; El-Awady, A.R.; Messer, R.L.; Sharawy, M.M.; White, G.; Lapp, C.A. Pneumatic pressure bioreactor for cyclic hydrostatic stress application: Mechanobiology effects on periodontal ligament cells. J. Appl. Physiol. (1985) 2011, 111, 1072–1079. [Google Scholar] [CrossRef]
- Yousefian, J.; Firouzian, F.; Shanfeld, J.; Ngan, P.; Lanese, R.; Davidovitch, Z. A new experimental model for studying the response of periodontal ligament cells to hydrostatic pressure. Am. J. Orthod. Dentofac. Orthop. 1995, 108, 402–409. [Google Scholar] [CrossRef]
- Jia, R.; Yi, Y.; Liu, J.; Pei, D.; Hu, B.; Hao, H.; Wu, L.; Wang, Z.; Luo, X.; Lu, Y. Cyclic compression emerged dual effects on the osteogenic and osteoclastic status of LPS-induced inflammatory human periodontal ligament cells according to loading force. BMC Oral Health. 2020, 20, 7. [Google Scholar] [CrossRef]
- Saminathan, A.; Sriram, G.; Vinoth, J.K.; Cao, T.; Meikle, M.C. Engineering the periodontal ligament in hyaluronan-gelatin-type I collagen constructs: Upregulation of apoptosis and alterations in gene expression by cyclic compressive strain. Tissue Eng. Part A 2015, 21, 518–529. [Google Scholar] [CrossRef]
- Nettelhoff, L.; Grimm, S.; Jacobs, C.; Walter, C.; Pabst, A.M.; Goldschmitt, J.; Wehrbein, H. Influence of mechanical compression on human periodontal ligament fibroblasts and osteoblasts. Clin. Oral Investig. 2016, 20, 621–629. [Google Scholar] [CrossRef]
- Saminathan, A.; Vinoth, K.J.; Low, H.H.; Cao, T.; Meikle, M.C. Engineering three-dimensional constructs of the periodontal ligament in hyaluronan-gelatin hydrogel films and a mechanically active environment. J. Periodontal Res. 2013, 48, 790–801. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Mohammed, A.; Oubaidin, M.; Evans, C.A.; Zhou, X.; Luan, X.; Diekwisch, T.G.; Atsawasuwan, P. Cyclic stretch and compression forces alter microRNA-29 expression of human periodontal ligament cells. Gene 2015, 566, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Shen, T.; Qiu, L.; Chang, H.; Yang, Y.; Jian, C.; Xiong, J.; Zhou, J.; Dong, S. Cyclic tension promotes osteogenic differentiation in human periodontal ligament stem cells. Int. J. Clin. Exp. Pathol. 2014, 7, 7872–7880. [Google Scholar] [PubMed]
- Wei, F.; Liu, D.; Feng, C.; Zhang, F.; Yang, S.; Hu, Y.; Ding, G.; Wang, S. microRNA-21 mediates stretch-induced osteogenic differentiation in human periodontal ligament stem cells. Stem Cells Dev. 2015, 24, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Feng, C.; Jin, Y.; Tan, W.; Wei, F. Identification and characterization of circular RNAs involved in mechanical force-induced periodontal ligament stem cells. J. Cell. Physiol. 2019, 234, 10166–10177. [Google Scholar] [CrossRef]
- Xi, X.; Zhao, Y.; Liu, H.; Li, Z.; Chen, S.; Liu, D. Nrf2 activation is involved in osteogenic differentiation of periodontal ligament stem cells under cyclic mechanical stretch. Exp. Cell Res. 2021, 403, 112598. [Google Scholar] [CrossRef]
- Meng, X.; Wang, W.; Wang, X. MicroRNA-34a and microRNA-146a target CELF3 and suppress the osteogenic differentiation of periodontal ligament stem cells under cyclic mechanical stretch. J. Dent. Sci. 2022, 17, 1281–1291. [Google Scholar] [CrossRef]
- Liu, J.; Li, Q.; Liu, S.; Gao, J.; Qin, W.; Song, Y.; Jin, Z. Periodontal Ligament Stem Cells in the Periodontitis Microenvironment Are Sensitive to Static Mechanical Strain. Stem Cells Int. 2017, 2017, 1380851. [Google Scholar] [CrossRef]
- Salim, C.; Muders, H.; Jager, A.; Konermann, A. Role of chaperone-assisted selective autophagy (CASA) in mechanical stress protection of periodontal ligament cells. J. Orofac. Orthop. 2022, 83, 1–12. [Google Scholar] [CrossRef]
- Ulbricht, A.; Eppler, F.J.; Tapia, V.E.; van der Ven, P.F.; Hampe, N.; Hersch, N.; Vakeel, P.; Stadel, D.; Haas, A.; Saftig, P.; et al. Cellular mechanotransduction relies on tension-induced and chaperone-assisted autophagy. Curr. Biol. 2013, 23, 430–435. [Google Scholar] [CrossRef]
- Ehrlicher, A.J.; Nakamura, F.; Hartwig, J.H.; Weitz, D.A.; Stossel, T.P. Mechanical strain in actin networks regulates FilGAP and integrin binding to filamin A. Nature 2011, 478, 260–263. [Google Scholar] [CrossRef]
- Huang, H.; Yang, R.; Zhou, Y.H. Mechanobiology of Periodontal Ligament Stem Cells in Orthodontic Tooth Movement. Stem Cells Int. 2018, 2018, 6531216. [Google Scholar] [CrossRef]
- Nemoto, T.; Kajiya, H.; Tsuzuki, T.; Takahashi, Y.; Okabe, K. Differential induction of collagens by mechanical stress in human periodontal ligament cells. Arch. Oral Biol. 2010, 55, 981–987. [Google Scholar] [CrossRef]
- Oortgiesen, D.A.; Yu, N.; Bronckers, A.L.; Yang, F.; Walboomers, X.F.; Jansen, J.A. A three-dimensional cell culture model to study the mechano-biological behavior in periodontal ligament regeneration. Tissue Eng. Part C Methods 2012, 18, 81–89. [Google Scholar] [CrossRef]
- Papadopoulou, A.; Iliadi, A.; Eliades, T.; Kletsas, D. Early responses of human periodontal ligament fibroblasts to cyclic and static mechanical stretching. Eur. J. Orthod. 2017, 39, 258–263. [Google Scholar] [CrossRef]
- Yu, W.; Su, X.; LI, M.; Wan, W.; Li, A.; Zhou, H.; Xu, F. Three-dimensional mechanical microenvironment enhanced osteogenic activity of mesenchymal stem cells-derived exosomes. Chem. Eng. J. 2021, 417, 128040. [Google Scholar] [CrossRef]
- Howard, P.S.; Kucich, U.; Taliwal, R.; Korostoff, J.M. Mechanical forces alter extracellular matrix synthesis by human periodontal ligament fibroblasts. J. Periodontal Res. 1998, 33, 500–508. [Google Scholar] [CrossRef]
- Xu, C.; Fan, Z.; Shan, W.; Hao, Y.; Ma, J.; Huang, Q.; Zhang, F. Cyclic stretch influenced expression of membrane connexin 43 in human periodontal ligament cell. Arch. Oral Biol. 2012, 57, 1602–1608. [Google Scholar] [CrossRef]
- Tang, N.; Zhao, Z.; Zhang, L.; Yu, Q.; Li, J.; Xu, Z.; Li, X. Up-regulated osteogenic transcription factors during early response of human periodontal ligament stem cells to cyclic tensile strain. Arch. Med. Sci. 2012, 8, 422–430. [Google Scholar] [CrossRef]
- Tang, M.; Peng, Z.; Mai, Z.; Chen, L.; Mao, Q.; Chen, Z.; Chen, Q.; Liu, L.; Wang, Y.; Ai, H. Fluid shear stress stimulates osteogenic differentiation of human periodontal ligament cells via the extracellular signal-regulated kinase 1/2 and p38 mitogen-activated protein kinase signaling pathways. J. Periodontol. 2014, 85, 1806–1813. [Google Scholar] [CrossRef]
- Zheng, L.; Chen, L.; Chen, Y.; Gui, J.; Li, Q.; Huang, Y.; Liu, M.; Jia, X.; Song, W.; Ji, J.; et al. The effects of fluid shear stress on proliferation and osteogenesis of human periodontal ligament cells. J. Biomech. 2016, 49, 572–579. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Zheng, L.; Na, J.; Li, X.; Yang, Z.; Chen, X.; Song, Y.; Li, C.; Zhou, L.; Fan, Y. Fluid shear stress promotes periodontal ligament cells proliferation via p38-AMOT-YAP. Cell. Mol. Life Sci. 2022, 79, 551. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.G.; Kim, S.G.; Viechnicki, B.; Kim, S.; Nah, H.D. Engineering of a periodontal ligament construct: Cell and fibre alignment induced by shear stress. J. Clin. Periodontol. 2011, 38, 1130–1136. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.H.; Chao, P.G.; Tai, W.C.; Chang, P.C. 3D-Printed Collagen-Based Waveform Microfibrous Scaffold for Periodontal Ligament Reconstruction. Int. J. Mol. Sci. 2021, 22, 7725. [Google Scholar] [CrossRef]
- Tsai, S.J.; Ding, Y.W.; Shih, M.C.; Tu, Y.K. Systematic review and sequential network meta-analysis on the efficacy of periodontal regenerative therapies. J. Clin. Periodontol. 2020, 47, 1108–1120. [Google Scholar] [CrossRef]
- Tassi, S.A.; Sergio, N.Z.; Misawa, M.Y.O.; Villar, C.C. Efficacy of stem cells on periodontal regeneration: Systematic review of pre-clinical studies. J. Periodontal Res. 2017, 52, 793–812. [Google Scholar] [CrossRef]
- Putame, G.; Gabetti, S.; Carbonaro, D.; Meglio, F.D.; Romano, V.; Sacco, A.M.; Belviso, I.; Serino, G.; Bignardi, C.; Morbiducci, U.; et al. Compact and tunable stretch bioreactor advancing tissue engineering implementation. Application to engineered cardiac constructs. Med. Eng. Phys. 2020, 84, 1–9. [Google Scholar] [CrossRef]
- Lim, D.; Renteria, E.S.; Sime, D.S.; Ju, Y.M.; Kim, J.H.; Criswell, T.; Shupe, T.D.; Atala, A.; Marini, F.C.; Gurcan, M.N.; et al. Bioreactor design and validation for manufacturing strategies in tissue engineering. Bio-Des. Manuf. 2022, 5, 43–63. [Google Scholar] [CrossRef]
- Gabetti, S.; Masante, B.; Cochis, A.; Putame, G.; Sanginario, A.; Armando, I.; Fiume, E.; Scalia, A.C.; Daou, F.; Baino, F.; et al. An automated 3D-printed perfusion bioreactor combinable with pulsed electromagnetic field stimulators for bone tissue investigations. Sci. Rep. 2022, 12, 13859. [Google Scholar] [CrossRef]



| Type of Scaffold | Cell Type | Production Method | Outcome | Reference |
|---|---|---|---|---|
| PGA or PCL+ βTCP | OBs, PDLSCs | Cell sheet technology | Periodontal complex | [50,51] |
| Human tooth root | PDLSCs, HUVEC | Cell sheet technology | PDL fibers | [52] |
| PCL | PDLSCs | 3D printing of fiber-guided scaffolds | Enhancement of the bone volume fraction and of tissue mineral density | [53,54] |
| collagen/bioactive glass/chitosan membrane | PDLSCs | electrospinning | Periodontal complex | [55] |
| PCL/PLGA+BMP-2,-7, CTGF | PDLSCs | 3D printing | Cementum-like layer formation | [56] |
| iTE scaffold (core/shell fibrous super-assembled framework+ BMP-2, bFGF) | PDLSCs | iTE | Periodontal complex | [57] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roato, I.; Masante, B.; Putame, G.; Massai, D.; Mussano, F. Challenges of Periodontal Tissue Engineering: Increasing Biomimicry through 3D Printing and Controlled Dynamic Environment. Nanomaterials 2022, 12, 3878. https://doi.org/10.3390/nano12213878
Roato I, Masante B, Putame G, Massai D, Mussano F. Challenges of Periodontal Tissue Engineering: Increasing Biomimicry through 3D Printing and Controlled Dynamic Environment. Nanomaterials. 2022; 12(21):3878. https://doi.org/10.3390/nano12213878
Chicago/Turabian StyleRoato, Ilaria, Beatrice Masante, Giovanni Putame, Diana Massai, and Federico Mussano. 2022. "Challenges of Periodontal Tissue Engineering: Increasing Biomimicry through 3D Printing and Controlled Dynamic Environment" Nanomaterials 12, no. 21: 3878. https://doi.org/10.3390/nano12213878
APA StyleRoato, I., Masante, B., Putame, G., Massai, D., & Mussano, F. (2022). Challenges of Periodontal Tissue Engineering: Increasing Biomimicry through 3D Printing and Controlled Dynamic Environment. Nanomaterials, 12(21), 3878. https://doi.org/10.3390/nano12213878







