Anion-Exchange Blue Perovskite Quantum Dots for Efficient Light-Emitting Devices
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of CsPbBr3 QD Solution
2.2. Device Fabrication
2.3. Measuring Instruments
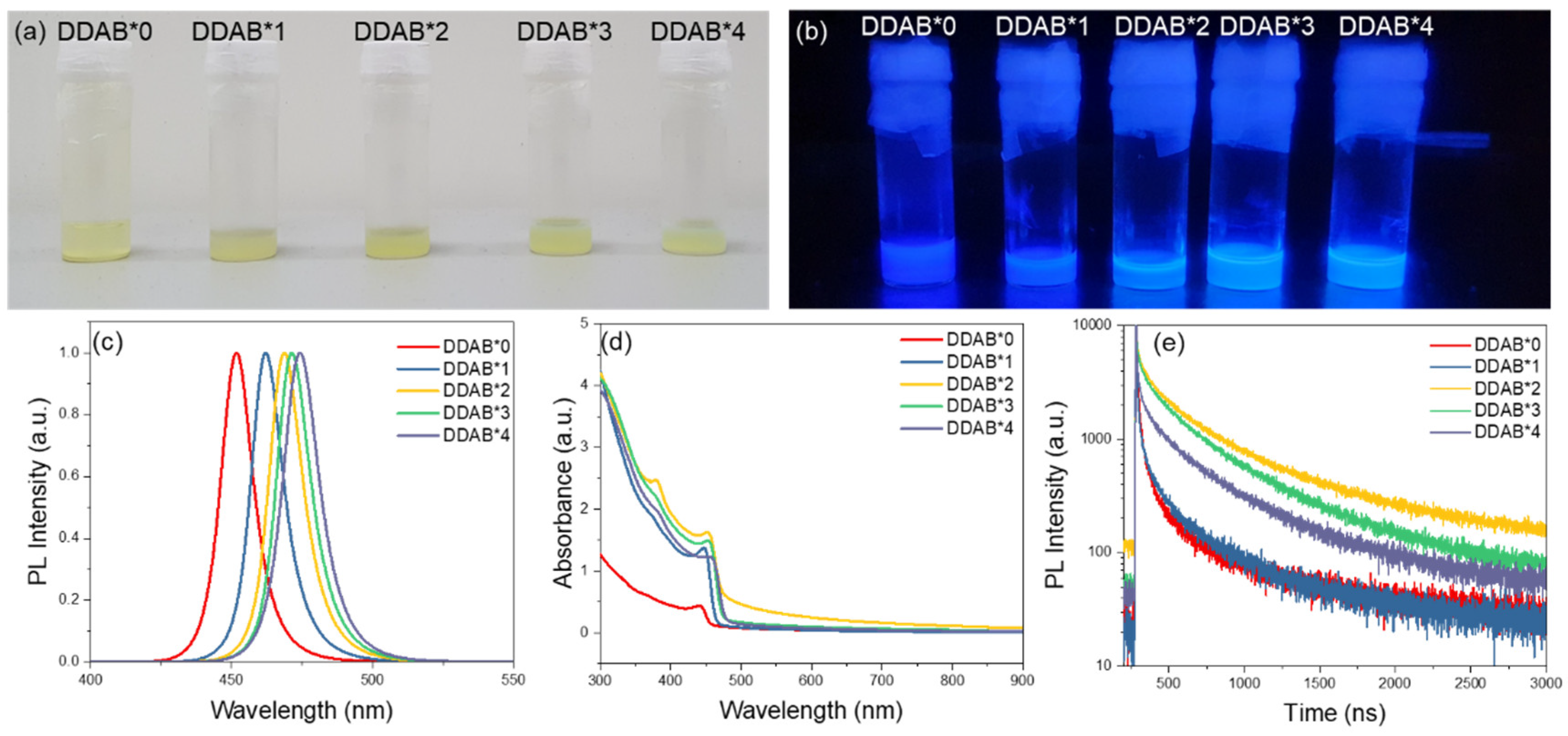
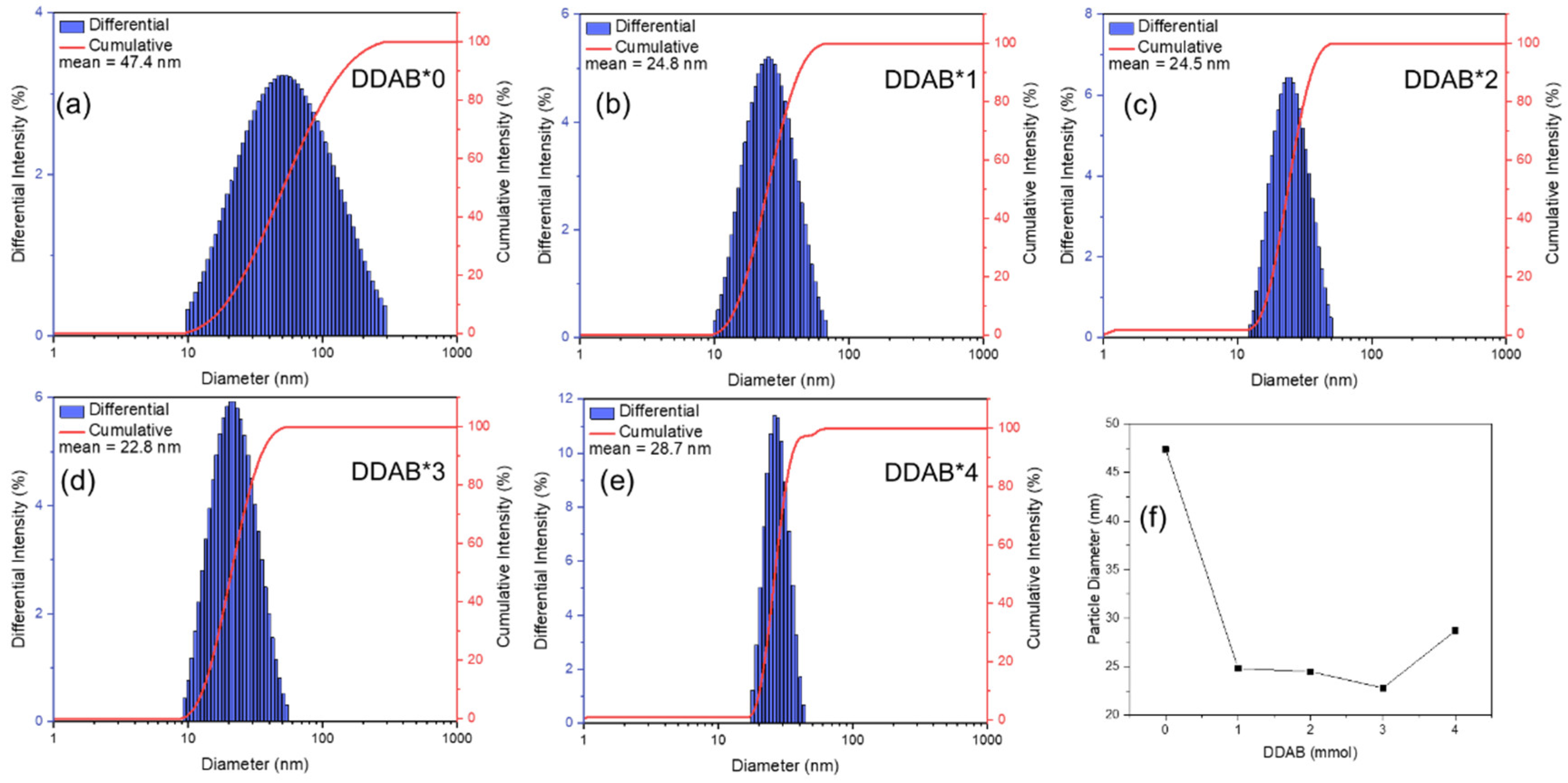
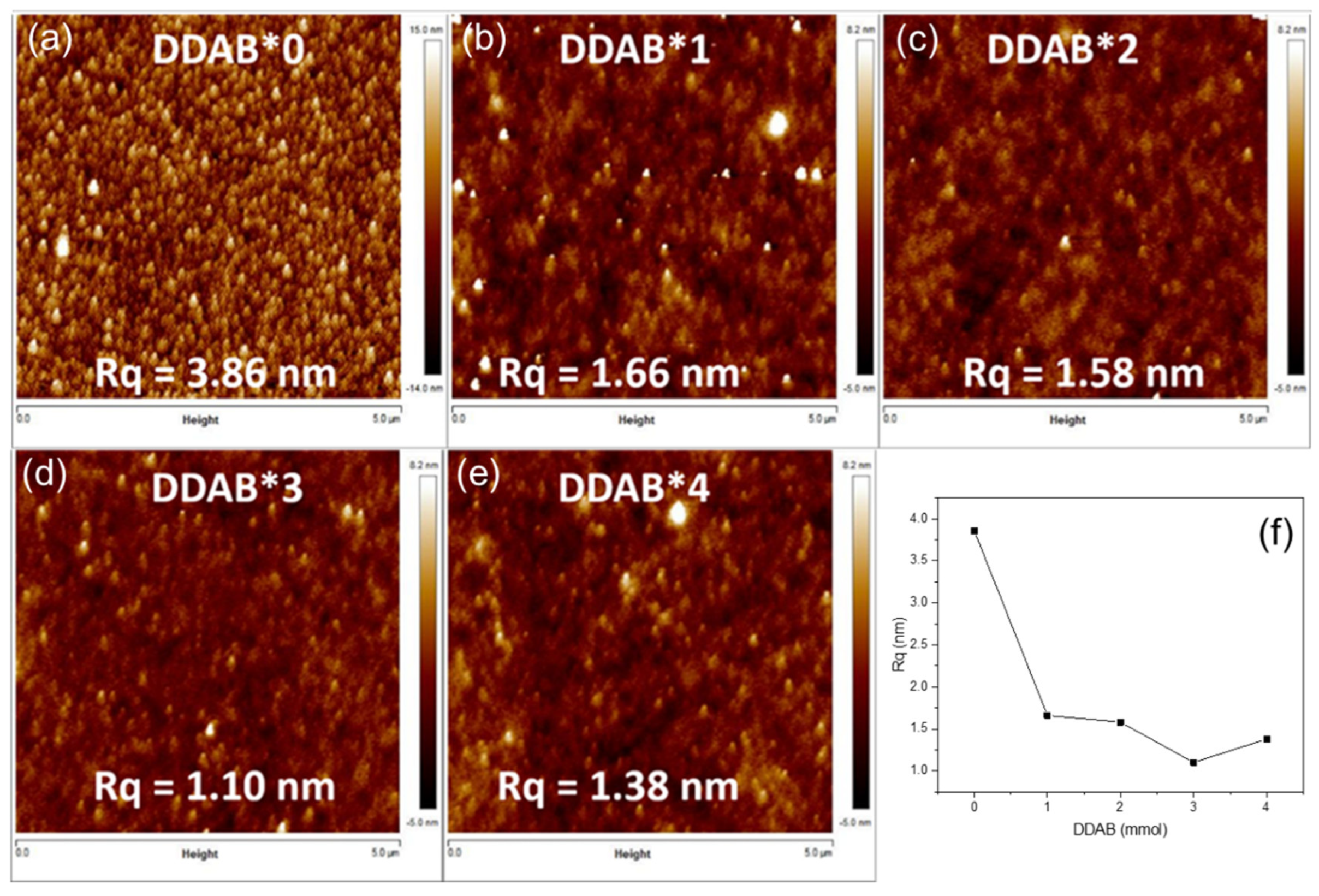
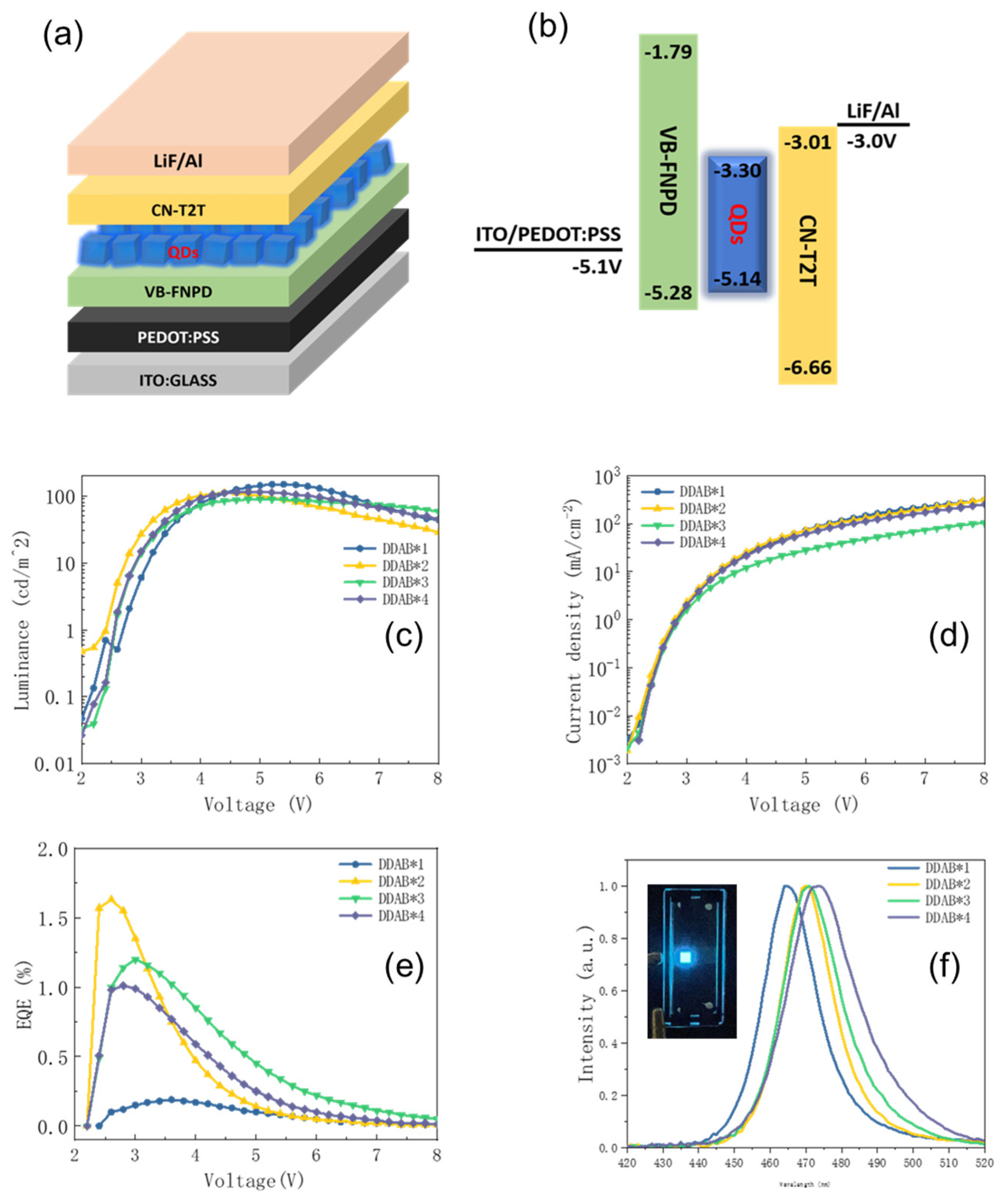
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Samuolienė, G.; Brazaitytė, A.; Urbonavičiūtė, A.; Šabajevienė, G.; Duchovskis, P. The Effect of Red and Blue Light Component on the Growth and Development of Frigo Strawberries. Žemdirbystė (Agric.) 2010, 97, 99–104. [Google Scholar]
- Avercheva, O.; Berkovich, Y.A.; Smolyanina, S.; Bassarskaya, E.; Pogosyan, S.; Ptushenko, V.; Erokhin, A.; Zhigalova, T. Biochemical, Photosynthetic and Productive Parameters of Chinese Cabbage Grown under Blue–Red LED Assembly Designed for Space Agriculture. Adv. Space Res. 2014, 53, 1574–1581. [Google Scholar] [CrossRef]
- Naznin, M.T.; Lefsrud, M.; Azad, M.O.K.; Park, C.H. Effect of Different Combinations of Red and Blue LED Light on Growth Characteristics and Pigment Content of In Vitro Tomato Plantlets. Agriculture 2019, 9, 196. [Google Scholar] [CrossRef] [Green Version]
- Pennisi, G.; Sanyé-Mengual, E.; Orsini, F.; Crepaldi, A.; Nicola, S.; Ochoa, J.; Fernandez, J.A.; Gianquinto, G. Modelling Environmental Burdens of Indoor-Grown Vegetables and Herbs as Affected by Red and Blue LED Lighting. Sustainability 2019, 11, 4063. [Google Scholar] [CrossRef] [Green Version]
- Hasperué, J.H.; Guardianelli, L.; Rodoni, L.M.; Chaves, A.R.; Martínez, G.A. Continuous White–Blue LED Light Exposition Delays Postharvest Senescence of Broccoli. LWT—Food Sci. Technol. 2016, 65, 495–502. [Google Scholar] [CrossRef]
- Lin, C.C.; Zheng, Y.S.; Chen, H.Y.; Ruan, C.H.; Xiao, G.W.; Liu, R.S. Improving Optical Properties of White LED Fabricated by a Blue LED Chip with Yellow/Red Phosphors. J. Electrochem. Soc. 2010, 157, H900. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Min, K.; Park, Y.; Cho, K.-S.; Jeon, H. Photonic Crystal Phosphors Integrated on a Blue LED Chip for Efficient White Light Generation. Adv. Mater. 2018, 30, 1703506. [Google Scholar] [CrossRef]
- Nakamura, M.; Yako, T.; Kuse, Y.; Inoue, Y.; Nishinaka, A.; Nakamura, S.; Shimazawa, M.; Hara, H. Exposure to Excessive Blue LED Light Damages Retinal Pigment Epithelium and Photoreceptors of Pigmented Mice. Exp. Eye Res. 2018, 177, 1–11. [Google Scholar] [CrossRef]
- Mosti, G.; Gasperini, S. Observations Made on Three Patients Suffering from Ulcers of the Lower Limbs Treated with Blue Light. CWCMR 2018, 5, 23–28. [Google Scholar] [CrossRef] [Green Version]
- Barros, D.; Alvares, C.; Alencar, T.; Baqueiro, P.; Marianno, A.; Alves, R.; Lenzi, J.; Rezende, L.F.; Lordelo, P. Blue LED as a New Treatment to Vaginal Stenosis Due Pelvic Radiotherapy: Two Case Reports. World J. Clin. Cases 2021, 9, 6839–6845. [Google Scholar] [CrossRef]
- Lv, Y.; Chen, Z.; Yang, Z.; Yang, W.; Chu, W.; Tu, Y.; Xie, J.; Cao, D. Evaluation of the Red & Blue LED Effects on Cutaneous Refractory Wound Healing in Male Sprague–Dawley Rat Using 3 Different Multi-Drug Resistant Bacteria. Lasers Surg. Med. 2022, 54, 725–736. [Google Scholar] [CrossRef]
- Hirose, M.; Yoshida, Y.; Horii, K.; Hasegawa, Y.; Shibuya, Y. Efficacy of Antimicrobial Photodynamic Therapy with Rose Bengal and Blue Light against Cariogenic Bacteria. Arch. Oral Biol. 2021, 122, 105024. [Google Scholar] [CrossRef]
- Prasad, A.; Gänzle, M.; Roopesh, M.S. Antimicrobial Activity and Drying Potential of High Intensity Blue Light Pulses (455 Nm) Emitted from LEDs. Food Res. Int. 2021, 148, 110601. [Google Scholar] [CrossRef]
- Reuss, A.M.; Groos, D.; Scholl, R.; Schröter, M.; Maihöfner, C. Blue-Light Treatment Reduces Spontaneous and Evoked Pain in a Human Experimental Pain Model. Pain Rep. 2021, 6, e968. [Google Scholar] [CrossRef]
- Schmidt, L.C.; Pertegás, A.; González-Carrero, S.; Malinkiewicz, O.; Agouram, S.; Mínguez Espallargas, G.; Bolink, H.J.; Galian, R.E.; Pérez-Prieto, J. Nontemplate Synthesis of CH3NH3PbBr3 Perovskite Nanoparticles. J. Am. Chem. Soc. 2014, 136, 850–853. [Google Scholar] [CrossRef]
- Baugher, B.W.H.; Churchill, H.O.H.; Yang, Y.; Jarillo-Herrero, P. Optoelectronic Devices Based on Electrically Tunable p–n Diodes in a Monolayer Dichalcogenide. Nat. Nanotechnol. 2014, 9, 262–267. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.-H.; Cho, H.; Heo, J.H.; Kim, T.-S.; Myoung, N.; Lee, C.-L.; Im, S.H.; Lee, T.-W. Multicolored Organic/Inorganic Hybrid Perovskite Light-Emitting Diodes. Adv. Mater. 2015, 27, 1248–1254. [Google Scholar] [CrossRef]
- Fang, T.; Wang, T.; Li, X.; Dong, Y.; Bai, S.; Song, J. Perovskite QLED with an External Quantum Efficiency of over 21% by Modulating Electronic Transport. Sci. Bull. 2021, 66, 36–43. [Google Scholar] [CrossRef]
- Li, P.; Duan, Y.; Lu, Y.; Xiao, A.; Zeng, Z.; Xu, S.; Zhang, J. Nanocrystalline Structure Control and Tunable Luminescence Mechanism of Eu-Doped CsPbBr3 Quantum Dot Glass for WLEDs. Nanoscale 2020, 12, 6630–6636. [Google Scholar] [CrossRef]
- Iwasaki, Y.; Motomura, G.; Tsuzuki, T. Pure-Colored Red, Green, and Blue Quantum Dot Light-Emitting Diodes Using Emitting Layers Composed of Cadmium-Free Quantum Dots and Organic Electron-Transporting Materials. Jpn. J. Appl. Phys. 2022, 61, 052004. [Google Scholar] [CrossRef]
- Park, Y.R.; Kim, H.H.; Eom, S.; Choi, W.K.; Choi, H.; Lee, B.R.; Kang, Y. Luminance Efficiency Roll-off Mechanism in CsPbBr3−xClx Mixed-Halide Perovskite Quantum Dot Blue Light-Emitting Diodes. J. Mater. Chem. C 2021, 9, 3608–3619. [Google Scholar] [CrossRef]
- Meng, F.; Liu, X.; Cai, X.; Gong, Z.; Li, B.; Xie, W.; Li, M.; Chen, D.; Yip, H.-L.; Su, S.-J. Incorporation of Rubidium Cations into Blue Perovskite Quantum Dot Light-Emitting Diodes via FABr-Modified Multi-Cation Hot-Injection Method. Nanoscale 2019, 11, 1295–1303. [Google Scholar] [CrossRef] [PubMed]
- Tseng, Z.-L.; Chen, L.-C.; Chao, L.-W.; Tsai, M.-J.; Luo, D.; Al Amin, N.R.; Liu, S.-W.; Wong, K.-T. Aggregation Control, Surface Passivation, and Optimization of Device Structure toward Near-Infrared Perovskite Quantum-Dot Light-Emitting Diodes with an EQE up to 15.4%. Adv. Mater. 2022, 34, 2109785. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Li, J.; Li, X.; Xu, L.; Dong, Y.; Zeng, H. Quantum Dot Light-Emitting Diodes Based on Inorganic Perovskite Cesium Lead Halides (CsPbX3). Adv. Mater. 2015, 27, 7162–7167. [Google Scholar] [CrossRef] [PubMed]
- Weng, S.; Yu, G.; Zhou, C.; Lin, F.; Han, Y.; Wang, H.; Huang, X.; Liu, X.; Hu, H.; Liu, W.; et al. Challenges and Opportunities for the Blue Perovskite Quantum Dot Light-Emitting Diodes. Crystals 2022, 12, 929. [Google Scholar] [CrossRef]
- Yan, D.; Shi, T.; Zang, Z.; Zhou, T.; Liu, Z.; Zhang, Z.; Du, J.; Leng, Y.; Tang, X. Ultrastable CsPbBr3 Perovskite Quantum Dot and Their Enhanced Amplified Spontaneous Emission by Surface Ligand Modification. Small 2019, 15, 1901173. [Google Scholar] [CrossRef]
- Chen, W.; Tang, X.; Wangyang, P.; Yao, Z.; Zhou, D.; Chen, F.; Li, S.; Lin, H.; Zeng, F.; Wu, D.; et al. Surface-Passivated Cesium Lead Halide Perovskite Quantum Dots: Toward Efficient Light-Emitting Diodes with an Inverted Sandwich Structure. Adv. Opt. Mater. 2018, 6, 1800007. [Google Scholar] [CrossRef]
- Jiang, Q.; Zhao, Y.; Zhang, X.; Yang, X.; Chen, Y.; Chu, Z.; Ye, Q.; Li, X.; Yin, Z.; You, J. Surface Passivation of Perovskite Film for Efficient Solar Cells. Nat. Photonics 2019, 13, 460–466. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, X.; Deng, J.; Chu, Z.; Jiang, Q.; Meng, J.; Wang, P.; Zhang, L.; Yin, Z.; You, J. Efficient Green Light-Emitting Diodes Based on Quasi-Two-Dimensional Composition and Phase Engineered Perovskite with Surface Passivation. Nat. Commun. 2018, 9, 570. [Google Scholar] [CrossRef] [Green Version]
- Haydous, F.; Gardner, J.M.; Cappel, U.B. The Impact of Ligands on the Synthesis and Application of Metal Halide Perovskite Nanocrystals. J. Mater. Chem. A 2021, 9, 23419–23443. [Google Scholar] [CrossRef]
- Wang, S.; Du, L.; Donmez, S.; Xin, Y.; Mattoussi, H. Polysalt Ligands Achieve Higher Quantum Yield and Improved Colloidal Stability for CsPbBr3 Quantum Dots. Nanoscale 2021, 13, 16705–16718. [Google Scholar] [CrossRef]
- Pan, J.; Quan, L.N.; Zhao, Y.; Peng, W.; Murali, B.; Sarmah, S.P.; Yuan, M.; Sinatra, L.; Alyami, N.M.; Liu, J.; et al. Highly Efficient Perovskite-Quantum-Dot Light-Emitting Diodes by Surface Engineering. Adv. Mater. 2016, 28, 8718–8725. [Google Scholar] [CrossRef]
- Li, T.; Zhang, H.; Yu, C.; Wang, P.; Wang, H.; Zhang, X.; Sun, Y.; Liu, D.; Wang, T. Conjugated Amidine Ligands Enhance the Performance of Perovskite Nanocrystal Blue Light-Emitting Diodes Prepared in Air with Green Solvents. J. Mater. Chem. C 2021, 9, 15488–15495. [Google Scholar] [CrossRef]
- Shin, Y.S.; Yoon, Y.J.; Lee, K.T.; Jeong, J.; Park, S.Y.; Kim, G.-H.; Kim, J.Y. Vivid and Fully Saturated Blue Light-Emitting Diodes Based on Ligand-Modified Halide Perovskite Nanocrystals. ACS Appl. Mater. Interfaces 2019, 11, 23401–23409. [Google Scholar] [CrossRef]
- Song, J.; Li, J.; Xu, L.; Li, J.; Zhang, F.; Han, B.; Shan, Q.; Zeng, H. Room-Temperature Triple-Ligand Surface Engineering Synergistically Boosts Ink Stability, Recombination Dynamics, and Charge Injection toward EQE-11.6% Perovskite QLEDs. Adv. Mater. 2018, 30, 1800764. [Google Scholar] [CrossRef]
- Hooper, T.J.N.; Fang, Y.; Brown, A.A.M.; Pu, S.H.; White, T.J. Structure and Surface Properties of Size-Tuneable CsPbBr3 Nanocrystals. Nanoscale 2021, 13, 15770–15780. [Google Scholar] [CrossRef]
- Vickers, E.T.; Xu, K.; Li, X.; Zhang, J.Z. Dependence of Stability and Electronic and Optical Properties of Perovskite Quantum Dots on Capping Ligand Chain Length. J. Chem. Phys. 2020, 152, 034701. [Google Scholar] [CrossRef]
- Tseng, Z.-L.; Huang, Y.-S.; Liu, Y.-L.; Wu, T.-L.; Wei, Y.-J. Tetraoctylammonium Bromide-Passivated CsPbI3−xBrx Perovskite Nanoparticles with Improved Stability for Efficient Red Light-Emitting Diodes. J. Alloys Compd. 2022, 897, 163182. [Google Scholar] [CrossRef]

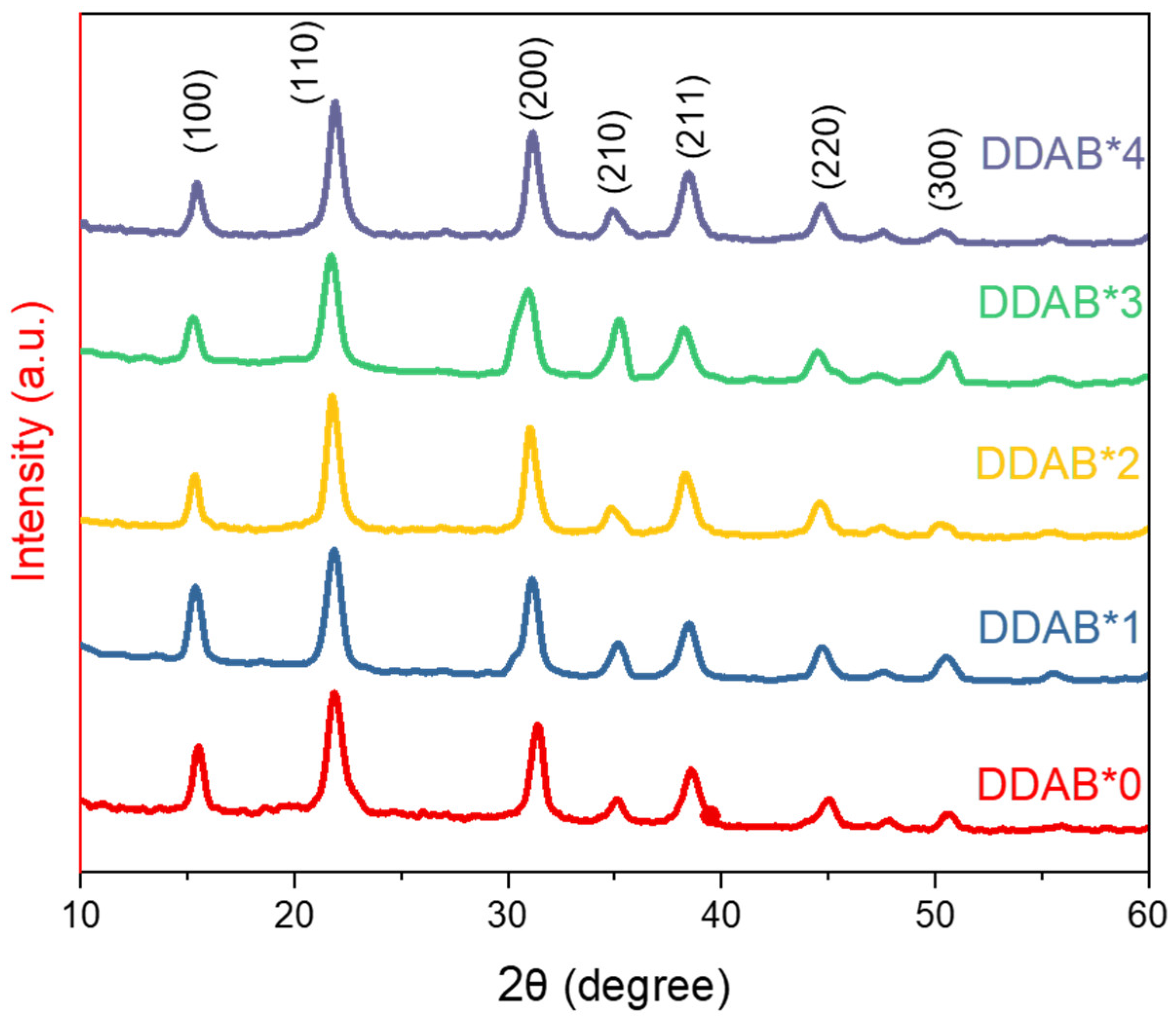
| (hkl) | (100) | (110) | (200) | (210) | (211) | (220) | (300) | |
|---|---|---|---|---|---|---|---|---|
| DDAB*0 | 2θ(degree) | 15.50 | 21.92 | 31.38 | 35.08 | 38.68 | 44.96 | 50.73 |
| d-spaceing(Å) | 5.712085 | 4.052382 | 2.84877 | 2.556268 | 2.326202 | 2.013899 | 1.798275 | |
| DDAB*1 | 2θ(degree) | 15.37 | 21.86 | 31.13 | 35.28 | 38.43 | 44.63 | 50.48 |
| d-spaceing(Å) | 5.760104 | 4.063371 | 2.871079 | 2.542231 | 2.34076 | 2.028879 | 1.806594 | |
| DDAB*2 | 2θ(degree) | 15.32 | 21.81 | 31.03 | 34.78 | 38.28 | 44.58 | 50.28 |
| d-spaceing(Å) | 5.778791 | 4.072576 | 2.880105 | 2.57763 | 2.349587 | 2.031038 | 1.813311 | |
| DDAB*3 | 2θ(degree) | 15.30 | 21.78 | 31.03 | 34.78 | 38.28 | 44.53 | 50.13 |
| d-spaceing(Å) | 5.7863 | 4.078119 | 2.880105 | 2.57763 | 2.349587 | 2.033203 | 1.818385 | |
| DDAB*4 | 2θ(degree) | 15.26 | 21.72 | 30.93 | 35.28 | 38.23 | 44.53 | 50.63 |
| d-spaceing(Å) | 5.801377 | 4.089251 | 2.88919 | 2.542231 | 2.352546 | 2.033203 | 1.801592 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hung, W.-K.; Tseng, Y.-H.; Lin, C.-C.; Chen, S.-A.; Hsu, C.-H.; Li, C.-F.; Chen, Y.-J.; Tseng, Z.-L. Anion-Exchange Blue Perovskite Quantum Dots for Efficient Light-Emitting Devices. Nanomaterials 2022, 12, 3957. https://doi.org/10.3390/nano12223957
Hung W-K, Tseng Y-H, Lin C-C, Chen S-A, Hsu C-H, Li C-F, Chen Y-J, Tseng Z-L. Anion-Exchange Blue Perovskite Quantum Dots for Efficient Light-Emitting Devices. Nanomaterials. 2022; 12(22):3957. https://doi.org/10.3390/nano12223957
Chicago/Turabian StyleHung, Wei-Kuan, Yi-Hsun Tseng, Chun-Cheng Lin, Sih-An Chen, Chih-Hung Hsu, Chen-Feng Li, Yen-Ju Chen, and Zong-Liang Tseng. 2022. "Anion-Exchange Blue Perovskite Quantum Dots for Efficient Light-Emitting Devices" Nanomaterials 12, no. 22: 3957. https://doi.org/10.3390/nano12223957





