Recent Advancements in Two-Dimensional Layered Molybdenum and Tungsten Carbide-Based Materials for Efficient Hydrogen Evolution Reactions
Abstract
:1. Introduction
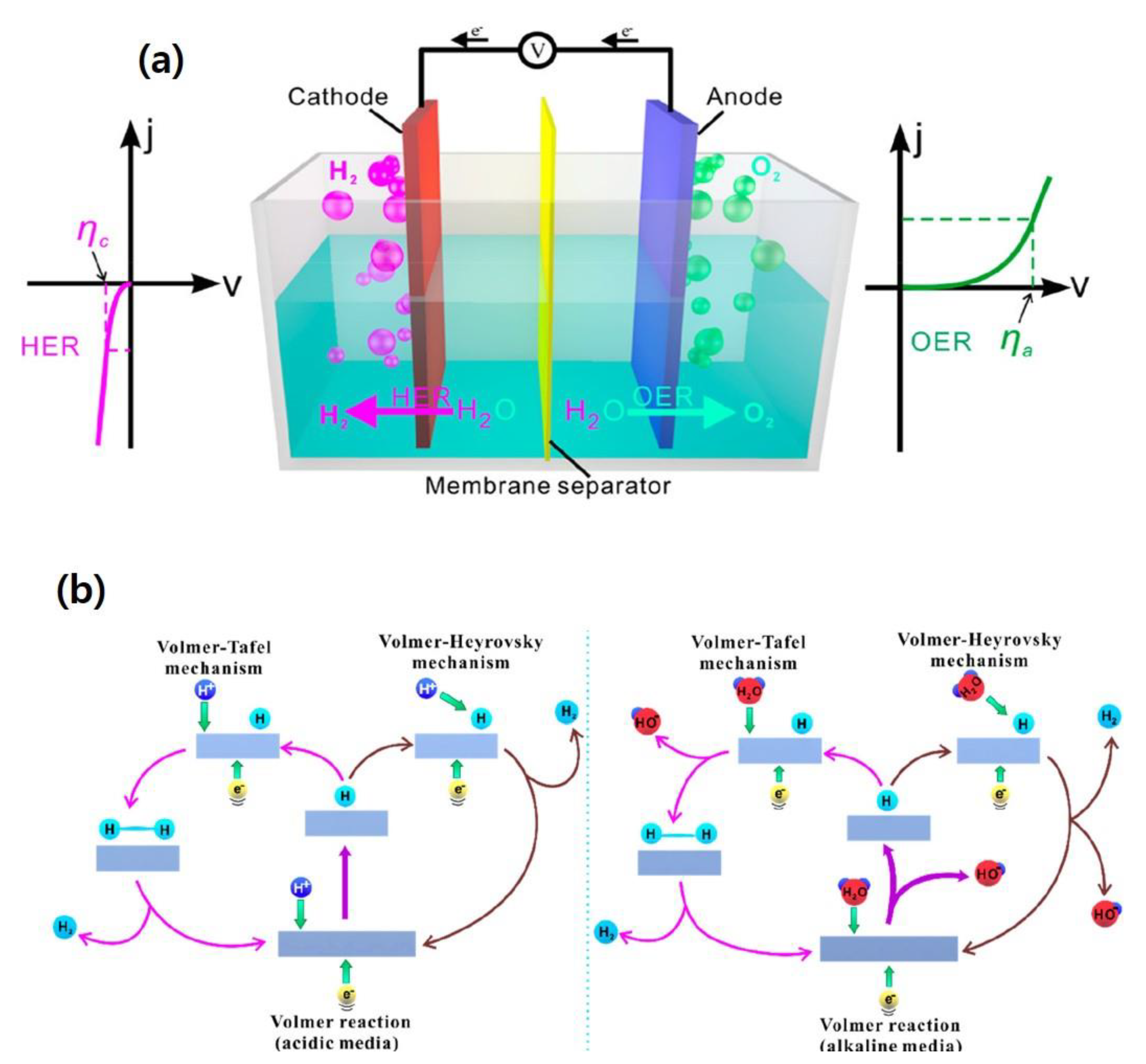
2. Basic Principles of Electrocatalytic HER
2.1. Parameters Governing the Electrocatalytic HER Process
2.1.1. Overpotential η
2.1.2. Faradic Efficiency
2.1.3. Tafel Plot
2.1.4. Turnover Frequency
2.1.5. Gibbs Free Energy (ΔGH)
2.1.6. Electrochemical Active Surface Area
2.2. Necessity of Noble-Metal Free Catalysts for HER
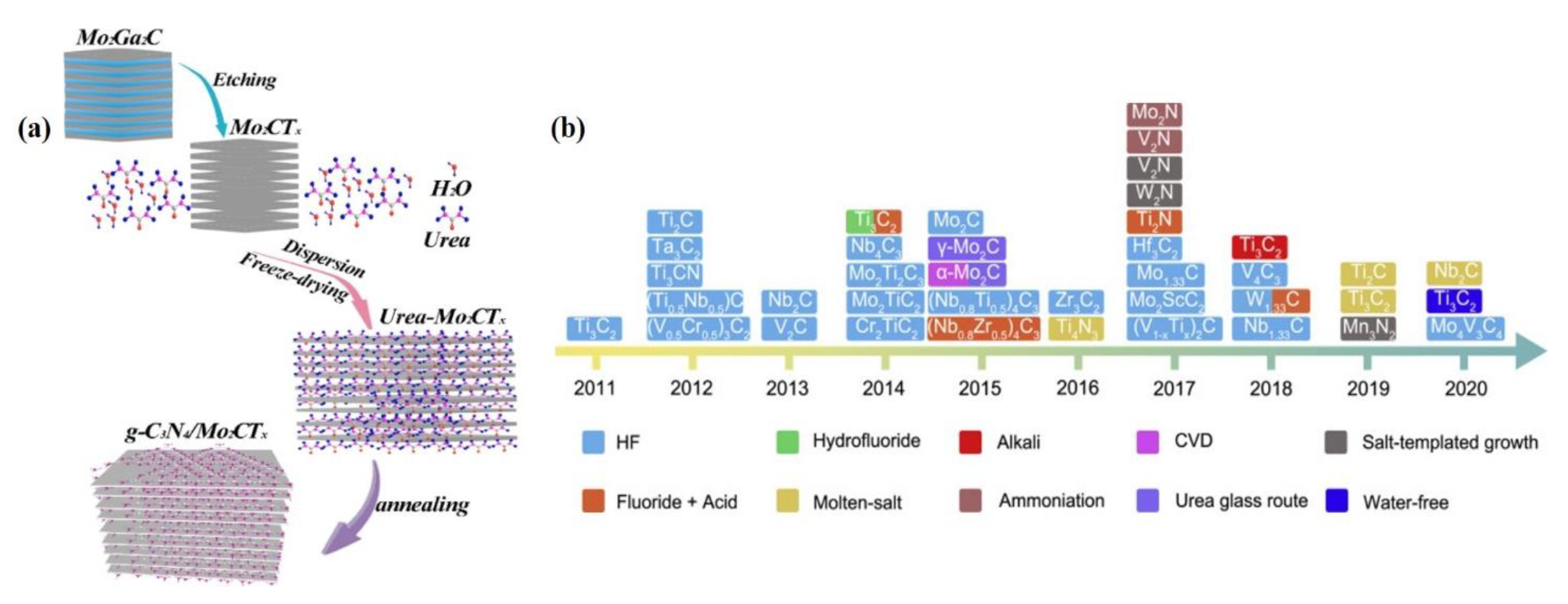
2.3. Synthesis of TMCs
2.3.1. Exfoliation Processes
2.3.2. CVD
2.3.3. Hydrothermal/Solvothermal Process
3. HER Activity of 2D TMCs
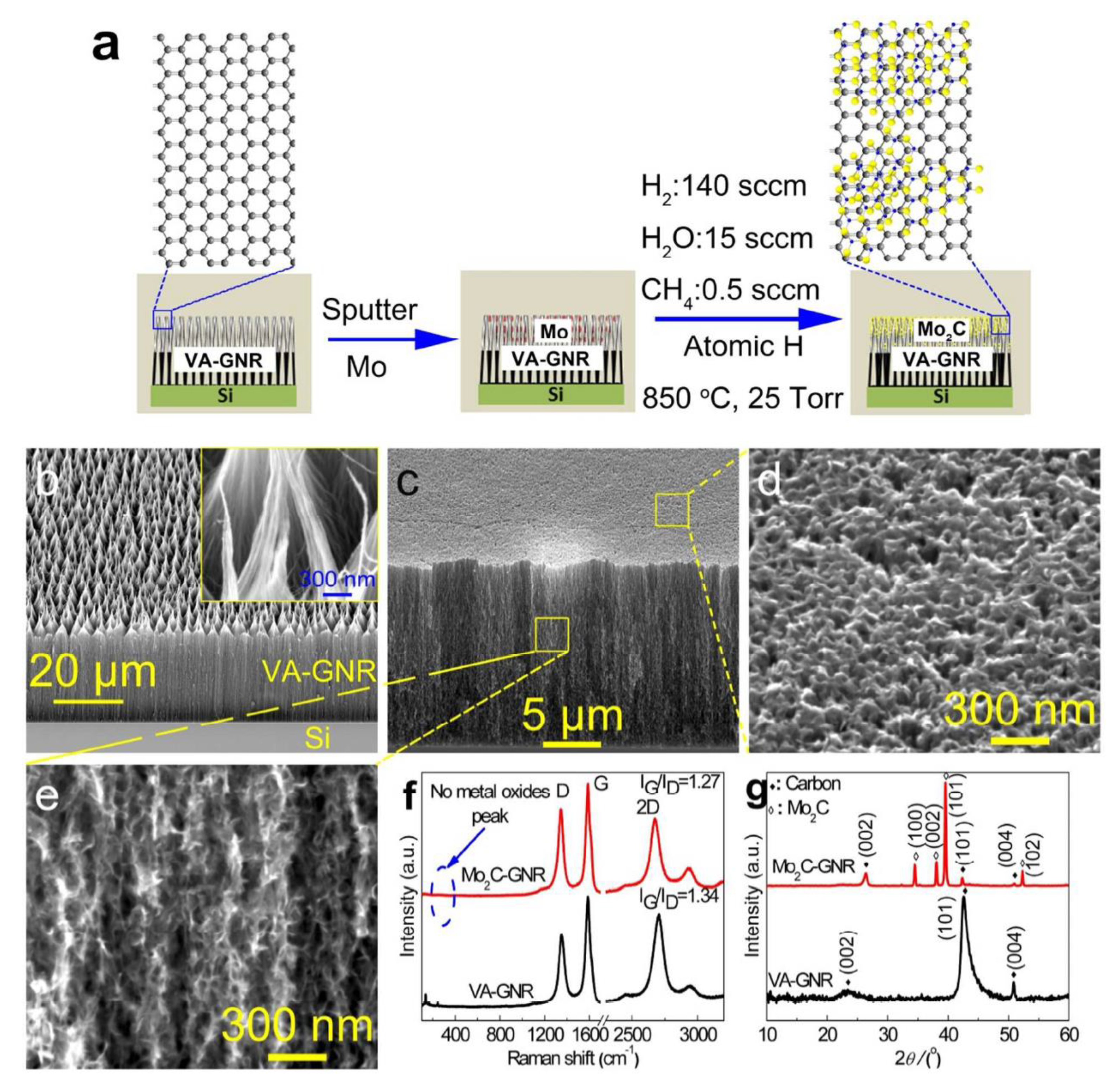
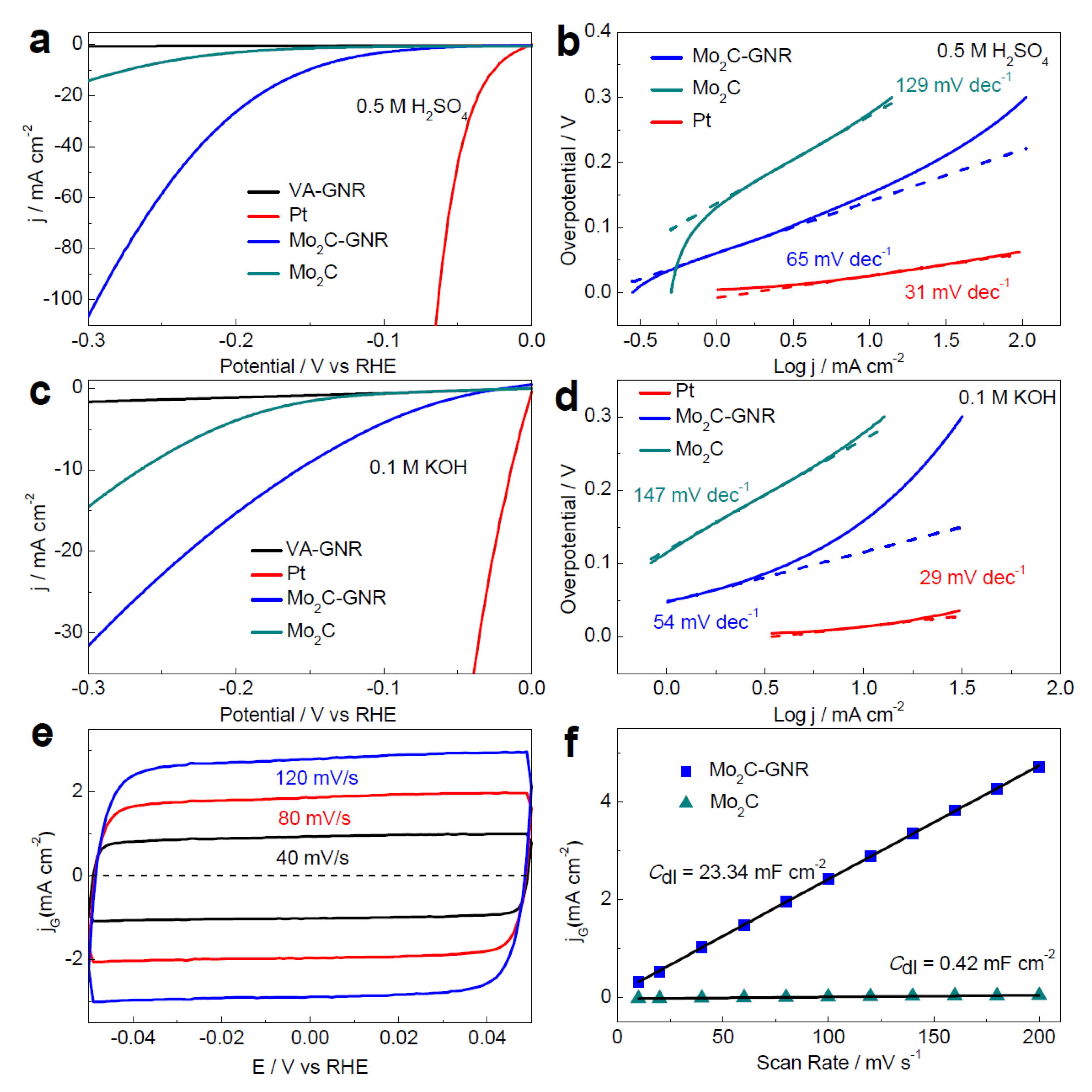
3.1. Molybdenum Carbide (Mo2C) and Its Composite
3.1.1. Molybdenum Carbide (Mo2C)
3.1.2. Mo2C and Carbon Composites
3.1.3. Heterometal Atom Doped Mo2C
3.2. Tungsten Carbide (WC/W2C)
3.2.1. Various Nanostructures of Tungsten Carbide Catalyst
3.2.2. Tungsten Carbide and Its Composites
4. Summary and Future Outlook
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Verger, L.; Xu, C.; Natu, V.; Cheng, H.-M.; Ren, W.; Barsoum, M.W. Overview of the synthesis of MXenes and other ultrathin 2D transition metal carbides and nitrides. Curr. Opin. Solid State Mater. Sci. 2019, 23, 149–163. [Google Scholar] [CrossRef]
- Vikraman, D.; Hussain, S.; Karuppasamy, K.; Feroze, A.; Kathalingam, A.; Sanmugam, A.; Chun, S.-H.; Jung, J.; Kim, H.-S. Engineering the novel MoSe2-Mo2C hybrid nanoarray electrodes for energy storage and water splitting applications. Appl. Catal. B Environ. 2020, 264, 118531. [Google Scholar] [CrossRef]
- Vikraman, D.; Hussain, S.; Truong, L.; Karuppasamy, K.; Kim, H.-J.; Maiyalagan, T.; Chun, S.-H.; Jung, J.; Kim, H.-S. Fabrication of MoS2/WSe2 heterostructures as electrocatalyst for enhanced hydrogen evolution reaction. Appl. Surf. Sci. 2019, 480, 611–620. [Google Scholar] [CrossRef]
- Vikraman, D.; Hussain, S.; Karuppasamy, K.; Kathalingam, A.; Jo, E.-B.; Sanmugam, A.; Jung, J.; Kim, H.-S. Engineering the active sites tuned MoS2 nanoarray structures by transition metal doping for hydrogen evolution and supercapacitor applications. J. Alloys Compd. 2022, 893, 162271. [Google Scholar] [CrossRef]
- Theerthagiri, J.; Karuppasamy, K.; Lee, S.J.; Shwetharani, R.; Kim, H.-S.; Pasha, S.K.K.; Ashokkumar, M.; Choi, M.Y. Fundamentals and comprehensive insights on pulsed laser synthesis of advanced materials for diverse photo- and electrocatalytic applications. Light Sci. Appl. 2022, 11, 250. [Google Scholar] [CrossRef] [PubMed]
- Mei, J.; Ayoko, G.A.; Hu, C.; Bell, J.M.; Sun, Z. Two-dimensional fluorine-free mesoporous Mo2C MXene via UV-induced selective etching of Mo2Ga2C for energy storage. Sustain. Mater. Technol. 2020, 25, e00156. [Google Scholar] [CrossRef]
- Jothi, V.R.; Karuppasamy, K.; Maiyalagan, T.; Rajan, H.; Jung, C.-Y.; Yi, S.C. Corrosion and Alloy Engineering in Rational Design of High Current Density Electrodes for Efficient Water Splitting. Adv. Energy Mater. 2020, 10, 1904020. [Google Scholar] [CrossRef]
- Huo, L.; Liu, B.; Zhang, G.; Zhang, J. Universal Strategy to Fabricate a Two-Dimensional Layered Mesoporous Mo2C Electrocatalyst Hybridized on Graphene Sheets with High Activity and Durability for Hydrogen Generation. ACS Appl. Mater. Interfaces 2016, 8, 18107–18118. [Google Scholar] [CrossRef]
- Du, C.; Li, P.; Zhuang, Z.; Fang, Z.; He, S.; Feng, L.; Chen, W. Highly porous nanostructures: Rational fabrication and promising application in energy electrocatalysis. Coord. Chem. Rev. 2022, 466, 214604. [Google Scholar] [CrossRef]
- Wang, C.; Yan, B.; Chen, Z.; You, B.; Liao, T.; Zhang, Q.; Lu, Y.; Jiang, S.; He, S. Recent advances in carbon substrate supported nonprecious nanoarrays for electrocatalytic oxygen evolution. J. Mater. Chem. A 2021, 9, 25773–25795. [Google Scholar] [CrossRef]
- Ligani Fereja, S.; Li, P.; Zhang, Z.; Guo, J.; Fang, Z.; Li, Z.; He, S.; Chen, W. W-doping induced abundant active sites in a 3D NiS2/MoO2 heterostructure as an efficient electrocatalyst for urea oxidation and hydrogen evolution reaction. Chem. Eng. J. 2022, 432, 134274. [Google Scholar] [CrossRef]
- Theerthagiri, J.; Durai, G.; Karuppasamy, K.; Arunachalam, P.; Elakkiya, V.; Kuppusami, P.; Maiyalagan, T.; Kim, H.-S. Recent advances in 2-D nanostructured metal nitrides, carbides, and phosphides electrodes for electrochemical supercapacitors—A brief review. J. Ind. Eng. Chem. 2018, 67, 12–27. [Google Scholar] [CrossRef]
- Vikraman, D.; Hussain, S.; Hailiang, L.; Karuppasamy, K.; Sivakumar, P.; Santhoshkumar, P.; Jung, J.; Kim, H.-S. Spinel-structured metal oxide-embedded MXene nanocomposites for efficient water splitting reactions. Inorg. Chem. Front. 2022. [Google Scholar] [CrossRef]
- Wan, L.; Tang, Y.; Chen, L.; Wang, K.; Zhang, J.; Gao, Y.; Lee, J.Y.; Lu, T.; Xu, X.; Li, J.; et al. In-situ construction of g-C3N4/Mo2CTx hybrid for superior lithium storage with significantly improved Coulombic efficiency and cycling stability. Chem. Eng. J. 2021, 410, 128349. [Google Scholar] [CrossRef]
- Li, N.; Peng, J.; Ong, W.-J.; Ma, T.; Arramel; Zhang, P.; Jiang, J.; Yuan, X.; Zhang, C. MXenes: An Emerging Platform for Wearable Electronics and Looking Beyond. Matter 2021, 4, 377–407. [Google Scholar] [CrossRef]
- Kang, Z.; Zheng, Z.; Wei, H.; Zhang, Z.; Tan, X.; Xiong, L.; Zhai, T.; Gao, Y. Controlled Growth of an Mo2C—Graphene Hybrid Film as an Electrode in Self-Powered Two-Sided Mo2C—Graphene/Sb2S0.42Se2.58/TiO2 Photodetectors. Sensors 2019, 19, 1099. [Google Scholar] [CrossRef] [Green Version]
- Burakov, V.S.; Butsen, A.V.; Brüser, V.; Harnisch, F.; Misakov, P.Y.; Nevar, E.A.; Rosenbaum, M.; Savastenko, N.A.; Tarasenko, N.V. Synthesis of tungsten carbide nanopowder via submerged discharge method. J. Nanoparticle Res. 2008, 10, 881–886. [Google Scholar] [CrossRef]
- Miao, M.; Pan, J.; He, T.; Yan, Y.; Xia, B.Y.; Wang, X. Molybdenum Carbide-Based Electrocatalysts for Hydrogen Evolution Reaction. Chem.—A Eur. J. 2017, 23, 10947–10961. [Google Scholar] [CrossRef]
- Kitchin, J.R.; Nørskov, J.K.; Barteau, M.A.; Chen, J.G. Trends in the chemical properties of early transition metal carbide surfaces: A density functional study. Catal. Today 2005, 105, 66–73. [Google Scholar] [CrossRef]
- Jun, H.; Kim, S.; Lee, J. Development strategies in transition metal carbide for hydrogen evolution reaction: A review. Korean J. Chem. Eng. 2020, 37, 1317–1330. [Google Scholar] [CrossRef]
- Eftekhari, A. Electrocatalysts for hydrogen evolution reaction. Int. J. Hydrog. Energy 2017, 42, 11053–11077. [Google Scholar] [CrossRef]
- Chen, J.G. Carbide and Nitride Overlayers on Early Transition Metal Surfaces: Preparation, Characterization, and Reactivities. Chem. Rev. 1996, 96, 1477–1498. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Hu, L.; Zhao, P.; Lee, L.Y.S.; Wong, K.-Y. Recent Advances in Electrocatalytic Hydrogen Evolution Using Nanoparticles. Chem. Rev. 2020, 120, 851–918. [Google Scholar] [CrossRef] [PubMed]
- Conway, B.; Tilak, B. Interfacial processes involving electrocatalytic evolution and oxidation of H2, and the role of chemisorbed H. Electrochim. Acta 2002, 47, 3571–3594. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, X.; Rui, Y.; Wang, R.; Li, X. Recent advances in non-precious metal electrocatalysts for pH-universal hydrogen evolution reaction. Green Energy Environ. 2021, 6, 458–478. [Google Scholar] [CrossRef]
- Sahoo, P.K.; Bisoi, S.R.; Huang, Y.-J.; Tsai, D.-S.; Lee, C.-P. 2D-Layered Non-Precious Electrocatalysts for Hydrogen Evolution Reaction: Fundamentals to Applications. Catalysts 2021, 11, 689. [Google Scholar] [CrossRef]
- Chen, Z.; Qing, H.; Zhou, K.; Sun, D.; Wu, R. Metal-organic framework-derived nanocomposites for electrocatalytic hydrogen evolution reaction. Prog. Mater. Sci. 2020, 108, 100618. [Google Scholar] [CrossRef]
- Ali, A.; Shen, P.K. Nonprecious metal’s graphene-supported electrocatalysts for hydrogen evolution reaction: Fundamentals to applications. Carbon Energy 2020, 2, 99–121. [Google Scholar] [CrossRef] [Green Version]
- Anantharaj, S.; Ede, S.R.; Karthick, K.; Sam Sankar, S.; Sangeetha, K.; Karthik, P.E.; Kundu, S. Precision and correctness in the evaluation of electrocatalytic water splitting: Revisiting activity parameters with a critical assessment. Energy Environ. Sci. 2018, 11, 744–771. [Google Scholar] [CrossRef]
- Anantharaj, S.; Karthik, P.E.; Noda, S. The Significance of Properly Reporting Turnover Frequency in Electrocatalysis Research. Angew. Chem. Int. Ed. 2021, 60, 23051–23067. [Google Scholar] [CrossRef]
- Connor, P.; Schuch, J.; Kaiser, B.; Jaegermann, W. The Determination of Electrochemical Active Surface Area and Specific Capacity Revisited for the System MnOx as an Oxygen Evolution Catalyst. Z. Phys. Chem. 2020, 234, 979–994. [Google Scholar] [CrossRef] [Green Version]
- McCrory, C.C.; Jung, S.; Ferrer, I.M.; Chatman, S.M.; Peters, J.C.; Jaramillo, T.F. Benchmarking hydrogen evolving reaction and oxygen evolving reaction electrocatalysts for solar water splitting devices. J. Am. Chem. Soc. 2015, 137, 4347–4357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shao, R.-Y.; Chen, L.-W.; Yan, Q.-Q.; Zeng, W.-J.; Yin, P.; Liang, H.-W. Is Pt/C More Electrocatalytic than Ru/C for Hydrogen Evolution in Alkaline Electrolytes? ACS Appl. Energy Mater. 2021, 4, 4284–4289. [Google Scholar] [CrossRef]
- Sheng, W.; Zhuang, Z.; Gao, M.; Zheng, J.; Chen, J.G.; Yan, Y. Correlating hydrogen oxidation and evolution activity on platinum at different pH with measured hydrogen binding energy. Nat. Commun. 2015, 6, 5848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Danilovic, N.; Subbaraman, R.; Strmcnik, D.; Stamenkovic, V.; Markovic, N. Electrocatalysis of the HER in acid and alkaline media. J. Serb. Chem. Soc. 2013, 78, 2007–2015. [Google Scholar] [CrossRef]
- Tian, X.; Zhao, P.; Sheng, W. Hydrogen evolution and oxidation: Mechanistic studies and material advances. Adv. Mater. 2019, 31, 1808066. [Google Scholar] [CrossRef] [PubMed]
- Vesborg, P.C.K.; Seger, B.; Chorkendorff, I. Recent Development in Hydrogen Evolution Reaction Catalysts and Their Practical Implementation. J. Phys. Chem. Lett. 2015, 6, 951–957. [Google Scholar] [CrossRef]
- Guo, Y.; Jin, S.; Wang, L.; He, P.; Hu, Q.; Fan, L.-Z.; Zhou, A. Synthesis of two-dimensional carbide Mo2CTx MXene by hydrothermal etching with fluorides and its thermal stability. Ceram. Int. 2020, 46, 19550–19556. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, Y.; Gao, F.; Wang, Y.; Shen, X.; He, N.; Zhu, J.; Chen, Y.; Wan, X.; Lian, X.; et al. Formation of new MXene film using spinning coating method with DMSO solution and its application in advanced memristive device. Ceram. Int. 2019, 45, 19467–19472. [Google Scholar] [CrossRef]
- Gkountaras, A.; Kim, Y.; Coraux, J.; Bouchiat, V.; Lisi, S.; Barsoum, M.W.; Ouisse, T. Mechanical Exfoliation of Select MAX Phases and Mo4Ce4Al7C3 Single Crystals to Produce MAXenes. Small 2020, 16, 1905784. [Google Scholar] [CrossRef]
- Xu, C.; Wang, L.; Liu, Z.; Chen, L.; Guo, J.; Kang, N.; Ma, X.L.; Cheng, H.M.; Ren, W. Large-area high-quality 2D ultrathin Mo2C superconducting crystals. Nat. Mater. 2015, 14, 1135–1141. [Google Scholar] [CrossRef] [PubMed]
- Geng, D.; Zhao, X.; Li, L.; Song, P.; Tian, B.; Liu, W.; Chen, J.; Shi, D.; Lin, M.; Zhou, W.; et al. Controlled growth of ultrathin Mo2C superconducting crystals on liquid Cu surface. 2D Mater. 2016, 4, 011012. [Google Scholar] [CrossRef]
- Fan, X.; Liu, Y.; Peng, Z.; Zhang, Z.; Zhou, H.; Zhang, X.; Yakobson, B.I.; Goddard, W.A.; Guo, X.; Hauge, R.H.; et al. Atomic H-Induced Mo2C Hybrid as an Active and Stable Bifunctional Electrocatalyst. ACS Nano 2017, 11, 384–394. [Google Scholar] [CrossRef] [Green Version]
- Jia, J.; Xiong, T.; Zhao, L.; Wang, F.; Liu, H.; Hu, R.; Zhou, J.; Zhou, W.; Chen, S. Ultrathin N-Doped Mo2C Nanosheets with Exposed Active Sites as Efficient Electrocatalyst for Hydrogen Evolution Reactions. ACS Nano 2017, 11, 12509–12518. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.F.; Li, Y.H.; Yang, S.; Liu, P.F.; Yu, M.Q.; Yang, H.G. Molybdenum carbide stabilized on graphene with high electrocatalytic activity for hydrogen evolution reaction. Chem. Commun. 2014, 50, 13135–13137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wan, C.; Regmi, Y.N.; Leonard, B.M. Multiple Phases of Molybdenum Carbide as Electrocatalysts for the Hydrogen Evolution Reaction. Angew. Chem. Int. Ed. 2014, 126, 6525–6528. [Google Scholar] [CrossRef]
- Fan, M.; Chen, H.; Wu, Y.; Feng, L.-L.; Liu, Y.; Li, G.-D.; Zou, X. Growth of molybdenum carbide micro-islands on carbon cloth toward binder-free cathodes for efficient hydrogen evolution reaction. J. Mater. Chem. A 2015, 3, 16320–16326. [Google Scholar] [CrossRef]
- Vrubel, H.; Hu, X. Molybdenum Boride and Carbide Catalyze Hydrogen Evolution in both Acidic and Basic Solutions. Angew. Chem. Int. Ed. 2012, 51, 12703–12706. [Google Scholar] [CrossRef] [Green Version]
- Qamar, M.; Adam, A.; Merzougui, B.; Helal, A.; Abdulhamid, O.; Siddiqui, M. Metal–organic framework-guided growth of Mo2C embedded in mesoporous carbon as a high-performance and stable electrocatalyst for the hydrogen evolution reaction. J. Mater. Chem. A 2016, 4, 16225–16232. [Google Scholar] [CrossRef]
- Chen, W.-F.; Wang, C.-H.; Sasaki, K.; Marinkovic, N.; Xu, W.; Muckerman, J.; Zhu, Y.; Adzic, R. Highly active and durable nanostructured molybdenum carbide electrocatalysts for hydrogen production. Energy Environ. Sci. 2013, 6, 943–951. [Google Scholar] [CrossRef]
- Tang, C.; Sun, A.; Xu, Y.; Wu, Z.; Wang, D. High specific surface area Mo2C nanoparticles as an efficient electrocatalyst for hydrogen evolution. J. Power Source 2015, 296, 18–22. [Google Scholar] [CrossRef]
- Lin, H.; Shi, Z.; He, S.; Yu, X.; Wang, S.; Gao, Q.; Tang, Y. Heteronanowires of MoC–Mo2C as efficient electrocatalysts for hydrogen evolution reaction. Chem. Sci. 2016, 7, 3399–3405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, P.; Qi, J.; Chen, X.; Li, C.; Wang, T.; Liang, C. New insights into high-valence state Mo in molybdenum carbide nanobelts for hydrogen evolution reaction. Int. J. Hydrog. Energy 2017, 42, 10880–10890. [Google Scholar] [CrossRef]
- Wu, H.B.; Xia, B.Y.; Yu, L.; Yu, X.-Y.; Lou, X.W.D. Porous molybdenum carbide nano-octahedrons synthesized via confined carburization in metal-organic frameworks for efficient hydrogen production. Nat. Commun. 2015, 6, 6512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, T.; Zheng, L.; Qin, J.; Zhao, D.; Cao, M. A three-dimensional hierarchically porous Mo2C architecture: Salt-template synthesis of a robust electrocatalyst and anode material towards the hydrogen evolution reaction and lithium storage. J. Mater. Chem. A 2017, 5, 20228–20238. [Google Scholar] [CrossRef]
- Pu, Z.; Wang, M.; Kou, Z.; Amiinu, I.S.; Mu, S. Mo 2 C quantum dot embedded chitosan-derived nitrogen-doped carbon for efficient hydrogen evolution in a broad pH range. Chem. Commun. 2016, 52, 12753–12756. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Gong, Q.; Song, X.; Feng, K.; Nie, K.; Zhao, F.; Wang, Y.; Zeng, M.; Zhong, J.; Li, Y. Mo2C Nanoparticles Dispersed on Hierarchical Carbon Microflowers for Efficient Electrocatalytic Hydrogen Evolution. ACS Nano 2016, 10, 11337–11343. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wang, J.; Luo, X.; Wu, Z.; Ye, L. In situ Preparation of Mo2C Nanoparticles Embedded in Ketjenblack Carbon as Highly Efficient Electrocatalysts for Hydrogen Evolution. ACS Sustain. Chem. Eng. 2018, 6, 983–990. [Google Scholar] [CrossRef]
- Wu, C.; Li, J. Unique Hierarchical Mo2C/C Nanosheet Hybrids as Active Electrocatalyst for Hydrogen Evolution Reaction. ACS Appl. Mater. Interfaces 2017, 9, 41314–41322. [Google Scholar] [CrossRef]
- Lv, C.; Huang, Z.; Yang, Q.; Wei, G.; Chen, Z.; Humphrey, M.G.; Zhang, C. Ultrafast synthesis of molybdenum carbide nanoparticles for efficient hydrogen generation. J. Mater. Chem. A 2017, 5, 22805–22812. [Google Scholar] [CrossRef]
- He, C.; Tao, J. Synthesis of nanostructured clean surface molybdenum carbides on graphene sheets as efficient and stable hydrogen evolution reaction catalysts. Chem. Commun. 2015, 51, 8323–8325. [Google Scholar] [CrossRef] [PubMed]
- Ojha, K.; Saha, S.; Kolev, H.; Kumar, B.; Ganguli, A.K. Composites of graphene-Mo2C rods: Highly active and stable electrocatalyst for hydrogen evolution reaction. Electrochim. Acta 2016, 193, 268–274. [Google Scholar] [CrossRef]
- Zhang, K.; Zhao, Y.; Fu, D.; Chen, Y. Molybdenum carbide nanocrystal embedded N-doped carbon nanotubes as electrocatalysts for hydrogen generation. J. Mater. Chem. A 2015, 3, 5783–5788. [Google Scholar] [CrossRef]
- Youn, D.H.; Han, S.; Kim, J.Y.; Kim, J.Y.; Park, H.; Choi, S.H.; Lee, J.S. Highly active and stable hydrogen evolution electrocatalysts based on molybdenum compounds on carbon nanotube–graphene hybrid support. ACS Nano 2014, 8, 5164–5173. [Google Scholar] [CrossRef]
- Xiao, J.; Zhang, Y.; Zhang, Z.; Lv, Q.-Y.; Jing, F.; Chi, K.; Wang, S. Self-Supported Biocarbon Fiber Electrode Decorated with Molybdenum Carbide Nanoparticles for Highly Active Hydrogen Evolution Reaction. ACS Appl. Mater. Interfaces 2017, 9, 22604–22611. [Google Scholar] [CrossRef]
- Jia, J.; Zhou, W.; Wei, Z.; Xiong, T.; Li, G.; Zhao, L.; Zhang, X.; Liu, H.; Zhou, J.; Chen, S. Molybdenum carbide on hierarchical porous carbon synthesized from Cu-MoO2 as efficient electrocatalysts for electrochemical hydrogen generation. Nano Energy 2017, 41, 749–757. [Google Scholar] [CrossRef]
- Fan, M.; Zheng, Y.; Li, A.; Ma, Y.; Huo, Q.; Qiao, Z.A.; Dai, S. Sprout-like Growth of Mesoporous Mo2C/NC Nanonetworks as Efficient Electrocatalysts for Hydrogen Evolution. ChemCatChem 2018, 10, 625–631. [Google Scholar] [CrossRef]
- Li, J.-S.; Wang, Y.; Liu, C.-H.; Li, S.-L.; Wang, Y.-G.; Dong, L.-Z.; Dai, Z.-H.; Li, Y.-F.; Lan, Y.-Q. Coupled molybdenum carbide and reduced graphene oxide electrocatalysts for efficient hydrogen evolution. Nat. Commun. 2016, 7, 11204. [Google Scholar] [CrossRef]
- Wan, J.; Wu, J.; Gao, X.; Li, T.; Hu, Z.; Yu, H.; Huang, L. Structure Confined Porous Mo2C for Efficient Hydrogen Evolution. Adv. Funct. Mater. 2017, 27, 1703933. [Google Scholar] [CrossRef]
- Lin, H.; Liu, N.; Shi, Z.; Guo, Y.; Tang, Y.; Gao, Q. Cobalt-Doping in Molybdenum-Carbide Nanowires Toward Efficient Electrocatalytic Hydrogen Evolution. Adv. Funct. Mater. 2016, 26, 5590–5598. [Google Scholar] [CrossRef]
- Xiong, K.; Li, L.; Zhang, L.; Ding, W.; Peng, L.; Wang, Y.; Chen, S.; Tan, S.; Wei, Z. Ni-doped Mo2C nanowires supported on Ni foam as a binder-free electrode for enhancing the hydrogen evolution performance. J. Mater. Chem. A 2015, 3, 1863–1867. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, G.; Li, G.D.; Sun, Y.; Asefa, T.; Chen, W.; Zou, X. Coupling Mo2C with Nitrogen-Rich Nanocarbon Leads to Efficient Hydrogen-Evolution Electrocatalytic Sites. Angew. Chem. Int. Ed. 2015, 54, 10752–10757. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, J.; Zhu, M.; Bao, X.; Xiao, B.; Su, D.; Li, H.; Wang, Y. Molybdenum-carbide-modified nitrogen-doped carbon vesicle encapsulating nickel nanoparticles: A highly efficient, low-cost catalyst for hydrogen evolution reaction. J. Am. Chem. Soc 2015, 137, 15753–15759. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Tang, Q.; He, B.; Yang, P. Carbide decorated carbon nanotube electrocatalyst for high-efficiency hydrogen evolution from seawater. RSC Adv. 2016, 6, 93267–93274. [Google Scholar] [CrossRef]
- Du, C.; Huang, H.; Wu, Y.; Wu, S.; Song, W. Ultra-efficient electrocatalytic hydrogen evolution at one-step carbonization generated molybdenum carbide nanosheets/N-doped carbon. Nanoscale 2016, 8, 16251–16258. [Google Scholar] [CrossRef]
- Zhao, Z.; Qin, F.; Kasiraju, S.; Xie, L.; Alam, M.K.; Chen, S.; Wang, D.; Ren, Z.; Wang, Z.; Grabow, L.C. Vertically Aligned MoS2/Mo2C hybrid Nanosheets Grown on Carbon Paper for Efficient Electrocatalytic Hydrogen Evolution. ACS Catal. 2017, 7, 7312–7318. [Google Scholar] [CrossRef]
- Griesser, C.; Li, H.; Wernig, E.-M.; Winkler, D.; Shakibi Nia, N.; Mairegger, T.; Götsch, T.; Schachinger, T.; Steiger-Thirsfeld, A.; Penner, S.; et al. True Nature of the Transition-Metal Carbide/Liquid Interface Determines Its Reactivity. ACS Catal. 2021, 11, 4920–4928. [Google Scholar] [CrossRef]
- Kuang, M.; Huang, W.; Hegde, C.; Fang, W.; Tan, X.; Liu, C.; Ma, J.; Yan, Q. Interface engineering in transition metal carbides for electrocatalytic hydrogen generation and nitrogen fixation. Mater. Horiz. 2020, 7, 32–53. [Google Scholar] [CrossRef]
- Hussain, S.; Faizan, M.; Vikraman, D.; Rabani, I.; Ali, B.; Kim, H.-S.; Jung, J.; Nam, K.-W. Eutectoid WxC embedded WS2 nanosheets as a hybrid composite anode for lithium-ion batteries. Ceram. Int. 2021, 47, 18646–18655. [Google Scholar] [CrossRef]
- Yang, C.; Zhao, R.; Xiang, H.; Wu, J.; Zhong, W.; Li, W.; Zhang, Q.; Yang, N.; Li, X. Ni-Activated Transition Metal Carbides for Efficient Hydrogen Evolution in Acidic and Alkaline Solutions. Adv. Energy Mater. 2020, 10, 2002260. [Google Scholar] [CrossRef]
- Li, Z.; Yu, L.; Milligan, C.; Ma, T.; Zhou, L.; Cui, Y.; Qi, Z.; Libretto, N.; Xu, B.; Luo, J.; et al. Two-dimensional transition metal carbides as supports for tuning the chemistry of catalytic nanoparticles. Nat. Commun. 2018, 9, 5258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levy, R.B.; Boudart, M. Platinum-like behavior of tungsten carbide in surface catalysis. Science 1973, 181, 547–549. [Google Scholar] [CrossRef] [PubMed]
- Gong, Q.; Wang, Y.; Hu, Q.; Zhou, J.; Feng, R.; Duchesne, P.N.; Zhang, P.; Chen, F.; Han, N.; Li, Y.; et al. Ultrasmall and phase-pure W2C nanoparticles for efficient electrocatalytic and photoelectrochemical hydrogen evolution. Nat. Commun. 2016, 7, 13216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, H.; Huang, J.; Wang, W.; Ma, C. Preparation of nano-crystalline tungsten carbide thin film electrode and its electrocatalytic activity for hydrogen evolution. Electrochem. Commun. 2005, 7, 1045–1049. [Google Scholar] [CrossRef]
- Ren, B.; Li, D.; Jin, Q.; Cui, H.; Wang, C. Novel porous tungsten carbide hybrid nanowires on carbon cloth for high-performance hydrogen evolution. J. Mater. Chem. A 2017, 5, 13196–13203. [Google Scholar] [CrossRef]
- Ko, Y.-J.; Cho, J.-M.; Kim, I.; Jeong, D.S.; Lee, K.-S.; Park, J.-K.; Baik, Y.-J.; Choi, H.-J.; Lee, W.-S. Tungsten carbide nanowalls as electrocatalyst for hydrogen evolution reaction: New approach to durability issue. Appl. Catal. B Environ. 2017, 203, 684–691. [Google Scholar] [CrossRef]
- Xu, Y.-T.; Xiao, X.; Ye, Z.-M.; Zhao, S.; Shen, R.; He, C.-T.; Zhang, J.-P.; Li, Y.; Chen, X.-M. Cage-Confinement Pyrolysis Route to Ultrasmall Tungsten Carbide Nanoparticles for Efficient Electrocatalytic Hydrogen Evolution. J. Am. Chem. Soc. 2017, 139, 5285–5288. [Google Scholar] [CrossRef]
- Ishii, T.; Yamada, K.; Osuga, N.; Imashiro, Y.; Ozaki, J.-i. Single-Step Synthesis of W2C Nanoparticle-Dispersed Carbon Electrocatalysts for Hydrogen Evolution Reactions Utilizing Phosphate Groups on Carbon Edge Sites. ACS Omega 2016, 1, 689–695. [Google Scholar] [CrossRef] [Green Version]
- Tang, C.; Wang, D.; Wu, Z.; Duan, B. Tungsten carbide hollow microspheres as electrocatalyst and platinum support for hydrogen evolution reaction. Int. J. Hydrog. Energy 2015, 40, 3229–3237. [Google Scholar] [CrossRef]
- Chen, Z.; Qin, M.; Chen, P.; Jia, B.; He, Q.; Qu, X. Tungsten carbide/carbon composite synthesized by combustion-carbothermal reduction method as electrocatalyst for hydrogen evolution reaction. Int. J. Hydrog. Energy 2016, 41, 13005–13013. [Google Scholar] [CrossRef]
- Zeng, M.; Chen, Y.; Li, J.; Xue, H.; Mendes, R.G.; Liu, J.; Zhang, T.; Rümmeli, M.H.; Fu, L. 2D WC single crystal embedded in graphene for enhancing hydrogen evolution reaction. Nano Energy 2017, 33, 356–362. [Google Scholar] [CrossRef]
- Han, L.; Xu, M.; Han, Y.; Yu, Y.; Dong, S. Core–Shell-Structured Tungsten Carbide Encapsulated within Nitrogen-Doped Carbon Spheres for Enhanced Hydrogen Evolution. ChemSusChem 2016, 9, 2784–2787. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.-F.; Pitkänen, O.; Mäklin, J.; Puskas, R.; Kukovecz, A.; Dombovari, A.; Toth, G.; Kordas, K. Synthesis of tungsten carbide and tungsten disulfide on vertically aligned multi-walled carbon nanotube forests and their application as non-Pt electrocatalysts for the hydrogen evolution reaction. J. Mater. Chem. A 2015, 3, 14609–14616. [Google Scholar] [CrossRef]
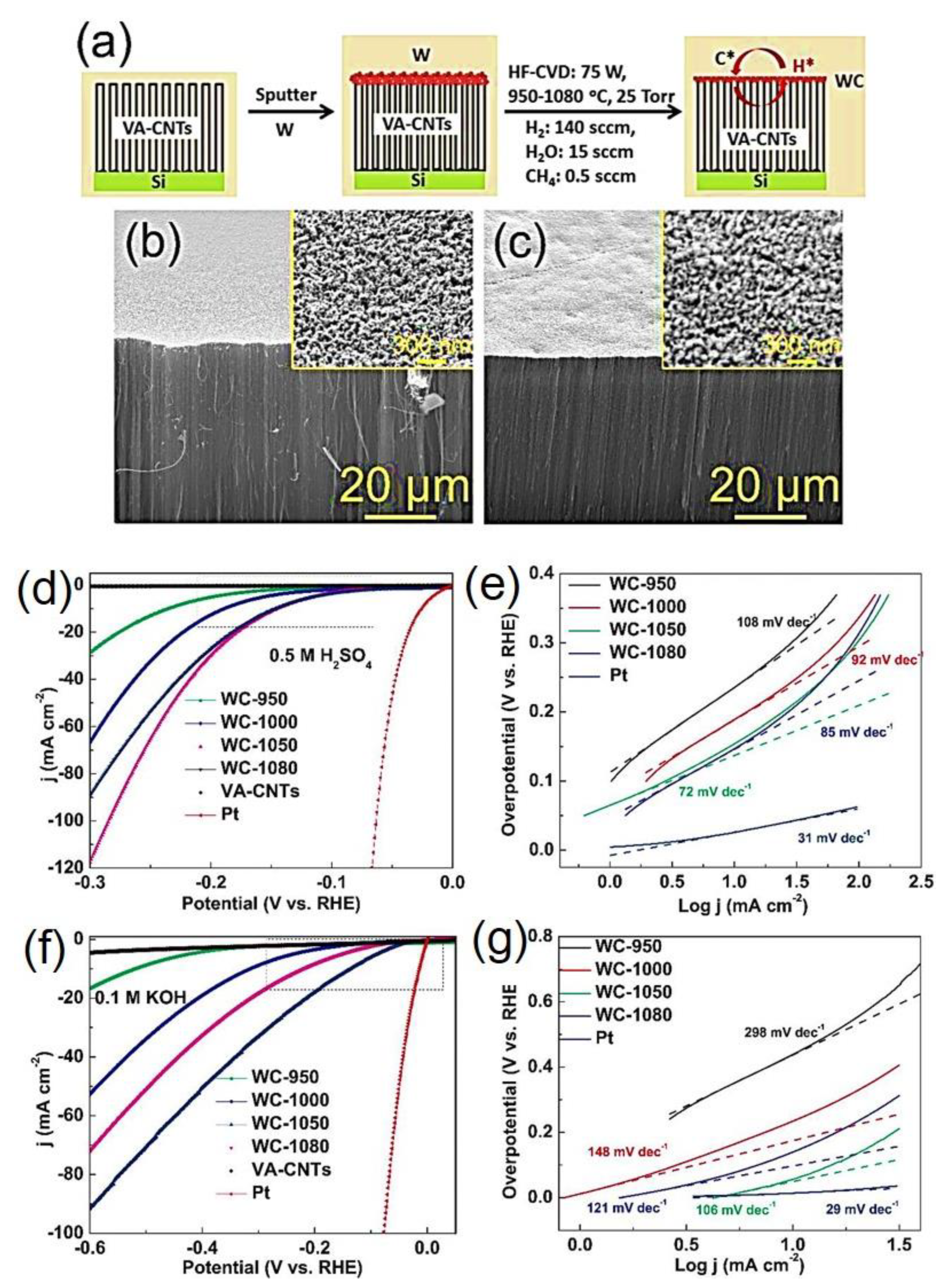
- Fan, X.; Zhou, H.; Guo, X. WC Nanocrystals Grown on Vertically Aligned Carbon Nanotubes: An Efficient and Stable Electrocatalyst for Hydrogen Evolution Reaction. ACS Nano 2015, 9, 5125–5134. [Google Scholar] [CrossRef]
- Li, Z.; Cui, Y.; Wu, Z.; Milligan, C.; Zhou, L.; Mitchell, G.; Xu, B.; Shi, E.; Miller, J.T.; Ribeiro, F.H. Reactive metal–support interactions at moderate temperature in two-dimensional niobium-carbide-supported platinum catalysts. Nat. Catal. 2018, 1, 349–355. [Google Scholar] [CrossRef]
- Bernal, S.; Calvino, J.; Gatica, J.; Larese, C.; López-Cartes, C.; Pérez-Omil, J. Nanostructural Evolution of a Pt/CeO2 Catalyst Reduced at Increasing Temperatures (473–1223 K): A HREM Study. J. Catal. 1997, 169, 510–515. [Google Scholar] [CrossRef]
- Sabnis, K.D.; Cui, Y.; Akatay, M.C.; Shekhar, M.; Lee, W.-S.; Miller, J.T.; Delgass, W.N.; Ribeiro, F.H. Water–gas shift catalysis over transition metals supported on molybdenum carbide. J. Catal. 2015, 331, 162–171. [Google Scholar] [CrossRef]

| Catalyst | Synthesis | Morphology | ηj(mV) | j(mA cm−2) | Tafel Slope (mV dec−1) | Ref |
|---|---|---|---|---|---|---|
| N-Mo2C NSs | CVD | nanosheets | 99 | 10 | 48.3 | [44] |
| Mo2C-RGO | Hydrothermal carbonization | nanoparticles | 70 | 10 | 57.3 | [45] |
| β-Mo2C | Hydrothermal/calcination | nanospheres | 240 | 10 | 120 | [46] |
| Mo2C/CC | Hydrothermal/calcination | Nano-island | 140 | 10 | 124 | [47] |
| Mo2C | Chemical activation process | nanoparticle | 240 | 20 | 56 | [48] |
| β-Mo2C/C | hydrothermal | Irregular ill-defined particles | 330 | 20 | 72 | [49] |
| Mo2C/CNT | In situ-carburization | nanoparticle | 64 | 1 | 63 | [50] |
| β-Mo2C | Hydrothermal/calcination | nanoparticle | 165 | 10 | 55 | [51] |
| MoC-Mo2C | Controlled carbonization | nanoparticle | 33 | 10 | 42 | [52] |
| MoxC | One-pot pyrolysis | nanobelts | 50 | 10 | 49.6 | [53] |
| 3DHP-Mo2C | Scalable salt-template process | Highly interconnected 3D porous network | 75 | 1 | 75 | [55] |
| Mo2C/NCF | High temperature calcination | nanoflowers | 144 | 10 | 65 | [57] |
| Mo2C/C | pyrolysis | nanosheets | 180 | 10 | 72 | [59] |
| Mo2C-G | In situ-carburization | nanoparticle | 150 | 10 | 57 | [61] |
| Mo2C/CLCN | Hydrothermal/calcination | nanorods | 155 | 10 | 48.2 | [66] |
| Mo2C/NC | Polymerization/carbonization | sprout | 140 | 10 | 114.4 | [67] |
| 1D Mo2C | In situ-carburization | nanosheets | 36 | 10 | 47 | [69] |
| Catalyst | Synthesis | Morphology | ηj(mV) | j(mA cm−2) | Tafel Slope (mV dec−1) | Ref |
|---|---|---|---|---|---|---|
| W2C/MWCNT | carburization | nanoparticles | 123 | 10 | 485 | [83] |
| W2C-thinfilm | CVD | nanograins | 263 | 10 | 42.2 | [84] |
| W2C | Plasma assisted carburization | nanowires | 118 | 10 | 55 | [85] |
| WC | Plasma assisted deposition | nanowall | 160 | 10 | 67 | [86] |
| WC | Cage confinement pyrolysis | nanoparticle | 51 | 10 | 49 | [87] |
| W2C | High temperature calcination | nanoparticles | 368 | 20 | 50 | [88] |
| Pt/WC | High temperature calcination | Spherical particles | 22 | 10 | 28.8 | [89] |
| WCx/C | Combustion reaction | nanoparticle | 264 | 10 | 85 | [90] |
| 2D WC-G | Liquid metal solvent-based co-segregation strategy | Single crystals | 120 | 10 | 38 | [91] |
| N-doped WC | In-situ polymerization/carburization | nanospheres | 290 | 10 | 110 | [92] |
| WC/CNT | CVD | nanoflakes | 435 | 10 | 103 | [93] |
| W2C | carburization | nanoparticles | 50 | 10 | 45 | [83] |
| W2C | HF-CVD | nanocrystal | 117.6 | 10 | 72 | [94] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karuppasamy, K.; Nichelson, A.; Vikraman, D.; Choi, J.-H.; Hussain, S.; Ambika, C.; Bose, R.; Alfantazi, A.; Kim, H.-S. Recent Advancements in Two-Dimensional Layered Molybdenum and Tungsten Carbide-Based Materials for Efficient Hydrogen Evolution Reactions. Nanomaterials 2022, 12, 3884. https://doi.org/10.3390/nano12213884
Karuppasamy K, Nichelson A, Vikraman D, Choi J-H, Hussain S, Ambika C, Bose R, Alfantazi A, Kim H-S. Recent Advancements in Two-Dimensional Layered Molybdenum and Tungsten Carbide-Based Materials for Efficient Hydrogen Evolution Reactions. Nanomaterials. 2022; 12(21):3884. https://doi.org/10.3390/nano12213884
Chicago/Turabian StyleKaruppasamy, K., A. Nichelson, Dhanasekaran Vikraman, Jun-Hyeok Choi, Sajjad Hussain, C. Ambika, Ranjith Bose, Akram Alfantazi, and Hyun-Seok Kim. 2022. "Recent Advancements in Two-Dimensional Layered Molybdenum and Tungsten Carbide-Based Materials for Efficient Hydrogen Evolution Reactions" Nanomaterials 12, no. 21: 3884. https://doi.org/10.3390/nano12213884





