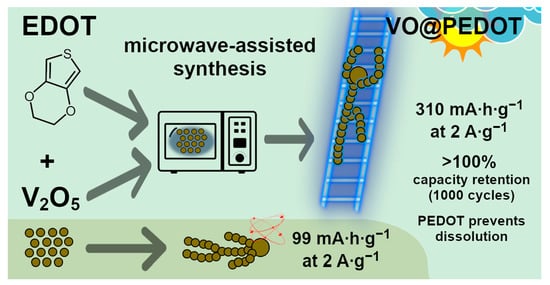
Vanadium Oxide-Poly(3,4-ethylenedioxythiophene) Nanocomposite as High-Performance Cathode for Aqueous Zn-Ion Batteries: The Structural and Electrochemical Characterization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of VO@PEDOT Composite
2.2. Characterization Methods
2.3. Electrochemical Characterization
3. Results and Discussion
3.1. Physical Characterization
3.2. Electrochemical Performance
3.3. Mechanism Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Armand, M.; Tarascon, J.M. Building better batteries. Nature 2008, 451, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Goodenough, J.B.; Park, K.S. The Li-ion rechargeable battery: A perspective. J. Am. Chem. Soc. 2013, 135, 1167–1176. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Hao, J.; Wang, Z.; Mao, J.; Guo, Z. Recent progress and perspectives on aqueous Zn-based rechargeable batteries with mild aqueous electrolytes. Energy Storage Mater. 2019, 20, 410–437. [Google Scholar] [CrossRef]
- He, P.; Chen, Q.; Yan, M.; Xu, X.; Zhou, L.; Mai, L.; Nan, C.W. Building better zinc-ion batteries: A materials perspective. EnergyChem 2019, 1, 100022. [Google Scholar] [CrossRef]
- Fang, G.; Zhou, J.; Pan, A.; Liang, S. Recent Advances in Aqueous Zinc-Ion Batteries. ACS Energy Lett. 2018, 3, 2480–2501. [Google Scholar] [CrossRef]
- Blanc, L.E.; Kundu, D.; Nazar, L.F. Scientific Challenges for the Implementation of Zn-Ion Batteries. Joule 2020, 4, 771–799. [Google Scholar] [CrossRef]
- Xu, C.; Li, B.; Du, H.; Kang, F. Energetic zinc ion chemistry: The rechargeable zinc ion battery. Angew. Chemie Int. Ed. 2012, 51, 933–935. [Google Scholar] [CrossRef]
- Zhang, N.; Cheng, F.; Liu, Y.; Zhao, Q.; Lei, K.; Chen, C.; Liu, X.; Chen, J. Cation-Deficient Spinel ZnMn2O4 Cathode in Zn(CF3SO3)2 Electrolyte for Rechargeable Aqueous Zn-Ion Battery. J. Am. Chem. Soc. 2016, 138, 12894–12901. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Z.; Xi, B.; Chen, W.; Jia, Y.; Feng, J.; Xiong, S. Advances and Perspectives of Cathode Storage Chemistry in Aqueous Zinc-Ion Batteries. ACS Nano 2021, 15, 9244–9272. [Google Scholar] [CrossRef]
- Chen, L.; An, Q.; Mai, L. Recent Advances and Prospects of Cathode Materials for Rechargeable Aqueous Zinc-Ion Batteries. Adv. Mater. Interfaces 2019, 6, 1900387. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, X. Review of vanadium-based electrode materials for rechargeable aqueous zinc ion batteries. J. Energy Chem. 2021, 56, 223–237. [Google Scholar] [CrossRef]
- Yi, T.F.; Qiu, L.; Qu, J.P.; Liu, H.; Zhang, J.H.; Zhu, Y.R. Towards high-performance cathodes: Design and energy storage mechanism of vanadium oxides-based materials for aqueous Zn-ion batteries. Coord. Chem. Rev. 2021, 446, 214124. [Google Scholar] [CrossRef]
- Zampardi, G.; La Mantia, F. Prussian blue analogues as aqueous Zn-ion batteries electrodes: Current challenges and future perspectives. Curr. Opin. Electrochem. 2020, 21, 84–92. [Google Scholar] [CrossRef]
- Li, C.; Zhang, X.; He, W.; Xu, G.; Sun, R. Cathode materials for rechargeable zinc-ion batteries: From synthesis to mechanism and applications. J. Power Sources 2020, 449, 227596. [Google Scholar] [CrossRef]
- Sun, T.; Fan, H.J. Understanding cathode materials in aqueous zinc–organic batteries. Curr. Opin. Electrochem. 2021, 30, 100799. [Google Scholar] [CrossRef]
- Zuo, S.; Xu, X.; Ji, S.; Wang, Z.; Liu, Z.; Liu, J. Cathodes for Aqueous Zn-Ion Batteries: Materials, Mechanisms, and Kinetics. Chem. A Eur. J. 2021, 27, 830–860. [Google Scholar] [CrossRef]
- Wu, Y.; Song, T.Y.; Chen, L.N. A review on recent developments of vanadium-based cathode for rechargeable zinc-ion batteries. Tungsten 2021, 3, 289–304. [Google Scholar] [CrossRef]
- Yang, G.; Li, Q.; Ma, K.; Hong, C.; Wang, C. The degradation mechanism of vanadium oxide-based aqueous zinc-ion batteries. J. Mater. Chem. A 2020, 8, 8084–8095. [Google Scholar] [CrossRef]
- Yang, Y.; Tang, Y.; Liang, S.; Wu, Z.; Fang, G.; Cao, X.; Wang, C.; Lin, T.; Pan, A.; Zhou, J. Transition metal ion-preintercalated V2O5 as high-performance aqueous zinc-ion battery cathode with broad temperature adaptability. Nano Energy 2019, 61, 617–625. [Google Scholar] [CrossRef]
- Sun, J.; Zhao, Y.; Liu, Y.; Jiang, H.; Huang, C.; Cui, M.; Hu, T.; Meng, C.; Zhang, Y. “Three-in-One” Strategy that Ensures V2O5·nH2O with Superior Zn2+ Storage by Simultaneous Protonated Polyaniline Intercalation and Encapsulation. Small Struct. 2022, 3, 2100212. [Google Scholar] [CrossRef]
- Zhang, W.; Zuo, C.; Tang, C.; Tang, W.; Lan, B.; Fu, X.; Dong, S.; Luo, P. The Current Developments and Perspectives of V2O5 as Cathode for Rechargeable Aqueous Zinc-Ion Batteries. Energy Technol. 2021, 9, 2000789. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, X.; Li, X.; Hu, X.; Cai, S.; Zheng, C. Recent progress in rate and cycling performance modifications of vanadium oxides cathode for lithium-ion batteries. J. Energy Chem. 2021, 59, 343–363. [Google Scholar] [CrossRef]
- Wang, W.; He, D.; Fang, Y.; Wang, S.; Zhang, Z.; Zhao, R.; Xue, W. Pillaring of a conductive polymer in layered V2O5 boosting ultra-fast Zn2+/H+ storage in aqueous media. Electrochim. Acta 2022, 416, 140270. [Google Scholar] [CrossRef]
- Dai, X.; Wan, F.; Zhang, L.; Cao, H.; Niu, Z. Freestanding graphene/VO2 composite films for highly stable aqueous Zn-ion batteries with superior rate performance. Energy Storage Mater. 2019, 17, 143–150. [Google Scholar] [CrossRef]
- Zhao, X.; Mao, L.; Cheng, Q.; Liao, F.; Yang, G.; Chen, L. Dual-cation preintercalated and amorphous carbon confined vanadium oxides as a superior cathode for aqueous zinc-ion batteries. Carbon 2022, 186, 160–170. [Google Scholar] [CrossRef]
- Zhang, G.; Wu, T.; Zhou, H.; Jin, H.; Liu, K.; Luo, Y.; Jiang, H.; Huang, K.; Huang, L.; Zhou, J. Rich Alkali Ions Preintercalated Vanadium Oxides for Durable and Fast Zinc-Ion Storage. ACS Energy Lett. 2021, 6, 2111–2120. [Google Scholar] [CrossRef]
- Khairy, M.; Tinet, D.; Van Damme, H. The synthesis of pillared vanadium oxide. J. Chem. Soc. Chem. Commun. 1990, 856–857. [Google Scholar] [CrossRef]
- Vujković, M.J.; Mladenović, D.; Milović, M.; Petrović, T.; Bajuk-Bogdanović, D.; Šljukić Paunković, B.; Mentus, S. Sodium-pillared vanadium oxides as next-gen materials: Does co-inserted water control the cyclic stability of vanadates in an aqueous electrolyte? Electrochim. Acta 2022, 425, 140603. [Google Scholar] [CrossRef]
- Dong, Y.; Deng, S.; Ma, Z.; Yin, G.; Li, C.; Yuan, X.; Tan, H.; Pan, J.; Mai, L.; Xia, F. Sodium vanadium oxides: From nanostructured design to high-performance energy storage materials. J. Mater. Sci. Technol. 2022, 121, 80–92. [Google Scholar] [CrossRef]
- Yan, M.; He, P.; Chen, Y.; Wang, S.; Wei, Q.; Zhao, K.; Xu, X.; An, Q.; Shuang, Y.; Shao, Y.; et al. Water-Lubricated Intercalation in V2O5·nH2O for High-Capacity and High-Rate Aqueous Rechargeable Zinc Batteries. Adv. Mater. 2018, 30, 1703725. [Google Scholar] [CrossRef]
- Zhu, K.; Wu, T.; Huang, K. Understanding the Dissolution and Phase Transformation Mechanisms in Aqueous Zn/α-V2O5 Batteries. Chem. Mater. 2021, 33, 4089–4098. [Google Scholar] [CrossRef]
- Lv, T.T.; Liu, Y.Y.; Wang, H.; Yang, S.Y.; Liu, C.S.; Pang, H. Crystal water enlarging the interlayer spacing of ultrathin V2O5·4VO2·2.72H2O nanobelts for high-performance aqueous zinc-ion battery. Chem. Eng. J. 2021, 411, 128533. [Google Scholar] [CrossRef]
- Wu, T.; Zhu, K.; Qin, C.; Huang, K. Unraveling the role of structural water in bilayer V2O5 during Zn2+-intercalation: Insights from DFT calculations. J. Mater. Chem. A 2019, 7, 5612–5620. [Google Scholar] [CrossRef]
- Zhu, K.; Wu, T.; Huang, K. NaCa0.6V6O16·3H2O as an Ultra-Stable Cathode for Zn-Ion Batteries: The Roles of Pre-Inserted Dual-Cations and Structural Water in V3O8 Layer. Adv. Energy Mater. 2019, 9, 1901968. [Google Scholar] [CrossRef]
- Wu, T.H.; Liang, W.Y. Reduced Intercalation Energy Barrier by Rich Structural Water in Spinel ZnMn2O4for High-Rate Zinc-Ion Batteries. ACS Appl. Mater. Interfaces 2021, 13, 23822–23832. [Google Scholar] [CrossRef]
- Shin, J.; Choi, D.S.; Lee, H.J.; Jung, Y.; Choi, J.W. Hydrated Intercalation for High-Performance Aqueous Zinc Ion Batteries. Adv. Energy Mater. 2019, 9, 1900083. [Google Scholar] [CrossRef]
- Dong, R.; Zhang, T.; Liu, J.; Li, H.; Hu, D.; Liu, X.; Xu, Q. Mechanistic Insight into Polypyrrole Coating on V2O5 Cathode for Aqueous Zinc-Ion Battery. ChemElectroChem 2022, 9, e202101441. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, L.; Jiang, H.; Liu, Y.; Meng, C. Polyaniline-expanded the interlayer spacing of hydrated vanadium pentoxide by the interface-intercalation for aqueous rechargeable Zn-ion batteries. J. Colloid Interface Sci. 2021, 603, 641–650. [Google Scholar] [CrossRef]
- Li, S.; Wei, X.; Wu, C.; Zhang, B.; Wu, S.; Lin, Z. Constructing Three-Dimensional Structured V2O5/Conductive Polymer Composite with Fast Ion/Electron Transfer Kinetics for Aqueous Zinc-Ion Battery. ACS Appl. Energy Mater. 2021, 4, 4208–4216. [Google Scholar] [CrossRef]
- Li, R.; Zhang, H.; Yan, J.; Zheng, Q.; Li, X. A novel 3-phenylpropylamine intercalated molecular bronze with ultrahigh layer spacing as a high-rate and stable cathode for aqueous zinc-ion batteries. Fundam. Res. 2021, 1, 425–431. [Google Scholar] [CrossRef]
- Xu, D.; Wang, H.; Li, F.; Guan, Z.; Wang, R.; He, B.; Gong, Y.; Hu, X. Conformal Conducting Polymer Shells on V2O5 Nanosheet Arrays as a High-Rate and Stable Zinc-Ion Battery Cathode. Adv. Mater. Interfaces 2019, 6, 1801506. [Google Scholar] [CrossRef]
- Liu, X.; Xu, G.; Zhang, Q.; Huang, S.; Li, L.; Wei, X.; Cao, J.; Yang, L.; Chu, P.K. Ultrathin hybrid nanobelts of single-crystalline VO2 and Poly(3,4-ethylenedioxythiophene) as cathode materials for aqueous zinc ion batteries with large capacity and high-rate capability. J. Power Sources 2020, 463, 228223. [Google Scholar] [CrossRef]
- Bin, D.; Huo, W.; Yuan, Y.; Huang, J.; Liu, Y.; Zhang, Y.; Dong, F.; Wang, Y.; Xia, Y. Organic-Inorganic-Induced Polymer Intercalation into Layered Composites for Aqueous Zinc-Ion Battery. Chem 2020, 6, 968–984. [Google Scholar] [CrossRef]
- Volkov, F.S.; Tolstopjatova, E.G.; Eliseeva, S.N.; Kamenskii, M.A.; Vypritskaia, A.I.; Volkov, A.I.; Kondratiev, V.V. Vanadium(V) oxide coated by poly(3,4-ethylenedioxythiophene) as cathode for aqueous zinc-ion batteries with improved electrochemical performance. Mater. Lett. 2022, 308, 131210. [Google Scholar] [CrossRef]
- Wan, F.; Zhang, L.; Dai, X.; Wang, X.; Niu, Z.; Chen, J. Aqueous rechargeable zinc/sodium vanadate batteries with enhanced performance from simultaneous insertion of dual carriers. Nat. Commun. 2018, 9, 1656. [Google Scholar] [CrossRef] [Green Version]
- Xia, C.; Guo, J.; Li, P.; Zhang, X.; Alshareef, H.N. Highly Stable Aqueous Zinc-Ion Storage Using a Layered Calcium Vanadium Oxide Bronze Cathode. Angew. Chem. Int. Ed. 2018, 57, 3943–3948. [Google Scholar] [CrossRef]
- Oberholzer, P.; Tervoort, E.; Bouzid, A.; Pasquarello, A.; Kundu, D. Oxide versus Nonoxide Cathode Materials for Aqueous Zn Batteries: An Insight into the Charge Storage Mechanism and Consequences Thereof. ACS Appl. Mater. Interfaces 2019, 11, 674–682. [Google Scholar] [CrossRef]
- Guo, J.; Ming, J.; Lei, Y.; Zhang, W.; Xia, C.; Cui, Y.; Alshareef, H.N. Artificial Solid Electrolyte Interphase for Suppressing Surface Reactions and Cathode Dissolution in Aqueous Zinc Ion Batteries. ACS Energy Lett. 2019, 4, 2776–2781. [Google Scholar] [CrossRef]
- Murugan, A.V.; Kwon, C.W.; Campet, G.; Kale, B.B.; Mandale, A.B.; Sainker, S.R.; Gopinath, C.S.; Vijayamohanan, K. A novel approach to prepare poly(3,4-ethylenedioxythiophene) nanoribbons between V2O5 layers by microwave irradiation. J. Phys. Chem. B 2004, 108, 10736–10742. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Sc, Ti, V., Cu and Zn. Appl. Surf. Sci. 2010, 257, 887–898. [Google Scholar] [CrossRef]
- Silversmit, G.; Depla, D.; Poelman, H.; Marin, G.B.; De Gryse, R. An XPS study on the surface reduction of V2O5 (0 0 1) induced by Ar+ ion bombardment. Surf. Sci. 2006, 600, 3512–3517. [Google Scholar] [CrossRef] [Green Version]
- Biesinger, M.C.; Payne, B.P.; Lau, L.W.M.; Gerson, A.; Smart, R.S.C. X-ray photoelectron spectroscopic chemical state Quantification of mixed nickel metal, oxide and hydroxide systems. Surf. Interface Anal. 2009, 41, 324–332. [Google Scholar] [CrossRef]
- Beamson, G.; Briggs, D. High Resolution XPS of Organic Polymers: The Scienta ESCA300 Database; Wiley: Hoboken, NJ, USA, 1992; ISBN 0471935921. [Google Scholar]
- Chen, H.; Huang, J.; Tian, S.; Liu, L.; Qin, T.; Song, L.; Liu, Y.; Zhang, Y.; Wu, X.; Lei, S.; et al. Interlayer Modification of Pseudocapacitive Vanadium Oxide and Zn(H2O)n2+ Migration Regulation for Ultrahigh Rate and Durable Aqueous Zinc-Ion Batteries. Adv. Sci. 2021, 8, 2004924. [Google Scholar] [CrossRef] [PubMed]
- Et Taouil, A.; Lallemand, F.; Hihn, J.Y.; Melot, J.M.; Blondeau-Patissier, V.; Lakard, B. Doping properties of PEDOT films electrosynthesized under high frequency ultrasound irradiation. Ultrason. Sonochem. 2011, 18, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Ushakova, E.E.; Frolov, A.; Reveguk, A.A.; Usachov, D.Y.; Itkis, D.M.; Yashina, L.V. Solid electrolyte interface formation between lithium and PEO-based electrolyte. Appl. Surf. Sci. 2022, 589, 153014. [Google Scholar] [CrossRef]
- Mitraka, E.; Jafari, M.J.; Vagin, M.; Liu, X.; Fahlman, M.; Ederth, T.; Berggren, M.; Jonsson, M.P.; Crispin, X. Oxygen-induced doping on reduced PEDOT. J. Mater. Chem. A 2017, 5, 4404–4412. [Google Scholar] [CrossRef] [Green Version]
- Lien, S.-Y.; Lin, P.-C.; Chen, W.-R.; Liu, C.-H.; Lee, K.-W.; Wang, N.-F.; Huang, C.-J. The Mechanism of PEDOT:PSS Films with Organic Additives. Crystals 2022, 12, 1109. [Google Scholar] [CrossRef]
- Zhan, L.; Song, Z.; Zhang, J.; Tang, J.; Zhan, H.; Zhou, Y.; Zhan, C. PEDOT: Cathode active material with high specific capacity in novel electrolyte system. Electrochim. Acta 2008, 53, 8319–8323. [Google Scholar] [CrossRef]
- Rabchinskii, M.K.; Saveliev, S.D.; Stolyarova, D.Y.; Brzhezinskaya, M.; Kirilenko, D.A.; Baidakova, M.V.; Ryzhkov, S.A.; Shnitov, V.V.; Sysoev, V.V.; Brunkov, P.N. Modulating nitrogen species via N-doping and post annealing of graphene derivatives: XPS and XAS examination. Carbon 2021, 182, 593–604. [Google Scholar] [CrossRef]
- Li, S.; Chen, Y.; He, X.; Mao, X.; Zhou, Y.; Xu, J.; Yang, Y. Modifying Reduced Graphene Oxide by Conducting Polymer Through a Hydrothermal Polymerization Method and its Application as Energy Storage Electrodes. Nanoscale Res. Lett. 2019, 14, 226. [Google Scholar] [CrossRef]
- Yan, Y.; Jamal, R.; Yu, Z.; Zhang, R.; Zhang, W.; Ge, Y.; Liu, Y.; Abdiryim, T. Composites of thiol-grafted PEDOT with N-doped graphene or graphitic carbon nitride as an electrochemical sensor for the detection of paracetamol. J. Mater. Sci. 2020, 55, 5571–5586. [Google Scholar] [CrossRef]
- Li, Y.; Huang, Z.; Kalambate, P.K.; Zhong, Y.; Huang, Z.; Xie, M.; Shen, Y.; Huang, Y. V2O5 nanopaper as a cathode material with high capacity and long cycle life for rechargeable aqueous zinc-ion battery. Nano Energy 2019, 60, 752–759. [Google Scholar] [CrossRef]
- Du, Y.; Wang, X.; Man, J.; Sun, J. A novel organic-inorganic hybrid V2O5@polyaniline as high-performance cathode for aqueous zinc-ion batteries. Mater. Lett. 2020, 272, 127813. [Google Scholar] [CrossRef]
- Feng, Z.; Sun, J.; Liu, Y.; Jiang, H.; Hu, T.; Cui, M.; Tian, F.; Meng, C.; Zhang, Y. Polypyrrole-intercalation tuning lamellar structure of V2O5·nH2O boosts fast zinc-ion kinetics for aqueous zinc-ion battery. J. Power Sources 2022, 536, 231489. [Google Scholar] [CrossRef]
- Li, Q.; Wei, T.; Ma, K.; Yang, G.; Wang, C. Boosting the Cyclic Stability of Aqueous Zinc-Ion Battery Based on Al-Doped V10O24·12H2O Cathode Materials. ACS Appl. Mater. Interfaces 2019, 11, 20888–20894. [Google Scholar] [CrossRef]
- Wu, W.; Wang, S.; Zhang, C.; Hou, S.; Zhang, L. Facile and Scalable Synthesis of 3D Structures of V10O24·12H2O Nanosheets Coated with Carbon toward Ultrafast and Ultrastable Zinc Storage. ACS Appl. Mater. Interfaces 2021, 13, 18704–18712. [Google Scholar] [CrossRef]
- Wei, T.; Li, Q.; Yang, G.; Wang, C. High-rate and durable aqueous zinc ion battery using dendritic V10O24·12H2O cathode material with large interlamellar spacing. Electrochim. Acta 2018, 287, 60–67. [Google Scholar] [CrossRef]
- Yan, H.; Ru, Q.; Gao, P.; Shi, Z.; Gao, Y.; Chen, F.; Chi-Chun Ling, F.; Wei, L. Organic pillars pre-intercalated V4+-V2O5·3H2O nanocomposites with enlarged interlayer and mixed valence for aqueous Zn-ion storage. Appl. Surf. Sci. 2020, 534, 147608. [Google Scholar] [CrossRef]
- Liu, W.; Dong, L.; Jiang, B.; Huang, Y.; Wang, X.; Xu, C.; Kang, Z.; Mou, J.; Kang, F. Layered vanadium oxides with proton and zinc ion insertion for zinc ion batteries. Electrochim. Acta 2019, 320, 134565. [Google Scholar] [CrossRef]
- Li, R.; Xing, F.; Li, T.; Zhang, H.; Yan, J.; Zheng, Q.; Li, X. Intercalated polyaniline in V2O5 as a unique vanadium oxide bronze cathode for highly stable aqueous zinc ion battery. Energy Storage Mater. 2021, 38, 590–598. [Google Scholar] [CrossRef]
- Li, S.; Wei, X.; Chen, H.; Lai, G.; Wang, X.; Zhang, S.; Wu, S.; Tang, W.; Lin, Z. A mixed-valent vanadium oxide cathode with ultrahigh rate capability for aqueous zinc-ion batteries. J. Mater. Chem. A 2021, 9, 22392–22398. [Google Scholar] [CrossRef]
- Chen, X.; Wang, L.; Li, H.; Cheng, F.; Chen, J. Porous V2O5 nanofibers as cathode materials for rechargeable aqueous zinc-ion batteries. J. Energy Chem. 2019, 38, 20–25. [Google Scholar] [CrossRef] [Green Version]
- Lin, Z.; Shi, H.Y.; Lin, L.; Yang, X.; Wu, W.; Sun, X. A high capacity small molecule quinone cathode for rechargeable aqueous zinc-organic batteries. Nat. Commun. 2021, 12, 4424. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.; Li, X.; Su, S.; Li, J.; Fang, J.; Liang, B.; Hou, J.; Luo, M. Hydrated lithium ions intercalated V2O5 with dual-ion synergistic insertion mechanism for high-performance aqueous zinc-ion batteries. J. Colloid Interface Sci. 2022, 606, 645–653. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Seo, H.R.; Lee, H.R.; Yoon, C.S.; Kim, J.H.; Chung, K.Y.; Cho, B.W.; Oh, S.H. Critical Role of pH Evolution of Electrolyte in the Reaction Mechanism for Rechargeable Zinc Batteries. ChemSusChem 2016, 9, 2948–2956. [Google Scholar] [CrossRef] [PubMed]
- Kundu, D.; Hosseini Vajargah, S.; Wan, L.; Adams, B.; Prendergast, D.; Nazar, L.F. Aqueous: Vs. nonaqueous Zn-ion batteries: Consequences of the desolvation penalty at the interface. Energy Environ. Sci. 2018, 11, 881–892. [Google Scholar] [CrossRef]
- Chen, S.; Li, K.; Hui, K.S.; Zhang, J. Regulation of Lamellar Structure of Vanadium Oxide via Polyaniline Intercalation for High-Performance Aqueous Zinc-Ion Battery. Adv. Funct. Mater. 2020, 30, 2003890. [Google Scholar] [CrossRef]
- Yin, C.; Pan, C.; Liao, X.; Pan, Y.; Yuan, L. Regulating the Interlayer Spacing of Vanadium Oxide by In Situ Polyaniline Intercalation Enables an Improved Aqueous Zinc-Ion Storage Performance. ACS Appl. Mater. Interfaces 2021, 13, 39347–39354. [Google Scholar] [CrossRef]
- Wang, Z.; Tang, X.; Yuan, S.; Bai, M.; Wang, H.; Liu, S.; Zhang, M.; Ma, Y. Engineering Vanadium Pentoxide Cathode for the Zero-Strain Cation Storage via a Scalable Intercalation-Polymerization Approach. Adv. Funct. Mater. 2021, 31, 2100164. [Google Scholar] [CrossRef]
- Yao, Z.; Wu, Q.; Chen, K.; Liu, J.; Li, C. Shallow-layer pillaring of a conductive polymer in monolithic grains to drive superior zinc storage: Via a cascading effect. Energy Environ. Sci. 2020, 13, 3149–3163. [Google Scholar] [CrossRef]











| Cathode | Day 1, mg∙dm−3 | Day 2, mg∙dm−3 | Day 3, mg∙dm−3 | Day 5, mg∙dm−3 | Day 10, mg∙dm−3 | Month 6, mg∙dm−3 |
|---|---|---|---|---|---|---|
| V2O5 | 0.72 | 1.14 | 1.34 | 1.86 | 2.42 | 57.8 |
| VO@PEDOT | 0.60 | 0.70 | 0.99 | 1.56 | 1.87 | 6.53 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Volkov, F.S.; Eliseeva, S.N.; Kamenskii, M.A.; Volkov, A.I.; Tolstopjatova, E.G.; Glumov, O.V.; Fu, L.; Kondratiev, V.V. Vanadium Oxide-Poly(3,4-ethylenedioxythiophene) Nanocomposite as High-Performance Cathode for Aqueous Zn-Ion Batteries: The Structural and Electrochemical Characterization. Nanomaterials 2022, 12, 3896. https://doi.org/10.3390/nano12213896
Volkov FS, Eliseeva SN, Kamenskii MA, Volkov AI, Tolstopjatova EG, Glumov OV, Fu L, Kondratiev VV. Vanadium Oxide-Poly(3,4-ethylenedioxythiophene) Nanocomposite as High-Performance Cathode for Aqueous Zn-Ion Batteries: The Structural and Electrochemical Characterization. Nanomaterials. 2022; 12(21):3896. https://doi.org/10.3390/nano12213896
Chicago/Turabian StyleVolkov, Filipp S., Svetlana N. Eliseeva, Mikhail A. Kamenskii, Alexey I. Volkov, Elena G. Tolstopjatova, Oleg V. Glumov, Lijun Fu, and Veniamin V. Kondratiev. 2022. "Vanadium Oxide-Poly(3,4-ethylenedioxythiophene) Nanocomposite as High-Performance Cathode for Aqueous Zn-Ion Batteries: The Structural and Electrochemical Characterization" Nanomaterials 12, no. 21: 3896. https://doi.org/10.3390/nano12213896
APA StyleVolkov, F. S., Eliseeva, S. N., Kamenskii, M. A., Volkov, A. I., Tolstopjatova, E. G., Glumov, O. V., Fu, L., & Kondratiev, V. V. (2022). Vanadium Oxide-Poly(3,4-ethylenedioxythiophene) Nanocomposite as High-Performance Cathode for Aqueous Zn-Ion Batteries: The Structural and Electrochemical Characterization. Nanomaterials, 12(21), 3896. https://doi.org/10.3390/nano12213896