Unraveling the Influence of the Electrolyte on the Polarization Resistance of Nanostructured La0.6Sr0.4Co0.2Fe0.8O3-δ Cathodes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials Preparation
2.2. Structural, Microstructural and Electrical Characterization
3. Results
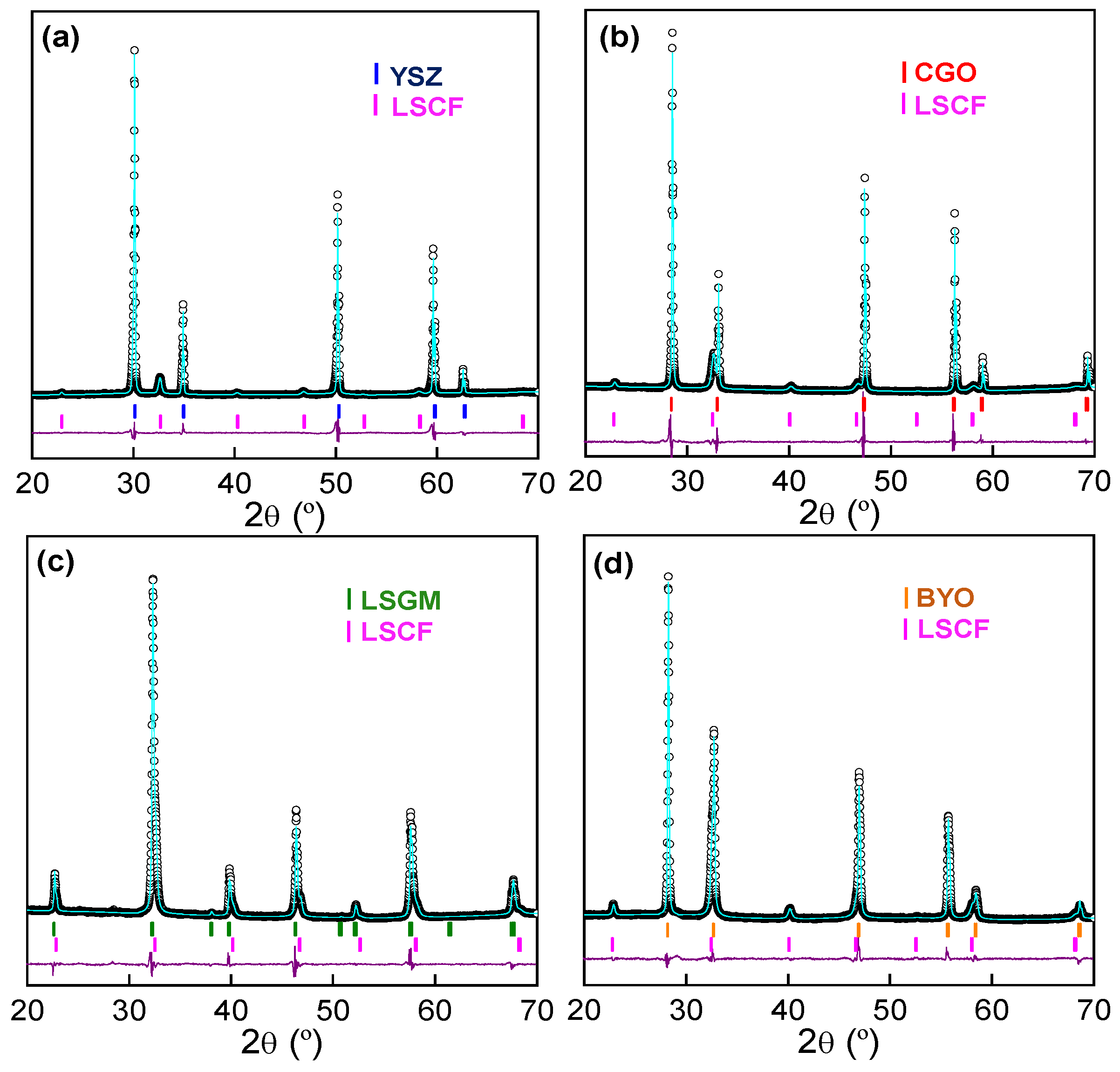
3.1. Phase Formation
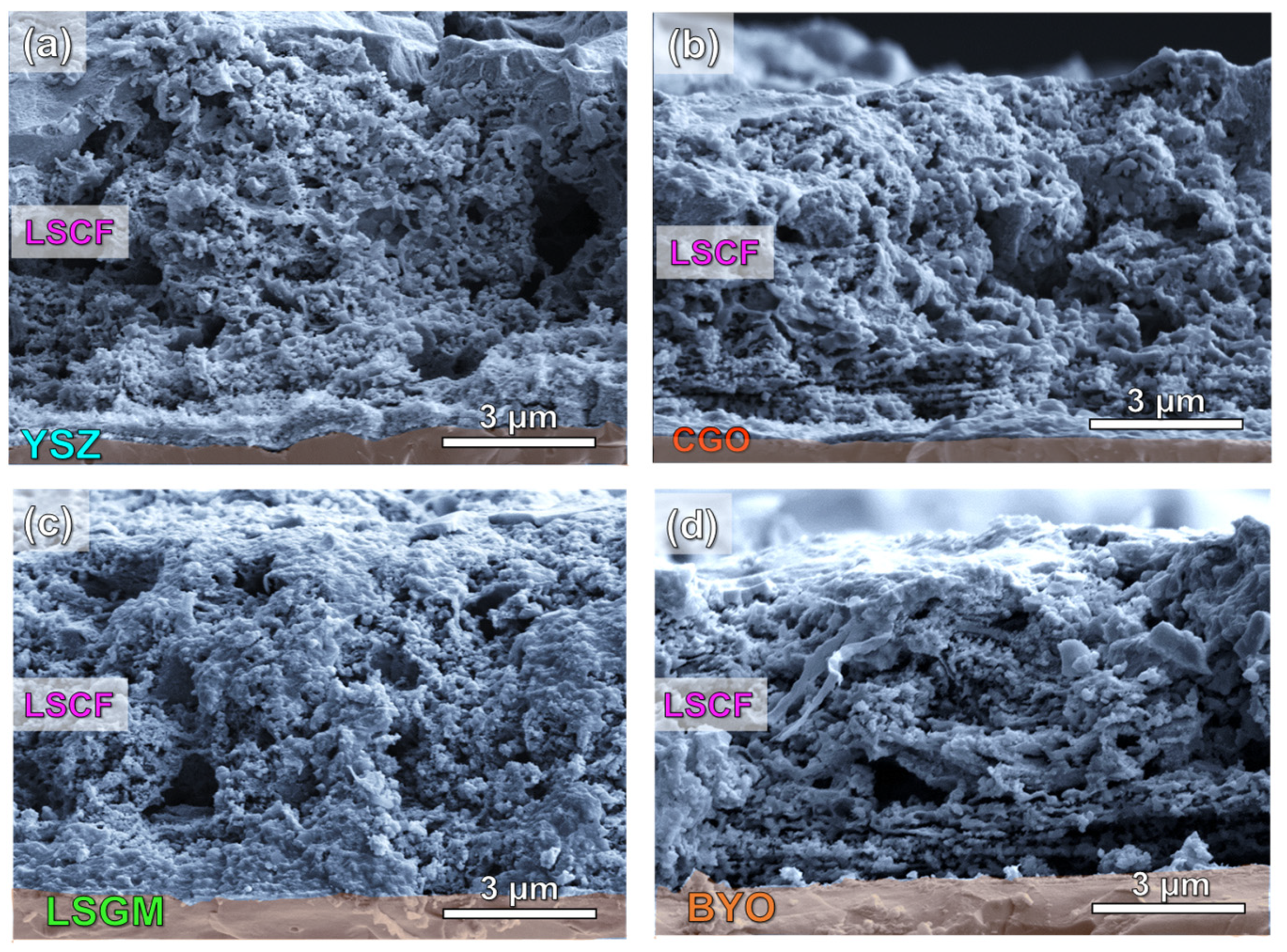
3.2. Microstructure
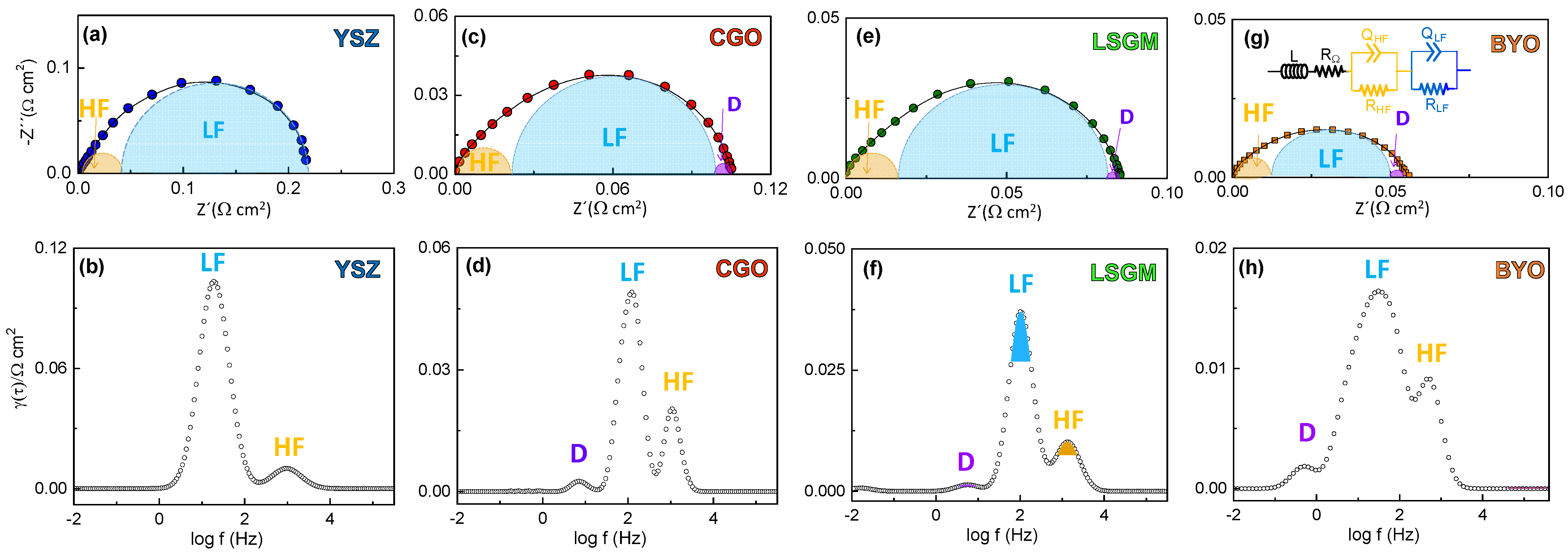
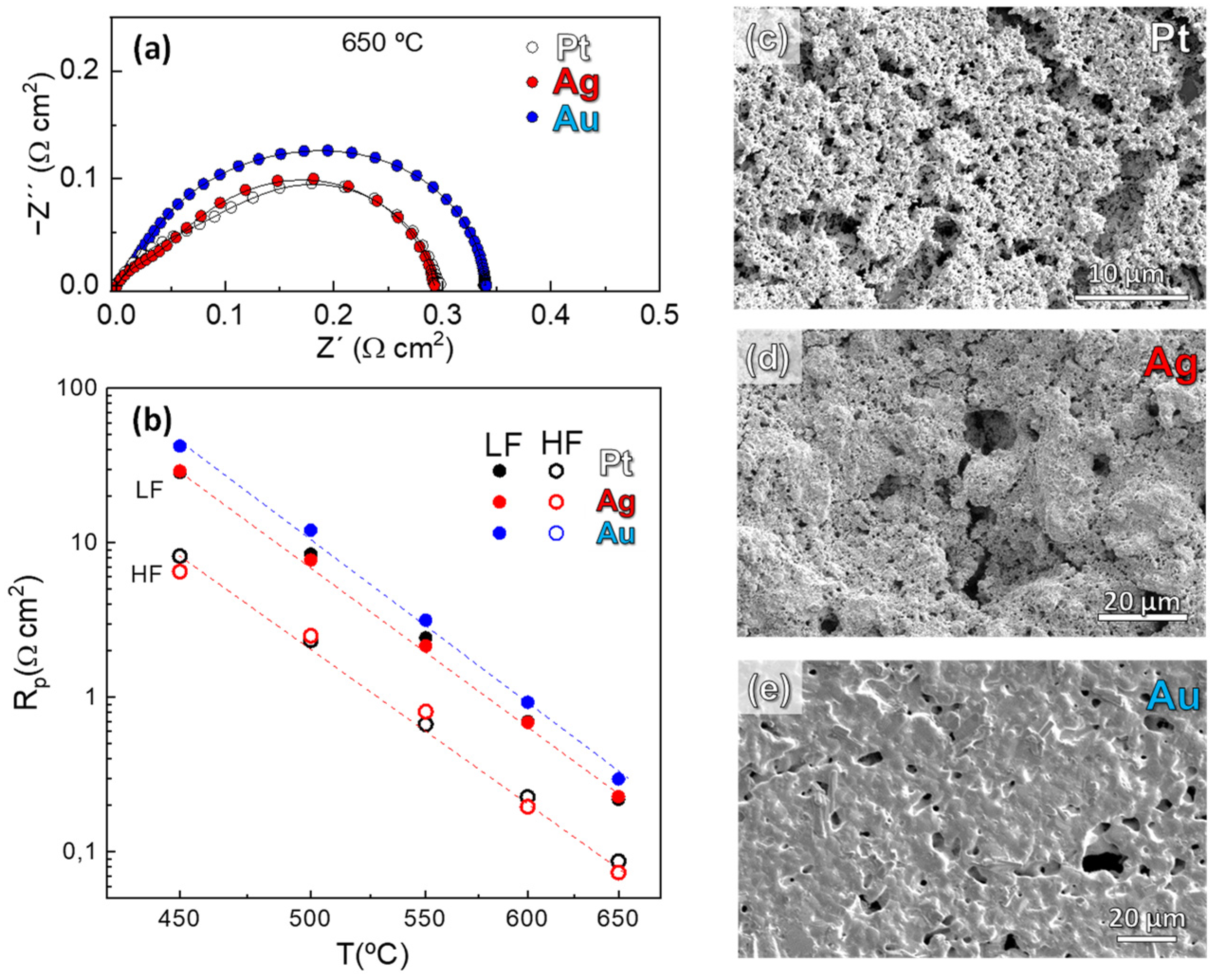
3.3. Electrochemical Characterization
3.4. Influence on the Current Collector Layer
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gür, T.M. Review of Electrical Energy Storage Technologies, Materials and Systems: Challenges and Prospects for Large-Scale Grid Storage. Energy Environ. Sci. 2018, 11, 2696–2767. [Google Scholar] [CrossRef]
- Gao, Z.; Mogni, L.V.; Miller, E.C.; Railsback, J.G.; Barnett, S.A. A Perspective on Low-Temperature Solid Oxide Fuel Cells. Energy Environ. Sci. 2016, 9, 1602–1644. [Google Scholar] [CrossRef]
- Boldrin, P.; Brandon, N.P. Progress and Outlook for Solid Oxide Fuel Cells for Transportation Applications. Nat. Catal. 2019, 2, 571–577. [Google Scholar] [CrossRef] [Green Version]
- Vinoth Kumar, R.; Khandale, A.P. A Review on Recent Progress and Selection of Cobalt-Based Cathode Materials for Low Temperature-Solid Oxide Fuel Cells. Renew. Sustain. Energy Rev. 2022, 156, 111985. [Google Scholar] [CrossRef]
- Lima, A.J.M.; Silva, V.D.; Raimundo, R.A.; Morales, M.A.; Simões, T.A.; Loureiro, F.J.A.; Fagg, D.P.; Macedo, D.A.; Nascimento, R.M. Fe-Doped Calcium Cobaltites as Electrocatalysts for Oxygen Evolution Reaction. Ceram. Int. 2021, 47, 26109–26118. [Google Scholar] [CrossRef]
- Yoo, S.; Shin, J.Y.; Kim, G. Thermodynamic and Electrical Characteristics of NdBaCo2O5+δ at Various Oxidation and Reduction States. J. Mater. Chem. 2011, 21, 439–443. [Google Scholar] [CrossRef]
- Švarcová, S.; Wiik, K.; Tolchard, J.; Bouwmeester, H.J.M.; Grande, T. Structural Instability of Cubic Perovskite BaxSr1- xCo1-yFeyO3-δ. Solid State Ionics 2008, 178, 1787–1791. [Google Scholar] [CrossRef]
- Jin, F.; Liu, J.; Shen, Y.; He, T. Improved Electrochemical Performance and Thermal Expansion Compatibility of LnBaCoFeO5+δ-Sm0.2Ce0.8O1.9 (Ln=Pr and Nd) Composite Cathodes for IT-SOFCs. J. Alloys Compd. 2016, 685, 483–491. [Google Scholar] [CrossRef]
- Jiang, S.P. A Comparison of O2 Reduction Reactions on Porous (La,Sr)MnO3 and (La,Sr)(Co,Fe)O3 Electrodes. Solid State Ionics 2002, 146, 1–22. [Google Scholar] [CrossRef]
- Kaur, P.; Singh, K. Review of Perovskite-Structure Related Cathode Materials for Solid Oxide Fuel Cells. Ceram. Int. 2020, 46, 5521–5535. [Google Scholar] [CrossRef]
- dos Santos-Gómez, L.; Zamudio-García, J.; Porras-Vázquez, J.M.; Losilla, E.R.; Marrero-López, D. Recent Progress in Nanostructured Electrodes for Solid Oxide Fuel Cells Deposited by Spray Pyrolysis. J. Power Sources 2021, 507. [Google Scholar] [CrossRef]
- Jiang, S.P. Development of Lanthanum Strontium Cobalt Ferrite Perovskite Electrodes of Solid Oxide Fuel Cells—A Review. Int. J. Hydrogen Energy 2019, 44, 7448–7493. [Google Scholar] [CrossRef]
- Plonczak, P.; Søgaard, M.; Bieberle-Hütter, A.; Hendriksen, P.V.; Gauckler, L.J. Electrochemical Characterization of La0.58Sr0.4Co0.2Fe0.8O3−δ Thin Film Electrodes Prepared by Pulsed Laser Deposition. J. Electrochem. Soc. 2012, 159, B471–B482. [Google Scholar] [CrossRef]
- Zamudio-García, J.; Caizán-Juanarena, L.; Porras-Vázquez, J.M.; Losilla, E.R.; Marrero-lópez, D. Boosting the Performance of La0.8Sr0.2MnO3-δ Electrodes by The Incorporation of Nanocomposite Active Layers. Adv. Mater. Interfaces 2022, 9, 2200702. [Google Scholar] [CrossRef]
- Nicollet, C.; Flura, A.; Vibhu, V.; Rougier, A.; Bassat, J.M.; Grenier, J.C. An Innovative Efficient Oxygen Electrode for SOFC: Pr6O11 Infiltrated into Gd-Doped Ceria Backbone. Int. J. Hydrogen Energy 2016, 41, 15538–15544. [Google Scholar] [CrossRef]
- Dos Santos-Gómez, L.; Losilla, E.R.; Martín, F.; Ramos-Barrado, J.R.; Marrero-López, D. Novel Microstructural Strategies to Enhance the Electrochemical Performance of La0.8Sr0.2MnO3-δ Cathodes. ACS Appl. Mater. Interfaces 2015, 7, 7197–7205. [Google Scholar] [CrossRef] [Green Version]
- Nicollet, C.; Flura, A.; Vibhu, V.; Rougier, A.; Bassat, J.-M.; Grenier, J.-C. Lanthanum Nickelate Infiltration into Porous Gd-Doped Ceria As Cathode for Solid Oxide Fuel Cells. ECS Trans. 2015, 68, 837–845. [Google Scholar] [CrossRef]
- Beckel, D.; Muecke, U.P.; Gyger, T.; Florey, G.; Infortuna, A.; Gauckler, L.J. Electrochemical Performance of LSCF Based Thin Film Cathodes Prepared by Spray Pyrolysis. Solid State Ionics 2007, 178, 407–415. [Google Scholar] [CrossRef]
- Haider, M.A.; McIntosh, S. The Influence of Grain Size on La0.6Sr0.4Co0.2Fe0.8O3-δ Thin Film Electrode Impedance. J. Electrochem. Soc. 2011, 158, B1128. [Google Scholar] [CrossRef]
- dos Santos-Gómez, L.; Porras-Vázquez, J.M.; Losilla, E.R.; Martín, F.; Ramos-Barrado, J.R.; Marrero-López, D. Stability and Performance of La0.6Sr0.4Co0.2Fe0.8O3-δ Nanostructured Cathodes with Ce0.8Gd0.2O1.9 Surface Coating. J. Power Sources 2017, 347, 178–185. [Google Scholar] [CrossRef]
- Marinha, D.; Dessemond, L.; Djurado, E. Electrochemical Investigation of Oxygen Reduction Reaction on La0.6Sr0.4Co0.2Fe0.8O3-δ Cathodes Deposited by Electrostatic Spray Deposition. J. Power Sources 2012, 197, 80–87. [Google Scholar] [CrossRef]
- Lu, M.Y.; Railsback, J.G.; Wang, H.; Liu, Q.; Chart, Y.A.; Zhang, S.L.; Barnett, S.A. Stable High Current Density Operation of La0.6Sr0.4Co0.2Fe0.8O3-δ: Oxygen Electrodes. J. Mater. Chem. A 2019, 7, 13531–13539. [Google Scholar] [CrossRef]
- Kim, Y.-M.; Pyun, S.-I.; Kim, J.-S.; Lee, G.-J. Mixed Diffusion and Charge-Transfer-Controlled Oxygen Reduction on Dense LaSrxCo0.2Fe0.8O3−δ Electrodes with Various Sr Contents. J. Electrochem. Soc. 2007, 154, B802. [Google Scholar] [CrossRef]
- Lee, J.W.; Liu, Z.; Yang, L.; Abernathy, H.; Choi, S.H.; Kim, H.E.; Liu, M. Preparation of Dense and Uniform La0.6Sr0.4Co0.2Fe0.8O3-δ (LSCF) Films for Fundamental Studies of SOFC Cathodes. J. Power Sources 2009, 190, 307–310. [Google Scholar] [CrossRef]
- Zhou, F.; Liu, Y.; Zhao, X.; Tang, W.; Yang, S.; Zhong, S.; Wei, M. Effects of Cerium Doping on the Performance of LSCF Cathodes for Intermediate Temperature Solid Oxide Fuel Cells. Int. J. Hydrogen Energy 2018, 43, 18946–18954. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, H.; Li, J.; Guo, X.; Hu, Q.; Yang, Z.; Yu, F.; Li, G. Modified La0.6Sr0.4Co0.2Fe0.8O3-δ Cathodes with the Infiltration of Er0.4Bi1.6O3 for Intermediate-Temperature Solid Oxide Fuel Cells. Int. J. Hydrogen Energy 2021, 46, 22932–22941. [Google Scholar] [CrossRef]
- Yoo, C.Y.; Yun, D.S.; Park, S.Y.; Park, J.; Joo, J.H.; Park, H.; Kwak, M.; Yu, J.H. Investigation of Electrochemical Properties of Model Lanthanum Strontium Cobalt Ferrite-Based Cathodes for Proton Ceramic Fuel Cells. Electrocatalysis 2016, 7, 280–286. [Google Scholar] [CrossRef]
- dos Santos-Gómez, L.; Hurtado, J.; Porras-Vázquez, J.M.; Losilla, E.R.; Marrero-López, D. Durability and Performance of CGO Barriers and LSCF Cathode Deposited by Spray-Pyrolysis. J. Eur. Ceram. Soc. 2018, 38, 3518–3526. [Google Scholar] [CrossRef]
- Angoua, B.F.; Slamovich, E.B. Single Solution Spray Pyrolysis of La0.6Sr0.4Co0.2Fe0.8O3-δ-Ce0.8Gd0.2O1.9 (LSCF-CGO) Thin Film Cathodes. Solid State Ionics 2012, 212, 10–17. [Google Scholar] [CrossRef]
- Bebelis, S.; Kotsionopoulos, N.; Mai, A.; Tietz, F. Electrochemical Characterization of Perovskite-Based SOFC Cathodes. J. Appl. Electrochem. 2006, 37, 15–20. [Google Scholar] [CrossRef]
- Perry Murray, E.; Sever, M.J.; Barnett, S.A. Electrochemical Performance of (La,Sr)(Co,Fe)O3–(Ce, Gd)O3 Composite Cathodes. Solid State Ionics 2002, 148, 27–34. [Google Scholar] [CrossRef]
- Nie, L.; Liu, M.; Zhang, Y.; Liu, M. La0.6Sr0.4Co0.2Fe0.8O3-δ Cathodes Infiltrated with Samarium-Doped Cerium Oxide for Solid Oxide Fuel Cells. J. Power Sources 2010, 195, 4704–4708. [Google Scholar] [CrossRef]
- Kournoutis, V.C.; Tietz, F.; Bebelis, S. AC Impedance Characterisation of a La0.8Sr0.2Co0.2Fe0.8O3-δ Electrode. Fuel Cells 2009, 9, 852–860. [Google Scholar] [CrossRef]
- Zhang, Q.; Hou, Y.; Chen, L.; Wang, L.; Chou, K. Enhancement of Electrochemical Performance for Proton Conductive Solid Oxide Fuel Cell by 30%GDC-LSCF Cathode. Ceram. Int. 2022, 48, 17816–17827. [Google Scholar] [CrossRef]
- Philippeau, B.; Mauvy, F.; Nicollet, C.; Fourcade, S.; Grenier, J.C. Oxygen Reduction Reaction in Pr2NiO4+δ/Ce0.9Gd0.1O1.95 and La0.6Sr0.4Co0.2Fe0.8O3−δ/La0.8Sr0.2Ga0.8Mg0.2O2.80 half cells: An electrochemical study. J. Solid State Electrochem. 2015, 19, 871–882. [Google Scholar] [CrossRef]
- X’Pert HighScore Plus Software, v3.0e; PANalytical B. V.: Amelo, The Netherlands, 2012.
- Larson, A.C.; Von Dreele, R.B. General Structure Analysis System (GSAS) Software; Rep. No. LAUR-86-748; LosAlamos National Lab: California, CA, USA, 2004.
- Dos Santos-Gómez, L.; Porras-Vázquez, J.M.; Losilla, E.R.; Marrero-López, D. Improving the Efficiency of Layered Perovskite Cathodes by Microstructural Optimization. J. Mater. Chem. A 2017, 5, 7896–7904. [Google Scholar] [CrossRef]
- Johnson, D. ZView, A Software Program for IES Analysis, Version 2.8; Scribner Assoc. Inc.: Southern Pines, NC, USA, 2002.
- Wan, T.H.; Saccoccio, M.; Chen, C.; Ciucci, F. Influence of the Discretization Methods on the Distribution of Relaxation Times Deconvolution: Implementing Radial Basis Functions with DRTtools. Electrochim. Acta 2015, 184, 483–499. [Google Scholar] [CrossRef]
- Lim, Y.; Park, J.; Lee, H.; Ku, M.; Kim, Y.B. Rapid Fabrication of Lanthanum Strontium Cobalt Ferrite (LSCF) with Suppression of LSCF/YSZ Chemical Side Reaction via Flash Light Sintering for SOFCs. Nano Energy 2021, 90, 106524. [Google Scholar] [CrossRef]
- Marrero-López, D.; Romero, R.; Martín, F.; Ramos-Barrado, J.R. Effect of the Deposition Temperature on the Electrochemical Properties of La0.6Sr0.4Co0.8Fe0.2O3-δ Cathode Prepared by Conventional Spray-Pyrolysis. J. Power Sources 2014, 255, 308–317. [Google Scholar] [CrossRef]
- Acuña, L.M.; Peña-Martínez, J.; Marrero-López, D.; Fuentes, R.O.; Nuñez, P.; Lamas, D.G. Electrochemical Performance of Nanostructured La0.6Sr0.4CoO3-δ and Sm0.5Sr0.5CoO3-δ Cathodes for IT-SOFCs. J. Power Sources 2011, 196, 9276–9283. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, G.; Dai, R.; Lv, X.; Yang, D.; Geng, S. A Review of the Chemical Compatibility between Oxide Electrodes and Electrolytes in Solid Oxide Fuel Cells. J. Power Sources 2021, 492. [Google Scholar] [CrossRef]
- Nikonov, A.V.; Kuterbekov, K.A.; Bekmyrza, K.Z.; Pavzderin, N.B. A Brief Review of Conductivity and Thermal Expansion of Perovskite-Related Oxides for SOFC Cathode. Eurasian J. Phys. Funct. Mater. 2018, 2, 274–292. [Google Scholar] [CrossRef] [Green Version]
- Zarabi Golkhatmi, S.; Asghar, M.I.; Lund, P.D. A Review on Solid Oxide Fuel Cell Durability: Latest Progress, Mechanisms, and Study Tools. Renew. Sustain. Energy Rev. 2022, 161, 112339. [Google Scholar] [CrossRef]
- Datta, P.; Majewski, P.; Aldinger, F. Thermal Expansion Behaviour of Sr- and Mg-Doped LaGaO3 Solid Electrolyte. J. Eur. Ceram. Soc. 2009, 29, 1463–1468. [Google Scholar] [CrossRef]
- Osinkin, D.A. An Approach to the Analysis of the Impedance Spectra of Solid Oxide Fuel Cell Using the DRT Technique. Electrochim. Acta 2021, 372, 137858. [Google Scholar] [CrossRef]
- Xia, J.; Wang, C.; Wang, X.; Bi, L.; Zhang, Y. A Perspective on DRT Applications for the Analysis of Solid Oxide Cell Electrodes. Electrochim. Acta 2020, 349, 136328. [Google Scholar] [CrossRef]
- Osinkin, D.A. Detailed Analysis of Electrochemical Behavior of High–Performance Solid Oxide Fuel Cell Using DRT Technique. J. Power Sources 2022, 527. [Google Scholar] [CrossRef]
- Hong, J.; Bhardwaj, A.; Bae, H.; Kim, I.; Song, S.-J. Electrochemical Impedance Analysis of SOFC with Transmission Line Model Using Distribution of Relaxation Times (DRT). J. Electrochem. Soc. 2020, 167, 114504. [Google Scholar] [CrossRef]
- Chen, Y.; Bu, Y.; Zhang, Y.; Yan, R.; Ding, D.; Zhao, B.; Yoo, S.; Dang, D.; Hu, R.; Yang, C.; et al. A Highly Efficient and Robust Nanofiber Cathode for Solid Oxide Fuel Cells. Adv. Energy Mater. 2017, 7, 1–7. [Google Scholar] [CrossRef]
- Zapata-Ramírez, V.; Dos Santos-Gómez, L.; Mather, G.C.; Marrero-López, D.; Pérez-Coll, D. Enhanced Intermediate-Temperature Electrochemical Performance of Air Electrodes for Solid Oxide Cells with Spray-Pyrolyzed Active Layers. ACS Appl. Mater. Interfaces 2020, 12, 10571–10578. [Google Scholar] [CrossRef]
- Araújo, A.J.M.; Loureiro, F.J.A.; Holz, L.I.V.; Graça, V.C.D.; Grilo, J.P.F.; Macedo, D.A.; Paskocimas, C.A.; Fagg, D.P. Creating New Surface-Exchange Pathways on the Misfit Ca-Cobaltite Electrode by the Addition of an Active Interlayer. J. Power Sources 2021, 510. [Google Scholar] [CrossRef]
- Dos Santos-Gómez, L.; Zamudio-García, J.; Porras-Vázquez, J.M.; Losilla, E.R.; Marrero-López, D. Nanostructured BaCo0.4Fe0.4Zr0.1Y0.1O3-δ Cathodes with Different Microstructural Architectures. Nanomaterials 2020, 10, 1055. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Lei, Z.; Ding, B.; Xia, C.; Zhao, F.; Chen, F. Electrochemical Characteristics of Solid Oxide Fuel Cell Cathodes Prepared by Infiltrating (La,Sr)MnO3 Nanoparticles into Yttria-Stabilized Bismuth Oxide Backbones. Int. J. Hydrogen Energy 2010, 35, 8322–8330. [Google Scholar] [CrossRef]
- Peng, R.; Wu, T.; Liu, W.; Liu, X.; Meng, G. Cathode Processes and Materials for Solid Oxide Fuel Cells with Proton Conductors as Electrolytes. J. Mater. Chem. 2010, 20, 6218–6225. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, D.; Li, Y. A Fundamental Comprehension and Recent Progress in Advanced Pt-based ORR Nanocatalysts. SmartMat 2021, 2, 56–75. [Google Scholar] [CrossRef]
- Simner, S.P.; Electrochem, J.; Soc, A.; Simner, S.P.; Anderson, M.D.; Pederson, L.R.; Stevenson, J.W. Performance Variability of La(Sr)FeO3 SOFC Cathode with Pt, Ag, and Au Current Collectors. J. Electrochem. Soc. 2005, 152, A1851. [Google Scholar] [CrossRef]



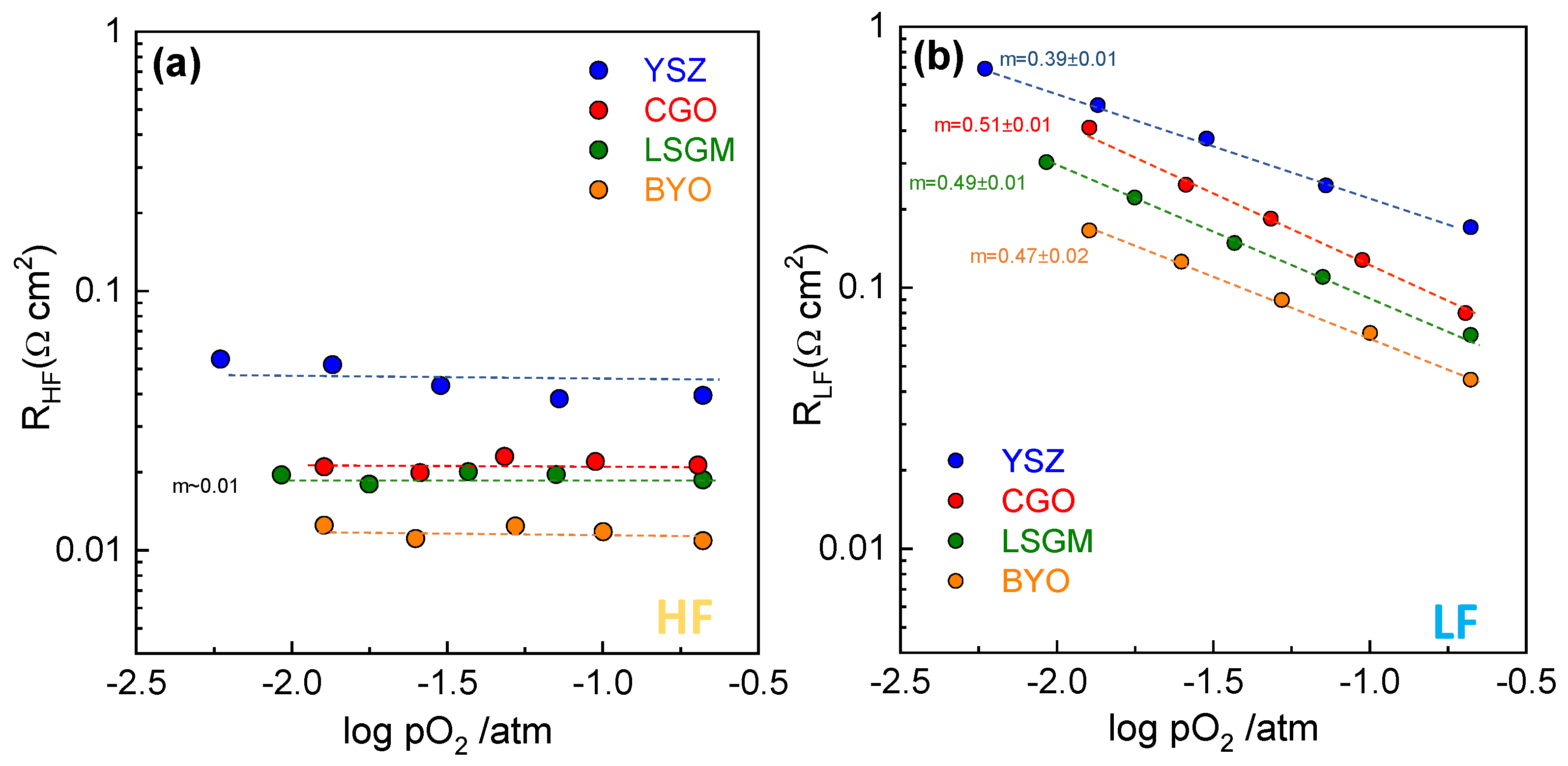
| Fabrication Technique | Electrolyte | Rp (Ω cm2) | m | Ref. |
|---|---|---|---|---|
| Spray-pyrolysis | Zr0.84Y0.16O1.92 | 0.21 | ~0 0.39 | This work |
| Spray-pyrolysis | Ce0.9Gd0.1O1.95 | 0.11 | ~0 0.51 | This work |
| Spray-pyrolysis | La0.9Sr0.1Ga0.8Mg0.2O3-δ | 0.089 | ~0 0.49 | This work |
| Spray-pyrolysis | Bi1.5Y0.5O3 | 0.058 | ~0 0.47 | This work |
| Spray-pyrolysis | Ce0.8Gd0.2O1.9 | 0.7650 °C | - | [18] |
| Spray-pyrolysis | Ce0.8Gd0.2O1.9 | 0.9 | - | [19] |
| Freeze-Drying | Ce0.8Gd0.2O1.9 | 0.3 | - | [20] |
| Spray-pyrolysis | Ce0.8Gd0.2O1.9 | 10600 °C | 0 0.20 0.71 | [21] |
| Screen-printing | Ce0.9Gd0.1O1.95 | 0.12 | - | [22] |
| Solid-state reaction | Ce0.9Gd0.1O1.95 | 15750 °C | 0 0.28 | [23] |
| Magnetron sputtering | Ce0.9Gd0.1O1.95 | 122 | - | [24] |
| Screen-printing | Ce0.9Gd0.1O1.95 | 0.21750 ºC | - | [25] |
| Screen-printing | Sm0.2Ce0.8O1.9 | 0.55600 °C | 0.24 0.44 | [26] |
| Co-precipitation | BaZr0.8Y0.2O3-δ | 0.70600 °C | 0.16 0.50 | [27] |
| Spray-pyrolysis | Zr0.84Y0.16O1.92 | 0.3650 °C | - | [28] |
| Spray-pyrolysis | Zr0.84Y0.16O1.92 | 3600 °C | - | [29] |
| Spray-drying | Zr0.84Y0.16O1.92 | 2 | - | [30] |
| Spin coating | Zr0.84Y0.16O1.92 | 0.1750 °C | - | [31] |
| Pulsed laser deposition | Zr0.84Y0.16O1.92 | 9750 °C | - | [13] |
| Tape casting | Zr0.84Y0.16O1.92 | 0.4 | - | [32] |
| Spray-drying | Zr0.84Y0.16O1.92 | 5 | ~0 0.25 | [33] |
| Spray-drying | La0.9Sr0.1Ga0.8Mg0.2O3-δ | 0.19 | - | [34] |
| Screen-printing | La0.8Sr0.2Ga0.8Mg0.2O3-δ | 0.08 | 0.10 0.82 | [35] |
| Electrolyte | Lattice Parameter (Å) | Cell Volume (Å3) | Rwp (%) | dLSCF (nm) |
|---|---|---|---|---|
| YSZ | 3.8828 (4) | 58.540 (2) | 4.57 | 24 |
| CGO | 3.8876 (4) | 58.540 (2) | 4.86 | 20 |
| LSGM | 3.8820 (4) | 58.540 (2) | 4.01 | - |
| BYO | 3.8883 (4) | 58.540 (2) | 3.01 | 22 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zamudio-García, J.; Caizán-Juanarena, L.; Porras-Vázquez, J.M.; Losilla, E.R.; Marrero-López, D. Unraveling the Influence of the Electrolyte on the Polarization Resistance of Nanostructured La0.6Sr0.4Co0.2Fe0.8O3-δ Cathodes. Nanomaterials 2022, 12, 3936. https://doi.org/10.3390/nano12223936
Zamudio-García J, Caizán-Juanarena L, Porras-Vázquez JM, Losilla ER, Marrero-López D. Unraveling the Influence of the Electrolyte on the Polarization Resistance of Nanostructured La0.6Sr0.4Co0.2Fe0.8O3-δ Cathodes. Nanomaterials. 2022; 12(22):3936. https://doi.org/10.3390/nano12223936
Chicago/Turabian StyleZamudio-García, Javier, Leire Caizán-Juanarena, José M. Porras-Vázquez, Enrique R. Losilla, and David Marrero-López. 2022. "Unraveling the Influence of the Electrolyte on the Polarization Resistance of Nanostructured La0.6Sr0.4Co0.2Fe0.8O3-δ Cathodes" Nanomaterials 12, no. 22: 3936. https://doi.org/10.3390/nano12223936








