Improved Dehydrogenation Properties of LiAlH4 by Addition of Nanosized CoTiO3
Abstract
:1. Introduction
2. Materials and Methods
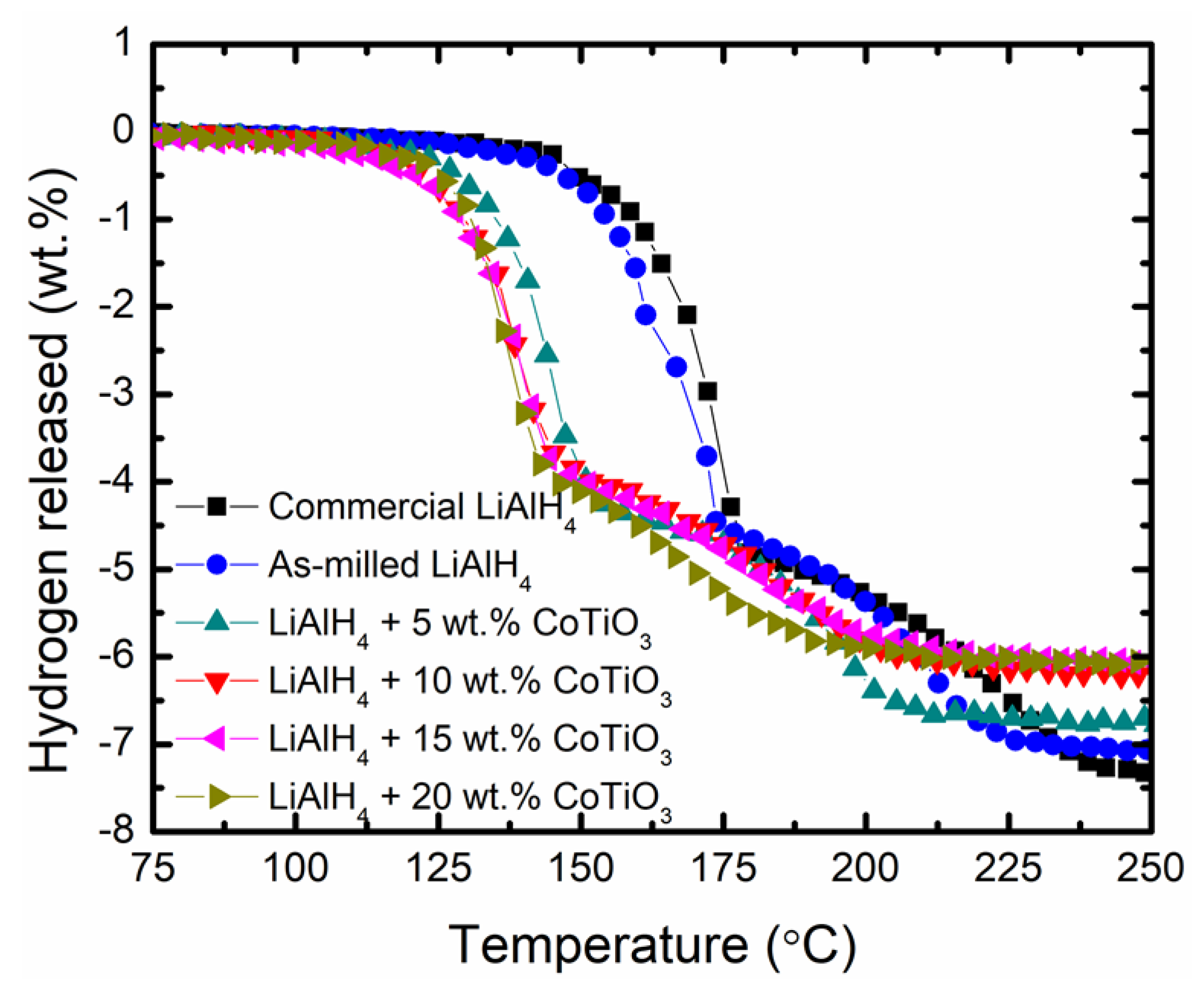
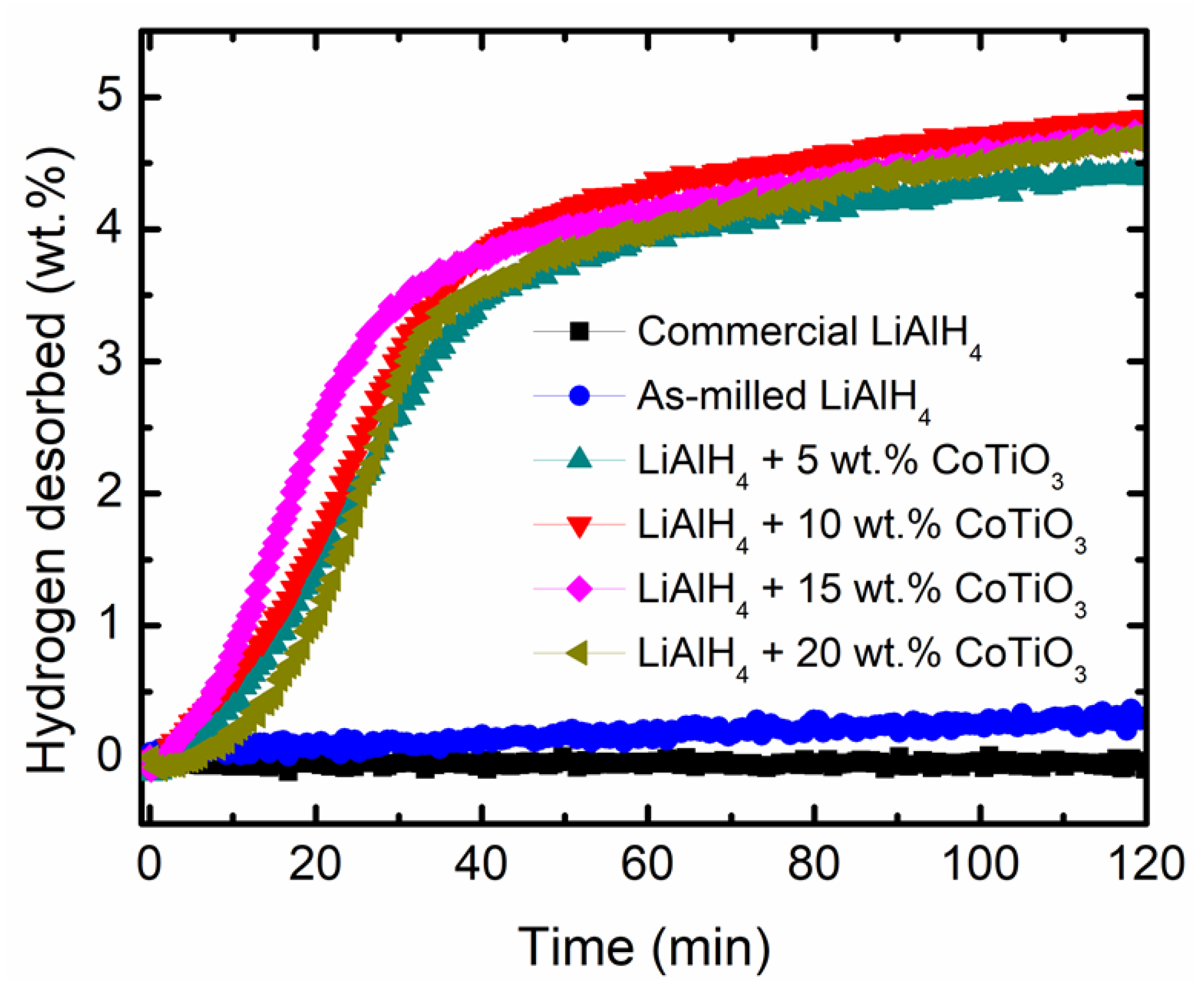
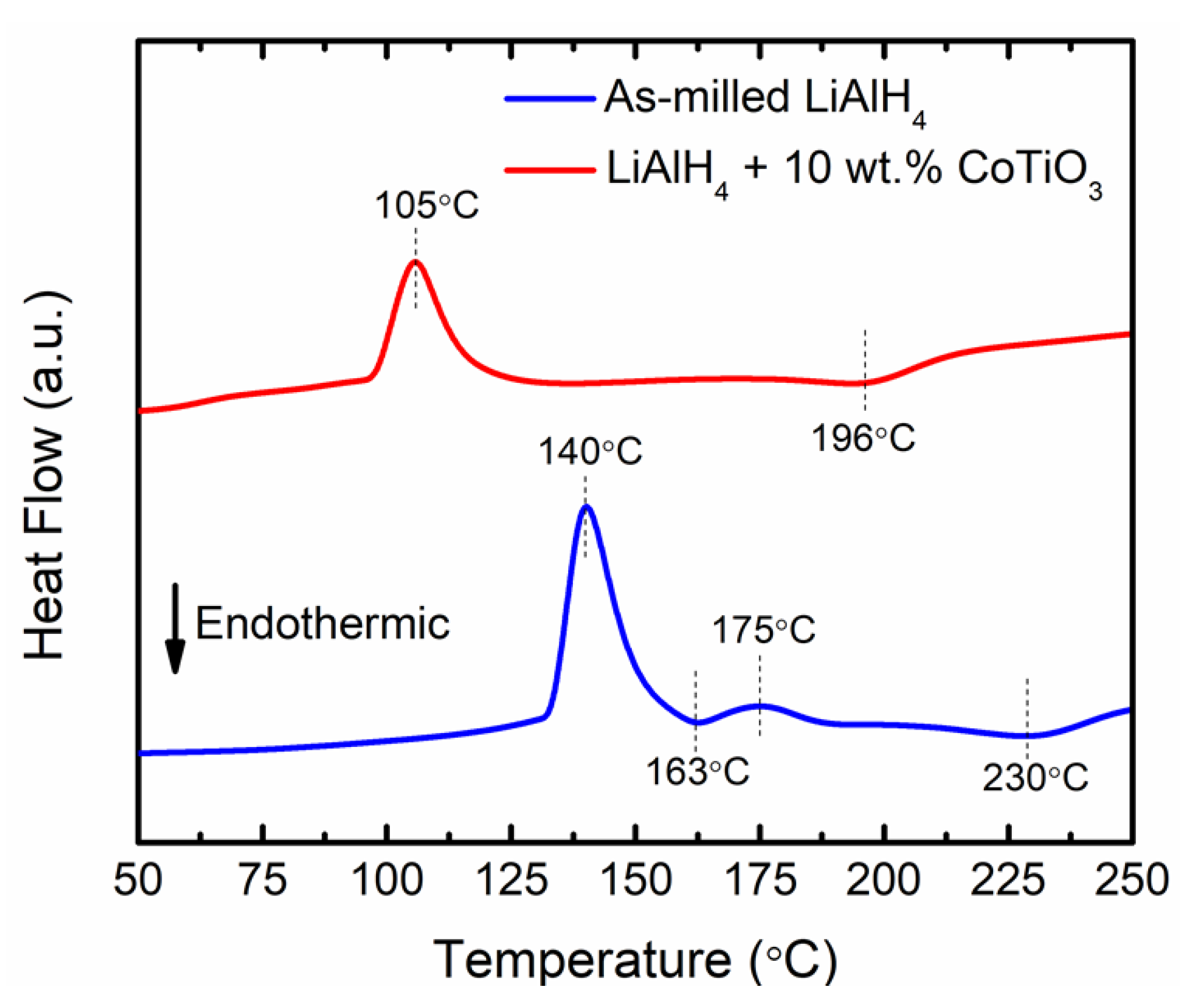
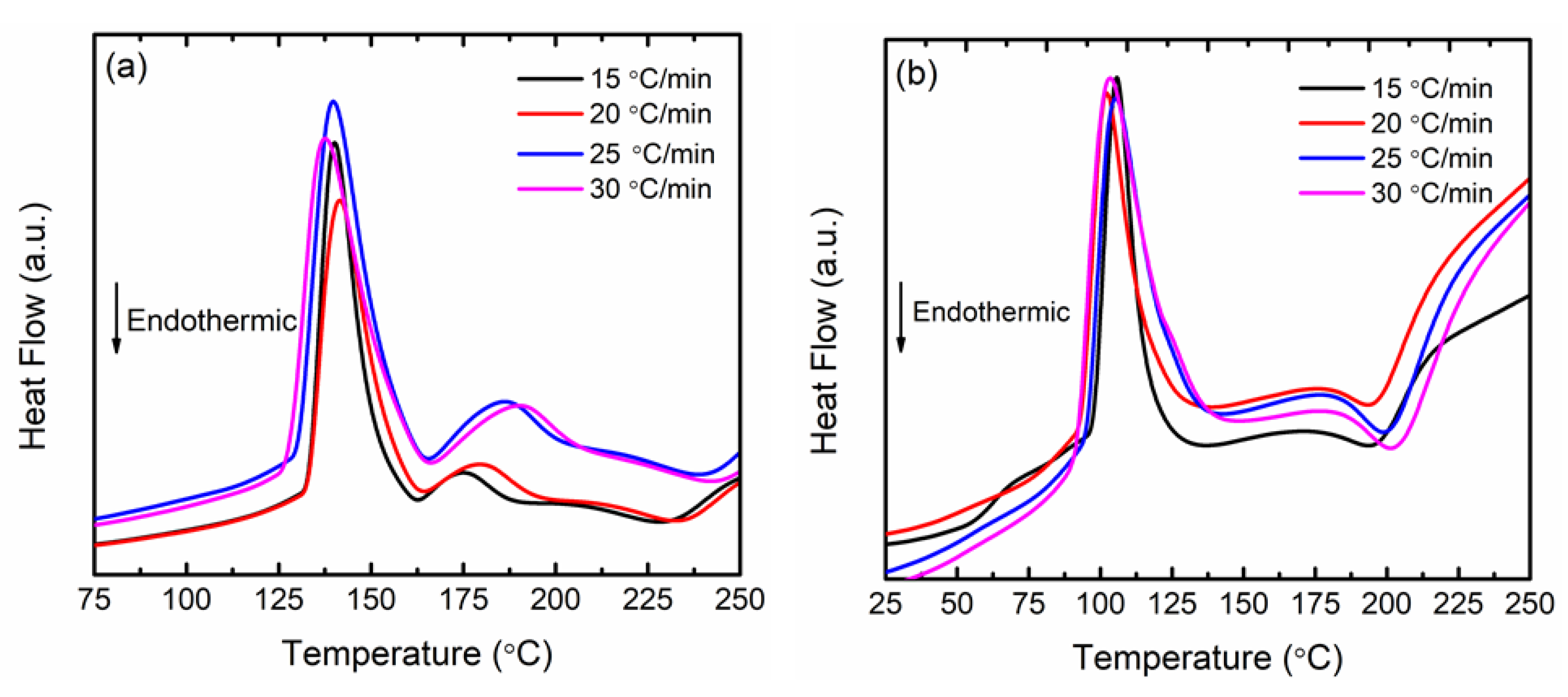
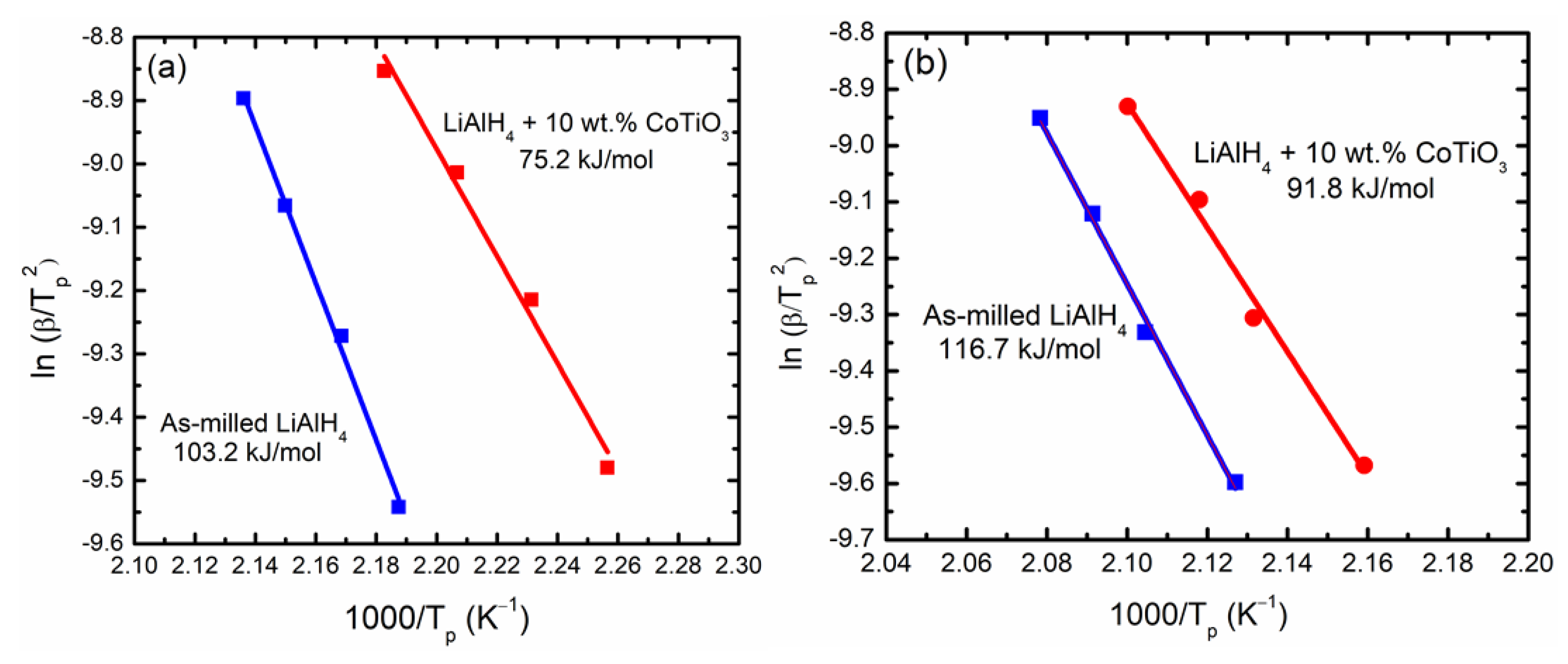
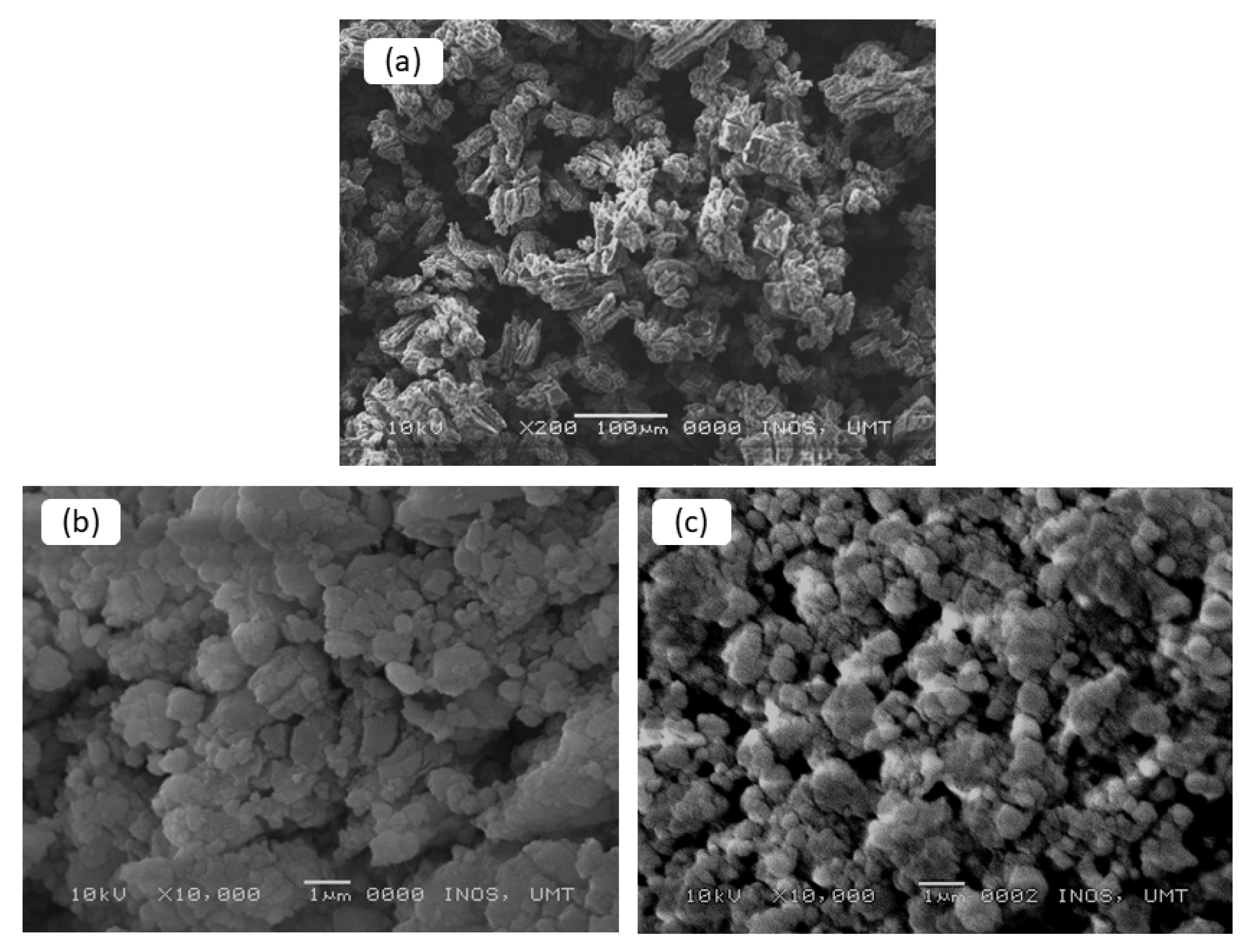
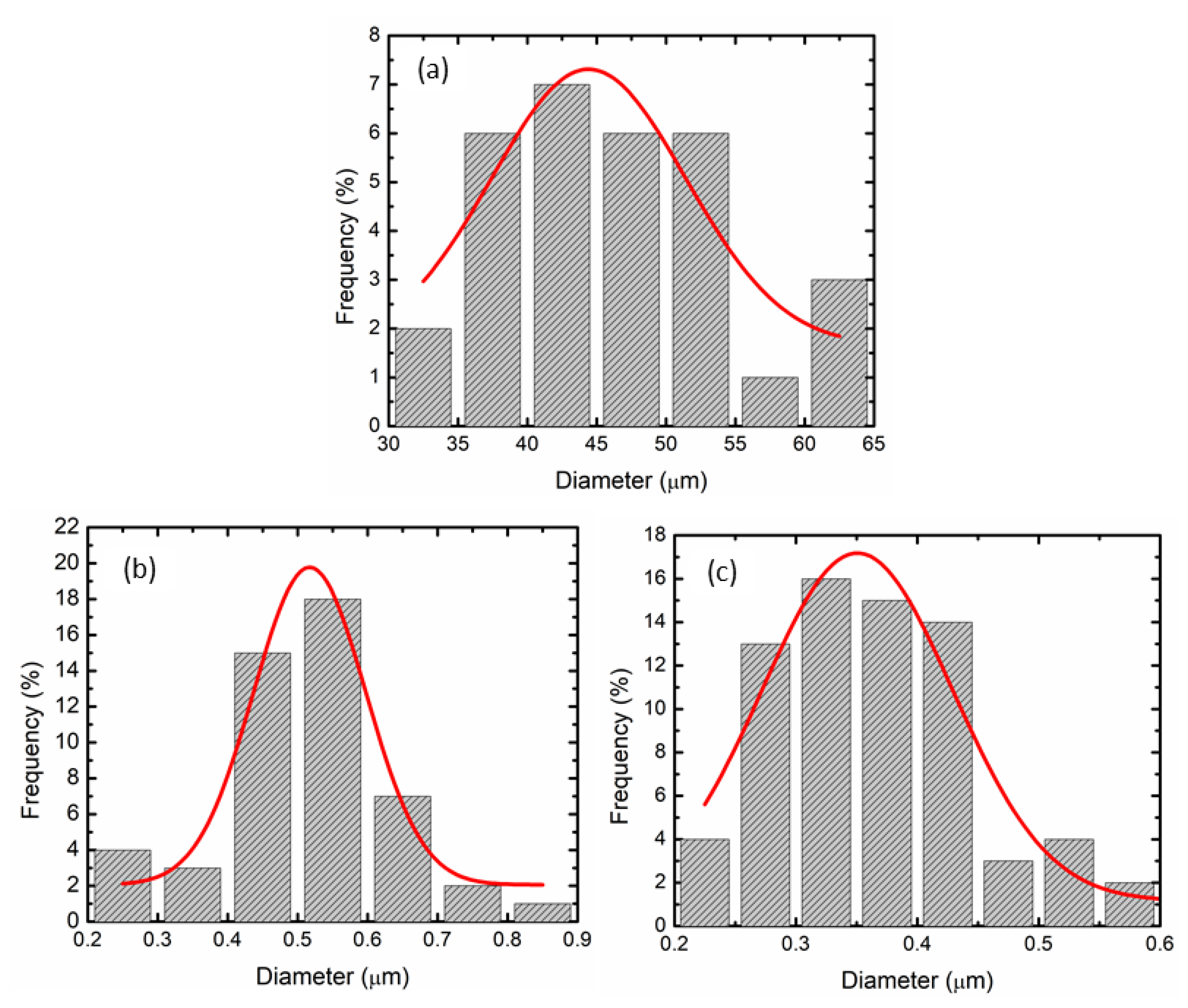
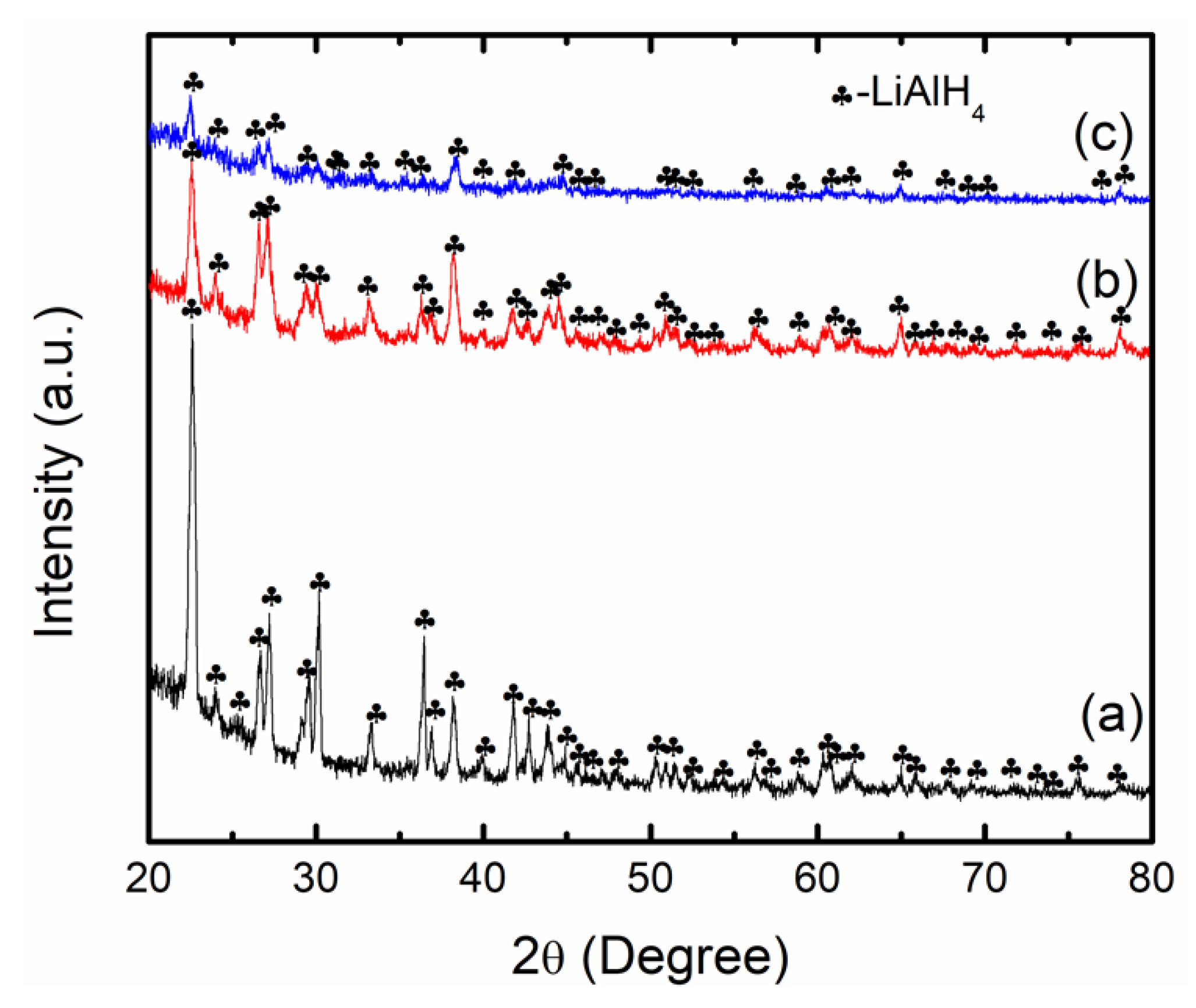
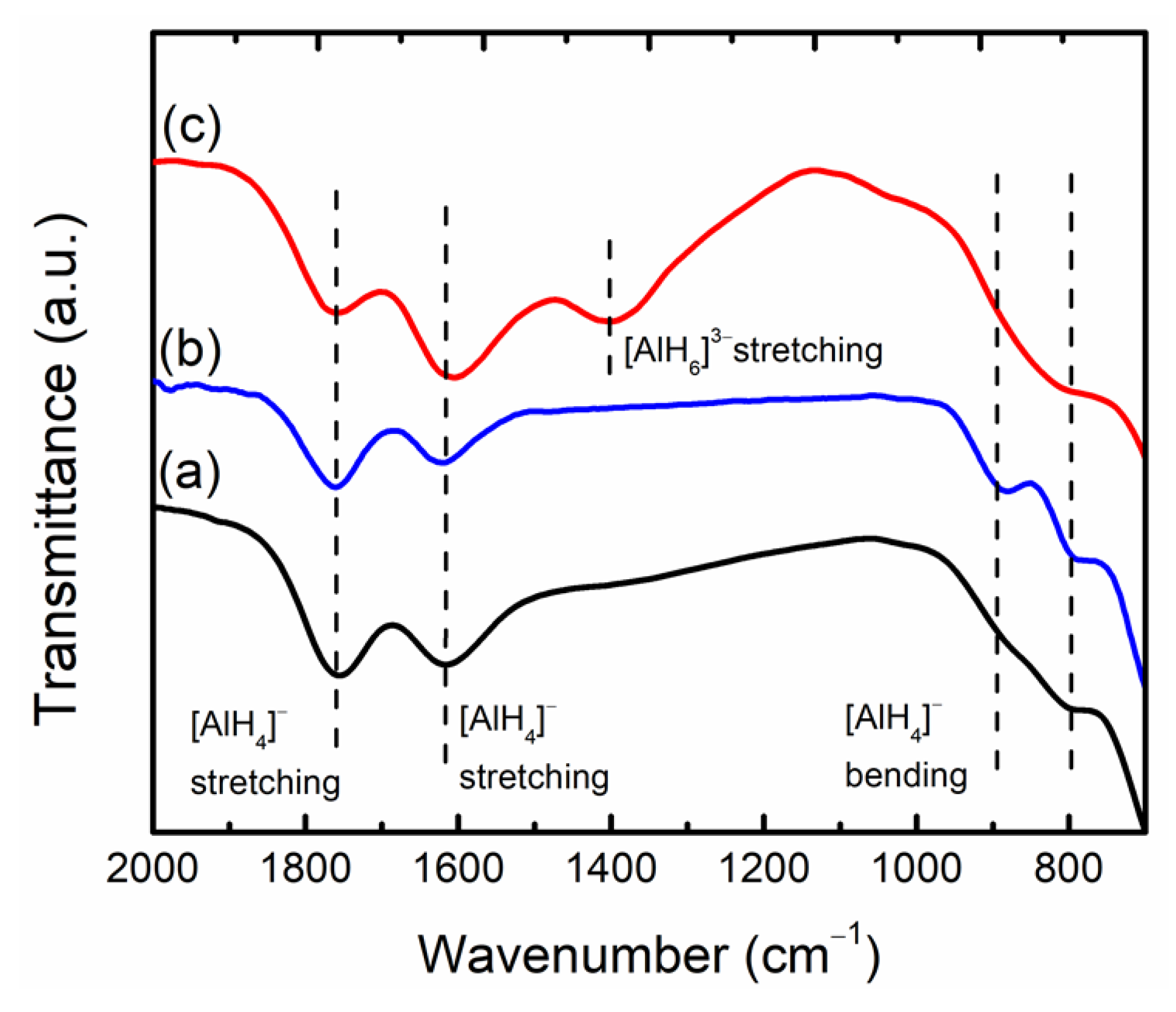
3. Results and Discussions
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tan, C.-Y.; Tsai, W.-T. Effects of Ni and Co-decorated MWCNTs addition on the dehydrogenation behavior and stability of LiAlH4. Int. J. Hydrog. Energy 2015, 40, 14064–14071. [Google Scholar] [CrossRef]
- Xia, Y.; Zhang, H.; Sun, Y.; Sun, L.; Xu, F.; Sun, S.; Zhang, G.; Huang, P.; Du, Y.; Wang, J.; et al. Dehybridization effect in improved dehydrogenation of LiAlH4 by doping with two-dimensional Ti3C2. Mater. Today Nano 2019, 8, 100054. [Google Scholar] [CrossRef]
- Zang, L.; Cai, J.; Zhao, L.; Gao, W.; Liu, J.; Wang, Y. Improved hydrogen storage properties of LiAlH4 by mechanical milling with TiF3. J. Alloys Compd. 2015, 647, 756–762. [Google Scholar] [CrossRef]
- Varin, R.; Zbroniec, L. Fast and slow dehydrogenation of ball milled lithium alanate (LiAlH4) catalyzed with manganese chloride (MnCl2) as compared to nanometric nickel catalyst. J. Alloys Compd. 2011, 509, S736–S739. [Google Scholar] [CrossRef]
- Humphries, T.D.; Birkmire, D.; McGrady, G.S.; Hauback, B.C.; Jensen, C.M. Regeneration of LiAlH4 at sub-ambient temperatures studied by multinuclear NMR spectroscopy. J. Alloys Compd. 2017, 723, 1150–1154. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Liu, S.; Si, X.; Zhang, J.; Jiao, C.; Wang, S.; Liu, S.; Zou, Y.-J.; Sun, L.; Xu, F. Significantly improved dehydrogenation of LiAlH4 destabilized by K2TiF6. Int. J. Hydrog. Energy 2012, 37, 3261–3267. [Google Scholar] [CrossRef]
- Fu, J.; Röntzsch, L.; Schmidt, T.; Tegel, M.; Weißgärber, T.; Kieback, B. Comparative study on the dehydrogenation properties of TiCl4-doped LiAlH4 using different doping techniques. Int. J. Hydrog. Energy 2012, 37, 13387–13392. [Google Scholar] [CrossRef]
- Li, L.; Wang, Y.; Jiao, L.; Yuan, H. Enhanced catalytic effects of Co@C additive on dehydrogenation properties of LiAlH4. J. Alloys Compd. 2015, 645, S468–S471. [Google Scholar] [CrossRef]
- Ali, N.A.; Sazelee, N.; Yahya, M.S.; Ismail, M. Influence of K2NbF7 catalyst on the desorption behavior of LiAlH4. Front. Chem. 2020, 8, 457. [Google Scholar] [CrossRef]
- Liu, S.-S.; Sun, L.-X.; Zhang, Y.; Xu, F.; Zhang, J.; Chu, H.-L.; Fan, M.-Q.; Zhang, T.; Song, X.-Y.; Grolier, J.P. Effect of ball milling time on the hydrogen storage properties of TiF3-doped LiAlH4. Int. J. Hydrog. Energy 2009, 34, 8079–8085. [Google Scholar] [CrossRef]
- Fu, J.; Röntzsch, L.; Schmidt, T.; Weißgärber, T.; Kieback, B. Improved dehydrogenation properties of lithium alanate (LiAlH4) doped by low energy grinding. J. Alloys Compd. 2012, 525, 73–77. [Google Scholar] [CrossRef]
- Varin, R.A.; Parviz, R. The effects of the micrometric and nanometric iron (Fe) additives on the mechanical and thermal dehydrogenation of lithium alanate (LiAlH4), its self-discharge at low temperatures and rehydrogenation. Int. J. Hydrog. Energy 2012, 37, 9088–9102. [Google Scholar] [CrossRef]
- Wang, L.; Rawal, A.; Quadir, M.Z.; Aguey-Zinsou, K.-F. Nanoconfined lithium aluminium hydride (LiAlH4) and hydrogen reversibility. Int. J. Hydrog. Energy 2017, 42, 14144–14153. [Google Scholar] [CrossRef]
- Zhou, S.; Zou, J.; Zeng, X.; Ding, W. Effects of REF3 (RE = Y, La, Ce) additives on dehydrogenation properties of LiAlH4. Int. J. Hydrog. Energy 2014, 39, 11642–11650. [Google Scholar] [CrossRef]
- Isobe, S.; Ikarashi, Y.; Yao, H.; Hino, S.; Wang, Y.; Hashimoto, N.; Ohnuki, S. Additive effects of TiCl3 on dehydrogenation reaction of LiAlH4. Mater. Trans. 2014, 55, 1138–1140. [Google Scholar] [CrossRef] [Green Version]
- Sazelee, N.A.; Ismail, M. Recent advances in catalyst-enhanced LiAlH4 for solid-state hydrogen storage: A review. Int. J. Hydrog. Energy 2021, 46, 9123–9141. [Google Scholar] [CrossRef]
- Li, L.; Qiu, F.; Wang, Y.; Xu, Y.; An, C.; Liu, G.; Jiao, L.; Yuan, H. Enhanced hydrogen storage properties of TiN–LiAlH4 composite. Int. J. Hydrog. Energy 2013, 38, 3695–3701. [Google Scholar] [CrossRef]
- Sulaiman, N.N.; Ismail, M.; Timmiati, S.N.; Lim, K.L. Improved hydrogen storage performances of LiAlH4+ Mg(BH4)2 composite with TiF3 addition. Int. J. Energy Res. 2020, 45, 2882–2898. [Google Scholar] [CrossRef]
- Meethom, S.; Kaewsuwan, D.; Chanlek, N.; Utke, O.; Utke, R. Enhanced hydrogen sorption of LiBH4–LiAlH4 by quenching dehydrogenation, ball milling, and doping with MWCNTs. J. Phys. Chem. Solids 2020, 136, 109202. [Google Scholar] [CrossRef]
- Chen, G.; Zhang, Y.; Cheng, H.; Zhu, Y.; Li, L.; Lin, H. Effects of two-dimension MXene Ti3C2 on hydrogen storage performances of MgH2-LiAlH4 composite. Chem. Phys. 2019, 522, 178–187. [Google Scholar] [CrossRef]
- Rattan Paul, D.; Sharma, A.; Panchal, P.; Chaudhary, S.; Patidar, D.; Nehra, S.P. Effect of ball milling and iron mixing on structural and morphological properties of magnesium for hydrogen storage application. Mater. Today 2021, 42, 1673–1677. [Google Scholar] [CrossRef]
- Alsabawi, K.; Gray, E.M.; Webb, C.J. The effect of ball-milling gas environment on the sorption kinetics of MgH2 with/without additives for hydrogen storage. Int. J. Hydrog. Energy 2019, 44, 2976–2980. [Google Scholar] [CrossRef]
- Ali, N.; Idris, N.; Sazelee, N.; Yahya, M.; Yap, F.H.; Ismail, M. Catalytic effects of MgFe2O4 addition on the dehydrogenation properties of LiAlH4. Int. J. Hydrog. Energy 2019, 44, 28227–28234. [Google Scholar] [CrossRef]
- Sazelee, N.; Yahya, M.; Idris, N.; Din, M.M.; Ismail, M. Desorption properties of LiAlH4 doped with LaFeO3 catalyst. Int. J. Hydrog. Energy 2019, 44, 11953–11960. [Google Scholar] [CrossRef]
- Rafi-ud-din; Xuanhui, Q.; Ping, L.; Zhang, L.; Ahmad, M. Hydrogen sorption improvement of LiAlH4 catalyzed by Nb2O5 and Cr2O3 nanoparticles. J. Phys. Chem. C 2011, 115, 13088–13099. [Google Scholar] [CrossRef]
- Liu, S.; Ma, Q.; Lü, H.; Zheng, X.; Feng, X.; Xiao, G.; Zheng, J. Study on hydrogen release capacity of LiAlH4 doped with CeO2. Rare Metal Mat. Eng. 2014, 43, 544–547. [Google Scholar] [CrossRef]
- Li, L.; An, C.; Wang, Y.; Xu, Y.; Qiu, F.; Wang, Y.; Jiao, L.; Yuan, H. Enhancement of the H2 desorption properties of LiAlH4 doping with NiCo2O4 nanorods. Int. J. Hydrog. Energy 2014, 39, 4414–4420. [Google Scholar] [CrossRef]
- Sulaiman, N.; Ismail, M. Catalytic effect of SrFe12O19 on the hydrogen storage properties of LiAlH4. Int. J. Hydrog. Energy 2017, 42, 19126–19134. [Google Scholar] [CrossRef]
- Li, Z.; Zhai, F.; Wan, Q.; Liu, Z.; Shan, J.; Li, P.; Volinsky, A.A.; Qu, X. Enhanced hydrogen storage properties of LiAlH4 catalyzed by CoFe2O4 nanoparticles. RSC Adv. 2014, 4, 18989–18997. [Google Scholar] [CrossRef] [Green Version]
- Ismail, M.; Zhao, Y.; Yu, X.B.; Nevirkovets, I.P.; Dou, S.X. Significantly improved dehydrogenation of LiAlH4 catalysed with TiO2 nanopowder. Int. J. Hydrog. Energy 2011, 36, 8327–8334. [Google Scholar] [CrossRef]
- Barkhordarian, G.; Klassen, T.; Bormann, R. Catalytic mechanism of transition-metal compounds on Mg hydrogen sorption reaction. J. Phys. Chem. B 2006, 110, 11020–11024. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Xu, F.; Zhang, C.; Wang, Z.; Ju, H.; Gao, X.; Zhang, X.; Sun, L.; Liu, Z. Enhanced hydrogen storage of alanates: Recent progress and future perspectives. ProG. Nat. Sci. Mater. 2021, 31, 165–179. [Google Scholar] [CrossRef]
- Ali, N.A.; Yahya, M.S.; Sazelee, N.; Din, M.F.M.; Ismail, M. Influence of nanosized CoTiO3 synthesized via a solid-state method on the hydrogen storage behavior of MgH2. Nanomaterials 2022, 12, 3043. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Wei, S.; Huang, Q.; Li, J.; Cen, X.; Zhang, H.; Chu, H.; Sun, L.; Xu, F.; Huang, P. Facile synthesis of NiCo2O4-anchored reduced graphene oxide nanocomposites as efficient additives for improving the dehydrogenation behavior of lithium alanate. Inorg. Chem. Front. 2020, 7, 1257–1272. [Google Scholar] [CrossRef]
- Li, L.; Xu, Y.; Wang, Y.; Wang, Y.; Qiu, F.; An, C.; Jiao, L.; Yuan, H. NbN nanoparticles as additive for the high dehydrogenation properties of LiAlH4. Dalton Trans. 2014, 43, 1806–1813. [Google Scholar] [CrossRef]
- Cao, Z.; Ma, X.; Wang, H.; Ouyang, L. Catalytic effect of ScCl3 on the dehydrogenation properties of LiAlH4. J. Alloys Compd. 2018, 762, 73–79. [Google Scholar] [CrossRef]
- Yap, F.H.; Ali, N.; Idris, N.; Ismail, M. Catalytic effect of MgFe2O4 on the hydrogen storage properties of Na3AlH6–LiBH4 composite system. Int. J. Hydrog. Energy 2018, 43, 20882–20891. [Google Scholar] [CrossRef]
- Aguey-Zinsou, K.-F.; Fernandez, J.A.; Klassen, T.; Bormann, R. Effect of Nb2O5 on MgH2 properties during mechanical milling. Int. J. Hydrog. Energy 2007, 32, 2400–2407. [Google Scholar] [CrossRef]
- Yuan, Z.; Sui, Y.; Zhai, T.; Yin, Y.; Luo, L.; Feng, D. Influence of CeO2 nanoparticles on microstructure and hydrogen storage performance of Mg-Ni-Zn alloy. Mater. Charact. 2021, 178, 111248. [Google Scholar] [CrossRef]
- Xu, X.; Zang, L.; Zhao, Y.; Zhao, Y.; Wang, Y.; Jiao, L. Hydrogen storage behavior of LiBH4 improved by the confinement of hierarchical porous ZnO/ZnCo2O4 nanoparticles. J. Power Sources 2017, 359, 134–141. [Google Scholar] [CrossRef]
- Ismail, M.; Sazelee, N.A.; Ali, N.A.; Suwarno, S. Catalytic effect of SrTiO3 on the dehydrogenation properties of LiAlH4. J. Alloys Compd. 2021, 855, 157475. [Google Scholar] [CrossRef]
- Zhang, T.; Isobe, S.; Wang, Y.; Liu, C.; Hashimoto, N.; Takahashi, K. Enhanced hydrogen desorption properties of LiAlH4 by doping lithium metatitanate. Phys. Chem. Chem. Phys. 2016, 18, 27623–27629. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Zang, L.; Zhao, L.; Liu, J.; Wang, Y. Dehydrogenation characteristics of LiAlH4 improved by in situ formed catalysts. J. Energy Chem. 2016, 25, 868–873. [Google Scholar] [CrossRef]
- Ismail, M.; Ali, N.A.; Sazelee, N.A.; Suwarno, S. Catalytic effect of Al2TiO5 on the dehydrogenation properties of LiAlH4. Int. J. Hydrog. Energy 2022, 47, 31903–31910. [Google Scholar] [CrossRef]
- Wohlwend, J.L.; Amama, P.B.; Shamberger, P.J.; Varshney, V.; Roy, A.K.; Fisher, T.S. Effects of titanium-containing additives on the dehydrogenation properties of LiAlH4: A computational and experimental study. J. Phys. Chem. C 2012, 116, 22327–22335. [Google Scholar] [CrossRef]
- Li, Z.; Li, P.; Wan, Q.; Zhai, F.; Liu, Z.; Zhao, K.; Wang, L.; Lü, S.; Zou, L.; Qu, X.; et al. Dehydrogenation improvement of LiAlH4 catalyzed by Fe2O3 and Co2O3 nanoparticles. J. Phys. Chem. C 2013, 117, 18343–18352. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, N.A.; Ahmad, M.A.N.; Yahya, M.S.; Sazelee, N.; Ismail, M. Improved Dehydrogenation Properties of LiAlH4 by Addition of Nanosized CoTiO3. Nanomaterials 2022, 12, 3921. https://doi.org/10.3390/nano12213921
Ali NA, Ahmad MAN, Yahya MS, Sazelee N, Ismail M. Improved Dehydrogenation Properties of LiAlH4 by Addition of Nanosized CoTiO3. Nanomaterials. 2022; 12(21):3921. https://doi.org/10.3390/nano12213921
Chicago/Turabian StyleAli, Nurul Amirah, Muhammad Amirul Nawi Ahmad, Muhammad Syarifuddin Yahya, Noratiqah Sazelee, and Mohammad Ismail. 2022. "Improved Dehydrogenation Properties of LiAlH4 by Addition of Nanosized CoTiO3" Nanomaterials 12, no. 21: 3921. https://doi.org/10.3390/nano12213921









