Improvement of Photocatalytic Performance by Building Multiple Heterojunction Structures of Anatase–Rutile/BiOI Composite Fibers
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Electrospinning and Annealing of TiO2 Fibers
2.3. Synthesis of TiO2/BiOI Composite Fibers
2.4. Structural Characterization
2.5. Photocatalytic Degradation Test
3. Results
3.1. Morphologies and Crystallization
3.2. Chemical Structure
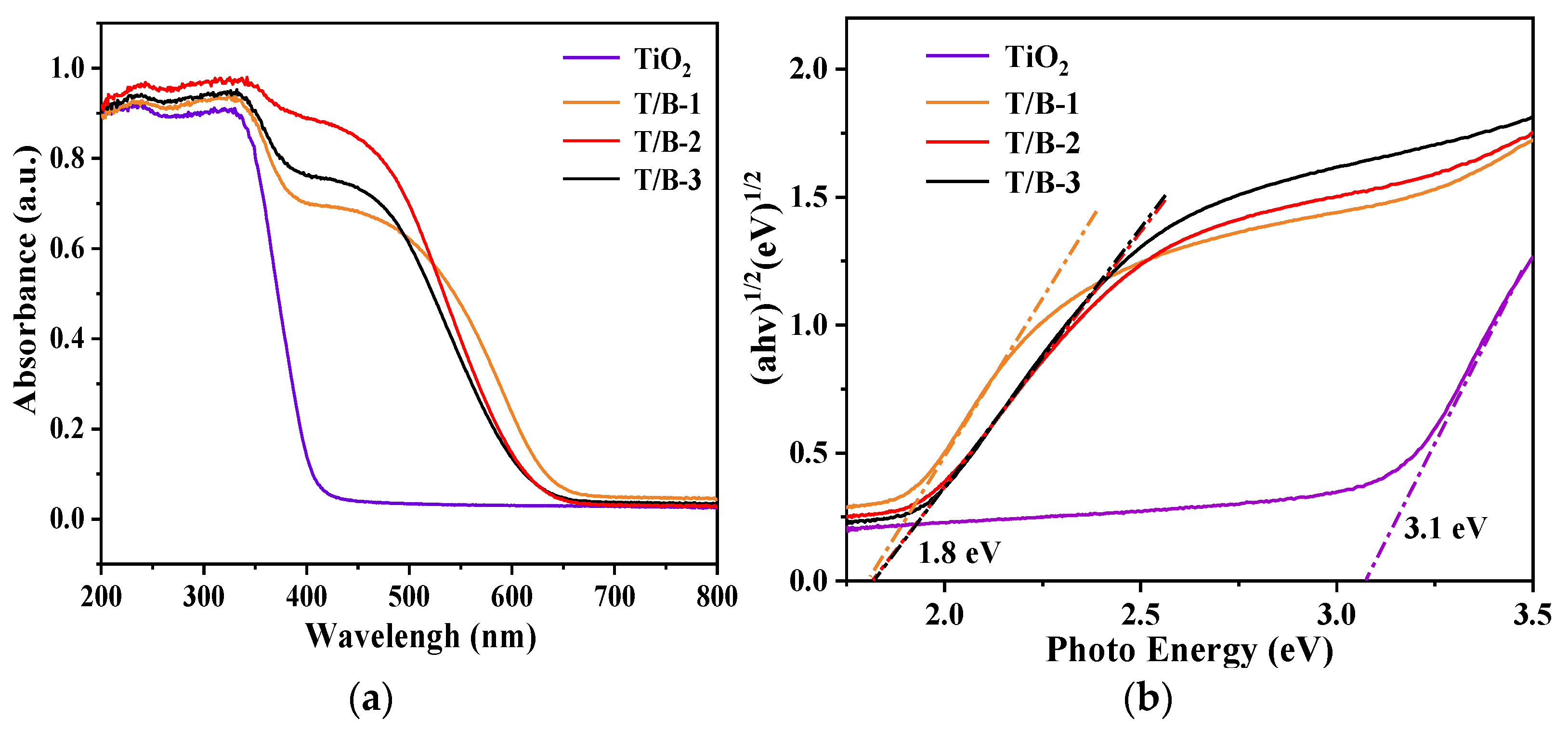
3.3. Calculation of UV–Vis Spectra and Bandgaps
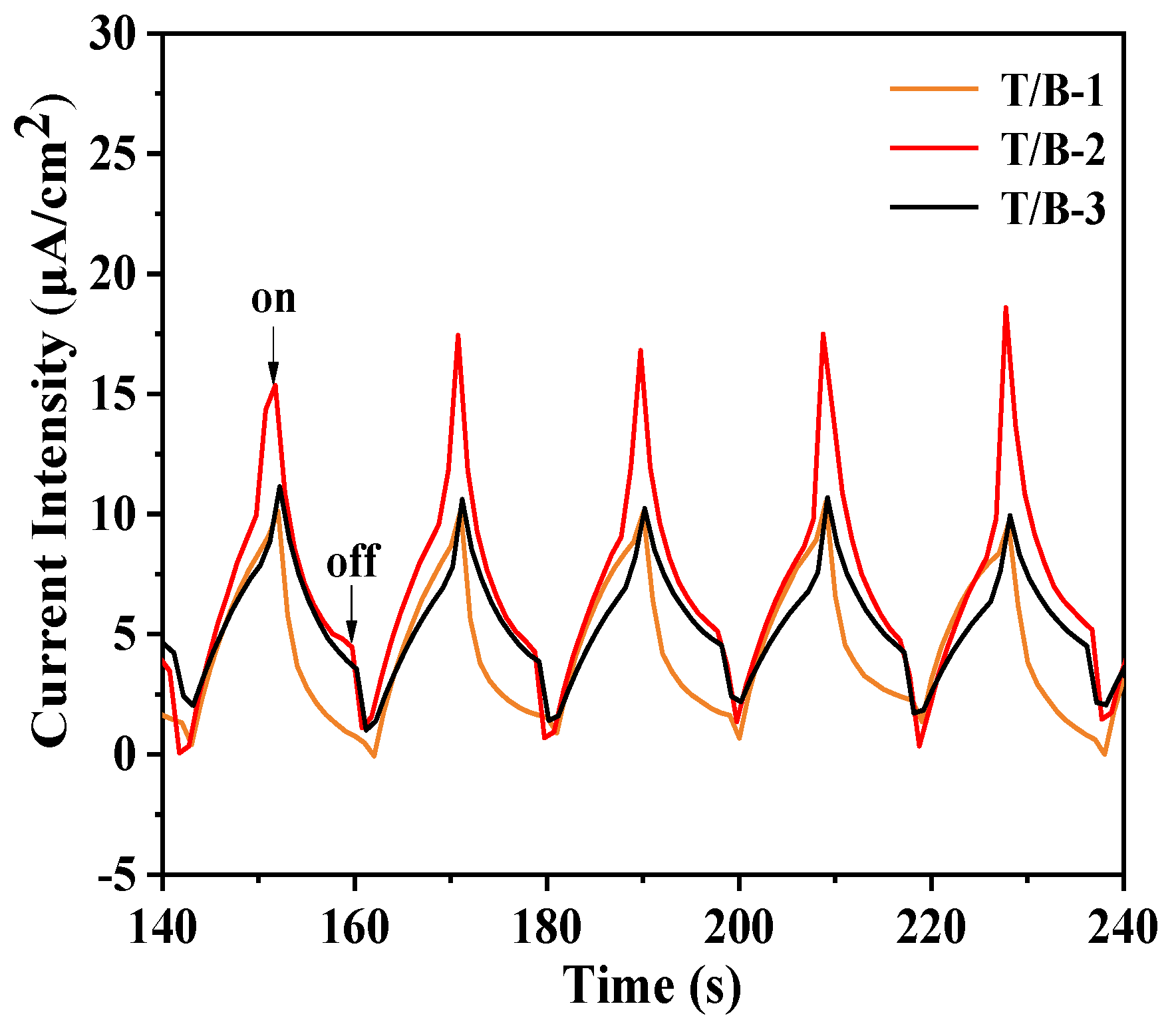
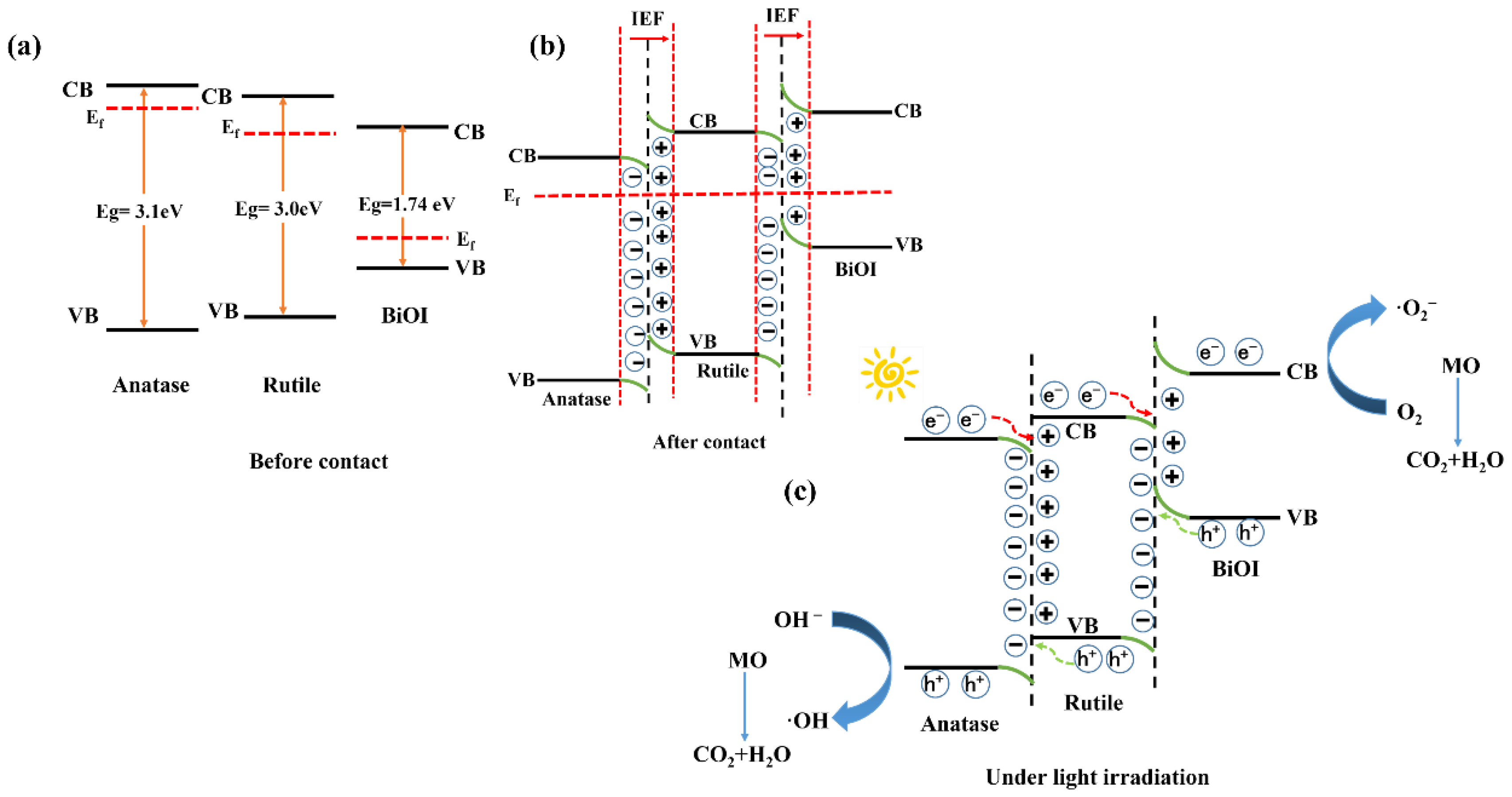
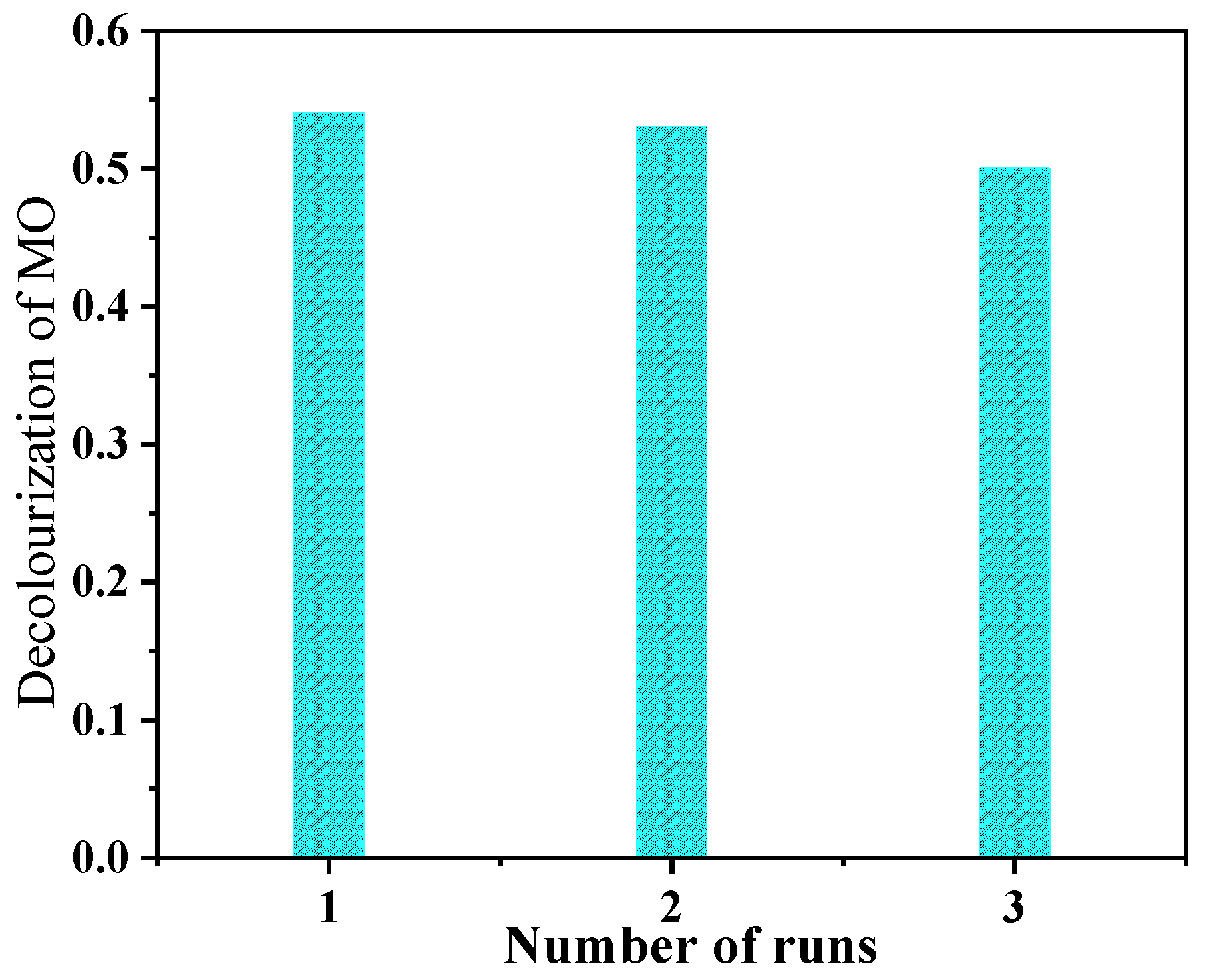
3.4. Photocatalytic Performance Test
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ivanuša, M.; Kumer, B.; Petrovčič, E.; Štular, D.; Zorc, M.; Jerman, I.; Gorjanc, M.; Tomšič, B.; Simončič, B. Eco-Friendly Approach to Produce Durable Multifunctional Cotton Fibres Using TiO2, ZnO and Ag NPs. Nanomaterials 2022, 12, 3140. [Google Scholar] [CrossRef] [PubMed]
- Čop, K.T.; Pavlović, D.; Kraljević, T. Photocatalytic Activity of TiO2 for the Degradation of Anticancer Drugs. Nanomaterials 2022, 12, 3532. [Google Scholar]
- Zhang, L.; Hu, Z.; Huang, J.; Chen, Z.; Li, X.; Feng, Z.; Yang, H.; Huang, S.; Luo, R. Experimental and DFT studies of flower-like Ni-doped Mo2C on carbon fiber paper: A highly efficient and robust HER electrocatalyst modulated by Ni(NO3)2 concentration. J. Adv. Ceram. 2022, 11, 1294–1306. [Google Scholar] [CrossRef]
- Zhang, L.; Huang, J.; Hu, Z.; Li, X.; Ding, T.; Hou, X.; Chen, Z.; Ye, Z.; Luo, R. Ni(NO3)2-induced high electrocatalytic hydrogen evolution performance of self-supported fold-like WC coating on carbon fiber paper prepared through molten salt method. Electrochim. Acta 2022, 422, 140553. [Google Scholar] [CrossRef]
- Khaliha, S.; Marforio, T.; Kovtun, A.; Mantovani, S.; Bianchi, A.; Navacchia, M.L.; Zambianchi, M.; Bocchi, L.; Boulanger, N.; Iakunkov, A.; et al. Defective graphene nanosheets for drinking water purification: Adsorption mechanism, performance, and recovery. Flat. Chem. 2021, 29, 100283. [Google Scholar] [CrossRef]
- Zhao, H.; Zhang, D.; Sun, H.; Zhao, Y.; Xied, M. Adsorption and detection of heavy metals fromaqueous water by PVDF/A TP-CDs composite membrane. Coll. Oid. Surface. A 2022, 641, 128573. [Google Scholar] [CrossRef]
- Frankowski, R.; Płatkiewicz, J.; Stanisz, E.; Grześkowiak, T.; Zgoła-Grześkowiak, A. Biodegradation and photo-Fenton degradation of bisphenol A, bisphenol S and fluconazole in water. Environ. Pollut. 2021, 289, 117947. [Google Scholar] [CrossRef]
- Babu, S.J.; Muniyappa, M.; Rao, V.N.; Mudike, R.; Shastri, M.; Tathagata, S.; Shivaramu, P.D.; Shankar, M.V.; Kumar, C.S.A.; Rangappa, D. Enhanced photocatalytic hydrogen evolution from reduced graphene oxide-defect rich TiO2-x nanocomposites. Int. J. Hydorgen Energ. 2022, in press.
- Alhaddad, M.; Ismail, A.A.; Alghamdi, Y.G.; Al-Khathami, N.D.; Mohamed, R.M. Co3O4 Nanoparticles Accommodated Mesoporous TiO2 framework as an Excellent Photocatalyst with Enhanced Photocatalytic Properties. Opt. Mater. 2022, 131, 112643. [Google Scholar] [CrossRef]
- Shen, H.; Zhang, W.; Guo, C.; Zhu, J.; Cui, J.; Xue, Z.; Chen, P. Natural Cotton Cellulose-Supported TiO2 Quantum Dots for the Highly Efficient Photocatalytic Degradation of Dyes. Nanomaterials 2022, 12, 3130. [Google Scholar] [CrossRef]
- Rutherford, B.X.; Dou, H.; Zhang, B.; He, Z.; Barnard, J.P.; Paldi, R.L.; Wang, H. Single-Step Fabrication of Au-Fe-BaTiO3 Nanocomposite Thin Films Embedded with Non-Equilibrium Au-Fe Alloyed Nanostructures. Nanomaterials 2022, 12, 3460. [Google Scholar] [CrossRef]
- Wang, N.; Li, J.; Lv, W.; Feng, J.; Yan, W. Synthesis of polyaniline/TiO2 composite with excellent adsorption performance on acid red G. RSC Adv. 2015, 5, 21132. [Google Scholar] [CrossRef]
- Bakhtiar, A.; Bouberka, Z.; Roussel, P.; Volkringer, C.; Addad, A.; Ouddane, B.; Pierlot, C.; Maschke, U. Development of a TiO2/Sepiolite Photocatalyst for the Degradation of a Persistent Organic Pollutant in Aqueous Solution. Nanomaterials 2022, 12, 3313. [Google Scholar] [CrossRef]
- Batista, W.V.F.C.; Cunha, R.; Santos, A.C.; Reis, P.M.; Furtado, C.A.; Silva, M.C.; Gorgulho, H.F. Synthesis of a reusable magnetic photocatalyst based on carbon xerogel/TiO2 composites and its application on acetaminophen degradation. Ceram. Int. 2022, in press.
- Zuarez-Chamba, M.; Tuba-Guamán, D.; Quishpe, M.; Vizuete, K.; Debut, A.; Herrera-Robledoa, M. Photocatalytic degradation of bisphenol A on BiOI nanostructured films under visible LED light irradiation. J. Photoch. Photobio. A 2022, 431, 114021. [Google Scholar] [CrossRef]
- Guan, Y.; Wu, J.; Hu, B.; Lin, Y.; Liu, Q.; Zhang, H.; Xiao, Y.; Huang, J.; Mao, X.; Zhao, S. Fabrication of flower-like BiOI/Bi5O7I heterojunction photocatalyst by pH adjustment method for enhancing photocatalytic performance. Mater. Lett. 2019, 250, 202–205. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, J.; Zhong, J.; Li, M.; Li, Z.; Yang, C. Enhanced photocatalytic performance of TiO2/BiOI heterojunctions benefited from effective separation of photogenerated carriers. Chem. Phys. Lett. 2021, 780, 138966. [Google Scholar] [CrossRef]
- Shakeri, M.; Delavari, H.H.; Montazerabadi, A.; Yourdkhani, A. Hyaluronic acid-coated ultrasmall BiOI nanoparticles as a potentially targeted contrast agent for X-ray computed tomography. Int. J. Biol. Macromol. 2022, 217, 668–676. [Google Scholar] [CrossRef]
- Liao, X.; Li, T.; Ren, H.; Zhang, X.; Shen, B.; Lin, J.; Lou, C. Construction of BiOI/TiO2 flexible and hierarchical S-scheme heterojunction nanofibers membranes for visible-light-driven photocatalytic pollutants degradation. Sci. Total. Environ. 2022, 806, 150698. [Google Scholar] [CrossRef]
- Luo, S.; Chen, J.; Huang, Z.; Liu, C.; Fang, M. Photocatalytic selective hydroxylation of phenol to dihydroxybenzene by BiOI/TiO2 p-n heterojunction photocatalysts for enhanced photocatalytic activity. ChemCatChem 2016, 8, 3780–3789. [Google Scholar] [CrossRef]
- Li, S.; Hu, S.; Xu, K.; Jiang, W.; Liu, J.; Wang, Z. A Novel Heterostructure of BiOI Nanosheets Anchored onto MWCNTs with Excellent Visible-Light Photocatalytic Activity. Nanomaterials 2017, 7, 22. [Google Scholar] [CrossRef]
- Zhang, G.; Ji, S.; Zhang, Y.; Wei, Y. Facile synthesis of p-n heterojunction of phosphorus doped TiO2 and BiOI with enhanced visible-light photocatalytic activity. Soild. State Commun. 2017, 259, 34–39. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, W.; Hu, D.; Xie, H.; Song, Y.; Luo, B.; Fang, Y.; Gao, W.; Zhong, Z. Design and in-situ construct BiOI/Bi/TiO2 photocatalysts with metal-mediated heterostructures employing oxygen vacancies in TiO2 nanosheets. Green Energy Environ. 2022, 7, 680–690. [Google Scholar] [CrossRef]
- Qiu, J.; Dai, D.; Zhang, L.; Zhou, Y.; Yang, L.; Yao, J. Inlaying metal-organic framework derived pancake-like TiO2 into three-dimensional BiOI for visible-light-driven generation of vanillin from sodium lignosulfonate. J. Colloid Interf. Sci. 2022, 605, 648–656. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, D.; Jiao, S.; Xu, Z.; Liu, Y.; Zhao, C.; Pan, J.; Liu, D.; Liu, G.; Jiang, B.; et al. TiO2-X mesoporous nanospheres/BiOI nanosheets S-scheme heterostructure for high efficiency, stable and unbiased photaocatalytic hydrogen production. Chem. Eng. J. 2022, 446, 137138. [Google Scholar] [CrossRef]
- Salehi, T.; Taherizadeh, A.; Bahrami, A.; Allafchian, A.; Ghafarinia, V. Towards a Highly Functional Hybrid ZnO Nanofiber-rGO Gas Sensor. Adv. Eng. Mater. 2020, 22, 2000005. [Google Scholar] [CrossRef]
- Çinar, B.; Kerïmoğlu, I.; Tönbül, B.; Demïrbüken, A.; Dursun, S.; Kaya, I.C.; Kalem, V.; Akyildiz, H. Hydrothermal/electrospinning synthesis of CuO plate-like particles/TiO2 fibers heterostructures for high-efficiency photocatalytic degradation of organic dyes and phenolic pollutants. Mat. Sci. Semicon. Proc. 2020, 109, 104919. [Google Scholar] [CrossRef]
- Xu, B.; Wang, X.; Huang, Y.; Liu, J.; Wang, D.; Feng, S.; Huang, X.; Wang, H. Electrospinning preparation of PAN/TiO2/PANI hybrid fiber membrane with highly selective adsorption and photocatalytic regeneration properties. Chem. Eng. J. 2020, 399, 125749. [Google Scholar]
- Zhang, J.; Kai, C.; Zhang, F.; Wang, Y. Novel PAN/Bi2MoO6/Ti3C2 ternary composite membrane via electrospinning with enhanced photocatalytic degradation of tetracycline. Colloid. Surface A 2022, 648, 129255. [Google Scholar] [CrossRef]
- Jian, S.; Tian, Z.; Hu, J.; Zhang, K.; Zhang, L.; Duan, G.; Yang, W.; Jiang, S. Enhanced visible light photocatalytic efficiency of La-doped ZnO nanofibers via electrospinning-calcination technology. Adv. Powder. Mater. 2022, 1, 1000004. [Google Scholar]
- Chen, L.; Liang, Z.; Li, L. Force analysis of unstable section of electrostatic spinning charged jet. J. King Saud. Univ. Sci. 2019, 31, 1528–1534. [Google Scholar] [CrossRef]
- Zhang, T.; Xu, H.; Zhang, Y.; Zhang, S.; Yang, X.; Wei, Y.; Huang, D.; Lian, X. Fabrication and characterization of double-layer asymmetric dressing through electrostatic spinning and 3D printing for skin wound repair. Mater. Design 2022, 218, 110711. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, A.; Xiong, M.; Macharia, D.K.; Liu, J.; Chen, Z.; Li, M.; Zhang, L. TiO2/BiOI p-n junction-decorated carbon fibers as weavable photocatalyst with UV–vis photoresponsive for efficiently degrading various pollutants. Chem. Eng. J. 2021, 415, 129019. [Google Scholar] [CrossRef]
- Yang, L.; Wang, R.; Zhou, N.; Jiang, L.; Liu, H.; He, Q.; Deng, C.; Chu, D.; Zhang, M.; Sun, Z. Construction of p-n heterostructured BiOI/TiO2 nanosheets arrays for improved photoelectrochemical water splitting performance. Appl. Surf. Sci. 2022, 601, 154277. [Google Scholar] [CrossRef]
- Debnath, K.; Majumder, T.; Mondal, S.P. Photoelectrochemical study of hydrothermally grown vertically aligned rutile TiO2 nanorods. Chem. Phys. 2022, 561, 111609. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, A.; Zhang, Q.; Mei, Y.; Wang, C.; Wang, Y.; Xiang, J.; Su, S.; Zhang, X.; Tan, Z. Facile synthesis of ternary AgBr/BiOI/Bi2O2CO3 heterostructures via BiOI self-sacrifice for efficient photocatalytic removal of gaseous mercury. Sep. Purif. Technol. 2022, 299, 121722. [Google Scholar] [CrossRef]
- Wang, H.; Chen, L.; Su, W.; Chung, J. Effect of the Compact TiO2 Layer on Charge Transfer between N3 Dyes and TiO2 Investigated by Raman Spectroscopy. J. Phys. Chem. C. 2010, 114, 3185–3189. [Google Scholar] [CrossRef]
- Karunadasa, K.S.P.; Manoratne, C.H. Microstructural view of anatase to rutile phase transformation examined by in-situ high-temperature X-ray powder diffraction. J. Solid State Chem. 2022, 314, 123377. [Google Scholar] [CrossRef]
- Jin, Y.; Li, C.; Zhang, Y. Preparation and visible-light driven photocatalytic activity of the rGO/TiO2/BiOI heterostructure for methyl orange degradation. New. Carbon Mater. 2022, 35, 394–400. [Google Scholar] [CrossRef]
- Bullen, J.; Lapinee, C.; Miller, L.; Bullough, F.; Berry, A.; Najorka, J.; Cibin, G.; Vilar, R.; Weiss, D. Spectroscopic (XAS, FTIR) investigations into arsenic adsorption onto TiO2/Fe2O3 composites: Evaluation of the surface complexes, speciation and precipitation predicted by modelling. Results Surf. Interfaces 2022, 9, 100084. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, Q.; Cao, D.; Wang, Y.; Jin, R.; Gao, S. Vertical grown BiOI nanosheets on TiO2 NTs/Ti meshes toward enhanced photocatalytic performances. J. Aalloy Compd. 2022, 820, 153109. [Google Scholar] [CrossRef]
- Hao, L.; Hu, Y.; Zhang, Y.; Ping, X.; Liu, T.; Zhao, Q.; Lu, Y. Flexible TiO2 nanograss array film decorated with BiOI nanoflakes and its greatly boosted photocatalytic activity. J. Liu, Ceram. Int. 2021, 47, 7845–7852. [Google Scholar] [CrossRef]
- Surovčík, J.; Medvecká, V.; Greguš, J.; Gregor, M.; Roch, T.; Annušová, A.; Ďurina, P.; Vojtekovád, T. Characterization of TiO2 nanofibers with enhanced photocatalytic properties prepared by plasma assisted calcination. Ceram. Int. 2022, in press. [CrossRef]
- Sedaghati, N.; Habibi-Yangjeh, A.; Pirhashemi, M.; Asadzadeh-Khaneghah, S.; Ghosh, S. Integration of BiOI and Ag3PO4 nanoparticles onto oxygen vacancy rich-TiO2 for efficient visible-light photocatalytic decontaminations. J. Photoch. Photobio. A 2022, 400, 112659. [Google Scholar] [CrossRef]
- Zhao, W.; Zhao, S.; Zhu, W.; Lv, F.; Zhang, Y. Facile fabrication of mesoporous SiO2@(cTiO2/BiOI) heterojunctions with enhanced photocatalytic properties due to large surface area for contact with pollutants. Results Surf. Interfaces 2022, 8, 100065. [Google Scholar] [CrossRef]
- Lal, M.; Sharma, P.; Ram, C. Synthesis and photocatalytic potential of Nd-doped TiO2 under UV and solar light irradiation using a sol-gel ultrasonication method. Results Mater. 2022, 15, 100308. [Google Scholar] [CrossRef]
- Hajipour, P.; Bahrami, A.; Mehr, M.Y.; Driel, W.; Zhang, K. Facile Synthesis of Ag Nanowire/TiO2 and Ag Nanowire/TiO2/GO Nanocomposites for Photocatalytic Degradation of Rhodamine B. Materials 2021, 14, 763. [Google Scholar] [CrossRef]
- Luo, S.; Tang, C.; Huang, Z.; Liu, C.; Chen, J.; Fang, M. Effect of different Bi/Ti molar ratios on visible-light photocatalytic activity of BiOI/TiO2 heterostructured nanofibers. Ceram. Int. 2016, 42, 15780–15786. [Google Scholar] [CrossRef]
- Zhao, L.; Fang, W.; Meng, X.; Wang, L.; Bai, H.; Li, C. In-situ synthesis of metal Bi to improve the stability of oxygen vacancies and enhance the photocatalytic activity of Bi4O5Br2 in H2 evolution. J. Aalloy. Compd. 2022, 910, 164883. [Google Scholar] [CrossRef]
- Kursawe, M.; Anselmann, R.; Hilarius, V.; Pfaff, G. Nano-Particles by Wet Chemical Processing in Commercial Applications. J. Sol-Gel. Sci. Technol. 2005, 33, 71–74. [Google Scholar] [CrossRef]
- Yan, Y.H.; Zhou, Z.X.; Cheng, Y.; Qiu, L.; Gao, C.; Zhou, J. Template-free fabrication of α-and β-Bi2O3 hollow spheres and their visible light photocatalytic activity for water purification. J. Alloy. Compd. 2014, 605, 102–108. [Google Scholar] [CrossRef]
- Hou, J.G.; Yang, C.; Wang, Z.; Zhou, W.; Jiao, S.; Zhu, H. In situ synthesis of α–β phase heterojunction on Bi2O3 nanowires with exceptional visible-light photocatalytic performance. Appl. Catal. B Environ. 2013, 142–143, 504–511. [Google Scholar] [CrossRef]
- Zhu, Y.; Xv, Q.; Wang, D.; Sun, B.; Wang, Y.; Han, Z.; Gou, Y.; Liu, J.; Li, B. Construction of a hollow BiOI/TiO2/ZIF-8 heterojunction: Enhanced photocatalytic performance for norfloxacin degradation and mechanistic insight. J. Alloy Compd. 2022, 914, 165326. [Google Scholar] [CrossRef]
- Sedaghati, N.; Habibi-Yangjeh, A.; Pirhashemi, M.; Vadivel, S. Boosted visible-light photocatalytic performance of TiO2-X decorated by BiOI and AgBr nanoparticles. J. Photoch. Photobi. A 2019, 384, 112066. [Google Scholar] [CrossRef]
- Li, Q.; Qin, H.; Zhao, H.; Zhao, X.; Cheng, X.; Fan, W. Facile fabrication of a BiOI/TiO2 p-n junction via a surface charge-induced electrostatic self-assembly method. Appl. Surf. Sci. 2018, 457, 59–68. [Google Scholar] [CrossRef]
- Zhou, H.; Zhong, S.; Shen, M.; Hou, J.; Chen, W. Formamide-assisted one-pot synthesis of a Bi/Bi2O2CO3 heterojunction photocatalyst with enhanced photocatalytic activity. J. Alloy. Compd. 2018, 769, 301–310. [Google Scholar] [CrossRef]
- Xu, Q.; Wageh, S.; Al-Ghamdi, A.A. Design principle of S-scheme heterojunction photocatalyst. J. Mater. Sci. Technol. 2022, 124, 171–173. [Google Scholar] [CrossRef]
- Li, X.; Kang, B.; Dong, F.; Zhang, Z.; Luo, X.; Han, L.; Huang, J.; Feng, Z.; Chen, Z.; Xu, J.; et al. Enhanced photocatalytic degradation and H2/H2O2 production performance of S-pCN/WO2.72 S-scheme heterojunction with appropriate surface oxygen vacancies . Nano Energy 2021, 81, 105671. [Google Scholar]
- Ji, R.; Zhang, Z.; Tian, L.; Jin, L.; Xu, Q.; Lu, J. Z- scheme heterojunction of BiOI nanosheets grown in situ on NH2-UiO-66 crystals with rapid degradation of BPA in real water. Chem. Eng. J. 2022, 453, 139897. [Google Scholar] [CrossRef]
- Yao, S.; Lai, M.; Yang, J.; Yong, H.; Huang, J.; Wang, X.; Ma, Y. Synthesis and photocatalytic properties of electrodeposited bismuth oxyiodide on rutile/anatase TiO2 heterostructure. Mater. Res. Express 2019, 6, 055905. [Google Scholar] [CrossRef]
- Song, F.; Sun, H.; Ma, H.; Gao, H. Porous TiO2/Carbon Dot Nanoflowers with Enhanced Surface Areas for Improving Photocatalytic Activity. Nanomaterials 2022, 12, 2536. [Google Scholar] [CrossRef] [PubMed]


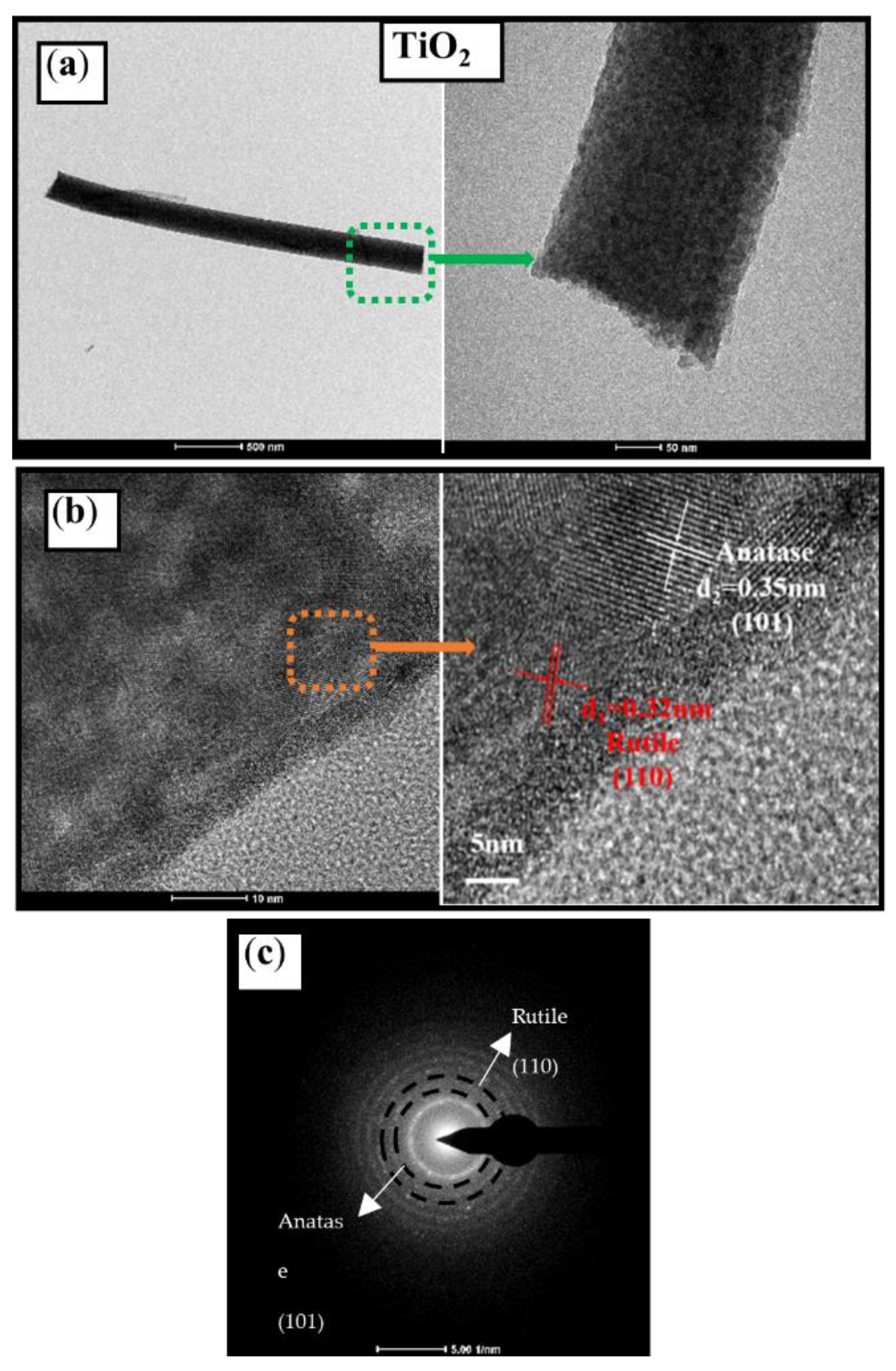

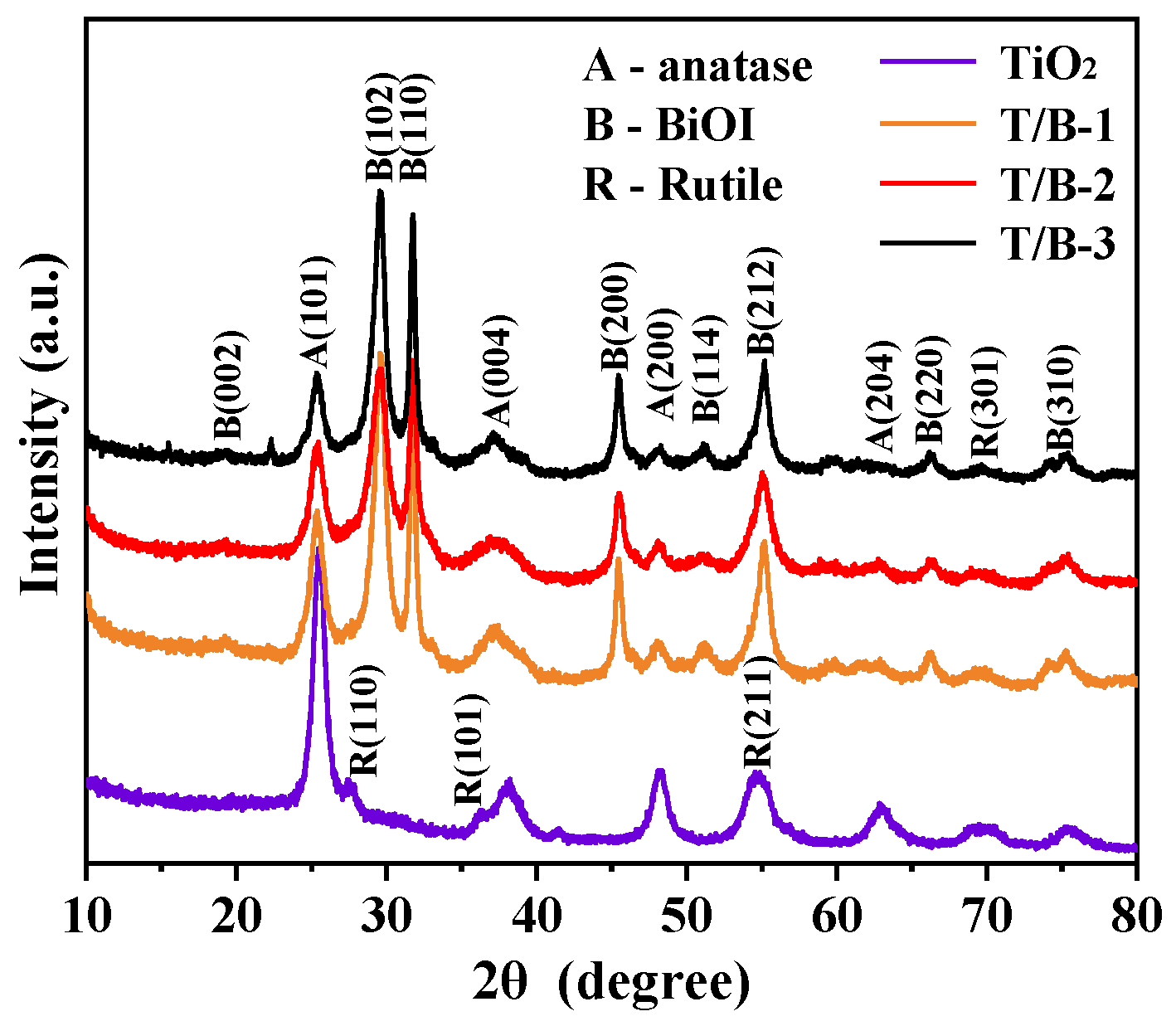

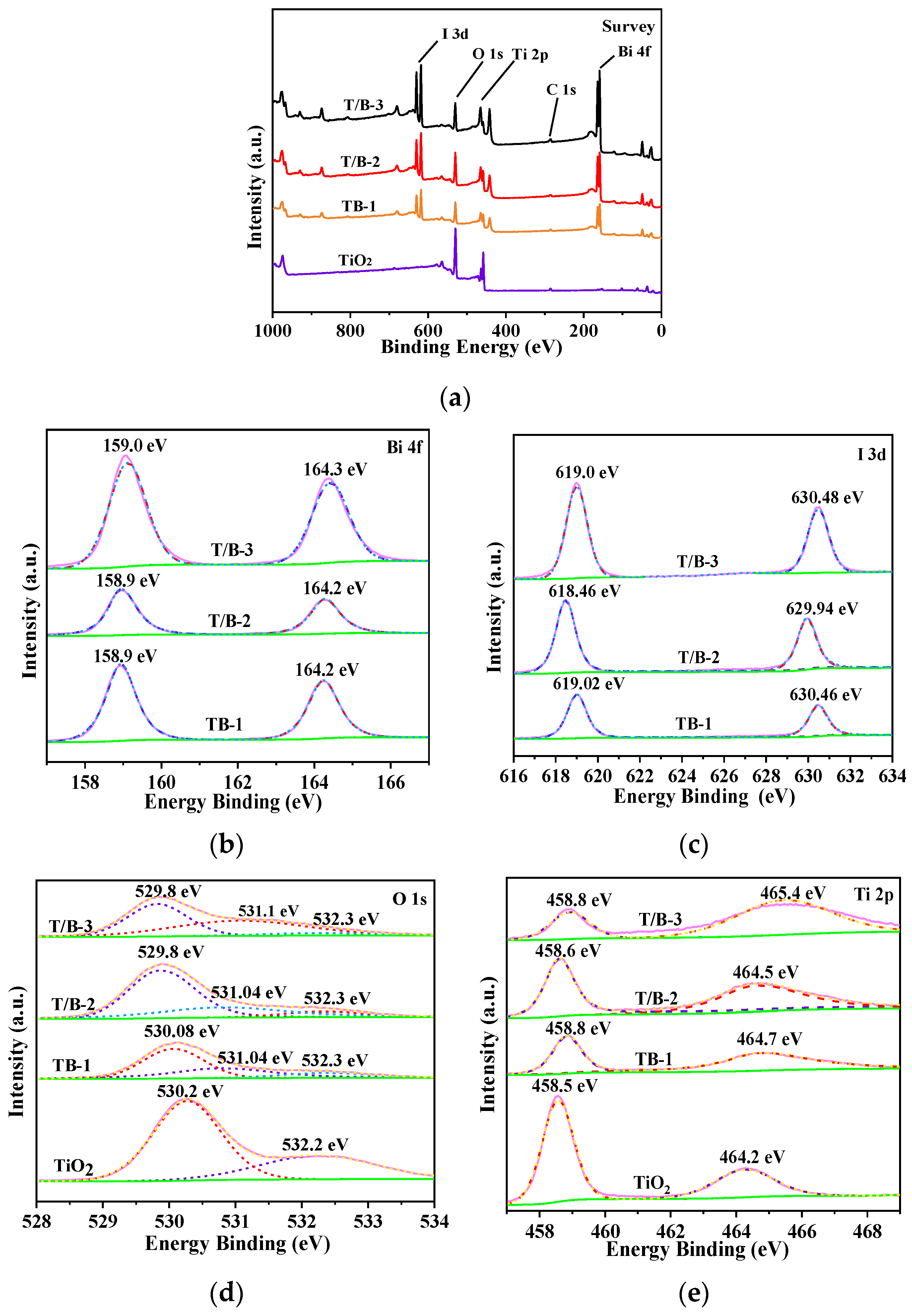
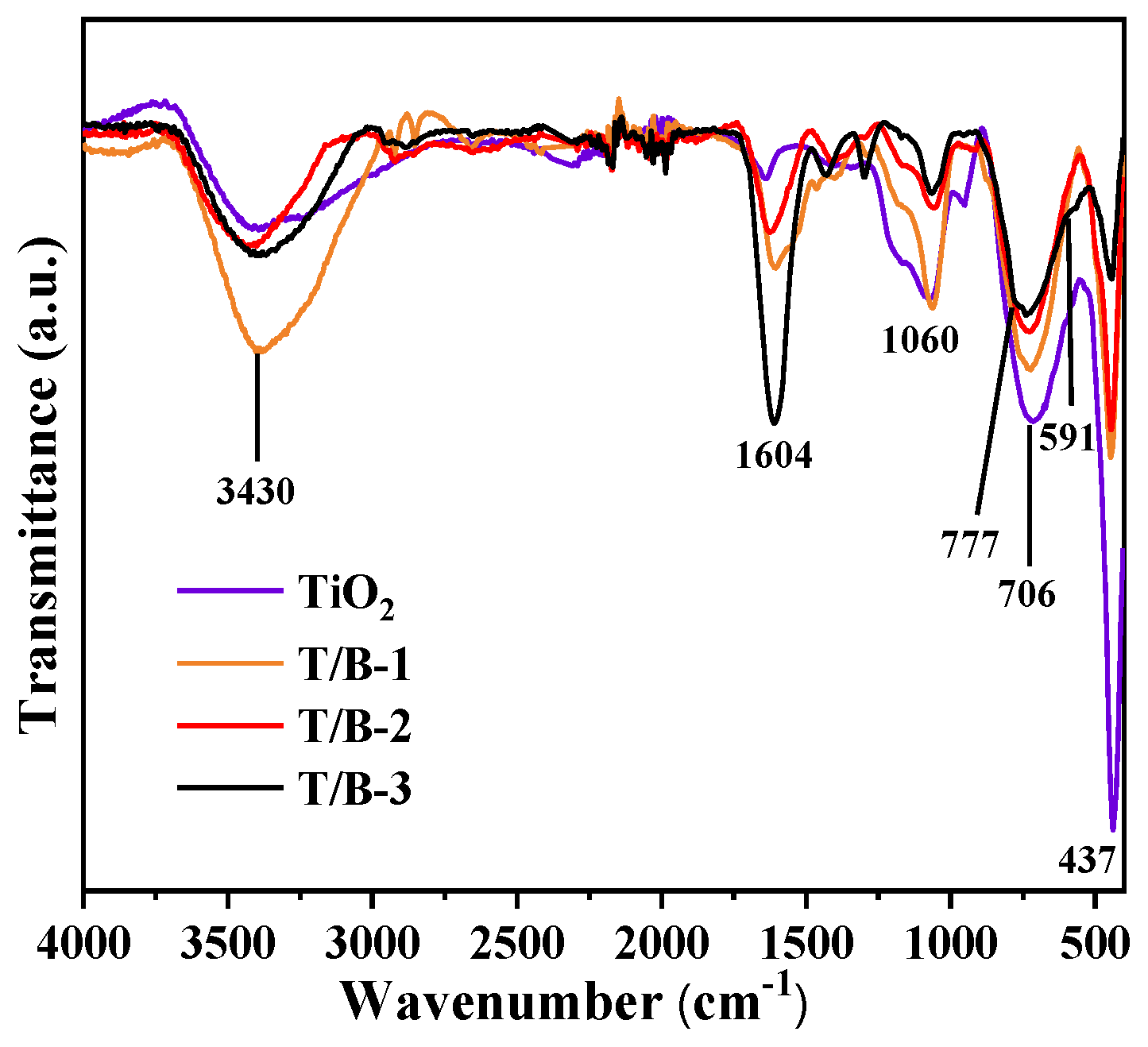

| Samples | Element Concentration | Ti/Bi | |||
|---|---|---|---|---|---|
| Ti (%) | O (%) | Bi (%) | I (%) | ||
| TiO2 | 21.90 | 64.41 | 0 | 0 | / |
| T/B-1 | 12.94 | 57.07 | 7.51 | 8.54 | 1.71 |
| T/B-2 | 13.59 | 61.99 | 8.63 | 5.64 | 1.57 |
| T/B-3 | 9.89 | 54.12 | 9.65 | 11.54 | 1.02 |
| Photocatalysts | Preparation Methods | Simulated Pollutant | The Degradation Rate Constant K Value | Ref |
|---|---|---|---|---|
| BiOI/TiO2/ZIF-8 | Hydrothermal synthesis | Norfloxacin (NOR) | 0.0058 min−1 | [53] |
| TiO2-x/BiOI/AgBr | Hydrothermal synthesis | Methylene blue (MB) | 0.0033 min−1 | [54] |
| BiOI/TiO2 | Electrostatic self-grouping induced by surface charge | MO | 0.0026 min−1 | [55] |
| * Anatase–rutile/BiOI | Hydrothermal synthesis | MO | 0.0062 min−1 | / |
| Materials | Eg (eV) | EVB (eV) | ECB (eV) |
|---|---|---|---|
| BiOI | 1.74 | 2.36 | 0.62 |
| Rutile TiO2 | 3.00 | 2.84 | −0.16 |
| Anatase TiO2 | 3.10 | 2.89 | −0.21 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, D.; Xu, K.; Zhang, C. Improvement of Photocatalytic Performance by Building Multiple Heterojunction Structures of Anatase–Rutile/BiOI Composite Fibers. Nanomaterials 2022, 12, 3906. https://doi.org/10.3390/nano12213906
Li D, Xu K, Zhang C. Improvement of Photocatalytic Performance by Building Multiple Heterojunction Structures of Anatase–Rutile/BiOI Composite Fibers. Nanomaterials. 2022; 12(21):3906. https://doi.org/10.3390/nano12213906
Chicago/Turabian StyleLi, Dayu, Kai Xu, and Chao Zhang. 2022. "Improvement of Photocatalytic Performance by Building Multiple Heterojunction Structures of Anatase–Rutile/BiOI Composite Fibers" Nanomaterials 12, no. 21: 3906. https://doi.org/10.3390/nano12213906
APA StyleLi, D., Xu, K., & Zhang, C. (2022). Improvement of Photocatalytic Performance by Building Multiple Heterojunction Structures of Anatase–Rutile/BiOI Composite Fibers. Nanomaterials, 12(21), 3906. https://doi.org/10.3390/nano12213906






